Abstract
Background
Tumor necrosis factor alpha (TNF-α) inhibitors are increasingly being used for treating refractory cardiac sarcoidosis. There is a theoretical risk, however, that these therapies can worsen heart failure, and reports on efficacy and safety are lacking.
Methods
We conducted a retrospective review of all cardiac sarcoidosis patients seen at Stanford University from 2009 to 2018. Data were collected on patient demographics, diagnostic testing, and treatment outcomes.
Results
We identified 77 cardiac sarcoidosis patients, of which 20 (26%) received TNF-α inhibitor treatment. The majority were treated for progressive heart failure or tachyarrhythmia, along with worsening imaging findings. All TNF-α inhibitor treated patients demonstrated meaningful benefit, as assessed by changes in advanced imaging, echocardiographic measures of cardiac function, and prednisone use.
Conclusions
A large cohort (n = 77) of cardiac sarcoidosis patients has been treated at Stanford University. Roughly one-fourth of these patients (n = 20) received TNF-α inhibitors. Of these patients, none had worsening heart failure and all saw clinical benefit. These results help support the use of TNF-α inhibitors for the treatment of cardiac sarcoidosis based on real-world evidence and highlight the need for future prospective studies.
Keywords: Sarcoidosis, Cardiac sarcoidosis, TNF-alpha, TNF-alpha antagonist, TNF-alpha inhibitor, Heart failure
1. INTRODUCTION
Sarcoidosis is a systemic inflammatory disease characterized by the presence of noncaseating granulomas. Granulomatous inflammation leads to tissue damage and fibrosis that can be life threatening. In particular, sarcoidosis can affect the myocardium, resulting in electrical conduction abnormalities, heart failure, and sudden death.
There are no FDA-approved treatments for cardiac sarcoidosis, but the standard of care includes glucocorticoids and steroid-sparing agents [1]. When the combination of a glucocorticoid and steroid-sparing agent is ineffective at controlling disease activity, biologic therapies such as TNF-α inhibitors are used. No formal clinical trials or large observational studies have been conducted in cardiac sarcoidosis to examine TNF-α inhibitor treatment outcomes, however these therapies do show benefit in patients with extracardiac sarcoidosis [2–12]. Despite their effectiveness, there is a concern that TNF-α inhibitors may potentially worsen heart failure in cardiac sarcoidosis patients.
Prior studies have demonstrated that non-sarcoidosis related heart failure patients (primarily with ischemic heart disease) have elevated serum levels of TNF-α and increased numbers of TNF-α producing cells in the myocardium [13, 14]. Based on these findings, TNF-α was thought to be harmful or pathogenic in patients with heart failure, and several clinical trials were conducted to study the efficacy of TNF-α inhibitors in this population [15–17]. The results of these trials, however, were conflicting. In several of these studies, TNF-α inhibitor treatment resulted in significantly worse outcomes, including a higher incidence of hospitalization for worsening heart failure and death. In contrast, the RECOVER study, in which patients were treated with etanercept 25mg weekly (instead of twice weekly like RENAISSANCE or RENEWAL), did not show an increased risk of heart failure and death [16]. On balance, however, these studies led the American Heart Association (AHA) to list TNF-α inhibitors as “Prescription Medications That May Cause or Exacerbate Heart Failure,” and to recommend avoiding use in patients with moderate to severe heart failure [18].
Despite the recommendations of the AHA, in patients with severe, active cardiac sarcoidosis who have not responded to glucocorticoids and steroid-sparing agents, TNF-α inhibitors are used. Although there are individual case reports of successful treatment of cardiac sarcoidosis with TNF-α inhibitors, no large cohort studies have been conducted to assess the safety and efficacy of this strategy [19, 20]. The aim of this retrospective review was to characterize the cardiac sarcoidosis patients at Stanford University and to evaluate the response to TNF-α inhibitor treatment.
2. METHODS
2.1. Study Design
This is a retrospective, single-center study conducted at Stanford University. A cohort of sarcoidosis patients was created using the STAnford medicine Research data Repository (STARR). This database draws from Epic “Clarity” data, as well as imaging, pathology, and genome sequencing data. Sarcoidosis patients were identified by searching all patients seen at Stanford University between 2009 and 2018 (allowing for complete electronic medical record data), ages 18 years and older, with an ICD-9 or ICD-10 code for sarcoidosis and at least one clinical note mentioning “sarcoidosis.” Each chart was then manually reviewed to verify the sarcoidosis diagnosis and to determine which patients had cardiac involvement. This study was approved by the Stanford University Institutional Review Board.
2.2. Patients
The diagnosis of non-cardiac sarcoidosis was determined based on the presence of noncaseating granulomas on pathology and notes documenting a clinical diagnosis of sarcoidosis and excluding alternative diagnoses. Cardiac sarcoidosis was determined using modified Heart Rhythm Society guidelines [21]. Patients were classified as having Definite Cardiac Sarcoidosis if they had a histological diagnosis from cardiac tissue (endomyocardial biopsy, tissue from left ventricular assist device placement, or post-transplant). Patients were classified as having Probable Cardiac Sarcoidosis if they had a histological diagnosis of extra-cardiac sarcoidosis AND characteristic 18-F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) or gadolinium-enhanced cardiac magnetic resonance imaging (CMR) of the heart AND other potential causes had been excluded. Patients were classified as having Possible Cardiac Sarcoidosis if they lacked histopathologic documentation but had characteristic 18F-FDG PET or CMR findings AND unexplained reduced LVEF (<40%) OR unexplained sustained ventricular tachycardia OR Mobitz type II 2nd degree heart block or 3rd degree heart block OR immunosuppressant responsive cardiomyopathy or heart block AND other potential causes had been excluded. Only patients with data from two or more time points were included in the analysis.
2.3. Studied Parameters
Data were collected on subject age at diagnosis, sex, race, ethnicity, date of diagnosis, duration of follow-up, initial cardiac manifestation at the time of presentation, cardiac biopsy history, extracardiac biopsy history, imaging (18F-FDG PET and CMR), echocardiography, treatment history, lowest prednisone dose within 6 months before and 6 months after TNF-α inhibitor treatment, lowest left ventricular ejection fraction within 12 months before and 12 months after TNF-α inhibitor treatment, smoking status (ever smoker at time of cardiac sarcoidosis diagnosis), body mass index (BMI) at time of cardiac sarcoidosis diagnosis, and serum angiotensin converting enzyme (ACE) level (ever elevated). For 18F-FDG PET, a scan was determined to be positive if focal or multifocal FDG uptake was present and the radiologist listed sarcoidosis in the differential diagnosis. For CMR, a scan was determined to be positive if subepicardial late gadolinium enhancement was present and the radiologist listed sarcoidosis in the differential diagnosis.
2.4. Statistical Analysis
Continuous variables were summarized as mean and standard deviation. Categorical variables were summarized as frequencies and percentage of the total. Group comparisons between patients with known extracardiac sarcoidosis and those with isolated cardiac sarcoidosis were made using the Student t-test. Group comparisons between patients treated with TNF-α inhibitors and patients not treated with TNF-α inhibitors were assessed with the Student t-test and Fisher exact test as appropriate.
3. RESULTS
3.1. Patient Selection
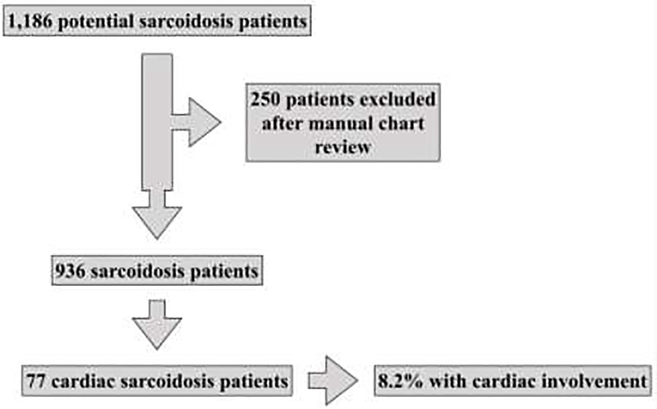
A total of 1,186 potential sarcoidosis patients were identified with the initial search (Figure 1). Of these, 250 patients (21%) were excluded, as they did not meet the criteria for the diagnosis of sarcoidosis. This left a total of 936 unique sarcoidosis patients (79%) seen at Stanford University over the 10-year time period. Of these, manual chart review identified 77 sarcoidosis patients with cardiac sarcoidosis (8.2% of all sarcoidosis patients).
Figure 1:
Sarcoidosis cohort patient selection
3.2. Baseline Characteristics
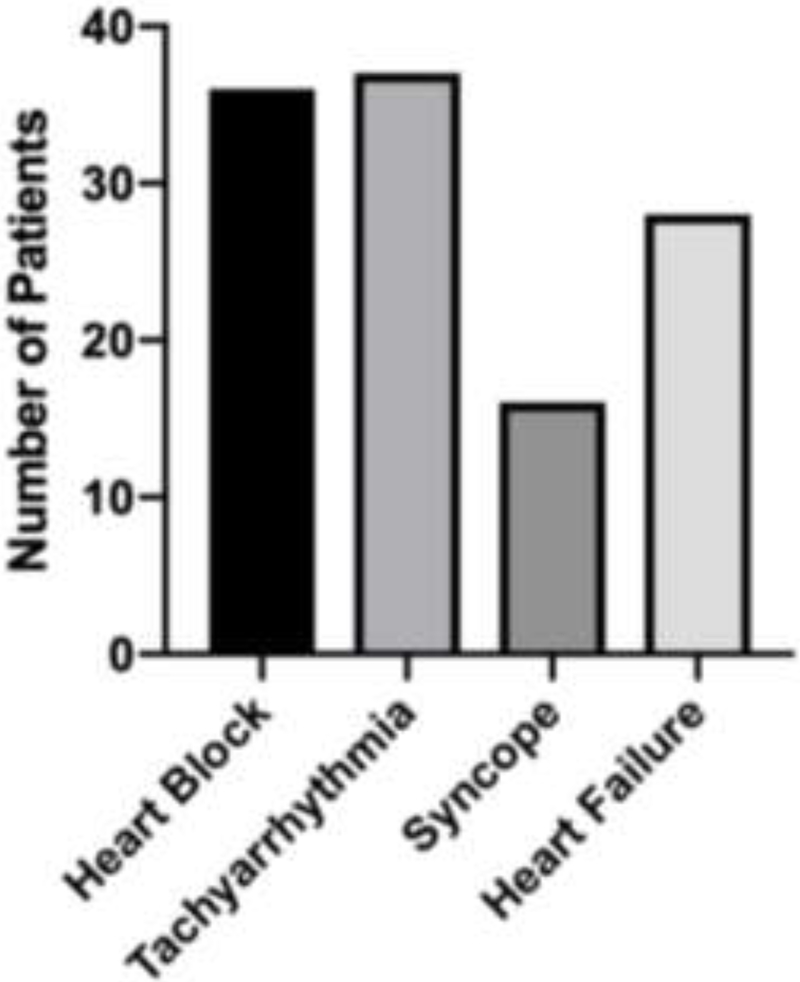
Baseline demographics and characteristics of the cardiac sarcoidosis cohort (n = 77) are presented in Table 1. The mean age at diagnosis of cardiac sarcoidosis was 55 years (median age 58 years) and 39% of patients were female. The cohort consists of 66% Caucasians (non-Hispanic), 16% African Americans (non-Hispanic), 9% Asians, and 9% Hispanics. Patients were considered ever smokers if they had greater than a 1-pack year history of smoking or more at the time of cardiac sarcoidosis diagnosis, which included 21% of the cohort. The mean BMI at time of cardiac sarcoidosis diagnosis was 28, with 38% of the cohort being obese and 39% overweight. The most common clinical presentations were heart block and tachyarrhythmia, which included both ventricular tachycardia and atrial fibrillation (Table 1 and Figure 2). A total of 11 patients (14%) had Definite Cardiac Sarcoidosis, 31 patients (40%) had Probable Cardiac Sarcoidosis, and 35 (46%) had Possible Cardiac Sarcoidosis. Of the 11 Definite Cardiac Sarcoidosis patients, 5 had no evidence of extracardiac disease, and of the 35 Possible Cardiac Sarcoidosis Patients, 15 had imaging findings of hilar and/or mediastinal lymphadenopathy whereas 20 had no evidence of extracardiac disease. Thus, a total of 25 patients (32%) had truly isolated cardiac sarcoidosis (ICS). The mean follow-up time for the 77 cardiac sarcoidosis patients in this cohort was 4.8 years, and the mean time to diagnosis of cardiac sarcoidosis from initial symptoms was 3.3 years. This was reduced to 2.7 years when patients were already known to have extracardiac sarcoidosis and increased to 4.6 years for those patients without a prior diagnosis of extracardiac sarcoidosis (P = 0.09, 2-sample t-test).
Table 1:
Baseline characteristics of 77 cardiac sarcoidosis patients seen at Stanford University between 2009 and 2018
| Clinical Characteristic | Full Cohort (n =77) | Definite CS (n = 11) | Probable CS (n = 31) | Possible CS (n = 35) |
|---|---|---|---|---|
| Age at diagnosis (mean), years | 55 | 49 | 58 | 54 |
| Women | 30 (39) | 5 (45) | 14 (45) | 11 (31) |
| Race | ||||
| White | 51 (66) | 8 (73) | 18 (58) | 25 (71) |
| Black | 12 (16) | 2 (18) | 6 (19) | 4 (11) |
| Asian | 7 (9) | 0 | 4 (13) | 3 (9) |
| Hispanic | 7 (9) | 1 (9) | 3 (10) | 3 (9) |
| Smoking (ever) | 16 (21) | 2 (18) | 6 (19) | 8 (23) |
| Body mass index at diagnosis (mean) | 28 | 27 | 28 | 29 |
| Normal weight (< 25) | 18 (23) | 6 (55) | 5 (16) | 6 (17) |
| Overweight (≥ 25 and < 30) | 30 (39) | 2 (18) | 16 (52) | 13 (37) |
| Obese (≥ 30) | 29 (38) | 3 (27) | 10 (32) | 16 (46) |
| Initial clinical presentation | ||||
| Heart block | 36 (47) | 6 (55) | 16 (52) | 14 (40) |
| 1st degree | 10 | 1 | 6 | 3 |
| 2nd degree | 3 | 1 | 1 | 1 |
| 3rd degree | 23 | 4 | 9 | 10 |
| Tachyarrhythmia | 37 (48) | 6 (55) | 14 (45) | 17 (49) |
| Palpitations | 22 (29) | 3 (27) | 10 (32) | 9 (26) |
| Heart failure | 28 (36) | 5 (45) | 8 (26) | 15 (43) |
| Syncope | 16 (21) | 4 (36) | 4 (13) | 8 (23) |
| Follow-up (mean), years | 4.8 | 9.1 | 5.5 | 2.8 |
| Time to diagnosis from first symptom (mean), years | 3.3 | 2.9 | 2.7 | 3.9 |
Data are number (%) except for age, body mass index, follow-up, and time to diagnosis, which are mean (SD). CS = cardiac sarcoidosis.
Figure 2:
Presenting signs and symptoms of 77 cardiac sarcoidosis patients
3.3. Diagnostic Characteristics
A total of 34 patients (44%) underwent extracardiac biopsies, of which 33 (97%) were positive for sarcoidosis. Cardiac biopsy was performed in 19 patients (25%), of which 5 (26%) were positive. Additionally, three patients had the apex of their heart surgically removed for left ventricular assist device placement, and the pathology from that tissue was positive in 2 out of 3 cases. Eleven patients underwent heart transplant, and in 6 of those cases the diagnosis of cardiac sarcoidosis was made upon examination of the explanted heart tissue. In total, 7 of the 11 explanted hearts showed active sarcoidosis, and the remaining 4 demonstrated extensive fibrosis consistent with end-stage sarcoidosis. With regard to the 72 patients who had imaging, all but one patient received an 18F-FDG PET, either alone or in combination with a CMR. In 5 cases, an initial CMR failed to show any evidence of cardiac sarcoidosis, but a subsequent 18F-FDG PET (within 6 months) confirmed the diagnosis. There were no examples of a 18F-FDG PET being negative, followed by a positive CMR. Serial echocardiograms were performed on all patients. The mean initial left ventricular ejection fraction (LVEF) at the time of cardiac sarcoidosis diagnosis was 43%. Serum ACE was checked in 42 patients (55%), and it was positive (ever elevated) in 24% of those patients. Immunoglobulins were also checked in the minority of patients (25%) and were positive (ever elevated) in 11% of those 19 patients in which they were checked.
3.4. Treatment Characteristics
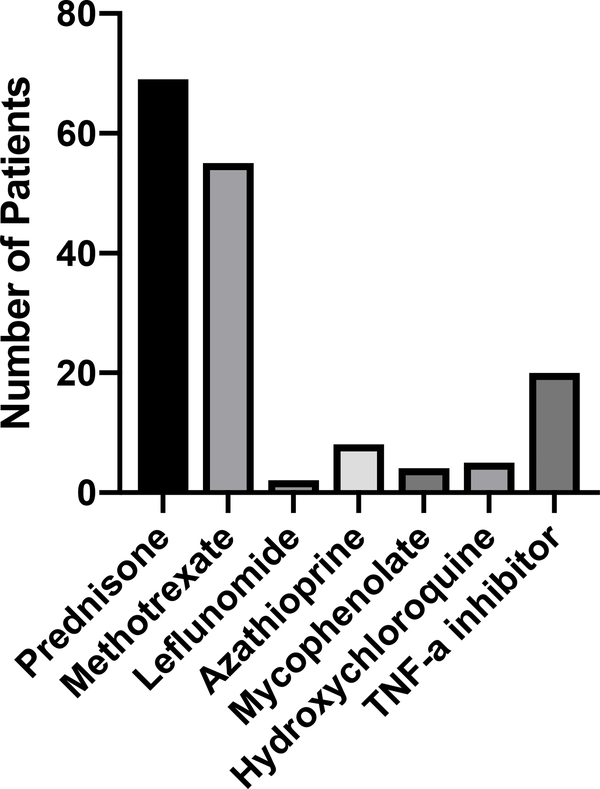
The majority of patients (90%) received prednisone at some point in the course of their treatment for cardiac sarcoidosis (Table 3 and Figure 3). Those who did not receive prednisone were either diagnosed with cardiac sarcoidosis after heart transplant (n = 6) or were started on methotrexate alone by an outside provider with resolution in FDG-PET before steroids could be started (n = 2). In addition, 74% of patients received a steroid-sparing agent, with the most common being methotrexate (71%). Twenty patients (26%) received a TNF-α inhibitor, with 10 patients receiving infliximab, 10 patients receiving adalimumab (one of whom had previously tried infliximab), and 1 patient receiving golimumab. No patients in our cohort were treated with any other class of biologic therapy.
Table 3:
Treatment characteristics of 77 cardiac sarcoidosis patients
| Treatment | Full Cohort (n =77) | Definite CS (n =ll) | Probable CS (n =31) | Possible CS (n = 35) |
|---|---|---|---|---|
| Glucocorticoids | ||||
| Prednisone | 69 (90) | 5 | 31 | 33 |
| Steroid-sparing agents | 57 (74) | 3 | 27 | 27 |
| Methotrexate | 55 (71) | 3 | 27 | 25 |
| Leflunomide | 2 (3) | 0 | 2 | 0 |
| Azathioprine | 8 (10) | 0 | 6 | 2 |
| Mycophenolate mofetil | 4 (5) | 0 | 3 | 1 |
| Hydroxycloroquine | 5 (7) | 0 | 5 | 0 |
| TNF-α inhibitors | 20 (26) | 3 | 10 | 7 |
| Infliximab | 10 (13) | 1 | 5 | 4 |
| Adalimumab | 10 (13) | 2 | 6 | 2 |
| Golimumab | 1 (1) | 0 | 0 | 1 |
Data are number (%). CS = cardiac sarcoidosis; TNF-α = tumor necrosis factor alpha.
Figure 3:
Treatments ever received by 77 cardiac sarcoidosis patients at Stanford University
3.5. TNF-α Inhibitors
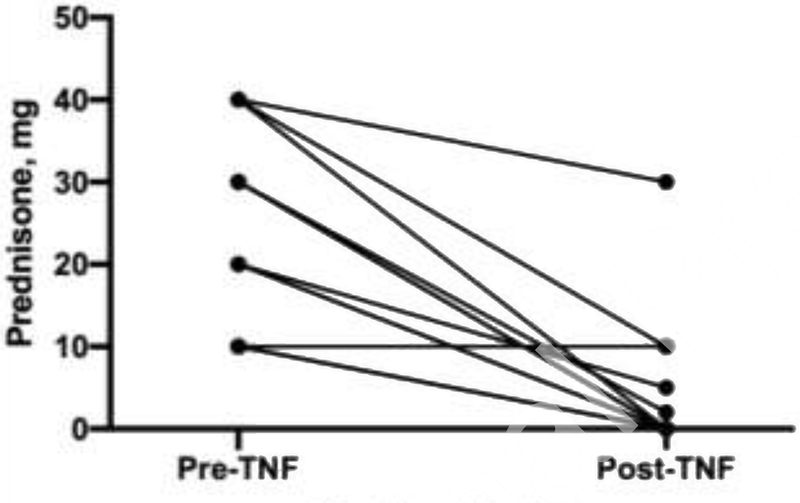
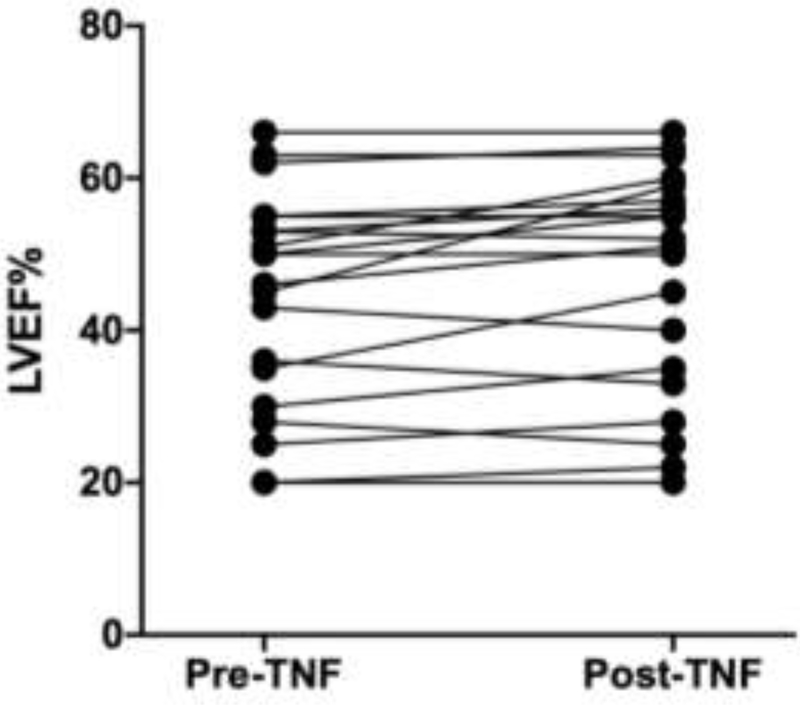
Twenty unique patients were started on TNF-α inhibitors in the cohort. Patients with Definite, Probable, or Possible disease were equally likely to receive TNF-α inhibitor therapy and had similar clinical responses (Table 3 and Table 5). Comparing patients who received TNF-α inhibitors to those who did not (Table 4), the only notable difference was that patients who presented with heart failure were less likely to be treated with a TNF-α inhibitor (however mean initial LVEF and mean worst ever LVEF were similar between the two groups). The majority (85%) of patients who received TNF-α inhibitors were started on this therapy for worsening imaging findings, and many had worsening of their heart failure or arrhythmia burden as well (Table 5). After starting a TNF-α inhibitor, all 17 patients with worsening imaging findings had resolution of disease activity on imaging within 12 months. The mean time from diagnosis of cardiac sarcoidosis to TNF-α inhibitor initiation in those patients who received a TNF-α inhibitor was 16 months. All 20 patients were on methotrexate at the time of TNF-α inhibitor initiation, and a total of 13 patients were also on prednisone at the time of initiating a TNF-α inhibitor (all had been treated with prednisone previously). The mean lowest dose of prednisone achieved in the 6 months before starting a TNF-α inhibitor was 23 milligrams (mg) daily (Figure 4). For these same 13 patients, the mean prednisone dose after treatment (the lowest dose achieved in the 6 months after starting a TNF-α inhibitor) was 4 mg daily. The mean time to completely stop prednisone after starting a TNF-α inhibitor was 9 months. Likewise, the mean LVEF before TNF-α inhibitor treatment (the worst LVEF in the 12 months before starting a TNF-α inhibitor) for all 20 patients who received TNF-α inhibitors was 44%, and after treatment (the worst LVEF in the 12 months after starting a TNF-α inhibitor) was 47%. This included 5 patients with an LVEF ≤ 30% at the time of TNF-α inhibitor initiation, and in 4 of these cases the LVEF improved and in 1 case the LVEF went from 28% to 25% (Figure 5). There were no serious infections, as defined by the need for intravenous antibiotics, antivirals, or antifungals or the need for hospitalization for an infection, seen in cardiac sarcoidosis patients receiving TNF-α inhibitors. There were 7 individuals (9%) treated with non-TNF-α inhibitor immunosuppression who did suffer from a serious infection. Overall, there were 10 deaths in this cohort, which did not include any patients who received a TNF-α inhibitor. Unfortunately, the causes of death were not recorded in the electronic medical record. Although there were no major differences between patients treated with INF-α inhibitors and those who were not (Table 4), it is possible that these two groups differed in ways that were not captured by the variables assessed.
Table 5:
Treatment outcomes for 20 cardiac sarcoidosis patients treated with a TNF-α inhibitor
| TNF- αInhibitor Indication/Outcome | Full Cohort (n= 20) | Definite CS (n=3) | Probable CS (n=10) | Possible CS (n=7) |
|---|---|---|---|---|
| Reason for initiating TNF-α inhibitor | ||||
| Worsening heart failure | 3 | 1 | 2 | 0 |
| Worsening arrhythmia | 9 | 2 | 3 | 4 |
| Worsening disease activity on FDG-PET | 17 | 2 | 9 | 6 |
| Improvement in FDG-PET after TNF- α inhibitor initiation | 17 | 2 | 9 | 6 |
| Time from diagnosis to TNF- α inhibitor initiation (mean), months | 16 | 21 | 15 | 15 |
| Prednisone dose 6 months before TNF- α inhibitor initiation (mean), mg | 23 | 20 | 27 | 22 |
| Prednisone dose 6 months after TNF- α inhibitor initiation (mean), mg | 4 | 0 | 4 | 6 |
| Time to stop prednisone after TNF- α inhibitor initiation (mean), months | 9 | 8 | 11 | 8 |
| LVEF within 12 months before TNF- α inhibitor initiation (mean), % | 41 | 39 | 47 | 44 |
| LVEF within 12 months after TNF- α inhibitor initiation (mean), % | 44 | 41 | 49 | 46 |
Data are number (%). CS = cardiac sarcoidosis; TNF-α = tumor necrosis factor alpha; LVEF = left ventricular ejection fraction.
Table 4:
Comparing baseline characteristics of cardiac sarcoidosis patients who did and did not receive TNF-α inhibitor treatment
| Clinical Characteristic | Non-TNF-α Inhibitor Treated (n = 57) | TNF- α Inhibitor Treated (n = 20) | P value |
|---|---|---|---|
| Age at diagnosis m years, mean (SD) | 54 (13) | 55 (7) | 0.741 |
| Women | 23 (40) | 7 (35) | 0.792 |
| Race | |||
| White | 35 (61) | 16 (80) | 0.173 |
| Black | 9 (16) | 3 (15) | 0.999 |
| Asian | 6 (11) | 1 (5) | 0.669 |
| Hispanic | 7 (12) | 0 (0) | 0.180 |
| Diagnostic category | |||
| Definite Cardiac Sarcoidosis | 8 (14) | 3 (15) | 0.999 |
| Probable Cardiac Sarcoidosis | 21 (37) | 10 (50) | 0.427 |
| Possible Cardiac Sarcoidosis | 28 (49) | 7 (35) | 0.308 |
| Smoking (ever) | 15 (26) | 1 (5) | 0.056 |
| Body mass index at diagnosis, mean (SD) | 28 (5) | 29 (5) | 0426 |
| Normal weight (< 25) | 15(26) | 3 (15) | 0.372 |
| Overweight (≥ 25 and < 30) | 22 (39) | 8 (40) | 0.999 |
| Obese (≥ 30) | 20 (35) | 9 (45) | 0.437 |
| Initial clinical presentation | |||
| Heart block | 36 (63) | 13 (65) | 0.999 |
| 1st degree | 7 (12) | 3 (15) | 0.714 |
| 2nd degree | 0 (0) | 3 (15) | 0.016 |
| 3rd degree | 16 (28) | 7 (35) | 0.580 |
| Tachyarrhythmia | 26 (46) | 11 (55) | 0.604 |
| Palpitations | 16 (28) | 6 (30) | 0.999 |
| heart failure | 25 (44) | 3 (15) | 0.030 |
| Syncope | 10 (18) | 6 (30) | 0.336 |
| Echocardiography | |||
| Initial LVEF at time of diagnosis, mean (SD) | 42 (15) | 48 (16) | 0.146 |
| Worst LVEF at any rime mean (SD) | 34 (14) | 39 (16) | 0.139 |
Data are number (%) except for age, BMI, and LVEF, which are mean (SD). Statistical analysis with Student t-test and Fisher exact test as appropriate. TNF-α = tumor necrosis factor alpha; LVEF = left ventricular ejection fraction.
Figure 4:
Change in prednisone dose after TNF-α inhibitor treatment in 13 cardiac sarcoidosis patients
Figure 5:
Change in left ventricular ejection fraction after TNF-α inhibitor treatment in 20 cardiac sarcoidosis patients
4. DISCUSSION
This retrospective review of cardiac sarcoidosis patients at Stanford University demonstrates clinical benefit from TNF-α inhibitor treatment without clear safety issues. The majority of patients in our cohort were treated with prednisone and methotrexate, which is in line with treatment recommendations by expert consensus [1, 22]. Twenty patients (26%) received a TNF-α inhibitor, with an even distribution of infliximab and adalimumab use, and one patient who received golimumab. Of the patients treated with a TNF-α inhibitor, most were started due to worsening imaging findings (n = 17), and in all cases the repeat imaging showed resolution of disease activity. Perhaps most importantly, despite concerns that TNF-α inhibitors can worsen heart failure, there were no patients with a notable decline in left ventricular ejection fraction (LVEF) within one year after treatment initiation, and most patients saw an improvement. This included five patients who had a LVEF ≤ 30% at the time of starting a TNF-α inhibitor. In addition, those patients who were on steroids at the time of TNF-α inhibitor initiation were all able to reduce their dose, from a mean of 23mg/day to 4mg/day within six months of starting the TNF-α inhibitor. Although the comparison of TNF-α inhibitor treated patients to non-TNF-α inhibitor treated patients did not reveal any major differences, it is possible that these two groups differed in ways that were not measured. For example, the patients who were not treated with TNF-α inhibitors may have been sicker at baseline with more comorbidities, and thus deemed too high risk to treat with a biologic therapy. This potential confounding by indication may explain why all serious infections and deaths were seen in the non-TNF-α inhibitor treated group.
We found that roughly 8% of sarcoidosis patients at Stanford University have clinical evidence of cardiac involvement, which is in line with prior reports [23, 24]. Our cohort of 77 cardiac sarcoidosis patients is one of the largest single-center cohorts described [25–28]. The cohort has a Caucasian majority but remains racially diverse, with a slight male predominance. On average, patients were followed for 4.8 years, allowing for longitudinal assessment and treatment outcomes. Isolated cardiac sarcoidosis (ICS) was seen in 25 subjects (32%), which is consistent with previous reports [26, 29]. ICS increased the time to diagnosis from 2.7 years in patients with known extracardiac disease to 4.6 years (P = 0.09).
This study confirms what has previously been reported about the yield of endomyocardial biopsy [30]. Of the 19 patients who underwent a cardiac biopsy, only 26% were positive. Similarly, serum ACE levels were only found to be ever elevated in 24% of patients in whom it was tested. Of the 10 patients with elevated ACE, only one patient had isolated cardiac sarcoidosis. This supports the notion that ACE levels may be even less sensitive for detecting sarcoidosis when disease is confined to the heart [26]. In our cohort, imaging was critical for making the diagnosis of cardiac sarcoidosis, and in 5 cases, CMR was negative while a subsequent 18F-FDG PET confirmed the diagnosis. Although the sensitivity and specificity of 18F-FDG PET and CMR are considered roughly equivalent, this remains an area of debate and active investigation [31].
There are several limitations to this study, including the retrospective design, the single-center experience that limits generalizability, and the relatively small number of patients studied. In addition, the definition we used for the diagnosis of cardiac sarcoidosis is not the formal 2014 expert consensus criteria proposed by the Heart Rhythm Society [21]. These criteria require either positive pathology from myocardial tissue with noncaseating granulomas or positive pathology from extracardiac tissue and at least one secondary factor: either steroid responsive cardiomyopathy or heart bock, unexplained LVEF < 40%, unexplained sustained ventricular tachycardia, Mobitz type II second- or third-degree heart block, patchy uptake on dedicated cardiac 18F-FDG PET, late gadolinium enhancement on CMR, or a positive gallium uptake, as well as the exclusion of other potential causes for the cardiac manifestations. These criteria, however, make it almost impossible to include isolated cardiac sarcoidosis, as the practice of obtaining endomyocardial biopsies is low, and the yield when done even lower. For this reason, we decided to include Possible Cardiac Sarcoidosis patients who had imaging findings on 18F-FDG PET or CMR that were highly suggestive of cardiac sarcoidosis along with one of the secondary factors listed above and the exclusion of other causes, but without the need for histopathology. This provides strength, in that we were able to include patients with isolated cardiac sarcoidosis who are most often excluded from such studies, but it is also a limitation, as it hinders our ability to compare some results with other published work.
5. CONCLUSIONS
Cardiac sarcoidosis is a life-threatening disease that requires significant immunosuppressive therapy to control. In some cases (26% of our cohort), conventional treatments such as glucocorticoids and steroid-sparing agents are ineffective or contraindicated. In such cases, TNF-α inhibitors are used, and a number of studies in extracardiac sarcoidosis demonstrate positive results. In cardiac sarcoidosis, however, the published experience with TNF-α inhibitors is limited to a few case reports. In addition, there is a hesitancy to use TNF-α inhibitors in patients with heart failure, due to the theoretical risk of worsening their structural heart disease. This study demonstrates that in a cohort of 77 cardiac sarcoidosis patients seen at Stanford University, the outcomes of the 20 patients who received TNF-α inhibitors were positive. In virtually every case, post-treatment imaging improved, glucocorticoids were reduced, and LVEF remained stable or improved. Importantly, in the five patients who started TNF-α inhibitors with LVEFs ≤ 30, they did not have worsening of their heart failure within 12 months after starting the TNF-α inhibitor. This supports the notion that patients with cardiac sarcoidosis who continue to have active disease despite conventional therapies could be treated with TNF-α inhibitors, and it provides a strong rationale to conduct larger, prospective studies examining this issue in the future.
Table 2:
Diagnostic characteristics of 77 cardiac sarcoidosis patients
| Diagnostic Test | Full Cohort | Delinite CS | Probable CS | Possible CS |
|---|---|---|---|---|
| (n = 77) | (n = 11) | (n =31) | (n = 35) | |
| Extracardiac pathology | ||||
| Extracardiac biopsy | 34 (44) | 2 | 31 | 1 |
| Positive cxtracardiac biopsy | 33 (97) | 2 | 31 | 0 |
| Cardiac pathology | ||||
| Cardiac biopsy | 19 (25) | 6 | 6 | 7 |
| Positive cardiac biopsy | 5 (26) | 5 | 0 | 0 |
| LVAD-related tissue | 3 (4) | 2 | 0 | 1 |
| Positive LVAD-rclated tissue | 2 (67) | 2 | 0 | 0 |
| Heart transplant | 11 (14) | 7 | 2 | 2 |
| Positive explanted cardiac tissue | 7 (64) | 7 | 0 | 0 |
| Diagnosis made after transplant | 6 (8) | 6 | 0 | 0 |
| Cardiac imaging | 72 (94) | 6 | 31 | 35 |
| FDG-PET only | 35 | 3 | 16 | 16 |
| CMR only | 1 | 1 | 0 | 0 |
| FDG-PET and CMR | 36 | 2 | 15 | 19 |
| FDG-PET negative. CMR positive | 0 | 0 | 0 | 0 |
| CMR negative, FDG-PET positive | 5 | 0 | 1 | 4 |
| Echocardiography | 77 (100) | 11 | 31 | 35 |
| Initial LVEF at time of diagnosis (mean), % | 43 | 38 | 48 | 43 |
| Worse LVEF at any time (mean), % | 35 | 22 | 38 | 37 |
| Most current LVEF (mean), % | 48 | 58 | 49 | 46 |
| Angiotensin converting enzyme checked (ever) | 42 (55) | 5 | 22 | 15 |
| Elevated ACE (ever) | 10 (24) | 1 | 6 | 3 |
| Immunoglobulin levels checked (ever) | 19 (25) | 3 | 7 | 9 |
| Elevated immunoglobulins (ever) | 2 (11) | 0 | 0 | 2 |
Data are number (%) except for LVEF, angiotensin converting enzyme, and immunoglobulins, which are mean (SD). CS = cardiac sarcoidosis; LVEF = left ventricular ejection fraction.
Acknowledgments
We thank John and Jacque Jarve for their generous support of this work.
Funding Sources: Matthew Baker received support for this work from the KL2 component of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083).
Footnotes
Conflicts of Interest: None
Contributor Information
Matthew C. Baker, Department of Medicine, Division of Immunology and Rheumatology, Stanford University
Khushboo Sheth, Department of Medicine, Division of Immunology and Rheumatology, Stanford University
Ronald Witteles, Division of Cardiology, Stanford University
Mark C. Genovese, Department of Medicine, Division of Immunology and Rheumatology, Stanford University
Stanford Shoor, Department of Medicine, Division of Immunology and Rheumatology, Stanford University
Julia F. Simard, Department of Medicine, Division of Immunology and Rheumatology, Stanford University Department of Health Research and Policy, Division of Epidemiology, Stanford University.
REFERENCES
- 1.Young L, Sperry BW, and Hachamovitch R, Update on Treatment in Cardiac Sarcoidosis. Curr Treat Options Cardiovasc Med, 2017. 19(6): p. 47. [DOI] [PubMed] [Google Scholar]
- 2.Judson MA, et al. , Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J, 2008. 31(6): p. 1189–96. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, et al. , Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med, 2006. 174(7): p. 795–802. [DOI] [PubMed] [Google Scholar]
- 4.Baughman RP, et al. , Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest, 2005. 128(2): p. 1062–47. [DOI] [PubMed] [Google Scholar]
- 5.Heidelberger V, et al. , Efficacy and Tolerance of Anti-Tumor Necrosis Factor alpha Agents in Cutaneous Sarcoidosis: A French Study of 46 Cases. JAMA Dermatol, 2017. 153(7): p. 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pariser RJ, et al. , A double-blind, randomized, placebo-controlled trial of adalimumab in the treatment of cutaneous sarcoidosis. J Am Acad Dermatol, 2013. 68(5): p. 765–73. [DOI] [PubMed] [Google Scholar]
- 7.Gelfand JM, et al. , Infliximab for the treatment of CNS sarcoidosis: A multi-institutional series. Neurology, 2017. 89(20): p. 2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamilloux Y, et al. , Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: A multicenter study of 132 patients. Semin Arthritis Rheum, 2017. 47(2): p. 288–294. [DOI] [PubMed] [Google Scholar]
- 9.Russell E, et al. , Long term follow-up of infliximab efficacy in pulmonary and extra-pulmonary sarcoidosis refractory to conventional therapy. Semin Arthritis Rheum, 2013. 43(1): p. 119–24. [DOI] [PubMed] [Google Scholar]
- 10.Schimmelpennink MC, et al. , Efficacy and safety of infliximab biosimilar Inflectra((R)) in severe sarcoidosis. Respir Med, 2018. 138S: p. S7–S13. [DOI] [PubMed] [Google Scholar]
- 11.Adler BL, et al. , Anti-tumor necrosis factor agents in sarcoidosis: A systematic review of efficacy and safety. Semin Arthritis Rheum, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Maneiro JR, et al. , Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review. Semin Arthritis Rheum, 2012. 42(1): p. 89–103. [DOI] [PubMed] [Google Scholar]
- 13.Levine B, et al. , Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med, 1990. 323(4): p. 236–41. [DOI] [PubMed] [Google Scholar]
- 14.Lisman KA, et al. , The role of tumor necrosis factor alpha blockade in the treatment of congestive heart failure. Congest Heart Fail, 2002. 8(5): p. 275–9. [DOI] [PubMed] [Google Scholar]
- 15.Chung ES, et al. , Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation, 2003. 107(25): p. 3133–40. [DOI] [PubMed] [Google Scholar]
- 16.Mann DL, et al. , Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation, 2004. 109(13): p. 1594–602. [DOI] [PubMed] [Google Scholar]
- 17.Bozkurt B, et al. , Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation, 2001. 103(8): p. 1044–7. [DOI] [PubMed] [Google Scholar]
- 18.Page RL 2nd, et al. , Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation, 2016. 134(6): p. e32–69. [DOI] [PubMed] [Google Scholar]
- 19.Barnabe C, et al. , Successful treatment of cardiac sarcoidosis with infliximab. J Rheumatol, 2008. 35(8): p. 1686–7. [PubMed] [Google Scholar]
- 20.Uthman I, Touma Z, and Khoury M, Cardiac sarcoidosis responding to monotherapy with infliximab. Clin Rheumatol, 2007. 26(11): p. 2001–3. [DOI] [PubMed] [Google Scholar]
- 21.Birnie DH, et al. , HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm, 2014. 11(7): p. 1305–23. [DOI] [PubMed] [Google Scholar]
- 22.Birnie DH, et al. , Cardiac Sarcoidosis. J Am Coll Cardiol, 2016. 68(4): p. 411–21. [DOI] [PubMed] [Google Scholar]
- 23.Perry A and Vuitch F, Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med, 1995. 119(2): p. 167–72. [PubMed] [Google Scholar]
- 24.Iwai K, et al. , Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn, 1993. 43(7–8): p. 372–6. [DOI] [PubMed] [Google Scholar]
- 25.Fussner LA, et al. , Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail, 2018. 20(12): p. 1713–1720. [DOI] [PubMed] [Google Scholar]
- 26.Kandolin R, et al. , Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation, 2015. 131(7): p. 624–32. [DOI] [PubMed] [Google Scholar]
- 27.Chapelon-Abric C, et al. , Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore), 2004. 83(6): p. 315–34. [DOI] [PubMed] [Google Scholar]
- 28.Silverman KJ, Hutchins GM, and Bulkley BH, Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation, 1978. 58(6): p. 1204–11. [DOI] [PubMed] [Google Scholar]
- 29.Tavora F, et al. , Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol, 2009. 104(4): p. 571–7. [DOI] [PubMed] [Google Scholar]
- 30.Bennett MK, et al. , Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circ Heart Fail, 2013. 6(4): p. 676–84. [DOI] [PubMed] [Google Scholar]
- 31.Bravo PE, et al. , Advanced cardiovascular imaging for the evaluation of cardiac sarcoidosis. J Nucl Cardiol, 2019. 26(1): p. 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]