Abstract
Background:
Apolipoprotein L1 gene (APOL1) G1 and G2 renal-risk alleles (RRAs) are associated with end-stage renal disease (ESRD) in blacks with lupus nephritis (LN). The present study determined frequencies of APOL1 RRAs in non-white Brazilian patients with LN and controls to assess association with renal outcomes.
Methods:
APOL1 RRAs were genotyped in 222 healthy blood donors (controls) and 201 cases with LN from three outpatient clinics. Two single nucleotide polymorphisms in the G1 (rs73885319; rs60910145) and an indel for the G2 (rs71785313) variant were genotyped.
Results:
The frequency of APOL1 RRAs in non-white Brazilian LN cases did not differ significantly from healthy controls, few participants had 2 RRAs. In the sample, 84.6% of LN cases and 82.9% of controls had 0 RRAs, 13.4% and 15.3% had 1 RRA, and 2.0% and 0.4% had 2 RRAs, respectively. LN cases with ≥1 APOL1 RRAs had similar baseline characteristics and renal responses to treatment, yet faced higher risk for progressive chronic kidney disease (CKD) to an eGFR <30 ml/min/1.732 compared to those with 0 RRAs (11.1% with 0, 29.6% with 1; 50% with 2 RRAs, p=0.005). Although glomerular lesions and activity scores on initial kidney biopsy did not differ significantly between individuals based on APOL1 genotype, chronicity scores, tubular atrophy and interstitial fibrosis were more severe in those with ≥1 RRA.
Conclusions:
Although initial kidney lesions and treatment responses were similar, a single APOL1 RRA in non-white Brazilians with LN was associated with increased risk of advanced CKD and possibly more tubulo-interstitial damage.
Introduction
Non-diabetic chronic kidney disease (CKD) is significantly more prevalent in those who possess recent African ancestry; a finding related in part to presence of apolipoprotein L1 gene (APOL1) renal risk alleles (RRAs). Two coding nephropathy variants in APOL1, G1 (rs73885319; rs60910145) and G2 (rs71785313), appear to have been selected for in sub-Saharan Africa because their circulating proteins provide resistance to Trypanosoma brucei rhodesiense and development of African sleeping sickness (1,2). Although 13% of African Americans possess APOL1 high-risk genotypes, defined as having two copies of the G1 and/or G2 allele, only a minority develops CKD. It appears likely that modifying factors are required to initiate APOL1 nephropathy.
High interferon (IFN) states, including HIV infection (producing HIV-associated nephropathy [HIVAN]) (3), exogenously administered IFN (4), and systemic lupus erythematosus (SLE) are linked with collapsing glomerulopathy in carriers of two APOL1 RRAs (autosomal recessive inheritance) (5). In addition, severe lupus nephritis (LN), LN-end-stage renal disease (ESRD), is associated with APOL1 in an autosomal recessive inheritance pattern (6,7). Effects of APOL1 on non-diabetic ESRD reveal odds ratios (ORs) for association of 3 in patients with LN-ESRD and 29–89 in those with HIVAN (3,6,8). A recent large genome-wide association study searching for modifying genes in APOL1 nephropathy failed to identify second genes or additional variants meeting genome-wide significance for association with LN ESRD, suggesting environmental modifiers often trigger APOL1 nephropathy (7).
Relative to Caucasians, African Americans and Hispanics develop more aggressive LN with earlier onset and poorer long-term renal outcomes (9). European ancestry is reportedly protective from LN in patients with SLE (10). Moreover, familial clustering of LN and CKD suggests a role for genetic factors (11) and African Americans with ≥1 APOL1 RRAs were reported to initiate renal replacement therapy earlier than those lacking APOL1 RRAs (12,13). South American populations have variable contributions of West African ancestry due to the slave trade that occurred 500 years ago (14,15). This should result in a range of APOL1 RRA frequencies in this relatively understudied population (16). Similar to other areas of the Latin America, the repeated forced migration of individuals of West African ancestry during the slave trade resulted in significant genetic admixture (i.e., interbreeding of two previously separated and distinct populations) (17). Brazilians are an admixed population, with differing proportions of Amerindian, African and European ancestry (14,15,18). Frequencies of APOL1 RRAs have been variable, depending on the region of Brazil. One study in Brazilians with LN revealed that approximately 30% of their genome was African; however, only 10% of cases had two APOL1 RRAs without significant association with CKD (19). Another report genotyped black and mixed Brazilian populations with ESRD; they detected 10-fold higher frequencies of APOL1 renal-risk genotypes (two RRAs) compared to related controls (20). The latter study reveals that APOL1 is associated with non-diabetic ESRD in Brazilians in autosomal recessive fashion; however, cases lacked LN.
The primary hypothesis of this study was to determine whether there was an association between APOL1 RRAs and development of progressive CKD defined as a sustained eGFR <30 mL/min/1.73m2 in this non-white (mixed) Brazilian population. Secondary analyses assessed the impact of APOL1 RRAs on additional kidney outcomes in LN, including kidney histology and long-term kidney function.
Materials and Methods
Cases with LN were enrolled from three outpatient clinics in Brazil specializing in treatment of glomerulonephritis (GN), Federal University of Pernambuco (UFPE) and Prof Fernando Figueira Integrative Medicine Institute – IMIP (Recife, Northeastern Brazil) and Federal University of São Paulo – EPM/UNIFESP (São Paulo, Southeastern Brazil). All cases provided written informed consent. The study was approved by the Brazilian National Committee for Ethics in Research (CONEP, report number: 2.568.450) and performed in accordance with the Declaration of Helsinki.
Overall, 309 patients with a previous diagnosis of LN were recruited between August 2015 and July 2018. All were ≥18 years of age, unrelated, met Systemic Lupus International Collaborating Clinics Classification Criteria, and had negative serologies for hepatitis B, hepatitis C, HIV and syphilis. All patients had a renal biopsy. Biopsies were analyzed by two renal pathologists, one from IMIP, Recife and one from EPM/UNIFESP, São Paulo. The classification and characteristics of LN were described according to the International Society of Nephrology/Renal Pathology Society guidelines. We excluded 30 patients with non-LN histologic patterns (including IgA nephropathy, vasculitis, post-infectious GN, idiopathic membranous GN, focal segmental glomerulosclerosis [FSGS] or collapsing GN) and those with <6 months of follow-up after diagnosis of LN. In addition, nine patients with inadequate DNA and 72 self-reporting their ancestry as white were excluded. The remaining 201 cases had LN on initial kidney biopsy. None had Class I or Class VI (>90% of glomeruli globally sclerosed) LN. We analyzed cases with Class II mesangial proliferative LN (pure mesangial hyper-cellularity and/or matrix expansion); Class III focal proliferative LN (involving <50% of the total number of glomeruli); Class IV diffuse proliferative or global LN (involving ≥50% of the total number of glomeruli) and Class V membranous LN (21). Eight of 201 cases (4%) did not have enough kidney tissue to classify LN, but were retained in the analyses based on appropriate clinical presentations with follow-up similar to the other LN cases (one had Class IV LN on a subsequent renal biopsy during a second lupus flare several months later). Those with a history of essential hypertension or with blood pressure readings ≥140 mmHg systolic and/or ≥90 mmHg diastolic on at least two occasions were considered to have hypertension.
Historical data regarding initial laboratory tests, first induction/maintenance therapy and treatment response were recorded from chart review. Thereafter; participants were followed prospectively during routine care through February 2019. During acute flares of nephritis, cases with LN underwent induction therapy with intravenous methylprednisolone, followed by oral prednisone and six boluses of intravenous cyclophosphamide 0.5–1 gram or mycophenolate mofetil (MMF) 2–3 gram/day. Post-induction, they received maintenance azathioprine or MMF, based on established protocols. At baseline, hydroxychloroquine was prescribed to more than 80% of LN cases. Changes in proteinuria and serum creatinine concentration (SCr) were recorded from chart reviews at 6, 12, 24 months and/or latest follow-up, according to Kidney Disease Improving Outcomes guidelines (22). Renal responses to therapy were classified as complete, partial or non-responsive (22). LN cases who developed CKD Stage 3 or Stage 4 (defined as a sustained eGFR <60 or <30 mL/min/1.73m2 using the CKD-Epidemiology Collaboration [EPI] equation, respectively) and ESRD defined as the need for renal replacement therapy or eGFR <10ml/min/1.73m2 were recorded. Refractory LN was defined as lack of a complete or partial response after two different induction treatments, including at least one course of cyclophosphamide (some may have received cyclosporine with MMF or rituximab).
A total of 222 unrelated, non-white, adult healthy blood donors from two Brazilian blood centers (Recife - PE and Ribeirão Preto - SP) were genotyped and served as non-SLE controls.
Genomic DNA was isolated from anti-coagulated whole blood collected in ethylenediaminetetraacetic acid blood tubes using the PureGene system, based on manufacturer instructions. Samples were shipped on ice to Wake Forest School of Medicine for APOL1 genotyping. Two single-nucleotide polymorphisms in the G1 nephropathy risk variant (rs73885319; rs60910145) and an indel for the G2 nephropathy risk variant (rs71785313) were genotyped using Taqman assays on the ViiA 7 platform (Applied Biosystems for Life Tech). APOL1 high-risk genotypes were present if participants had 2 RRAs (G1G2, G1G1 or G2G2).
Participant characteristics were compared using a student’s t-test or Mann-Whitney U test (i.e., Wilcoxon rank-sum test) as distributionally appropriate or Fisher’s exact test for categorical variables. Given the low frequency of 2 APOL1 RRAs, Kaplan Meier survival curves were computed separately for APOL1 RRA=0 and RRA≥1, and differences were computed using the logrank test. Cox proportional hazards models were computed to estimate a hazard ratio (HR) for RRA=0 vs. RRA≥1. The comparison of RRA=0 vs. RRA≥1 on development of progressive CKD (defined based on sustained eGFR <30 mL/min/1.73m2) was the primary a priori inference. Significance was set at p<0.05. Additional outcomes, including renal histologic changes and long-term clinical parameters, were considered secondary outcomes.
We computed three power analyses to quantify the effect size detectable with 0.80 power and a type 1 error rate of α=0.05. For binary outcomes (e.g., ESKD) between LN cases and controls, the study had 0.80 power to detect effects with odds ratios of 1.78. For continuous outcomes the study has 0.80 power to detect differences between cases and controls that explain 1.9% of the variation (i.e., r2=0.019), and case-only continuous traits that explain 3.9% of the variation.
Results
APOL1 genotypes and demographic characteristics in self-reported non-white LN cases and non-SLE controls are displayed in Table 1. As expected, cases with LN had more females than non-SLE controls (90% vs 36%). APOL1 allele frequencies did not differ significantly between LN cases and controls. Among the 72 self-described white LN cases excluded from the analyses, three had 1 APOL1 RRA (4%) and none had 2 RRAs. Thus, white non-SLE controls were not genotyped.
Table 1.
Demographic characteristics of non-white Brazilian cases with lupus nephritis and non-SLE controls.
| LN cases n=201 | Non-SLE controls n=222 | P-value | |
|---|---|---|---|
| Mean±SD age, years | 35.0±11.0 | 33.6±10.4 | 0.17 |
| Female sex, n (%) | 179 (89.0%) | 80 (36.0%) | <0.0001 |
| APOL1 | |||
| 0 RRA | 170 (84.6%) | 187 (84.2%) | 0.30** |
| 1 RRA | 27 (13.4%) | 34 (15.3%) | |
| 2 RRA | 4 (2.0%) | 1 (0.4%) | |
| Genotype frequency | |||
| G0G0 | 170 (84.6%) | 187 (84.2%) | 0.44** |
| G0G1 | 17 (8.4%) | 19 (8.6%) | |
| G0G2 | 10 (5.0%) | 15 (6.8%) | |
| G1G1 | 4 (2.0%) | 1 (0.4%) | |
| G1G2 | 0 | 0 | |
| G2G2 | 0 | 0 | |
Abbreviations: SLE, systemic lupus erythematosus; LN, lupus nephritis; RRA, renal risk alleles.
Chi-square test
Race was categorized as self-reported white and non-white (including mixed or black); Asians and Amerindians were not present (23). Household income was not analyzed because more than 90% of the national public health system (Sistema Único de Saúde) users earn less than US$ 100 monthly, and immunosuppressive medications are provided by the State government (24). Demographic characteristics, baseline laboratory results, kidney biopsy findings and long-term outcomes in non-white LN cases are displayed in Table 2, stratified by APOL1 genotype. Because only 4 LN cases (2%) possessed 2 APOL1 RRAs, groups were analyzed based on the presence of ≥1 APOL1 RRAs. Although not statistically significant, cases with 1 or 2 APOL1 RRAs tended to be younger and have shorter LN durations than cases with 0 RRAs (p=0.09 and 0.36, respectively). However, higher frequencies of CKD Stage 4 and 5 (ESRD) were present in LN cases with ≥1 APOL1 RRA (p=0.005 and 0.007, respectively). This occurred despite similar baseline demographic characteristics, CKD risk factor profiles, eGFR, proteinuria and histologic class of LN. In addition, prescribed treatments were similar in LN cases regardless of APOL1 genotype. Although no differences were observed in the initial clinical response between genotype groups, LN cases with ≥1 APOL1 RRA more often developed sustained eGFR<60mL/min/1.73m2 six months after induction therapy, compared to those with 0 RRA (21.7% vs 4.4%; p=0.018, OR=5.12, 95% confidence interval [95% CI]=1.6–17.6) (Table 3).
Table 2.
Non-white Brazilian lupus nephritis case characteristics, based on APOL1 genotype.
| 0 APOL1 RRA n=170 | 1 APOL1 RRA n=27 | 2 APOL1 RRA n=4 | P-value 0 vs ≥1 RRA | |
|---|---|---|---|---|
| Characteristic | ||||
| Age at enrollment, years | 35.5±10.8 | 32.1±12.1 | 35.0±10.8 | 0.09 |
| Mean±SD (Median) | (34.5) | (28.0) | (30.5) | |
| Age at onset, years | 30.0±10.2 | 26.6±8.8 | 30.5±12.4 | 0.14 |
| Mean±SD (Median) | (29.0) | (26.0) | (27.0) | |
| Female sex, n (%) | 149(87.6) | 26(96.3) | 4(100.0) | 0.21 |
| Less than high school graduate, n (%) | 58(36.9) | 10(45.4) | 1(33.3) | 0.51 |
| Mean±SD BMI, kg/m2 | 25.4±4.9 | 26.3±5.4 | 26.0±6.3 | 0.41 |
| Hypertension, n (%) | 104(61.2) | 20(74.1) | 2(50.0) | 0.32 |
| Diabetes, n (%) | 6(3.5) | 2(7.4) | 0(0.0) | 0.36 |
| Active smoker, n (%) | 5(3.8) | 1(5.3) | 0(0.0) | 0.58 |
| Mean±SD SLICC | 6.8±1.8 | 6.3±1.5 | 6.2±2.1 | 0.10 |
| Median duration SLE at last FU (25–75th), mos | 78.0(43.8–138.8) | 66.0(28.0–128.0) | 89.0(52.7–126.0) | 0.52 |
| Median duration LN at last FU (25–75th), mos | 60.0(30.0–252.0) | 36.0(14.0–128.0) | 58.5(43.3–107.5) | 0.36 |
| Initial laboratory results | ||||
| C3 <90mg/dL, n (%) | 81(79.4) | 10(62.5) | 1(100.0) | 0.21 |
| C4 <10mg/dL, n (%) | 57 (60.0) | 6 (40.0) | 0 (0) | 0.11 |
| Median SCr (25–75th), mg/dL | 1.20(0.70–2.00) | 0.85(0.55–1.85) | 4.18(0.77–6.00) | 0.45 |
| Median CKD-EPI eGFR (25–75th), ml/min/1.73m2 | 66(36.0–115.3) | 86(30.9–127.4) | 12(10.2–125.3) | 0.60 |
| Mean±SD SAlb, mg/dL | 2.7±0.76 | 2.4±0.83 | 2.7±0.85 | 0.21 |
| Median Proteinuria (25–75th), g/day | 3.40(1.60–6.20) | 2.20(0.97–7.65) | 3.21(2.20–4.20) | 0.73 |
| Initial LN kidney biopsy | ||||
| Proliferative lesion, % | 81.7 | 84.0 | 75.0 | 1.00 |
| Class (overall test) | 0.89** | |||
| Class II, n (%) | 3(1.8) | 0 | 0 | 1.00 |
| Class III (±V), n (%) | 41(25.0) | 8(32.0) | 0 | 0.82 |
| Class IV (±V), n (%) | 93(56.7) | 13(52.0) | 3(75.0) | 1.00 |
| Class V, n (%) | 27(16.5) | 4(16.0) | 1(25.0) | 1.00 |
| Median # glomeruli (25–75th), n | 15(9–21) | 13(9–22) | 13(6–18) | 0.68 |
| Median Global glomerular sclerosis (25–75th), % | 0.0(0.0–12.5) | 6.0(0.0–20.0) | 25.0(6.2-36.8) | 0.055 |
| Crescents, n (%) | 64(43.4) | 15(65.2) | 1(25.0) | 0.14 |
| Median % crescents (25–75th), % | 0.0(0.0–18.1) | 12.8(0–48.6) | 0.0(0–10) | 0.08 |
| Synechia to BC, n (%) | 54(54.0) | 12(70.6) | 2(50.0) | 0.34 |
| Fibrinoid necrosis, n (%) | 6(4.0) | 1(4.3) | 0(0.0) | 1.00 |
| Hyaline thrombi, n (%) | 20(13.4) | 4(17.4) | 1(25.0) | 0.55 |
| TMA, n (%) | 7(4.7) | 1(4.3) | 0(0.0) | 1.00 |
| Membranous component, n (%) | 76 49.7) | 12(50.0) | 3(75.0) | 0.84 |
| TIN, n (%) | 15(10.1) | 0(0.0) | 1(25.0) | 0.47 |
| Tubular atrophy >25%, n (%) | 17(11.6) | 14(63.6) | 2(50.0) | 0.002 |
| Interstitial fibrosis >25%, n (%) | 25(17.0) | 8(36.3) | 2(50.0) | 0.018 |
| Mean±SD activity index | 5.4±3.3 | 6.1±5.4 | 3.0±3.0 | 0.92 |
| Mean±SD chronicity index | 2.8±1.6 | 4.1±2.3 | 4.0±2.0 | 0.011 |
Chi-square test
T-test used for normally distributed continuous variables and reported as mean and standard deviation (±SD); Mann-Whitney test used for non-normally distributed continuous variables and displayed as median and 25–75th percentile; Fisher’s exact test used for categorical variables.
Abbreviations: CKD, chronic kidney disease; BMI, body mass index; SLICC, Systemic Lupus International Collaborating Clinics Classification Criteria; SLE, systemic lupus erythemathosus, LN, lupus nephritis; FU, follow-up; mos, months; RRA, renal-risk allele; SCr, serum creatinine; eGFR, estimated glomerular filtration rate; CKD-EPI, SAlb, serum albuminaemia; BC, Bowman capsule; TMA, thrombotic microangiopathy; TIN, tubule-interstitial nephritis.
Table 3.
Non-white Brazilian lupus nephritis case treatment and outcomes, based on APOL1 genotype.
| 0 APOL1 RRA n=170 | 1 APOL1 RRA n=27 | 2 APOL1 RRA n=4 | P-value 0 vs ≥1 RRA | |
|---|---|---|---|---|
| Treatment | ||||
| First Induction: Cyclophophamide, n (%) | 118(70.2) | 18(66.7) | 3(75.0) | 0.83 |
| Maintenance: Mycophenolate Mofetil, n (%) | 141(86.0) | 19(76.0) | 3(75.0) | 0.17 |
| Hydroxychloroquine at enrollment, n (%) | 142(84.0) | 20(74.1) | 4(100.0) | 0.43 |
| ACEi/ARB at enrollment, n (%) | 118(71.1) | 16(64.0) | 3(75.0) | 0.66 |
| Response after induction | ||||
| Complete or partial response at 6mos, n (%) | 85 (65.9) | 13(56.5) | 1(33.3) | 0.66 |
| Sustained eGFR <60 ml/min/1.73m2 at 6 mos, n (%) | 6(4.4) | 5(21.7) | 0 | 0.018 |
| Complete or partial response at 12mos, n (%) | 104(77.0) | 12(57.1) | 2(66.7) | 0.075 |
| Sustained eGFR <60 ml/min/1.73m2 at 12 mos, n (%) | 9(6.7) | 4(19.0) | 0 | 0.11 |
| Complete or partial response at 24mos, n (%) | 91(82.0) | 8(61.5) | 3(100) | 0.31 |
| Sustained eGFR <60 ml/min/1.73m2 at 24 mos, n (%) | 8(7.2) | 3(23.1) | 0 | 0.14 |
| Outcomes at last follow-up | ||||
| Median Scr (25–75th), mg/dL | 0.80(0.68–1.20) | 0.80(0.60–3.00) | 1.45(0.62–2.58) | 0.69 |
| Median eGFR (25–75th) | 97(64.8–116.1) | 95(20.9–118.9) | 72(22.3–135.6) | 0.63 |
| Mean SAlb, mg/dL | 3.8±0.54 | 3.8±0.55 | 3.7±0.48 | 0.47 |
| Median Proteinuria (25–75th), g/day | 0.40(0.15–1.20) | 0.49(0.20–1.67) | 0.13(0.11–0.76) | 0.74 |
| Complete or partial response, n (%) | 121(71.1) | 17(63.0) | 2(50.0) | 0.29 |
| Flare after response, n (%) | 65(47.8) | 8(44.4) | 1(33.3) | 0.82 |
| Refractory nephritis, n (%) | 23(13.6) | 8(29.6) | 0 | 0.10 |
| eGFR <60 ml/min/1.73m2, n(%) | 40(23.5) | 8(29.6) | 2(50.0) | 0.37 |
| eGFR <30 ml/min/1.73m2, n (%) | 19(11.2) | 8(29.6) | 2(50.0) | 0.005 |
| ESRD, n (%) | 10(5.9) | 7(25.9) | 0 | 0.007 |
| Median time to ESRD (25–75th), mos | 114(36–220) | 14(9–22) | 0 | 0.002 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; ESRD, end stage kidney disease.
In secondary analyses, a trend towards higher percentages of glomeruli with global glomerulosclerosis and crescents were seen in LN cases with ≥1 APOL1 RRAs; although, types of LN-related glomerular lesions were not different between genotypes (Table 2). Interstitial damage, measured by the percentage of tubular atrophy (TA) and interstitial fibrosis (IF), was more severe in LN cases with ≥1 APOL1 RRA (p=0.002 and p=0.018, respectively). The activity index was similar between genotype groups (p=0.92), but chronicity index on the initial kidney biopsy was significantly higher in LN cases with ≥1 APOL1 RRA (4.1±2.3), versus 0 RRA (2.8±1.6; p=0.011). Fifty of 201 LN cases (43 with 0 APOL1 RRAs and 7 with ≥1 RRAs) received a second kidney biopsy (Table 4). There was no statistically significant difference in renal histology between genotype groups, except that median percentage of crescents (not presence) was higher on the second biopsy in LN patients with ≥1 APOL1 RRA (p=0.03). It is difficult to estimate the value of the second biopsy done during relapses from only a quarter of participants.
Table 4.
Results of second kidney biopsy for LN cases.
| 0 APOL1 RRA n=43 | 1 APOL1 RRA n=7 | 2 APOL1 RRA n=0 | P-value 0 vs ≥1 RRA | |
|---|---|---|---|---|
| Proliferative lesion, % | 81.4 | 71.4 | 0.0 | 0.62 |
| Classes (overall test) | 0.28** | |||
| Class II | 1(2.3) | 0(0.0) | 0.0 | 1.00 |
| Class III (±V) | 15(34.9) | 0(0.0) | 0.0 | 0.09 |
| Class IV (±V) | 20(46.5) | 5(71.4) | 0.0 | 0.42 |
| Class V | 7(16.3) | 2(28.6) | 0.0 | 0.60 |
| Median # glomeruli (25–75th) | 12(8–16) | 18(10–22) | 0.0 | 0.11 |
| Median Global glomerular | 8.3 | 4.0 | 0.0 | 0.55 |
| sclerosis (25–75th), % | (0.0–28.2) | (0.0–13.3) | ||
| Crescents, n (%) | 10(23.8) | 4(57.1) | 0.0 | 0.09 |
| Median % crescents (25–75th), % | 0(0–22) | 18.2(5–75) | 0.0 | 0.03 |
| Synechia to BC, n (%) | 24(70.6) | 2(40.0) | 0.0 | 0.31 |
| Fibrinoid necrosis, n (%) | 2(4.8) | 0(0.0) | 0.0 | 1.00 |
| Hyaline thrombi, n (%) | 6(4.7) | 1(14.3) | 0.0 | 1.00 |
| TMA, n (%) | 2(4.7) | 0(0.0) | 0.0 | 1.00 |
| Membranous component, n (%) | 26(61.9) | 5(71.4) | 0.0 | 1.00 |
| TIN, n (%) | 4(9.5) | 3(42.9) | 0.0 | 0.05 |
| Tubular atrophy >25%, n (%) | 13(37.1) | 2(33.3) | 0.0 | 1.00 |
| Interstitial fibrosis >25%, n (%) | 16(45.7) | 4(66.7) | 0.0 | 0.41 |
| Mean±SD activity index | 3.9±2.8 | 5.7±3.5 | 0.0 | 0.32 |
| Mean±SD chronicity index | 4.4±1.9 | 4.7±0.6 | 0.0 | 0.80 |
Chi-square test
Abbreviations: RRA, renal-risk allele; BC, Bowman capsule; TMA, thrombotic microangiopathy; TIN, tubule-interstitial nephritis.
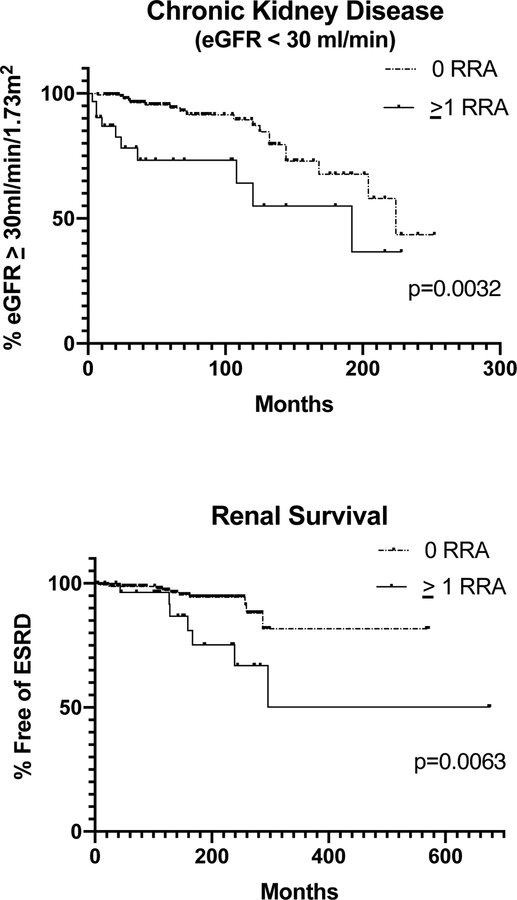
Figure 1 displays Kaplan-Meier renal survival curves for CKD, eGFR<30 ml/min/1.73m2 (p=0.003, HR=2.97, 95% CI=1.1–8.2) and ESRD (p=0.006, HR=3.49, 95% CI=1.0–12.5).
Figure1:
Kaplan-Meier survival curves, based on APOL1 genotype.
P-values represent results from logrank test.
The time from initial diagnosis of LN to ESRD was significantly shorter in LN cases with >1 APOL1 RRA, compared to those with 0 RRAs (14, [25–75th=9–22] vs 114 [25–75th=36–220] months, p=0.0023). Thus, faster progression to ESRD was present in those with ≥1 RRA (Table 3).
Table 5 displays the outcomes in the four LN cases with 2 APOL1 RRAs. Despite the small sample, half progressed to CKD Stage 4 (eGFR <30 ml/min/1.73m2) and one has persistent proteinuria after three rounds of induction therapy.
Table 5.
Characteristics and outcomes of LN cases with two APOL1 risk alleles.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age at recruitment | 28 | 51 | 29 | 32 |
| Sex | Female | Female | Female | Female |
| Ancestry | Black | Mixed | Black | Mixed |
| SLICC criteria | 8 | 5 | 4 | 8 |
| LN duration, months | 120 | 47 | 70 | 42 |
| Kidney biopsy | LN class IV-S | LN class IV-G/V | LN class IV-S/V | LN class V |
| Crescents, % | 10 | 0 | 0 | 0 |
| Global sclerosis, % | 36.8 | 25 | 25 | 0 |
| TA/IF, % | 50–75 | 50–75 | <25 | <25 |
| AI/CI | 3/6 | NA | 6/4 | 0/2 |
| Treatment | CF, MMF, steroids | CF, MMF, steroids | CF, MMF, CsA, steroids | CF, MMF, steroids |
| eGFR, mL/min (CKD-EPI) last follow-up | 28 | 18 | 140 | 113 |
| Outcome | CKD stage 4 | CKD stage 4 | Partial response | Complete response |
| APOL1 genotype | G1G1 | G1G1 | G1G1 | G1G1 |
Abbreviations: SLICC, Systemic Lupus International Collaborating Clinics Classification Criteria; LN, lupus nephritis; TA, tubular atrophy; IF, interstitial fibrosis; AI, activity index; CI, chronicity index; NA, not available; CF, cyclophosphamide; MMF, mycophenolate mofetil; CsA, cyclosporine; CKD, chronic kidney disease.
Discussion
The results of this study in Brazilians with LN demonstrate that participants with ≥1 APOL1 RRA had more severe kidney disease at initial diagnosis and higher stages of CKD after six months of therapy compared to those with 0 APOL1 RRA. Populations with mixed ancestry are not typically screened for APOL1 RRAs; frequencies are expected to vary based on extent of recent African ancestry (16). The Brazilian population is heterogeneous as a result of interethnic mating of peoples from three continents: European colonizers (mainly Portuguese), African slaves, and local Amerindians (14,15). This study genotyped self-reported non-white healthy controls and cases with LN. Cases and controls had similar and low frequencies of APOL1 high-risk genotypes (two RRAs), 0.4% and 2.0% respectively. A study in the Brazilian city Salvador genotyped 45 ESRD cases and identified only one (2.0%) with 2 APOL1 RRAs (25). In contrast, Riella et al. reported a higher prevalence of APOL1 2 RRA (12.4%) and 1 RRA carriers (17.5%) among 274 self-declared Brazilian mixed-race and black patients with ESRD; those with autoimmune kidney disease were excluded (20). They also analyzed 106 matched first-degree relatives of cases and found lower frequencies of APOL1 2 RRA carriers (0.9%) and similar frequencies with 1 RRA (13.2%) (20). The APOL1 frequencies in their controls appear similar to those in healthy blood donor controls from the present study.
A study from São Paulo genotyped APOL1 in 196 female outpatients with LN; participants had 30% African ancestry based on ancestry informative markers (AIMs) (19). Of these, 10% possessed 2 APOL1 RRAs and there was no significant association of APOL1 with doubling of the baseline Scr in a recessive genetic model (19). In the present cohort of LN cases and controls, AIMs were lacking due to a paucity of DNA. Although skin color is not an accurate predictor of AIMs in such an admixed population, those self-described as black or mixed Brazilians reportedly have a higher African ancestry index (AAI) (14). We detected no significant difference in genetic ancestry based on skin pigmentation in Brazilians; participants from Recife had 59.7% European ancestry, 23.0% African ancestry and 17.3% Amerindian ancestry (18). Other studies using AIMs from different Brazilian regions revealed similar patterns of European dominance, followed by African, and to a lesser extent Amerindian genetic ancestry (15,26).
A study comparing the AAI among black and white Brazilians from each region of the country found similar AAIs between individuals from the Northeast and Southeast regions of Brazil, but lower AAI in original Africans (and higher than in the founding Portuguese) (14). The prevailing hypothesis is that APOL1 G1 and G2 RRAs arose in the past 10,000 years in sub-Saharan Africa, likely in West Africa where they were subjected to intense positive selection since circulating APOL1 RRA proteins provide resistance to Trypanosoma brucei rhodesiense (1,27). South America was likely colonized around 15,000 years ago, likely by a single wave of migration (28) and before positive selection for APOL1. This suggests that APOL1 RRAs came from the trans-Atlantic slave trade during the 16th to 19th centuries.
Asian, Native American, and Caucasian populations with CKD generally have very low frequencies of APOL1 RRAs (29–32). Among American Indians, African-derived risk alleles in the DNA sequence of APOL1 coding regions were absent, providing additional evidence that these risk variants are only present in those with recent African ancestry (33). However, among admixed (with African ancestry) Hispanic and Latin Americans, APOL1 two RRA genotypes were present in 2% of individuals (31). This is similar to the present study, with low rates of CKD.
The low frequency of APOL1 2 RRA carriers in our Brazilian LN cohort did not permit performance of outcome analyses using the traditional autosomal recessive model. However, presence of even one APOL1 RRA demonstrated significant association with advanced CKD during follow-up. Presence of ≥1 APOL1 RRAs confers immunity against Trypanosoma brucei rhodesiense (34). APOL1 cellular toxicity may arise from the same trypanolytic factors that produce chloride channels in lysosomes, producing damage to cell membranes, mitochondria and cell death (35,36).
Genetic risk for APOL1-associated CKD in humans is autosomal recessive; animal models are complicated by the lack of APOL1. Few animal models have tested the heterozygous state, typically a disease-free condition in humans (37). Zebrafish embryos with APOL1 CRISPR/Cas9 genome editing revealed podocyte loss and glomerular filtration defects that could be rescued by expression of wild-type APOL1 mRNA (38). However, the APOL1 G1 RRA did not ameliorate defects caused by suppression of APOL1, nor did G2, which was deleterious to protein function (38). African Americans with 1 or 2 APOL1 RRAs are known to require dialysis an average of five years and nine years earlier than those with 0 RRAs (13). Moreover, as the number of APOL1 RRAs increased in the present study, duration from SLE onset to ESRD decreased (6).
Untreated patients with HIV who carry 2 APOL1 RRAs have among the highest ORs for CKD (29–89); however, even 1 RRA was associated with HIVAN in Africans (OR: 5.49) (8). A single APOL1 RRA also confers a 1.7-fold increased risk for FSGS, although 2 RRAs confer ten-fold higher risk (3). These findings support the influence of a single APOL1 RRA in kidney injury. Chromosome 22q is also enriched for gene duplications in the APOL1–4 gene cluster and copy number variation may change gene dosage and expression. Additional copies of APOL1 were observed more frequently in CKD cases than controls, possibly increasing susceptibility to CKD in heterozygotes (39). Association between null variants in APOL3 and ESRD has been reported (40), irrespective of APOL1 genotype status and percentage African ancestry. This supports the concept that other APOL proteins (besides APOL1) may influence risk for non-diabetic CKD.
The spectrum of APOL1 nephropathy has known mediating factors in those with 2 APOL1 RRAs, including HIV infection and interferons in collapsing glomerulopathy (3–5). Interferons are up regulated in patients with active SLE. Thus, this milieu might trigger APOL1 nephropathy even in cases with 1 RRA. α-Interferon increases APOL1 mRNA expression in endothelial cells (4) and LN reflects a chronic type I interferon-induced state. The present study identified a higher chronicity index and more frequent moderate to severe tubular atrophy and interstitial fibrosis on initial kidney biopsies in cases with LN with ≥1 APOL1 RRAs, versus 0 RRAs. However, significant differences in the type of glomerular lesion were not seen between genotypic groups, except a trend towards more global glomerulosclerosis and crescent formation in those with ≥1 APOL1 RRAs. As in Larsen et al., we did not detect differences among histologic classes of LN based on APOL1 genotypes, but saw a trend toward higher chronicity index in the ≥1 RRA group (41), with an increased risk for progression to ESRD in cases with at least 1 RRA.
This study has strengths and limitations. Strengths include longitudinal follow-up in a relatively large sample of Brazilians with LN. A weakness included the lack of AIMs in self-described non-white cases and controls due to insufficient DNA; instead, we relied on self-reported ancestry. We note that the “non-white” cases and controls were from the same geographic region, self-reported ancestry was obtained in the same fashion in each group, and APOL1 RRA frequencies were generally consistent with those expected. We note that Parra et al. also found that Brazilians self-reporting as black or mixed had higher proportions of African ancestry (14). Therefore, we restricted our sample to those self-reporting as non-white. Another limitation was absence of SLE controls without LN. However, when comparing LN cases with SLE controls lacking LN, it is possible that some “non-nephropathy controls” may develop LN given longer follow-up. A large number of the LN cases in our cohort first developed kidney disease five (or more) years after their diagnosis of SLE. The infrequent presence of 2 APOL1 RRAs in this cohort and few cases with LN-ESRD did not permit evaluation of APOL1 associations in an autosomal recessive model. However, among the 4 Brazilian LN cases with 2 APOL1 RRAs (Table 5), the only case that had a complete response initially presented with Class V (non-proliferative) membranous LN on kidney biopsy, a less aggressive lesion known to have lower Th1 lymphocytes response (42).
We conclude that frequencies of APOL1 RRAs in non-white Brazilians with LN are not significantly different from those in healthy non-white Brazilians; but participants with ≥1 APOL1 RRA had more severe kidney disease at presentation and higher stages of CKD after therapy compared to those with 0 APOL1 RRA. However, results do not preclude a recessive model. Our sample lacked sufficient numbers of individuals with two APOL1 RRAs needed to detect such an effect. Regardless of treatment for LN, presence of ≥1 APOL1 RRAs is associated with higher rates of chronic tubulo-interstitial injury and increased risk for advanced Stage 4 CKD and ESRD; there was no difference in the type of renal glomerular lesion. APOL1 genotyping in this admixed South American population sheds new light on the role of precision medicine in LN. Treatment approaches may need to be more aggressive or directly target the APOL1 gene in order to reduce rates of ESRD due to LN in the non-white Brazilian population.
Acknowledgements
The authors thank all the patients enrolled in the study, Michelle Tiveron for processing the samples at EPM/UNIFESP and the administrative staff from the Division of Nephrology/Federal University of Pernambuco: Mrs. Poliana Cassia and Mrs. Ivanize Souza.
Funding
B.I.F. received NIH grants: R01 DK084149, R01 DK070941.
Footnotes
Disclosures:Wake Forest University Health Sciences and BIF have rights to an issued US patent related to APOL1 genetic testing (www.apol1genetest.com). Dr. Freedman is a consultant for AstraZeneca Pharmaceuticals and Renalytix AI.
Disclosure
Wake Forest University Health Sciences and B.I.F. have rights to an issued United States patent related to APOL1 genetic testing. B.I.F. is a consultant for AstraZeneca and Renalytix AI Pharmaceuticals. None of the other authors declare competing interests.
References
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of Trypanolytic ApoL1 Variants with Kidney Disease in African Americans. Science 2010;329:841 LP–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsa A, Kao WHL, Xie D, Astor BC, Li M, Hsu C, et al. APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease. N Engl J Med 2013;369:2183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, Agati VD, et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 2015;87:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen CP, Beggs ML, Saeed M, Ambruzs JM, Cossey LN, Messias NC, et al. Histopathologic findings associated with APOL1 risk variants in chronic kidney disease. Mod Pathol 2014;28:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman BI, Langefeld CD, Andringa KK, Croker J a, Williams AH, Garner NE, et al. End-Stage Renal Disease in African Americans With Lupus Nephritis Is Associated With APOL1. Arthritis Rheumatol 2014;66:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langefeld CD, Comeau ME, Ng MCY, Guan M, Dimitrov L, Mudgal P, et al. Genome-wide association studies suggest that APOL1-environment interactions more likely trigger kidney disease in African Americans with nondiabetic nephropathy than strong APOL1–second gene interactions. Kidney Int 2018;94:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, et al. APOL1 Risk Variants Are Strongly Associated with HIV-Associated Nephropathy in Black South Africans. J Am Soc Nephrol 2015;26:2882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pons-Estel GJ, Alarcón GS, Burgos PI, Hachuel L, Boggio G, Wojdyla D, et al. Mestizos with systemic lupus erythematosus develop renal disease early while antimalarials retard its appearance: data from a Latin American cohort. Lupus 2013;22:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richman IB, Taylor KE, Chung SA, Trupin L, Petri M, Yelin E, et al. European Genetic Ancestry Is Associated With a Decreased Risk of Lupus Nephritis. Arthritis Rheum 2012;64:3374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman BI, Wilson CH, Spray BJ, Tuttle AB, Olorenshaw IM, Kammer GM. Familial clustering of end-stage renal disease in blacks with lupus nephritis. Am J Kidney Dis 1997;29:729–32. [DOI] [PubMed] [Google Scholar]
- 12.Kanji Z, Powe CE, Wenger JB, Huang C, Ankers E, Sullivan DA, et al. Genetic Variation in APOL1 Associates with Younger Age at Hemodialysis Initiation. J Am Soc Nephrol 2011;22:2091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzur S, Rosset S, Skorecki K, Wasser WG. APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant 2012;27:1498–505. [DOI] [PubMed] [Google Scholar]
- 14.Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SDJ. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA 2003;100:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lins TC, Vieira RG, Abreu BS, Grattapaglia D, Pereira RW. Genetic composition of Brazilian population samples based on a set of twenty eight ancestry informative SNPs. Am J Hum Biol 2010;22:187–92. [DOI] [PubMed] [Google Scholar]
- 16.Nadkarni GN, Gignoux CR, Sorokin EP, Daya M, Rahman R, Barnes KC, et al. Worldwide Frequencies of APOL1 Renal Risk Variants. N Engl J Med 2018;379:2571–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adhikari K, Mendoza-Revilla J, Chacón-Duque JC, Fuentes-Guajardo M, Ruiz-Linares A. Admixture in Latin America. Curr Opin Genet Dev 2016;41:106–14. [DOI] [PubMed] [Google Scholar]
- 18.Coelho AVC, Moura RR, Cavalcanti CAJ, Guimarães RL. A rapid screening of ancestry for genetic association studies in an admixed population from Pernambuco, Brazil. Genet Mol Res 2015;14:2876–84. [DOI] [PubMed] [Google Scholar]
- 19.Colares VS, Titan SMDO, Pereira ADC, Malafronte P, Cardena MM, Santos S, et al. MYH9 and APOL1 Gene Polymorphisms and the Risk of CKD in Patients with Lupus Nephritis from an Admixture Population. PLoS One 2014;9:e87716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riella C, Siemens TA, Wang M, Campos RP, Moraes TP, Riella LV., et al. APOL1-Associated Kidney Disease in Brazil. Kidney Int Reports 2019;4:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004;15:241–50. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2012;2:139. [Google Scholar]
- 23.CENSO-Instituto Brasileiro de Geografia e Estatística. [Internet]. IBGE 2016. [cited 2017 Jan 1]. Available from: http://www.ibge.gov.br/estadosat/perfil.php?sigla=pe [Google Scholar]
- 24.Silva ZP da, Ribeiro MCS de A, Barata RB, Almeida MF De. Socio-demographic profile and utilization patterns of the public healthcare system (SUS), 2003–2008. [Article in Portuguese] Cien Saude Colet 2011;16:3807–16. [DOI] [PubMed] [Google Scholar]
- 25.Alladagbin DJ, Fernandes PN, Tavares MB, Brito JT, Oliveira GGS, Silva LK, et al. The sickle cell trait and end stage renal disease in Salvador, Brazil. PLoS One 2018;13:e0209036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pena SDJ, Bastos-Rodrigues L, Pimenta JR, Bydlowski SP. DNA tests probe the genomic ancestry of Brazilians. Brazilian J Med Biol Res 2009;42:870–6. [DOI] [PubMed] [Google Scholar]
- 27.Limou S, Dummer PD, Nelson GW, Kopp JB, Winkler C a. APOL1 toxin, innate immunity, and kidney injury. Kidney Int 2015;88:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothhammer F, Dillehay TD. The late pleistocene colonization of South America: An interdisciplinary perspective. Ann Hum Genet 2009;73:540–9. [DOI] [PubMed] [Google Scholar]
- 29.Peng T, Wang L, Li G. The analysis of APOL1 genetic variation and haplotype diversity provided by 1000 Genomes project. BMC Nephrol 2017;18:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav AK, Kumar V, Sinha N, Jha V. APOL1 risk allele variants are absent in Indian patients with chronic kidney disease. Kidney Int 2016;90:906–7. [DOI] [PubMed] [Google Scholar]
- 31.Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, et al. Effect of Genetic African Ancestry on eGFR and Kidney Disease. J Am Soc Nephrol 2015;26:1682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 Kidney Risk Alleles: Population Genetics and Disease Associations. Adv Chronic Kidney Dis; 2014;21:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franceschini N, Haack K, Almasy L, Laston S, Lee ET, Best LG, et al. Generalization of Associations of Kidney-Related Genetic Loci to American Indians. Clin J Am Soc Nephrol 2014;9:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toole JFO, Bruggeman LA, Madhavan S, Sedor JR. The Cell Biology of APOL1. Semin Nephrol 2017;37:538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanwalleghem G, Fontaine F, Lecordier L, Tebabi P, Klewe K, Nolan DP, et al. Coupling of lysosomal and mitochondrial membrane permeabilization in trypanolysis by APOL1. Nat Commun 2015;6:8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma L, Chou JW, Snipes JA, Bharadwaj MS, Craddock AL, Cheng D, et al. APOL1 Renal-Risk Variants Induce Mitochondrial Dysfunction. J Am Soc Nephrol 2016;28:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruggeman LA, Toole JFO, Sedor JR. APOL1 polymorphisms and kidney disease: Loss-of-function or gain-of-function? Am J Physiol Ren Physiol 2019;316:F1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson BR, Howell DN, Soldano K, Garrett ME, Katsanis N, Telen MJ, et al. In vivo Modeling Implicates APOL1 in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress. PLOS Genet 2015;11:e1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruchi R, Genovese G, Lee J, Charoonratana VT, Bernhardy AJ, Alper SL, et al. Copy number variation at the APOL1 locus. PLoS One 2015;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skorecki KL, Lee JH, Langefeld CD, Rosset S, Tzur S, Wasser WG, et al. A null variant in the apolipoprotein L3 gene is associated with non-diabetic nephropathy. Nephrol Dial Transplant 2018;33:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen CP, Beggs ML, Saeed M, Walker PD. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 2013;24:722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaveri SV, Mouthon L, Bayry J. Basophils and Nephritis in Lupus. N Engl J Med 2010;363:1080–2. [DOI] [PubMed] [Google Scholar]