Abstract
Palatable taste can stimulate appetite in the absence of hunger, and individual differences in hedonic eating may be critical to overeating. Women are more prone to obesity and binge eating than men, which warrants comparisons of hedonic versus physiological consumption and the underlying neural substrates in both sexes. The current study examined palatable (high-sugar) food consumption in male and female rats under physiological hunger and satiety, and the role of the neuropeptide orexin/hypocretin (ORX). Across multiple tests, females consistently consumed similar amounts of palatable food regardless of whether they were sated or hungry prior to testing. In contrast, males typically adjusted their consumption according to their hunger/satiety state. This difference was specific to palatable food consumption, as both sexes ate standard chow according to their hunger state. ORX is important in food motivation and reward behaviors. Thus, to begin to determine the neuronal mechanisms of hedonic eating, we examined activation and signaling of ORX neurons. We systematically characterized Fos induction patterns of ORX neurons across the entire rostrocaudal extent of the lateral hypothalamus and found that they were activated by food and by fasting in both sexes. Then, we showed that systemic blockade of ORX receptor 1 signaling with SB-334867 decreased palatable food consumption in hungry and sated rats of both sexes. These results demonstrate sex differences in hedonic eating; increased susceptibility in females to overeat palatable food regardless of hunger state, and that ORX is a critical neuropeptide mechanism of hedonic eating in both sexes.
Keywords: hedonic, consumption, orexin, overeating, palatability, sex differences
Introduction
Hunger and palatable taste of food both drive appetite and consumption and could work in accord or independently. Palatability can stimulate appetite through reward mechanisms regardless of physiological hunger, and this form of overeating contributes to obesity and binge eating disorder (Cota et al., 2006, Berridge et al., 2010, Stice et al., 2013). Many modern environments are saturated with easily accessible high-calorie, palatable foods that are innately liked across mammalian species (Berridge, 2000). Cues for these foods can further amplify the drive to eat in the absence of hunger (Weingarten, 1983, Boggiano et al., 2009, Berthoud, 2012, Petrovich, 2013, Kendig et al., 2018).
How individuals respond to sweet taste and other appetite triggers when sated may be an important difference between those who are vulnerable and those who are resistant to overeating (Small, 2009, Reppucci and Petrovich, 2012, Sun et al., 2015). Women are more susceptible than men to weight gain and obesity, as well as binge eating and other disorders (Hudson et al., 2007, Mitchell and Shaw, 2015). These differences warrant comparisons of the neural substrates mediating hedonic versus physiological consumption in both sexes. Here, we compared palatable food consumption in male and female rats and examined the activation and signaling of the neuropeptide orexin/hypocretin (ORX) (de Lecea et al., 1998, Sakurai et al., 1998). ORX is important in the motivation to eat without physiological incentive (Choi et al., 2010, Mahler et al., 2014, Petrovich, 2019), but whether ORX neurons are similarly driving hedonic eating under sated and hungry states in males and females has not been examined.
In order to characterize behavioral and neural substrates in both sexes, in two studies we compared consumption of palatable, sweet-tasting food (high-sugar Test Diet pellets) under the physiological conditions of hunger and satiety. In the first study, we examined male and female rats that were either deprived of food for 20 hours or had ad libitum access to standard rat chow prior to consumption tests. Then we characterized Fos induction in ORX neurons during these tests in rats that had access to high-sugar pellets, along with controls without access to food. We systematically characterized patterns of Fos induction in ORX neurons across the entire rostrocaudal extent of the lateral hypothalamic area (LHA).
In the second study, we tested whether Fos induction patterns in ORX neurons observed in the first experiment indicate that ORX signaling is causal to hedonic eating. We systemically blocked ORX signaling via receptor 1 with SB-334867 in male and female rats that were either fasted or not fasted prior to tests with high-sugar pellets. Additionally, to determine if observed sex differences were due to palatability, rather than differences in hunger/satiety, we tested consumption of another, less palatable, familiar food (standard chow) in rats that were hungry or sated. First, we tested rats in two separate tests: one with high-sugar pellets and the other with standard chow. Then, after completion of ORX signaling blockade experiments we re-tested rats with both foods in a within-subjects design across two tests, in order to examine how individual rats that are hungry or sated consume palatable food versus chow.
Experimental Procedures
Subjects
Ninety-six, experimentally naive, male and female Sprague Dawley rats (48 male, 48 female) that were obtained from Envigo at 250–275g were used in these experiments. There were two sets of experiments, with n=64 in the first (Palatable Food Consumption and Fos Induction in Orexin Neurons) and n=32 in the second (Orexin Receptor 1 Signaling Blockade). Rats were individually housed and maintained on a 12h light/dark cycle (lights on 06:00). Behavioral testing was conducted during the light phase between 09:00 and 16:00. After arrival, rats were given 24h to acclimate to the colony room with ad libitum access to water and standard laboratory chow (Purina Lab Diet Prolab RMH 3000; 3.47 kcal/g: 26% protein, 15% fat, 59% carbohydrates (89% starch)) before any handling or habituation procedures began. All housing and testing procedures were in compliance with the National Institute of Health’s Guidelines for Care and Use of Laboratory Animals and were approved by the Boston College Institution Animal Care and Use Committee.
Palatable Food
The palatable food used here was Test Diet (TD) pellets (5TUL 45mg Scott Pharma: cat#1811155; 3.44 kcal/g; 20% protein, 13% fat, 67% carbohydrates—all from sucrose). Previously, we compared rats’ preference for TD pellets to other high-sugar/high-fat (Oreos, Nabisco), high-sugar/low-fat (Lucky Charms, General Mills), and low-sugar/ high-fat (Cheetos, Frito Lay) foods in adult, sated male rats (Reppucci, 2010). Rats’ preferences were evaluated based on their consumption during 30min tests (5g of single food given, test order counterbalanced). Rats preferred all high-sugar foods to the low-sugar/high-fat food and the highest preference score was for TD and the high-sugar/ high-fat food (identical mean values), which was higher than the score for high-sugar /low-fat food (but not statistically significant).
Habituation
After the 24h acclimation period, all subjects were handled daily for 5–6 days and were habituated to all experimental conditions prior to testing. All subjects received 1g of TD pellets at the bottom of their home cages. On a separate occasion, all subjects received empty ceramic testing dishes for a 30min exposure. Next, all subjects received 10g TD in ceramic dishes and were allowed to consume it for 30min (no chow was given) after ad libitum access to chow, and after 20h food deprivation (the order of these two habituation sessions was counterbalanced). Subjects were also habituated to being transported on a cart to and from the procedure room on two separate occasions.
Palatable Food Consumption and Fos Induction in Orexin Neurons
Design
Following a full factorial experimental design, male and female rats were assigned to either ad libitum or 20h food deprivation eating schedules (n=16 per sex). Within each group, half of the rats (Food condition) received 15g TD in ceramic testing dishes during testing, and half of the rats (NoFood condition) received empty dishes during testing (n=8 per group). Testing occurred in home cages and consisted of a 100min exposure to the dish with food (Food condition) or empty dish (NoFood condition) during which rats were left undisturbed. Rats were perfused immediately after the 100min testing session to measure test-specific Fos induction. All remaining food was collected and weighed to determine consumption, including any food that was moved from the dishes by rats during the testing sessions. Cages were thoroughly checked by hand for any pellets that may have been buried in the bedding, to ensure correct consumption calculations.
Histological Procedures
100min after the start of the testing session, rats were briefly anaesthetized with isoflurane (Henry Schein, Pittsburgh, PA), and then intraperitoneally injected with tribromoethanol (375mg/kg; Sigma Aldrich, St.Louis, MO) and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1M borate buffer. The brains were stored for 20–24h at 4°C in a paraformaldehyde and 12% sucrose mixture and then rapidly frozen in hexanes cooled with dry ice and stored at −80°C. Brains were cut into 30μm coronal sections using a microtome and were collected into four adjacent series. One tissue series was processed with ORX immunohistochemistry followed by Fos immunohistochemistry, described below. Another series was mounted and stained with thionin for identification of cytoarchitectonic borders, and the remaining two were stored in cryoprotectant in a −20°C freezer.
ORX and Fos Double-label Immunohistochemistry
Free-floating sections were incubated for 1h at room temperature in a blocking solution (0.02M potassium phosphate-buffered saline [KPBS] containing 2% normal horse serum [NHS], 0.3% Triton X-100 and 10% milk), and then incubated with mouse monoclonal antibody against ORX-A (1:500, MAB763; R&D Biosystems, Minneapolis, MN) in the blocking solution for 72h at 4°C with gentle agitation. Sections were rinsed with KPBS, 2% NHS and 10% milk, incubated with biotinylated secondary antibody against mouse (1:500, BA-2001; Vector Laboratories) in the blocking solution, rinsed in KPBS, incubated in avidin biotin complex (ABC, PK-6100; Vector Laboratories), rinsed in KPBS and recycled through the secondary antibody and ABC solutions with KPBS rinses in between. Finally, the tissue was processed with 3,3’-diaminobenzidine (SK-4100; Vector Laboratories) to visualize ORX immunoreactivity. Brain sections were then immediately processed again in a similar manner but using anti-c-Fos antibody raised in rabbit (ABE457; Millipore, 1:5,000 lot:3059066) and biotinylated secondary against rabbit (1:500, BA-1000; Vector Laboratories). Nuclei for Fos were visualized as gray after a two-minute incubation in nickel-intensified 3,3’- diaminobenzidine (SK-4100; Vector Laboratories). Sections were rinsed, mounted on SuperFrost slides (Fisher Scientific), dried at 45°C, dehydrated through graded alcohols, cleared in xylenes, and coverslipped with DPX Mountant (Electron Microscopy Services; Hatfield, PA).
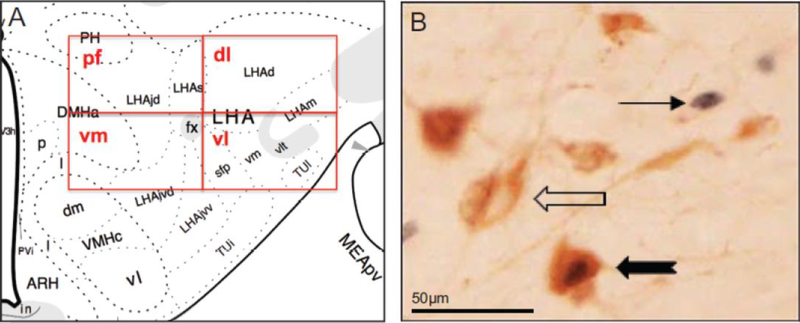
Image Acquisition and Analysis
Processed tissue was imaged using the 20x objective on an Olympus BX51 light microscope with attached Olympus DP74 camera, using DP2-BSW software (Olympus America Inc., Center Valley, PA, USA). Images of the tissue were taken bilaterally throughout the rostrocaudal extent of the lateral hypothalamus containing ORX neurons (Swanson, Sanchez-Watts & Watts 2005); atlas levels 27–31 (Swanson, 2004). At each atlas level, four images were taken on each side (total 8 images per level), one for each of 4 adjacent quadrants surrounding the fornix (with the fornix placed in the dorsolateral corner of the ventromedial image) as shown in Fig. 1A. The perifornical area (pf) was defined by the quadrant located dorsomedial to the fornix, and the remaining quadrants were placed to capture areas located clockwise dorsolateral (dl), ventrolateral (vl), and ventromedial (vm) to the fornix. Three types of neurons were manually identified and counted in the acquired images: single-labeled ORX-positive, single-labeled Fos-positive, and double-labeled ORX and Fos-positive neurons (Fig. 1B). ORX-positive neurons were characterized by distinct cytoplasmic staining (brown) and Fos protein-positive neurons were characterized by distinct nuclear staining (gray). Double-labeled neurons (ORX + Fos) had both cytoplasmic (ORX) and nuclear (Fos) labeling (Fig. 1B). An ORX-containing neuron was counted as positive only if both the cell body and the nucleus were clearly visible in the image. Fos-labeled neurons were counted as positive if the Fos-labeling intensity was clearly above background and contained within the nucleus. Two experimenters conducted cell identification and count analysis. They were blind to the experimental condition of the tissue images observed and images were counter-balanced across experimental conditions and sex. Total counts from images of the left and right hemispheres were pooled to calculate the total number of Fos-positive neurons, the total number of ORX-positive neurons, and the total number of double-labeled (ORX + Fos) neurons within each sampling region (pf, dl, vl, vm) for each brain. To perform a more accurate analysis of the degree of ORX neuron recruitment the percentage of the total number of ORX-specific neurons that were double-labeled with Fos were then calculated for each sampling region. Due to tissue damage or poor tissue quality, six brains were excluded from all neural analyses (one from each of these groups: Female Deprived NoFood, Male Deprived NoFood, Female Ad Lib Food, Male Ad Lib Food, Female Deprived Food, Female Ad Lib NoFood).
Figure 1.
Image acquisition and analysis A. Representation of the sampling regions is shown on a modified rat brain atlas template (level 29, Swanson, 2004). Images were taken in four sampling regions arranged in respect to the fornix (fx): perifornical area (pf), located dorsomedial to the fornix, dorsolateral (dl), ventromedial (vm), and ventrolateral (vl). B. Image shows representative types of labeled neurons. Arrows point to a representative of each type of labeled neuron: single-labeled Fos (gray), single-labeled ORX (brown), and double-labeled ORX and Fos. The scale bar (B) = 50 pm
Orexin Receptor 1 Signaling Blockade
Drugs
SB-334867 (SB; Tocris Bioscience; Minneapolis, MN, USA) was suspended in a solution consisting of 2% dimethylsulfoxide and 10% 2-hydroxypropyl-6-cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA) in sterile water. Vehicle was 2% dimethylsulfoxide and 10% 2-hydroxypropyl-6-cyclodextrin (Sigma-Aldrich, St. Louis, MO, USA) in sterile water. Both SB and vehicle were administered via intraperitoneal injection at a volume of 4 ml/kg. SB dose was 20 mg/kg and was injected at a concentration of 20mg/4ml. Injection was given 30 min prior to Test 3 and Test 4 (see below).
Design
Rats were first tested for consumption of palatable food (TD) under sated and food deprived states to replicate behavioral findings from the first study (Test 1). Then they were tested with standard chow under the same conditions of hunger and satiety to establish whether differences between males and females are due to food palatability (sweet taste) or differences in physiological hunger state (Test 2). The TD and chow have similar caloric densities (3.44 and 3.47 kcal/g, respectively), however, all carbohydrates in TD are from sucrose and 89% of carbohydrates in chow are from starch. Accordingly, TD tastes much sweeter than chow. Next, rats were tested for palatable food consumption (TD) after receiving SB or Vehicle across two counterbalanced tests (Tests 3&4). Because the order of Tests 1 and 2 was not counterbalanced, rats were re-tested for chow and TD consumption at the end of experiments (Tests 5&6).
Half of the male and female rats were food deprived for 20h (Deprived condition) while the other half had ad libitum access to rat chow (Ad Lib condition) (n=8). All testing sessions consisted of a 60min exposure to either TD or standard rat chow in ceramic dishes as indicated, after which food was weighed to determine consumption. Experimenters were careful to include food that remained in the dish, as well as any food that was displaced to other areas of the cage during testing in these calculations. Cages were thoroughly checked by hand after each test for any pellets that may have fallen out of the dish or been buried in the bedding by rats, to ensure correct consumption calculations. Rats were provided with sufficiently large quantities of food to ensure unrestricted eating (15–20g, consistent within each test). Only one type of food was given during the tests. Thirty minutes prior to tests 3 and 4, rats received either an SB or Vehicle intraperitoneal injection in a within-subjects design (SB and Veh order was counterbalanced across the two tests). For tests 5 and 6 rats were also tested in a within-subjects design with TD and chow (order was counterbalanced across tests). All testing sessions were conducted 2–4 days apart.
Statistical Analyses
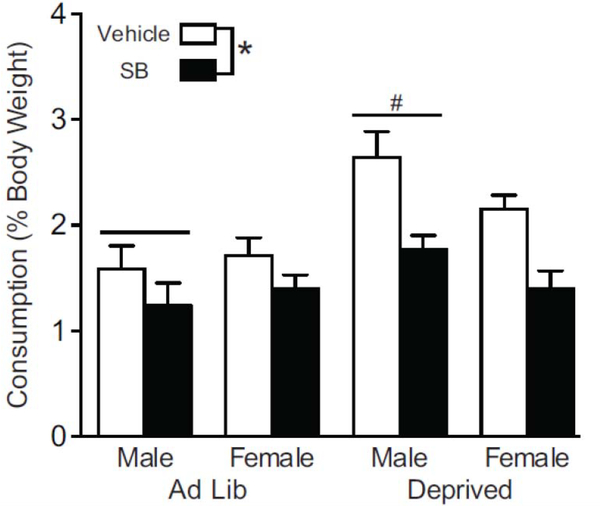
Data were analyzed using analysis of variances (ANOVAs) and t-Tests. Main effects were followed by post hoc Tukey HSD analyses. Statistical analyses were performed using SPSS. Data are presented as mean ± SEM. In all cases, p < 0.05 was considered significant. The data were tested for normal distributions and equal variances. If these criteria were not met, log and square root transformations were used to verify the results. In one analysis, after square root transformation the p value was 0.051 and that is reported in the Results. In the Orexin Receptor 1 Signaling Blockade study (Tests 3 and 4), the data were not normally distributed due to an outlier. The outlier was identified by a box plot (Male Deprived) and was removed from the data (Figure 6) and the statistical values are reported in the Results for both the analysis with and without the outlier.
Figure 6.
Consumption tests after ORX-R1 antagonist blockade. Graph shows consumption after rats received SB or Veh in a within-subjects design across two counterbalanced tests (Test 3 & 4). * indicates a difference (p< 0.005) between Vehicle and SB (n=15–16 per condition, n=7–8 per sex), # indicates a difference between Ad Lib and Deprived males (p = 0.002 n=7–8 per group).
Results
Palatable Food Consumption and Fos Induction in Orexin Neurons
Consumption
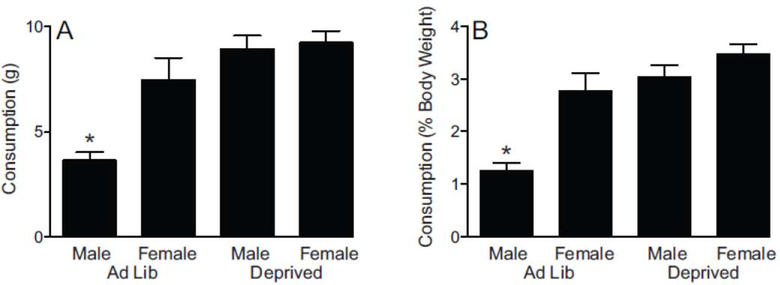
Males and females exhibited different patterns of TD consumption during the testing session (Figure 2A). Males ate more when they were in a deprivation state than ad lib, while females ate similar, large amounts regardless of their physiological state. ANOVA of consumption with sex and deprivation state (Ad Lib, Deprived) confirmed significant between-subjects main effects of sex (F1,28 ( = 8.496, p = 0.007), deprivation state (F1,28 = 25.254, p < 0.0001), and interaction of sex with deprivation state (F1,28 = 6.245, p = 0.019). Post hoc tests showed that Ad Lib males ate significantly less than any of the other groups (p < 0.005 in all cases). Ad Lib females ate similar amounts as deprived females and deprived males (p = 0.301 and p = 0.455, respectively).
Figure 2.
Consumption during testing session A. Consumption of TD shown in grams (mean±SEM) B. Consumption of TD expressed as a percentage of rat’s body weight (mean±SEM). * indicates Male Ad Lib different (p < 0.005) from each other group (n=8 per group).
The consumption patterns were similar when calculated relative to body weight (2b). ANOVA of consumption as a percentage of body weight with sex and deprivation state (Ad Lib, Deprived) found significant main effects of sex (F1,28 = 16.619, p < 0.0005), deprivation state (F1,28 = 27.260, p < 0.0001), and interaction of sex with deprivation state (F1,28 = 5.156, p = 0.031). Post hoc tests confirmed that Ad Lib males ate significantly less TD than any of the other groups (p < 0.005 in all cases), while Ad Lib females ate similar amounts as deprived females and deprived males (p = 0.182 and p = 0.850, respectively).
The average body weights for each group of rats prior to testing are shown in Table 1. All subjects arrived at the same weight range; however males gained weight much more rapidly and thus had higher body weights than females prior to testing (F1,55 = 74.635, p < 0.0001). There were no body weight differences based on deprivation state or food access conditions within each sex (p > 0.05 for all main effects and interactions).
Table 1.
Body weights (mean ± SEM) prior to testing
| Ad Lib | Deprived | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| No Food | 285 ± 4 | 262 ± 5 | 289 ± 2 | 261 ± 4 |
| Food | 290 ± 2 | 265 ± 7 | 294 ± 3 | 266 ± 4 |
Total ORX neurons
Table 2 displays the total number of ORX neurons (mean ± SEM) in each sampling region for sex and condition. No main effects or interactions were observed in the total number of ORX neurons in the LHA. Similarly, there were no effects or interactions when each sampling region was analyzed separately. The pf region had the highest number of ORX neurons compared to all sampling quadrants. ANOVA of total ORX neurons with sex, deprivation state (Ad Lib, Deprived), food access (Food, No Food), and region (pf, dl, vl, vm) found a main effect of region (F330 = 209.309, p < 0.0001). Follow up tests found that pf and dl differed from all other regions (p < 0.0001 in all cases), while vm and vl were similar (p = 0.401).
Table 2.
Total numbers of ORX neurons observed in the LHA. Displayed are values for total number of ORX neurons counted within each sampling quadrant for each group (mean ± SEM)
| Ad Lib | Deprived | |||||||
|---|---|---|---|---|---|---|---|---|
| LHA Region | Male No Food | Male Food | Female No Food | Female Food | Male No Food | Male Food | Female No Food | Female Food |
| All | 117 ± 11 | 126 ± 14 | 136 ± 11 | 124 ± 11 | 123 ± 17 | 150 ± 12 | 137 ± 14 | 148 ± 23 |
| pf | 61 ± 8 | 63 ± 9 | 72 ± 7 | 65 ± 5 | 69 ± 11 | 76 ± 7 | 80 ± 9 | 79 ± 11 |
| dl | 30 ± 3 | 33 ± 4 | 34 ± 3 | 32 ± 5 | 27 ± 4 | 37 ± 3 | 28 ± 3 | 39 ± 8 |
| vm | 11 ± 2 | 13 ± 2 | 11 ± 1 | 11 ± 2 | 11 ± 2 | 20 ± 2 | 12 ± 2 | 13 ± 3 |
| vl | 14 ± 1 | 16 ± 2 | 19 ± 2 | 16 ± 3 | 15 ± 3 | 21 ± 3 | 17 ± 3 | 17 ± 3 |
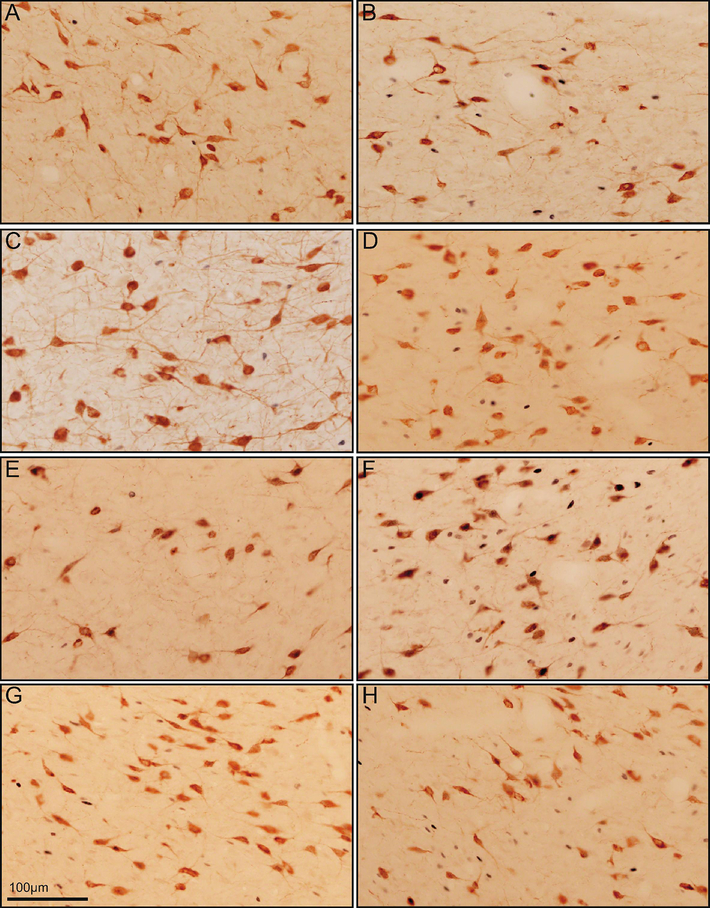
Fos induction in ORX neurons
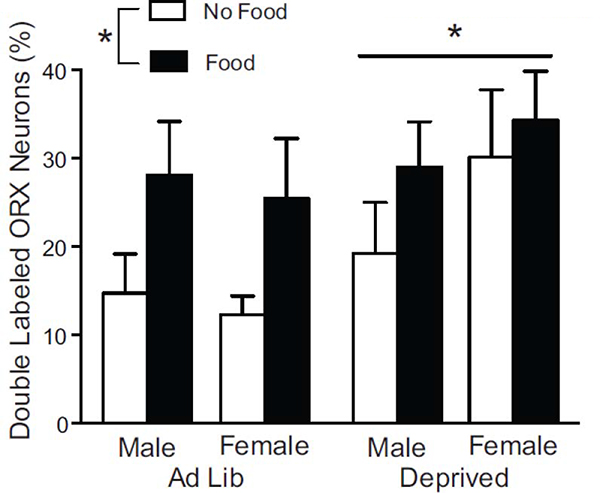
Images in Fig. 3 show Fos induction in ORX neurons in each group. The graphs in Fig. 4 show that Fos induction in ORX neurons was the lowest in Ad Lib groups without food access, and that food consumption and deprivation state induced Fos in both sexes. ANOVA of the percentage of ORX neurons that were double-labeled with Fos with sex, food access (Food, No Food), and deprivation condition (Ad Lib, Deprived) found significant main effects of food access (more Fos in the Food compared to No Food groups; F1,50 = 6.491, p = 0.014; after square root transformation F1,50 = 7.160, p = 0.010) and deprivation state, (more Fos in the Deprived compared to Ad Lib groups; F1,50 = 4.116, p = 0.048; after square root transformation F1,50 = 4.002, p = 0.051), but no significant effect of sex (F1,50 = 0.497, p = 0.484; after square root transformation F1,50 = 0.519, p = 0.475) or interactions.
Figure 3.
Images of Fos induction in ORX neurons. Images show induction of Fos (gray) in ORX neurons (brown) in ad libitum-fed male groups that were either tested without food (A) or given food (B), fasted males groups that were either tested without food (C) or given food (D), ad libitum-fed females groups that were either tested without food (E) and with food (F), and fasted female groups that were either tested without food (G) and with food (H). All images are from perifornical area. The scale bar (G) = 100 μm
Figure 4.
Fos induction in ORX neurons. Graphs show the percentage of total ORX neurons that were labeled with Fos in male and female Ad Lib and Deprived groups that either received food or no food during testing (mean ± SEM). * indicates a difference (p < 0.05) between Ad Lib and Deprived and between Food and No Food conditions (N=7–8/group; N=29 for each Ad Lib, Deprived, Food, and NoFood conditions).
Table 3 displays the percent of ORX neurons that were labeled with Fos (mean ± SEM) in each sampling region for each sex and condition. Analyses of each quadrant separately, found that pf exhibited main effects of both deprivation state and food access (more Fos in the Food compared to No Food group: F1,50 = 5.043, p = 0.029 and more Fos in the Deprived compared to Ad Lib groups: F1,50 = 6.113, p = 0.017) but no effects of sex or interactions. There were main effects of food access only for dl and vl regions (more Fos in the Food compared to No Food groups in dl: F1,50 = 12.579, p = 0.001 and vl: F1,50 = 4.617, p = 0.037) and no effects for the vm region (p > 0.05 for all).
Table 3.
Fos induction in ORX neurons. The table shows the percentage of ORX neurons that were labeled with Fos (mean ± SEM) across LHA (all) and in each sampling region for each sex and condition. See text for statistical differences
| Ad Lib | Deprived | |||||||
|---|---|---|---|---|---|---|---|---|
| LHA Region | Male No Food | Male Food | Female No Food | Female Food | Male No Food | Male Food | Female No Food | Female Food |
| All | 14.7 ± 4.4 | 28.1 ± 6.1 | 12.3 ± 2.1 | 25.4 ± 6.8 | 19.2 ± 5.8 | 29.0 ± 5.1 | 30.2 ± 7.6 | 34.3 ± 5.6 |
| pf | 17.3 ± 4.8 | 33.6 ± 7.1 | 14.1 ± 3.0 | 31.6 ± 7.7 | 25.6 ± 7.1 | 36.1 ± 5.9 | 37.5 ± 9.1 | 39.1 ± 6.4 |
| dl | 9.7 ± 3.3 | 20.9 ± 6.1 | 8.5 ± 2.9 | 17.9 ± 6.0 | 5.1 ± 2.4 | 18.6 ± 3.1 | 14.1 ± 5.3 | 24.9 ± 5.5 |
| vm | 24.3 ± 10.1 | 37.2 ± 8.3 | 19.9 ± 5.5 | 24.9 ± 8.1 | 23.5 ± 9.0 | 36.6 ± 7.8 | 50.5 ± 9.8 | 39.5 ± 8.3 |
| vl | 8.0 ± 3.6 | 14.6 ± 2.4 | 5.0 ± 1.9 | 8.8 ± 4.1 | 8.5 ± 3.8 | 12.3 ± 4.2 | 12.0 ± 5.6 | 23.4 ± 6.3 |
Total Fos induction
We analyzed the total number of Fos-positive neurons in the LHA (sum across four quadrants). ANOVA of total number of Fos neurons in the LHA found the main effects of food access (more Fos in the Food compared to No Food group; F1,50 = 10.544, p = 0.002) and deprivation (more Fos in the Deprived compared to Ad Lib groups; F1,50 = 7.252, p = 0.010), but no effect of sex (F1,50 = 0.111, p = 0.741) or interactions.
Table 4 displays the values of total number of Fos-positive neurons (mean ± SEM) across LHA and in each sampling region for each sex and condition. Analyses of each quadrant separately, found that within pf, vm and vl regions there were main effects of deprivation state (more Fos in the Deprived compared to Ad Lib groups; F1,50 = 4.982, p = 0.030 for pf, F1,50 = 15.115, p < 0.0005 for vm, and F(1,50) = 4.751, p = 0.034 for vl) and food access (more Fos in the Food compared to No Food group; F1,50 = 9.030, p = 0.004 for pf, F1,50 = 19.219, p < 0.0001 for vm, and F1,50 = 4.537, p = 0.038 for vl). There was only a main effect of food access within dl region (more Fos in the Food compared to No Food group; F1,50 = 4.112, p = 0.048). There were no main effects of sex or interactions within any region.
Table 4.
Total number of Fos-positive neurons in the LHA. The table shows total numbers of Fos-positive neurons (mean ± SEM) across LHA (all) and in each sampling region for each sex and condition
| Region | Male No Food | Male Food | Female No Food | Female Food | Male No Food | Male Food | Female No Food | Female Food |
|---|---|---|---|---|---|---|---|---|
| All | 1286 ± 182 | 1572 ± 194 | 1133 ± 185 | 1777 ± 236 | 1472 ± 245 | 2185 ± 187 | 1718 ± 193 | 2070 ± 194 |
| pf | 555 ± 71 | 635 ± 86 | 490 ± 94 | 712 ± 94 | 569 ± 97 | 811 ± 68 | 688 ± 73 | 851 ± 84 |
| dl | 251 ± 32 | 275 ± 45 | 189 ± 30 | 298 ± 50 | 259 ± 57 | 342 ± 35 | 281 ± 29 | 293 ± 37 |
| vm | 296 ± 48 | 377 ± 45 | 234 ± 35 | 435 ± 55 | 366 ± 57 | 605 ± 67 | 409 ± 49 | 542 ± 55 |
| vl | 247 ± 45 | 284 ± 34 | 208 ± 37 | 332 ± 58 | 278 ± 53 | 357 ± 41 | 341 ± 59 | 383 ± 43 |
Orexin Receptor 1 Signaling Blockade
Consumption
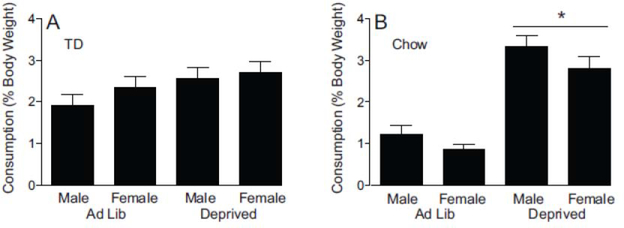
In two separate tests, we measured the consumption of TD and chow (Figure 5). In the test with TD (Fig. 5A) all rats ate similar high amounts regardless of sex or deprivation. This observation was supported by ANOVA which found no main effects of sex (F1,28 = 1.136, p = 0.296), deprivation state F1,28 = 3.866, p = 0.059), or sex by deprivation state interaction (F1,28 = 0.297, p = 0.590).
Figure 5.
Consumption tests with TD (Test 1) and chow (Test 2) A. Consumption of TD expressed as a percentage of rat’s body weight (mean ± SEM). B. Consumption of regular chow expressed as a percentage of rat’s body weight (mean ± SEM). indicates a difference (p < 0.0001) between Deprived and Ad lib (n=16 per condition; n=8 per sex).
In the test with chow (Fig. 5B), both male and female rats in the deprived condition ate more than Ad Lib. There was a main effect of deprivation state (F1,28 = 86.839, p < 0.0001) and no effect of sex (F1,28 = 4.053, p = 0.054) or sex by deprivation state interaction (F1,28 = 0.162, p = 0.690).
ORX-R1 Antagonist
In Tests 3 & 4, rats were tested for palatable food intake after receiving SB or Vehicle (order counterbalanced). Rats in all groups consumed less TD during the test following an SB injection compared to their consumption during the test following a Veh injection (Fig. 6). In addition, males in deprived state consumed more than males tested Ad Lib, while females consumed similar amounts. A mixed design repeated measures ANOVA (repeated: drug treatment (SB, Veh) and between: sex and deprivation groups) of consumption across tests 3 & 4 found a main effect of treatment (F1,28 = 19.992, p < 0.0005) but no effect of sex or interactions. In the same analysis, there was a between-subject effect of deprivation state (F1,28 = 14.793, p = 0.001), and a sex by deprivation state interaction (F1,28 = 5.937, p = 0.021). To follow up on the sex by deprivation interaction effect, a repeated measures ANOVA was performed for each sex separately. Consistent with the findings of repeated ANOVA with both sexes, there was an effect of treatment in males (F1,13 = 8.544, p = 0.012; with an outlier: F1,14 = 7.879, p = 0.014) and females (F1,14 = 14.339, p = 0.002) but no interaction effects. There was also a between subject effect of deprivation state for males (F1,13 = 14.248, p = 0.002; with an outlier: F1,14 = 13.401, p = 0.003), but not females (F1,14 = 1.884, p = 0.191).
Consumption of TD and chow
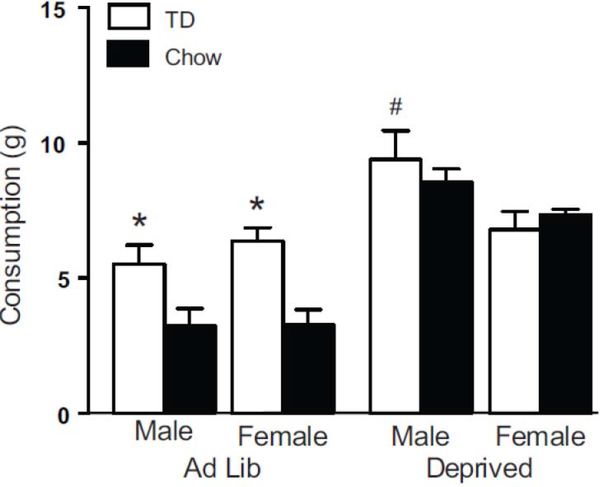
As shown in Figure 7, male and female rats in the Ad Lib condition consumed much less chow than rats in the Deprived condition but all rats consumed similar, large amounts of TD. A mixed design repeated measures ANOVA (repeated: food type (TD, Chow) and between: sex and deprivation groups) found within-subject main effects of food type (F1,28 = 8.266, p = 0.008) and deprivation state by food type interaction (F1,28 = 6.865, p = 0.014), as well as between-subjects effects of deprivation (F1,28 = 64.443, p < 0.0001) and sex by deprivation interaction (F1,28 = 7.503, p = 0.011). To follow up on the sex by deprivation interaction effect, a repeated measures ANOVA was performed for each sex separately. In females, there was both an effect of food type (F1,14 = 7.533, p = 0.016) and a food type by deprivation interaction (F1,14 = 15.958, p = 0.001), as well as a between-subjects effect of deprivation (F1,14 = 15.417, p = 0.002). In males, there were no effects of food type (F1,14 = 3.231, p = 0.094) or food type by deprivation interaction (F1,14 = 0.713, p = 0.413), but there was a between-subjects effect of deprivation (F1,14 = 53.033, p < 0.0001).
Figure 7.
Consumption tests with TD and chow. Graph shows consumption across two tests (Test 5 & 6) with TD and regular rat chow in a within-subjects design (the order of tests was counterbalanced). * indicates a difference between TD and Chow for Ad Lib males (p = 0.038; n=8) and females (p = 0.001 n=8), # indicates a difference in TD consumption between Ad Lib and Deprived males (p = 0.001 n=8 per group).
A paired samples t-test confirmed that both male and female groups in the ad lib condition consumed more TD than chow (t7 = 2.554, p = 0.038; t7 = 5.146, p = 0.001, respectively), while groups in the deprived condition consumed TD and chow similarly (females t7 = −0.827, p= 0.436; males t7 = 0.557, p= 0.595).
A follow up ANOVA of TD consumption only found a main effect of deprivation state (F1,28 = 7.801, p = 0.009) and a sex by deprivation state interaction (F1,28 = 5.022, p = 0.033). Pairwise comparisons indicated that the male group in the deprived condition consumed more TD than the male group in ad lib condition (F1,28 = 12.671, p = 0.001), while females in the deprived and ad lib conditions ate similar amounts (F1,28 = 0.125, p = 0.699).
Discussion
The current study examined hedonic eating and the role of ORX in male and female rats. We compared consumption of the same palatable food under different physiological conditions of hunger and satiety in both sexes. In each of four tests in two studies with different animals, females consistently consumed similar amounts of high-sugar (TD) pellets regardless of whether they were fasted or not prior to testing. In contrast, males typically adjusted consumption according to their physiological hunger; rats that were not fasted ate less than those that were fasted prior to testing, except during one test in which both groups ate similar amounts. Both males and females ate standard chow according to their satiety/hunger state, indicating that the observed sex differences were specific to hedonic eating.
In order to replicate behavioral sex differences observed in the first experiment, a new group of rats underwent testing under the same conditions in the second experiment. Consistent with the original observation, female consumption was driven by palatability, and was not adjusted according to their satiety state. Both hungry and sated females ate similar amounts of TD pellets. Males in sated condition in this experiment consumed similar amounts as hungry males, which was also similar to both female groups. Thus males behaved differently across the first and second experiments, as their consumption followed physiological state in the first and palatability in the second. In contrast, females consistently ate according to food palatability in both experiments and during additional tests, as discussed below.
In order to determine whether the observed sex differences were due to palatability or differences in hunger/satiety, we measured consumption of standard rat chow under the same conditions as during the TD tests. Both male and female rats adjusted their consumption of standard chow according to their hunger/satiety state. Rats of both sexes that were fasted prior to testing ate much larger amounts of chow than rats that had ad libitum access to food prior to testing. Thus, we found sex differences in the consumption of TD pellets but not in that of standard chow. However, these tests were not counterbalanced in order, and the test with standard chow occurred after TD testing. Thus, to confirm these findings, we re-tested these rats at the end of second study (after SB treatment, discussed below), with TD and standard chow across two counterbalanced tests, in a within-subjects design. Sated rats of both sexes ate more TD than chow, while fasted rats ate similar amounts of TD and chow. Furthermore, consistent with the patterns observed in the first experiment, females in fasted and sated groups ate similar amounts of TD, while males in sated condition ate less TD than males in the fasted condition.
The findings in the current study are in agreement with prior evidence, in Sprague Dawley rats, that females may have enhanced drive for palatable food compared to males (Freund et al., 2015, Reichelt et al., 2016). In a binge-eating model, Sisk and colleagues have demonstrated that females are more likely to be classified as binge eating prone than males (Klump et al., 2013). In another study with a conditioned place preference task, they found that females had a more pronounced shift in preference for the chamber that was paired with palatable food during training compared to males. Females also had longer feeding bouts and consumed more food than males (standardized per body weight) during training sessions when food was available in the chamber (Sinclair et al., 2017). Another study recently compared operant responding and consumption of sucrose pellets in male and female rats after acute (24h) deprivation or ad libitum food access (Tapia et al., 2019). That study found that female rats had higher responding to obtain sucrose pellets in a progressive ratio schedule of reinforcement and consumed more pellets than males in fasted and sated states. Current results add to that prior work by revealing that differences exist between the sexes in hedonic eating before any training, and that females differ from males in consumption of palatable foods when sated. Together, prior and current findings in animals are relevant to human eating behavior. Collectively the findings suggest that female biological vulnerability to hedonic eating may be casual to binge and other forms of overeating and associated disorders.
ORX Neurons: Activation and Signaling During Palatable Feeding Tests
Fos induction in ORX neurons during palatable feeding tests
To begin to determine the neural substrates underlying hedonic eating and sex differences, we characterized Fos induction in ORX neurons during a palatable feeding test in males and females. We systematically counted ORX neurons across the entire rostro-caudal extent of the LHA, within four adjacent quadrants that were placed dorsomedially, dorsolaterally, ventromedially and ventrolaterally in respect to fornix. This parcellation was chosen in order to reliably compare different groups of ORX neurons across all conditions. In terms of density of ORX neurons, each quadrant differed from the others. The pf quadrant was the densest, followed by dl, vl, and vm quadrants. These patterns generally match prior observations (de Lecea et al., 1998, Sakurai et al., 1998, Swanson et al., 2005, Yoshida et al., 2006).
We analyzed total number of ORX neurons across the four quadrants together, as well as within each quadrant separately. There were no differences in the number of ORX neurons across the LHA, or in any of the four quadrants analyzed, across any groups. Previous research found higher ORX-A content and mRNA in the hypothalamus in females compared to males, as well as higher body weight in female ORX-knockouts (Taheri et al., 1999, Ramanathan and Siegel, 2014, Grafe et al., 2017). Here, we identified ORX neurons with the immunohistochemistry method, which does not linearly correspond to the substrate quantities beyond the threshold for labeling. Thus, we cannot rule out the possibility that males and females produce different amounts of ORX. Furthermore, levels of ORX and its receptors in the brain tissue were shown to vary in female rats across estrous cycle ((Russell et al., 2001, Wang et al., 2003, Porkka-Heiskanen et al., 2004); for a review see (Grafe and Bhatnagar, 2018)). We did not monitor estrous cycling, in order to avoid potentially stressful effects of that procedure that could impact food intake.
Food and fasting independently impacted Fos induction in ORX neurons. Rats that had access to TD pellets during testing had higher numbers of ORX neurons with Fos compared to rats that were not given food during testing. That recruitment of ORX neurons could be due to anticipation of feeding (sight of food) and associated induction of the drive to eat, as well as the ingestive (palatability) and postingestive effects of consumption (Cai et al., 1999, González et al., 2016). Nevertheless, these patterns suggest that ORX neurons may be driving palatable intake in hungry and sated states, which we tested in the second study.
Fasting also increased Fos induction in ORX neurons. Rats that were fasted prior to testing had more Fos induction in ORX neurons than rats that had ad libitum access to food. These findings are in agreement with the original observations that fasting upregulates ORX mRNA and that ORX mediates activity/arousal associated with energy balance changes ((Sakurai et al., 1998, Cai et al., 1999, Yamanaka et al., 2003); for a review see (Barson and Leibowitz, 2017)). Previously, Funabashi et al., (Funabashi et al., 2009) found that fasting activated more ORX neurons in females compared to males. The current study found fasting-induced activation was similar in both sexes. The discrepancy could be due to a longer fast in Funabashi et al., study (48h versus 20h) or differences in the marker of neural activity used (pCREB versus Fos).
Interestingly, we found different activation patterns based on location of ORX neurons. ORX neurons that were responsive to both food and fasting were located within the pf (dorsomedial) quadrant. Food, but not fasting, activated dorsolateral and ventrolateral quadrants (dl, vl), while neither activated vm quadrant. An important task for future research is to determine the connectional targets within the reward system of these different groups of ORX neurons, and how they are engaged during hedonic eating (Harris et al., 2005, Zheng et al., 2007, Ho and Berridge, 2013, Castro et al., 2016, Ferrario et al., 2016).
In addition to the analysis of ORX neurons, we counted total Fos induction in all neurons within the LHA and found similar patterns. Groups that had food access and groups that were fasted prior to testing had higher numbers of neurons with Fos than corresponding controls. The pf, vm and vl quadrants had more Fos induction in the food and fasted conditions, while within the dl region only food access impacted Fos induction.
The food and fasting associated Fos induction patterns within the LHA in the current study are in agreement with prior work. Most prior work was exclusively in males but a study that compared males and females found no sex differences in hypothalamic activation, as in the current study (Sinclair et al., 2017). However, another study found more Fos induction in ORX neurons in females compared to males under control and repeated restraint stress conditions (Grafe et al., 2017). Food anticipation in food-entrained rats has also been shown to induce Fos within the LHA, including in ORX neurons (Johnstone et al., 2006). In the current study rats were not trained to expect feeding, however, they were familiar with the pellets and Fos may be due to some anticipatory activity in addition to consumption.
Our findings are well matched to the patterns of Zseli et al., study (Zseli et al., 2016) that examined satiety network recruitment during refeeding (for 2h) after fasting (for 40h) in male rats and found dense Fos induction across the LHA. They found dense Fos in both fasted and refed rats within the suprafornical (LHAs) and juxtadorsomedial (LHAjd) regions (within our pf quadrant), the dorsal zone (LHAjvd) (within our vm quadrant), and posterior (LHAsfp) and medial (LHAvm) zones (within our vl quadrant), as well as the dorsal (LHAd) region (within our dl quadrant). We found that in all quadrants with these regions (pf, vl, vm), food access and fasting increased Fos induction, except in dl (which includes LHAd) where only food, but not fasting, increased the number of neurons with Fos. However, Zseli et al., study did not have a non-fasted condition, which precludes direct comparisons of fasting-induced activation across the two studies.
ORX signaling during palatable feeding tests
The first study showed that ORX neurons were activated in both fasted and sated food groups. To follow up on these findings, we tested whether ORX receptor 1 signaling mediates palatable food intake regardless of hunger/satiety state. Rats of both sexes in fasted and sated conditions consumed less TD after receiving SB prior to testing, compared to the amounts they consumed after receiving a vehicle. These data indicate that ORX signaling via receptor 1 drives hedonic eating regardless of physiological hunger in both sexes. This is in agreement with prior work in males, and with the notion that ORX is critical in driving non-homeostatic hunger and the overconsumption of food and drugs (Rodgers et al., 2001, Choi et al., 2010, Barson and Leibowitz, 2017).
Previously, Cason & Aston-Jones (Cason and Aston-Jones, 2014) found that SB decreased sucrose self-administration only in fasted but not in ad libitum-fed females. In the current study there was an overall effect of SB across fasted and ad libitum-fed rats of both sexes. A difference in the amount of effort needed to obtain palatable food in the two studies—free feeding versus lever-pressing—may be the reason for different findings in the two studies.
Furthermore, in these tests, consumption patterns of males and females were consistent with sex differences we found in the first experiment. Fasted males consumed more than sated males, while both fasted and fed females consumed similar amounts. Thus, across four tests in two studies with different animals, females consistently consumed based on palatability, while males consumed according to their physiological need in three tests.
Sex Differences
Interestingly, there were no sex differences in Fos induction in ORX neurons during palatable feeding tests. Furthermore, blockade of ORX signaling decreased consumption in both sexes. These results indicate that ORX is important for hedonic eating in both sexes, and may not be key to sex differences. However, there are methodological limitations that may have precluded the detection of the role of ORX in behavioral sex differences. As discussed above, the immunohistochemistry methods used here to identify ORX neurons and Fos induction are semi-quantitative methods that do not linearly track the quantities of substrates. Males and females may have different receptor sensitivities and a lower dose of SB may be needed to detect sex differences (Cason and Aston-Jones, 2014, Barson, 2018). The dose used in the current study was higher than the lowest dose (3mg/kg) shown to impact palatable intake (Barson, 2018) but it did not produce non-specific locomotor effects (Cole et al., 2015, Keefer et al., 2016). Nevertheless, this dose could have potentially activated orexin 2 receptors in addition to ORXR1 and could have even affected other neurochemicals (Barson, 2018, Scammell and Winrow, 2011). Similarly, systemic pharmacological manipulations do not address specific targets of action. Thus, different amounts of ORX may be released in males and females from the same number of neurons. Furthermore, differences in ORX targets and distribution of ORX receptors may mediate hedonic eating differently in males and females. Supporting this idea, more Fos was found within the infralimbic cortex and the dorsal shell of the nucleus accumbens in females compared to males after consumption of palatable food (Sinclair et al., 2017). These areas contain ORX fibers and receptors (Peyron et al., 1998, Marcus et al., 2001, Baldo et al., 2003) and ORX infusions into the nucleus accumbens enhanced hedonic responses to sucrose and consumption (Castro et al., 2016). Furthermore, sex differences have been shown in a prominent ORX target, the paraventricular nucleus of the thalamus, during renewal of responding to cues for the same palatable food used in the current study (Anderson and Petrovich, 2017).
In conclusion, this comprehensive behavioral and neural analysis in intact, adult males and females is a first step in characterizing similarities and differences between the sexes in hedonic eating (McCarthy et al., 2012). These findings reveal important sex differences in feeding behavior that warrant further investigation. Of notable interest are potential targets of ORX signaling as substrates underlying eating dysregulation in males and females.
Palatable (high-sugar) food consumption was compared in adult, male and female rats under physiological hunger and satiety.
Females consistently consumed similar amounts of palatable food regardless of whether they were sated or hungry.
Males typically adjusted their consumption of palatable food according to their hunger/satiety state.
Fos induction in ORX neurons during palatable feeding tests was systematically characterized in both sexes.
Systemic blockade of ORX receptor 1 signaling decreased palatable food consumption in hungry and sated rats of both sexess
Acknowledgments
This work was supported by the National Institutes of Health, NIDDK grant R01DK085721 to GDP. A portion of the research reported here partially fulfilled the requirements for the Senior Honors Thesis awarded to JM by Boston College. We thank Dr. Ehri Ryu and Dr. Amanda Madden for helpful advice regarding statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson LC, Petrovich GD (2017) Sex specific recruitment of a medial prefrontal cortex-hippocampal-thalamic system during context-dependent renewal of responding to food cues in rats. Neurobiol Learn Mem 139:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003) Overlaping distribution of orexin/hypocretin- and dopamine-b-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. Journal of Comparative Neurology 464:220–237. [DOI] [PubMed] [Google Scholar]
- Barson JR, Leibowitz SF (2017) Orexin/hypocretin system: Role in food and drug overconsumption. Int Rev Neurobiol 136:199–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR (2018) Orexin/hypocretin and dysregulated eating: Promotion of foraging behavior. Brain Res In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2000) Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24:173–198. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG (2010) The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research 1350:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R (2012) The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc 71:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano MM, Dorsey JR, Thomas JM, Murdaugh DL (2009) The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obes 33:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JRS, Tadayyon M, Clapham JC, Wilding J, Williams G (1999) Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes 48:2132–2137. [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G (2014) Role of orexin/hypocretin in conditioned sucrose-seeking in female rats. Neuropharmacology 86:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Terry RA, Berridge KC (2016) Orexin in rostral hotspot of nucleus accumbens enhances sucrose ‘liking’ and intake but scopolamine in caudal Shell shifts ‘liking’ toward ‘disgust’ and ‘fear’. Neuropharmacology 41:2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzerald ME, Benoit SC (2010) The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats.Neuroscience 167:11–20. [DOI] [PubMed] [Google Scholar]
- Cole S, Mayer HS, Petrovich GD (2015) Orexin/hypocretin-1 receptor antagonism selectively reduces cue-induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep 5:16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Tschop MH, Horvath TL, Levine AS (2006) Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Research Reviews 51:85–107. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM,Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouébe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC (2016) Homeostasis meets motivation in the battle to control food intake. J Neurosci 36:11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund N, Thompson BS, Norman KJ, Einhorn P, Andersen SL (2015) Developmental emergence of an obsessive-compulsive phenotype and binge behavior in rats. Psychopharmacology 232:3173–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Hagiwara H, Mogi K, Mitsushima D, Shinohara K, Kimura F (2009) Sex differences in the responses of orexin neurons in the lateral hypothalamic area and feeding behavior to fasting. Neurosci Lett 463:31–34. [DOI] [PubMed] [Google Scholar]
- González JA, Jensen LT, Iordanidou P, Strom M, Fugger L, Burdakov D (2016) Inhibitory interplay between orexin neurons and eating. Curr Bio 26:2486–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Bhatnagar S (2018) The contribution of orexins to sex differences in the stress response. Brain Res In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S (2017) Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol Psychiatry 81:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC (2013) An orexin hotspot in Ventral Pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology 38:1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, H.G. P, Kessler RC (2007) The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry 61:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM, Leng G (2006) Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metabolism 4:313–321. [DOI] [PubMed] [Google Scholar]
- Keefer SE, Cole S, Petrovich GD (2016) Orexin/hypocretin receptor 1 signaling mediates Pavlovian cue-food conditioning and extinction. Physiol Behav 162:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig MD, Boakes RA, Corbit LH (2018) Variety overcomes the specificity of cue-potentiated feeding in rats. Journal of Experimental Psychology: Animal Learning and Cognition 44:56–66. [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine S, Hildebrandt B, Sisk CL (2013) Sex differences in binge eating patterns in male and female adult rats. Int J Eat Disord 46:729–736. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. Journal of Comparative Neurology 435:6–25. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ (2012) Sex differences in the brain: the not so inconvenient truth. J Neurosci 32:2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Shaw D (2015) The worldwide epidemic of female obesity. Best Pract Res Clin Obstet Gynaecol 29:289–299. [DOI] [PubMed] [Google Scholar]
- Petrovich GD (2013) Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav 121:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD (2019) Orexins and Control of Feeding by Learned Cues In: The Orexins/Hypocretins System: Functional Roles and Therapeutic Potential (Fadel JR and Burk JA, eds), pp 85–98: Academic Press. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D (2004) Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. Eur J Endocrinol 150:737–742. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Siegel JM (2014) Gender differences between hypocretin/orexin knockout and wild type mice: age, body weight, body composition, metabolic markers, leptin and insulin resistance. J Neurochem 131:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, Abbott KN, Westbrook RF, Morris MJ (2016) Differential motivational profiles following adolescent sucrose access in male and female rats. Physiol Behav 157:13–19. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ (2010) Chronic Stress, Food Consumption, and Emotional Behavior in Rats. Master’s thesis, Boston College. [Google Scholar]
- Reppucci CJ, Petrovich GD (2012) Learned food-cue stimulates persistent feeding in sated rats. Appetite 59:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JCG, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JRS, Upton N, Porter RA, Blundell JE (2001) SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci 13:1444–1452. [DOI] [PubMed] [Google Scholar]
- Russell SH, Small CJ, Kennedy AR, Stanley SA, Seth A, Murphy KG, Taheri S, Ghatei MA, Bloom SR (2001) Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology 142:5294–5302. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Winrow CJ (2011) Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol 51:243–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair EB, Hildebrandt BA, Culbert KM, Klump KL, Sisk CL (2017) Preliminary evidence of sex differences in behavioral and neural responses to palatable food reward in rats. Physiol Behav 176:165–173. [DOI] [PubMed] [Google Scholar]
- Small DM (2009) Individual differences in the neurophysiology of reward and the obesity epidemic. International Journal of Obesity 33:S44–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE (2013) The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev 37:2047–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Kroemer N, B., Veldhuizen M, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, R. S, Small DM (2015) Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci 35:7964–7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sanchez-Watts G, Watts AG (2005) Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett 387:80–84. [DOI] [PubMed] [Google Scholar]
- Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, Bloom SR (1999) Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Letters 457:157–161. [DOI] [PubMed] [Google Scholar]
- Tapia MA, Lee JR, Weise VN, Tamasi AM, Will MJ (2019) Sex differences in hedonic and homeostatic aspects of palatable food motivation. Behav Brain Res 359:396–400. [DOI] [PubMed] [Google Scholar]
- Wang JB, Murata T, Narita K, Honda K, Higuchi T (2003) Variation in the expression of orexin and orexin receptors in the rat hypothalamus during the estrous cycle, pregnancy, parturition, and lactation. Endocrine 22:127–134. [DOI] [PubMed] [Google Scholar]
- Weingarten HP (1983) Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science 220:431–433. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T (2003) Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38:701–713. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell T (2006) Afferents to the orexin neurons of the rat brain. J Comp Neurol 494:845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR (2007) Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27:11075–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zséli G, Vida B, Martinez A, Lechan RM, Khan AM, Fekete C (2016) Elucidation of the anatomy of a satiety network: Focus on connectivity of the parabrachial nucleus in the adult rat. J Comp Neurol 524:2803–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]