Abstract
Identifying and reducing TB-related costs is necessary for achieving the End TB Strategy’s goal that no family is burdened with catastrophic costs. This study explores costs during the pre-diagnosis period and assesses the potential for using coping costs as a proxy indicator for catastrophic costs when comprehensive surveys are not feasible.
Detailed interviews about TB-related costs and productivity losses were conducted with 196 recently diagnosed adult pulmonary TB patients in Kampala, Uganda. The threshold for catastrophic costs was defined as 20% of household income. Multivariable regression analyses were used to assess the influence of patient characteristics on economic burden, and the positive predictive value (PPV) of coping costs was estimated.
Over 40% of patients in the study experienced catastrophic costs, with average (median) pre-diagnosis costs making up 30.6% (14.1%) of household income. Low-income status (AOR=2.91, 95% CI=1.29, 6.72), hospitalization (AOR=8.66, 95% CI=2.60; 39.54), and coping costs (AOR=3.84, 95% CI=1.81; 8.40) were significantly associated with the experience of catastrophic costs. The PPV of coping costs as a proxy indicator for catastrophic costs was estimated to be 73% (95% CI=58%, 84%).
Pulmonary TB patients endure a substantial economic burden during the pre-diagnosis period, and identifying households that experience coping costs may be a useful proxy measure for identifying catastrophic costs.
Introduction
In 1992, the first study examining the costs of tuberculosis (TB) from the patient perspective was conducted in rural Uganda, concluding that the disease produced a ‘financial shock’ to patients and their families due in large part to the monetary costs and income losses incurred before the patient was able to obtain a diagnosis (Saunderson, 1995). One of the most comprehensive assessments of the economic burden faced by TB-affected households is a systematic review of 49 studies, reporting that total TB-related costs constituted an average 58% of annual income, and that approximately half of the patient costs occur during the time period between symptom onset and diagnosis (Squire et al., 2015; Tanimura, Jaramillo, Weil, Raviglione, & Lonnroth, 2014). Individuals experiencing TB symptoms tend to seek care from multiple non-TB providers, such as pharmacies and traditional healers, before being referred to an appropriate facility for TB screening, and costs accrue at each step of the care-seeking process (Abimbola et al., 2015; Needham, Foster, Tomlinson, & Godfrey-Faussett, 2001; Sekandi et al., 2015; Sreeramareddy, Qin, Satyanarayana, Subbaraman, & Pai, 2014). A recent study in rural Uganda found that the majority of patients first sought care for TB symptoms from a health facility that did not provide TB diagnostic services (Shete et al., 2015). Another study of the TB diagnostic pathway in urban Uganda revealed that 30% of patients sought care from non-TB providers (Sekandi et al., 2015).
Uganda’s National Tuberculosis and Leprosy Programme provides TB screening, diagnosis, and treatment free of charge at public health facilities throughout the country, but the economic burden of obtaining a TB diagnosis persists. Symptomatic individuals who first seek care from private clinics or non-TB providers before being referred to a public health facility incur direct medical costs in the form of consultation fees and non-TB drugs. Individuals also accrue direct non-medical costs for transportation to and from facilities, as well as food and caregiver costs during care-seeking; productivity losses in the form of income lost from missing work; and coping costs. Coping costs, also referred to as dissaving, are defined as borrowing money, selling assets, or requiring a child to quit school as a result of TB disease (Squire et al., 2015; Wingfield et al., 2016).
The measurement and understanding of the economic burden associated with TB have become increasingly more important since the World Health Organization added a milestone addressing catastrophic costs to the End TB Strategy in 2014. The milestone states that 0% of TB-affected families should face catastrophic costs as a result of TB disease (WHO, 2016). While a metric for ‘catastrophic costs’ is not specified in the End TB Strategy, the WHO’s suggested definition for ‘catastrophic’ includes both direct costs and income losses equal to or exceeding 20% of annual household income over the entire duration of TB disease (WHO, 2017). The 20% threshold is evidence-based, as costs above this threshold have been linked to adverse TB outcomes (Wingfield et al., 2014). In an effort to standardise the measurement of patient costs, global TB partners developed the Tool to Estimate Patients’ Costs, a detailed questionnaire designed to collect all TB-related patient costs (direct costs and productivity losses) during both stages (pre-diagnosis and treatment) of TB disease (Stop TB Partnership, 2008). Additionally, several studies propose that coping costs, also known as dissaving, can serve as a proxy indicator for catastrophic costs in circumstances where conducting extensive cost surveys may not be feasible (Madan, Lönnroth, Laokri, & Squire, 2015; Squire et al., 2015; Wingfield et al., 2014; Wingfield et al., 2016).
The purpose of this study was to use the globally accepted Tool to Estimate Patients’ Costs to identify the costs incurred by patients during the pre-diagnosis time period in the high burden TB setting of urban Uganda. We describe the care-seeking pathway of patients, estimate and categorise the costs incurred, and explore the relationship of patient characteristics to economic burden. We also identify the proportion of patients experiencing catastrophic costs and assess the accuracy of using coping costs as a proxy indicator for catastrophic costs. The results of this study contribute to the global understanding of TB-related economic burden and may be useful for interventions aligned with the End TB Strategy goals.
Methods
A retrospective cohort study was conducted among recently diagnosed adult TB patients attending five public health clinics administered under the Kampala Capital City Authority in Uganda. All five health facilities were a part of Uganda’s National Tuberculosis and Leprosy Programme (NTLP), which provides free diagnosis through either Gene Xpert or smear microscopy and free treatment when TB disease is confirmed (NTLP, 2017). Newly diagnosed adult TB patients were invited to participate in the study from July to September 2017. Patients were eligible if they were 18 years or older, spoke Luganda or English, and had a recently confirmed diagnosis of active pulmonary TB. To accurately and consistently identify the duration of the pre-diagnosis period, the sample was limited to patients with pulmonary TB. Trained research personnel conducted in-person interviews with the study cohort to collect information on all costs involved in the pre-diagnosis period, defined as the time from self-reported symptom onset to diagnosis. To minimise the effect of recall bias, interviews were conducted within 30 days of TB diagnosis. Patients were interviewed with an adapted version of the Tool to Estimate Patients’ Costs, translated into Luganda, the dominant dialect in Kampala (Stop TB Partnership, 2008). All participants in the study provided informed written consent, and all study protocols were approved by the Institutional Review Boards at the University of Georgia, the Makerere School of Public Health, and the Uganda National Council of Science and Technology.
Costs
Costs were assessed from the patient perspective in 2017 Uganda Shillings (UGX) and converted into 2017 US Dollars at an exchange rate of 3,600 UGX per $1 (XE Currency Converter, 2017). Costs were assigned one of the following cost categories: direct medical costs (non-TB medications, lab tests, and consultation fees); direct non-medical costs (transportation, phone calls, food, and caregiver costs); and productivity losses (missed time from work). Direct costs were measured by asking the respondent to detail each step of care-seeking behaviour in the pre-diagnosis time period and to describe all out of pocket expenditures attributable to TB symptoms. Direct costs also included the associated caregiver expenses when the participant was accompanied to health facilities. Each step of the care-seeking pathway was placed into one of four direct cost categories: medical facility (which includes TB providers and private non-TB providers), pharmacy, traditional healer/herbalist, or social contact (e.g., a parent or friend). Additionally, direct costs included instances in which the patient purchased extra fruit or protein in an effort to treat TB symptoms, and these costs are categorized as ‘self’.
Productivity losses were estimated by deriving a daily wage from the respondents’ self-reported average monthly income and multiplying this daily wage by the reported days of work missed due to TB disease prior to diagnosis. For unemployed participants, an opportunity cost of time approach was used to assign a daily income equivalent to that of the unskilled wage rate in Uganda ($1.39/day) (Drummond, Sculpher, Claxton, Stoddart, & Torrance, 2015; Kafeero, 2017; Mesfin et al., 2010). To determine household income, patients were asked: “What is the combined monthly income of all household members, including you?”. Total household income during the pre-diagnosis period was estimated by multiplying household monthly income by the number of months the patient spent seeking TB diagnosis. The threshold for catastrophic costs was defined as 20% of total household income during the pre-diagnosis period. Patients were identified as experiencing coping costs if anyone in the household had to borrow money or sell assets to finance TB disease, or if a child had to quit school.
Data analysis
Surveys were scanned into a database using optical scanning software and verified by research assistants for quality control. Direct medical costs, direct non-medical costs, and productivity losses were summed to produce total costs, and total costs were divided by the total household income during the pre-diagnosis period to produce an estimate of costs as a proportion of household income. A variable was created to identify low-income respondents (earning less than $47.53/month), as described in the 2013 Uganda National Household Survey and inflated to 2017 values using the Uganda Consumer Price Index, Kampala Low Income group (Uganda Bureau of Statistics, 2017; Uganda Bureau of Statistics, 2014).
Multivariable regression analyses were used to assess the influence of patient characteristics and diagnostic delay on patient costs. Specifically, patient characteristics of gender, age, educational attainment, HIV status, and income status were included in order to produce results comparable to existing and future TB cost studies, especially those using the Tool the Estimate Patient Costs (Fuady, Houweling, Mansyur, & Richardus, 2018; Kemp, Mann, Simwaka, Salaniponi, & Squire, 2007; Mauch et al., 2011; Ukwaja, Alobu, Lgwenyi, & Hopewell, 2013). Generalized models were estimated for four dependent variables of interest: direct costs, productivity losses, total costs, and total costs as a percentage of household income. Due to the right skew of the cost data, models using a log-linked gamma function were fitted. The majority of respondents did not incur productivity losses; therefore, two models consisting of a logistic regression (in which the dependent variable is the binary indicator of productivity losses) and a gamma regression (in which the dependent variable is the continuous amount of income lost) were constructed for this variable. The binary outcomes of coping costs and catastrophic costs were analysed with logistic regression. For the model of catastrophic costs, additional independent variables identified as being important predictors of catastrophic costs (experiencing coping costs, being hospitalized, and quitting/losing one’s job) were included (Fuady et al., 2018; Wingfield et al., 2016), and the multiple regression was optimized using AIC stepwise selection in order to focus on the estimates of best fit. Lastly, the positive predictive value (PPV) and its corresponding 95% confidence interval were estimated to assess the accuracy of using coping costs as a proxy indicator of catastrophic costs. All data were analysed using R Statistics (R Core Team, 2014).
Results
Study sample
A total of 224 patients presented to study sites during the study period, and all were invited to participate in the study. Seven patients declined to participate, and sixteen patients did not fulfil the eligibility criteria. Additionally, five patients were dropped from the sample due to receiving care in multiple countries with multiple currencies and incomparable cost data.
Study participants
In the sample of 196 participants, most were male (60.2%), and the mean age was 31.7 years (Table 1). Nearly half of the study participants (43.4%) did not complete more than the primary level of education, and 21.4% of participants were unemployed. HIV status was known for 98.5% of participants, and 32.7% were HIV-positive. When comparing the distribution of household income groups of the participants to that of Kampala, the study sample tended to have a higher proportion of households in the lower income groups, and 17.3% of the sample were categorized as low-income. The average (median) monthly income for those in the low-income group was $30 ($31), and the average (median) income of the higher income group was $171 ($111).
Table 1:
Participant characteristics, N=196
| Participant characteristics | % | n | |
| Sex | |||
| Female | 39.8 | 78 | |
| Male | 60.2 | 118 | |
| Age | |||
| 18–25 | 35.7 | 70 | |
| 26–40 | 44.9 | 88 | |
| >40 | 19.4 | 38 | |
| HIV status | |||
| Positive | 32.7 | 64 | |
| Negative | 65.8 | 129 | |
| Unknown | 1.5 | 3 | |
| Educationa | |||
| None | 4.1 | 8 | |
| Primary level | 39.3 | 77 | |
| Secondary level | 41.8 | 82 | |
| Post-secondary level | 10.7 | 21 | |
| University degree | 3.6 | 7 | |
| Occupation | |||
| Employed | 78.6 | 154 | |
| Not employed | 21.4 | 42 | |
| Low-income for Kampalab | 17.3 | 34 | |
| Household income groups | Study sample | Kampala (%) | |
| Up to $28 | 8.7 | 17 | 4.0 |
| >$28 – $56 | 21.4 | 42 | 11.2 |
| >$56 – $83 | 16.3 | 32 | 11.6 |
| >$83 – $139 | 23.5 | 46 | 24.9 |
| >$139 | 30.1 | 59 | 47.4 |
Primary education refers to the first seven years of school; Secondary refers to years 8–14; Post-secondary refers to more than 14 years of schooling.
Defined as earning below the value of mean consumption in Kampala ($47.53 per month)
Care-seeking behaviour and pre-diagnosis costs
The average (median) length of time between symptom onset and diagnosis was 2.5 (2) months, with 68.4% of patients having a delay between 1 and 3 months. The 196 patients in the sample took a total of 1,239 “steps” in their path toward diagnosis, with an average (median) of 6 (6) steps. The majority (62%) of patient steps involved visits to a medical facility, and the direct costs of these visits made up nearly 75% of total direct costs (Table 2). Nearly half (48%) of first steps were to a medical facility, but only 3.6% of the sample was diagnosed by TB providers during this first step. Female patients tended to take, on average, one more step than male patients (see online supplement).
Table 2:
Summary of care-seeking pathways and associated direct costs for 196 patients
| Step category | n | % | Mean $ | % Direct Costs |
|---|---|---|---|---|
| Medical facility | 769 | 62.1% | $32 | 74.4% |
| Pharmacy | 137 | 11.1% | $3 | 7.0% |
| Traditional healer/herbalist | 117 | 9.4% | $5 | 11.6% |
| Social contact | 216 | 17.4% | $1 | 2.3% |
| Self | -- | -- | $2 | 4.7% |
| Total | 1239 | 100.0% | $43 | 100.0% |
NOTES: Medical facilities include both public TB providers and private non-TB providers. Social contacts include family members, friends, co-workers, and other acquaintances. Self refers to patient expenditures on fruit or protein in an effort to treat TB symptoms. The ‘n’ column refers to the number of steps/visits to each step category.
Less than half of the sample experienced productivity losses (48%), and the average (median) days of work missed due to TB disease was 6 (0). For the subset of patients who did report productivity losses, the average (median) days missed was 13 (7). Approximately 26% of patients experienced coping costs.
Patients incurred an average of $62 in total TB-related costs from the time of symptom onset to diagnosis, corresponding to an average of 30.6% of household income during the care-seeking period (Table 3). Direct medical costs comprised the largest percentage of total costs (54.8%), followed by productivity losses (30.7%) and direct non-medical costs (14.5%).
Table 3:
Patient costs during the pre-diagnosis period, N=196
| Cost category | Mean | Median (IQR) | % of Total Costs |
|---|---|---|---|
| Total Direct Costs | $43 | $25 ($11, $49) | 69.3% |
| Direct Medical | $34 | $19 ($7, $41) | 54.8% |
| Lab/X-ray | $6 | $3 ($0, $11) | |
| Non-TB medicationa/Fees | $28 | $11 ($5, $30) | |
| Direct Non-medical | $9 | $4 ($1, $10) | 14.5% |
| Transportation | $5 | $2 ($1, $5) | |
| Food | $3 | $0 ($0, $3) | |
| Phone calls | $0.24 | $0 ($0, $0.28) | |
| Caregiver | $1 | $0 ($0, $.35) | |
| Productivity Losses | $19 | $0 ($0, $15) | 30.7% |
| Patients reporting Productivity Losses (n=94) | $39 | $17 ($6, $40) | |
| Total | $62 | $31 ($12, $62) | 100% |
| % HH income | 30.6% | 14.1% (5.3%, 35.4%) | |
Non-TB medication refers to non-TB medications purchased by the patient for the treatment of TB symptoms (e.g., cough suppressants). This category does not include non-TB medications purchased to treat comorbid illnesses or non-TB symptoms.
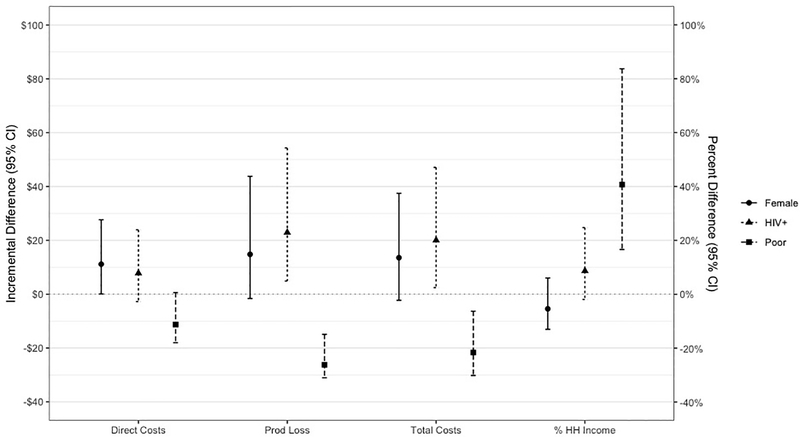
Figure 1 presents the results of the multivariable regression analysis for each cost category, with coefficients of the log-linked gamma regression analysis transformed into dollar values or percentage points for interpretable group comparisons. Differences between groups with regard to direct costs, productivity losses, and total costs should be interpreted using the dollar values (left axis). Differences in total costs as a percentage of household income should be interpreted using the percentage points (right axis). Low-income status, sex, and HIV status are significantly associated with economic burden. Additionally, patients with longer pre-diagnosis periods incurred significantly higher direct and total costs (see online supplement).
Figure 1: Differences in costs between groups, by cost category.
Figure 1 transforms the coefficients for sex, HIV, and low-income status into dollar values for the incremental difference (and 95% confidence interval) between groups. The left axis presents the difference in dollars and should be used to interpret differences in direct costs, productivity losses, and total costs. The right axis presents the difference in percentage points and should be used to interpret differences in total costs as a percentage of household income.
Low-income status is strongly associated with costs, as poorer patients incur consistently lower costs while simultaneously losing a higher proportion of household income to pre-diagnosis TB care. Total costs for low-income patients were, on average, $22 (95% CI = -$30, -$6) lower than the total costs of higher income patients, while the percentage of household income lost by poor patients was nearly 41 percentage points (95% CI = 17, 84) higher. Productivity losses are significantly lower for low-income patients when valued in dollars (incremental difference = -$26; 95% CI = -$31, -$15). However, further analysis revealed that the number of days missed from work did not differ across income groups (see online supplement).
Sex and HIV status were also significantly associated with costs. Female patients spent an average of $11 more on direct costs (95% CI = $0, $28), while HIV-positive patients incurred an average of $23 more in productivity losses (95% CI = $5, $54) and $20 more in total costs (95% CI = $2, $47). Age and education level did not appear to influence costs.
In logistic regression analyses, low-income status (AOR = 3.05; 95% CI = 1.26, 7.32) and hospitalization (AOR = 9.32; 95% CI = 3.37, 28.2) were significantly associated with the occurrence of coping costs, while there were no significant predictors of whether or not a patient would incur productivity losses (see online supplement).
Catastrophic Costs
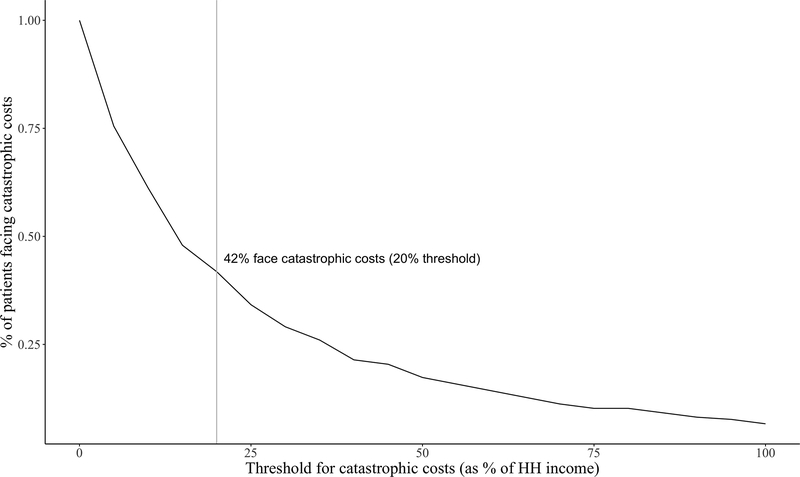
The threshold for catastrophic costs was defined as 20% of estimated household income during the pre-diagnosis period. Almost half (41.8%) of TB patients in this sample experienced catastrophic costs during the pre-diagnosis period at this threshold (Figure 2).
Figure 2: Proportion of patients experiencing catastrophic costs, by threshold.
Figure 2 depicts the proportion of patients in the sample experiencing catastrophic costs at various thresholds, with the grey line highlighting the 20% threshold for this study.
The results of the multiple logistic regression analysis (Table 4) revealed hospitalization to have the strongest impact on the odds of experiencing catastrophic costs (AOR = 8.66, 95% CI = 2.60; 39.54), followed by the experience of coping costs (AOR = 3.84, 95% CI = 1.81; 8.40) and low-income status (AOR = 2.91, 95% CI = 1.29, 6.72). When the 23 hospitalised patients were removed from the sample, coping costs remained significant in the multiple regression model (AOR = 3.71, 95% CI = 1.69; 8.41; see online supplement).
Table 4:
Predictors of catastrophic costs
| Variable | Univariate Logistic Regression | Multiple Logistic Regression | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Coping costs >0 | 5.87 (2.95, 12.26) | 0.000 | 3.84 (1.81, 8.40) | 0.001 |
| Poora | 2.68 (1.26, 5.85) | 0.011 | 2.91 (1.29, 6.72) | 0.011 |
| Hospitalized | 11.94 (3.90, 52.10) | 0.000 | 8.66 (2.60, 39.54) | 0.001 |
| Quit job | 4.81 (1.77, 15.38) | 0.004 | -- | -- |
| Female | 1.03 (0.58, 1.84) | 0.914 | -- | -- |
| HIV+ | 1.15 (0.63, 2.11) | 0.648 | -- | -- |
| Age | 0.87 (0.58, 1.28) | 0.470 | -- | -- |
| Education | 0.95 (0.67, 1.32) | 0.744 | -- | -- |
NOTES: Univariate logistic regressions were used to identify contributory variables (p<0.2), which were then subjected to AIC forward selection to produce an optimized multiple logistic regression model. The adjusted odds ratio, 95% confidence interval, and p-value are shown for the three selected variables.
Earning less than $47.53 per month
Accuracy of Coping Costs as a proxy indicator of Catastrophic Costs
The proportion of individuals experiencing coping costs who also experienced catastrophic costs is shown in Table 5 as a positive predictive value (PPV) of 73% (95% CI = 58%, 84%), and the proportion of patients who experienced neither coping costs nor catastrophic costs is shown as a negative predictive value (NPV) of 69% (95% CI = 61%, 76%). In a sensitivity analysis removing all patients who were hospitalised, the PPV was 63% (95% CI = 45%, 79%) and the NPV was 71% (63%, 78%; see online supplement).
Table 5:
Predictive value of coping costs as a proxy indicator for catastrophic costs
| Test | Estimate | 95% CI |
|---|---|---|
| Positive predictive value (PPV) | 0.73 | (0.58, 0.84) |
| Negative predictive value (NPV) | 0.69 | (0.61, 0.76) |
| Sensitivity | 0.45 | (0.34, 0.57) |
| Specificity | 0.88 | (0.80, 0.93) |
NOTES: The estimate and 95% confidence interval are shown for each measure of predictive value.
Discussion
The results of this retrospective study suggest that TB patients in a high-burden setting incur a substantial economic burden in the pre-diagnosis period. Average (median) pre-diagnosis costs made up 30.6% (14.1%) of household income, and nearly half of patients experienced catastrophic costs. The length of the pre-diagnosis period was associated with care-seeking costs, which is likely due to patients visiting multiple non-TB providers before receiving a referral to appropriate facilities (Mesfin et al., 2010; Sekandi et al., 2015; Vassall, Seme, Compernolle, & Meheus, 2010). Over two thirds (68.4%) of patients in this study experienced pre-diagnosis periods between one and three months, and a number of studies have presented pre-diagnosis periods of similar length (Mauch et al., 2011; Needham, Godfrey-Faussett, & Foster, 1998; Sekandi et al., 2015; Ukwaja et al., 2013). The composition of the care-seeking pathway (pharmacies, herbalists, medical facilities, and social contacts) was similar to prior studies in both urban and rural Uganda, and patients in this study took a median of six steps during care-seeking, which was slightly greater than these studies (Sekandi et al., 2015; Shete et al., 2015).
A large portion of total costs (54.8%) included direct medical expenditures in the form of health services fees, non-TB medications, and laboratory tests. Direct non-medical costs accounted for a relatively low proportion of total costs, perhaps because the study took place in an urban setting where patients did not require lengthy transport to access public health facilities. Overall, average costs due to productivity losses were also low, as fewer than half of patients in the sample missed work due to TB illness. However, for the 96 patients who did miss work, the losses were substantial. Other studies examining productivity losses due to TB illness find that this cost category often makes up the largest portion of total costs over the entire course of the disease, due to the length of the treatment phase and treatment methods requiring patients to frequently travel to clinics (Aspler et al., 2008; John, Daley, Kincler, Oxlade, & Menzies, 2009; Mauch et al., 2011; Squire et al., 2015).
Patients with higher household incomes incurred higher total costs (higher direct costs and higher productivity losses) in absolute terms, but low-income patients spent a higher proportion of their household income during the pre-diagnosis period. Poor patients were also over 2.5 times more likely to experience coping costs. These findings are consistent with other studies examining costs by socioeconomic status (Kemp et al., 2007; Lönnroth, Aung, Maung, Kluge, & Uplekar, 2007; Ukwaja et al., 2013; Wingfield et al., 2016). Female patients in this sample incurred marginally higher direct costs, but sex did not remain a significant predictor of total costs. A review of the literature produces no established associations between TB costs and the age, sex, or education level of the patient (Mauch et al., 2011; Tanimura et al., 2014; Ukwaja et al., 2013). TB/HIV patients were no more likely to incur productivity losses than their HIV-negative counterparts; however, in the subsample of patients reporting lost income, HIV-positive patients realized significantly higher losses. Being HIV-positive was also associated with higher total costs, and this added economic burden has been documented in similar studies (Mauch et al., 2011; Prasanna et al., 2018; Ukwaja et al., 2013; Vassall et al., 2010).
Over 40% of patients in this study experienced catastrophic costs during the pre-diagnosis period. This metric is difficult to compare to other studies due to differing definitions of ‘catastrophic’ across varying time periods but is nonetheless informative. A review of eight studies found that the pre-diagnosis period accounted for, on average, only half of the total costs for the entire duration of TB disease, revealing that the pre-diagnosis cost burden is likely to be sustained throughout the treatment phase as well (Squire et al., 2015; Tanimura et al., 2014). Previous studies have explored the possibility of using coping costs, or dissaving, as a proxy indicator for catastrophic costs when extensive cost surveys are infeasible (Squire et al., 2015; Wingfield et al., 2014), and the results found here support their findings. Patients in this study who experienced coping costs were significantly more likely to also have experienced catastrophic costs, and coping costs appear to serve as a reliable barometer for the assessment of catastrophic costs in studies that may not cover the entire duration of TB disease.
Limitations
A potential limitation of this study is the reliance on patients’ self-reported income data, as measures of consumption expenditure are regarded as more theoretically appropriate in depicting the impact of TB costs on household resources (Sweeney, et al., 2018). Most study participants had difficulty answering the income question, as many do not receive a consistent monthly salary, and it is possible that TB symptoms during the recall period may have resulted in under-reporting. A number of similar studies also report challenges with income data; some use a standard of living survey combined with national income groups to proxy income (Kemp et al., 2007; Lönnroth et al., 2007; Mauch et al., 2011), while others assign a constant value to all respondents’ time (Steffen et al., 2010; Umar, Fordham, Abubakar, & Bachmann, 2012). However, for this study, sufficient data were not obtained to utilise the standard of living proxy or to measure consumption expenditures, and the constant value approach was used only for those reporting no household income.
The definition of ‘catastrophic costs’ used in this study is not readily comparable to other studies with similar purposes. The definition in this study considers the time period over which the costs are occurring and therefore reveals the projected economic burden on the patient if costs were to remain at this level for a sustained period of time. For an examination of pre-diagnosis costs only, this definition seemed most appropriate.
Additionally, there is speculation that the economic burden associated with obtaining TB diagnosis is insurmountable for some, and this study only included participants who were able to afford a diagnosis. It is therefore likely that this study sample has a higher average household income than the average household income of all individuals with TB in Kampala. Finally, there is potential for recall bias. Patients were interviewed within 30 days of receiving a TB diagnosis to minimise such bias, but it is possible that patients either under- or over-reported care-seeking costs. Despite these limitations, this study presents an informative description of the economic burden incurred by TB patients during the pre-diagnosis period in a high-burden setting.
Implications
These findings have important implications for tuberculosis policy and program implementation in low-income countries. First, because patients incur significant costs before diagnosis, the current policy of free TB diagnosis and treatment is insufficient for achieving the End TB Strategy’s goal that 0% of families face catastrophic costs as a result of TB disease (WHO, 2016). Various social protection initiatives, such as direct cash transfers and temporary disability grants, show potential for reducing the economic burden of disease; however, the financial, ethical, and operational challenges of such programs are considerable (Boccia et al., 2016; Richter et al., 2014; Richterman et al., 2018; Rudgard et al., 2017). Second, policies aimed at shortening the length of diagnostic delay are likely to reduce care-seeking costs. Public health community outreach and education campaigns can increase awareness among potential TB patients as well as among non-TB health providers, leading to more rapid referrals to appropriate health facilities. The implementation and effectiveness of comprehensive TB strategies that combine community health education, affordable diagnosis and care, and social protection measures are a fitting topic for future TB policy research.
Conclusions
The economic burden of accessing TB diagnosis can be catastrophic for patients and their households. Social protection for the very poor and strategies for shortening the length of diagnostic delay may serve to reduce the incidence of economic catastrophe. Finally, identifying households that experience coping costs appears to be a useful proxy measure for assessing catastrophic costs, especially when a comprehensive evaluation of costs is not feasible.
Supplementary Material
Acknowledgements
We would like to express our gratitude to the COHSONET research study team, Damalie Nakkonde, Denis Kimbugwe, Jonah Lubega, Nicolas Kawuki, Mustafah Mubiru, and Joan Namatovu, for their valuable role in data collection. We would also like to thank Robert Kakaire and Hannah Southall from the University of Georgia College of Public Health for assisting us with data management.
Funding: This work was partly supported by the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (R01 AI093856).
Footnotes
Disclosure statement: No potential conflict of interest was reported by the authors.
Ethics: All study procedures were approved by the Institutional Review Boards at the University of Georgia, the Makerere School of Public Health, and the Uganda National Council of Science and Technology.
Contributor Information
Rebecca L Walcott, University of Washington, Evans School of Public Policy & Governance, Seattle, WA USA.
Justin B Ingels, University of Georgia, College of Public Health, Athens, GA USA.
Phaedra S Corso, Kennesaw State University, Office of Research, Kennesaw, GA USA.
Sarah Zalwango, Kampala Capital City Authority, Directorate of Public Health and Environment, Kampala, Uganda.
Christopher C Whalen, University of Georgia, Global Health Institute, Athens, GA USA.
Juliet N Sekandi, University of Georgia, Global Health Institute, Athens, GA USA.
References
- Abimbola S, Ukwaja KN, Onyedum CC, Negin J, Jan S, & Martiniuk AL (2015). Transaction costs of access to health care: Implications of the care-seeking pathways of tuberculosis patients for health system governance in Nigeria. Glob Public Health, 10(9), 1060–1077. doi: 10.1080/17441692.2015.1007470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspler A, Menzies D, Oxlade O, Banda J, Mwenge L, Godfrey-Faussett P, & Ayles H (2008). Cost of tuberculosis diagnosis and treatment from the patient perspective in Lusaka, Zambia. Int J Tuberc Lung Dis, 12(8), 928–935. [PubMed] [Google Scholar]
- Boccia D, Pedrazzoli D, Wingfield T, Jaramillo E, Lonnroth K, Lewis J,… Evans CA (2016). Towards cash transfer interventions for tuberculosis prevention, care and control: key operational challenges and research priorities. BMC Infect Dis, 16(1), 307. doi: 10.1186/s12879-016-1529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, & Torrance GW (2015). Methods for the economic evaluation of health care programmes: Oxford university press. [Google Scholar]
- Fuady A, Houweling TAJ, Mansyur M, & Richardus JH (2018). Catastrophic total costs in tuberculosis-affected households and their determinants since Indonesia’s implementation of universal health coverage. Infectious diseases of poverty, 7(1), 3. doi: 10.1186/s40249-017-0382-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John KR, Daley P, Kincler N, Oxlade O, & Menzies D (2009). Costs incurred by patients with pulmonary tuberculosis in rural India. Int J Tuberc Lung Dis, 13(10), 1281–1287. [PubMed] [Google Scholar]
- Kafeero S (2017, June 2). Government sets Shs130,000 minimum wage. Daily Monitor. Retrieved from http://www.monitor.co.ug/News/National/Government-sets-Shs130000-minimum-wage/688334-3952236-dtv5xs/index.html
- Kemp JR, Mann G, Simwaka BN, Salaniponi FM, & Squire SB (2007). Can Malawi’s poor afford free tuberculosis services? Patient and household costs associated with a tuberculosis diagnosis in Lilongwe. Bulletin of the World Health Organization, 85(8), 580–585. doi: 10.2471/blt.06.033167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth K, Aung T, Maung W, Kluge H, & Uplekar M (2007). Social franchising of TB care through private GPs in Myanmar: an assessment of treatment results, access, equity and financial protection. Health Policy and Planning, 22(3), 156–166. doi: 10.1093/heapol/czm007 [DOI] [PubMed] [Google Scholar]
- Madan J, Lönnroth K, Laokri S, & Squire SB (2015). What can dissaving tell us about catastrophic costs? Linear and logistic regression analysis of the relationship between patient costs and financial coping strategies adopted by tuberculosis patients in Bangladesh, Tanzania and Bangalore, India. BMC health services research, 15(1), 476. doi: 10.1186/s12913-015-1138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch V, Woods N, Kirubi B, Kipruto H, Sitienei J, & Klinkenberg E (2011). Assessing access barriers to tuberculosis care with the tool to Estimate Patients’ Costs: pilot results from two districts in Kenya. BMC Public Health, 11(1), 43. doi: 10.1186/1471-2458-11-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesfin MM, Newell JN, Madeley RJ, Mirzoev TN, Tareke IG, Kifle YT,… Walley JD (2010). Cost implications of delays to tuberculosis diagnosis among pulmonary tuberculosis patients in Ethiopia. BMC Public Health, 10(1), 173. doi: 10.1186/1471-2458-10-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Tuberculosis and Leprosy Program (2017). Manual for Management and Control of Tuberculosis and Leprosy. Uganda Ministry of Health, 2017. [Google Scholar]
- Needham DM, Foster SD, Tomlinson G, & Godfrey-Faussett P (2001). Socio-economic, gender and health services factors affecting diagnostic delay for tuberculosis patients in urban Zambia. Trop Med Int Health, 6(4), 256–259. doi: 10.1046/j.1365-3156.2001.00709.x [DOI] [PubMed] [Google Scholar]
- Needham DM, Godfrey-Faussett P, & Foster SD (1998). Barriers to tuberculosis control in urban Zambia: the economic impact and burden on patients prior to diagnosis. Int J Tuberc Lung Dis, 2(10), 811–817. [PubMed] [Google Scholar]
- Prasanna T, Jeyashree K, Chinnakali P, Bahurupi Y, Vasudevan K, & Das M (2018). Catastrophic costs of tuberculosis care: a mixed methods study from Puducherry, India. Glob Health Action, 11(1), 1477493. doi: 10.1080/16549716.2018.1477493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Richter LM, Lonnroth K, Desmond C, Jackson R, Jaramillo E, & Weil D (2014). Economic support to patients in HIV and TB grants in rounds 7 and 10 from the global fund to fight AIDS, tuberculosis and malaria. PloS one, 9(1), e86225. doi: 10.1371/journal.pone.0086225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richterman A, Steer-Massaro J, Jarolimova J, Luong Nguyen LB, Werdenberg J, & Ivers LC (2018). Cash interventions to improve clinical outcomes for pulmonary tuberculosis: systematic review and meta-analysis. Bull World Health Organ, 96(7), 471–483. doi: 10.2471/BLT.18.208959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudgard WE, Evans CA, Sweeney S, Wingfield T, Lonnroth K, Barreira D, & Boccia D (2017). Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study. PLoS Med, 14(11), e1002418. doi: 10.1371/journal.pmed.1002418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunderson PR (1995). An economic evaluation of alternative programme designs for tuberculosis control in rural Uganda. Soc Sci Med, 40(9), 1203–1212. doi: 10.1016/0277-9536(94)00240-t [DOI] [PubMed] [Google Scholar]
- Sekandi JN, Zalwango S, Martinez L, Handel A, Kakaire R, Nkwata AK,… Whalen CC (2015). Four Degrees of Separation: Social Contacts and Health Providers Influence the Steps to Final Diagnosis of Active Tuberculosis Patients in Urban Uganda. BMC Infect Dis, 15(1), 361. doi: 10.1186/s12879-015-1084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shete PB, Haguma P, Miller CR, Ochom E, Ayakaka I, Davis JL,… Cattamanchi A (2015). Pathways and costs of care for patients with tuberculosis symptoms in rural Uganda. Int J Tuberc Lung Dis, 19(8), 912–917. doi: 10.5588/ijtld.14.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire S, Thomson R, Namakhoma I, El Sony A, Kritski A, & Madan J (2015). Catastrophic care-seeking costs as an indicator for lung health. BMC Proceedings, 9(Suppl 10): S4. doi: 10.1186/1753+6561-9-S10-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeramareddy CT, Qin ZZ, Satyanarayana S, Subbaraman R, & Pai M (2014). Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis, 18(3), 255–266. doi: 10.5588/ijtld.13.0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen R, Menzies D, Oxlade O, Pinto M, de Castro AZ, Monteiro P, & Trajman A (2010). Patients’ costs and cost-effectiveness of tuberculosis treatment in DOTS and non-DOTS facilities in Rio de Janeiro, Brazil. PloS one, 5(11), e14014. doi: 10.1371/journal.pone.0014014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stop TB Partnership. (2008). The Tool to Estimate Patients’ Costs. Retrieved from http://stoptb.org/wg/dots_expansion/tbandpoverty/assets/documents/TooltoestimatePatients%27Costs.pdf
- Sweeney S, Mukora R, Candfield S, Guinness L, Grant AD, & Vassall A. (2018). Measuring income for catastrophic cost estimates: Limitations and policy implications of current approaches. Social Science & Medicine, 215:7–15. doi: 10.1016/j.socscimed.2018.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura T, Jaramillo E, Weil D, Raviglione M, & Lonnroth K (2014). Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J, 43(6), 1763–1775. doi: 10.1183/09031936.00193413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda Bureau of Statistics. Uganda Consumer Price Index: 2009/10=100. (2017). Kampala, Uganda: Uganda Bureau of Statistics; Retrieved from https://www.ubos.org/wpcontent/uploads/publications/CPI%20Publication%20for%20August%202017.pdf [Google Scholar]
- Uganda Bureau of Statistics. Uganda National Household Survey 2012/2013. (2014). Kampala, Uganda: Uganda Bureau of Statistics; Retrieved from https://www.ubos.org/onlinefiles/uploads/ubos/UNHS_12_13/2012_13%20UNHS%20Final%20Report.pdf [Google Scholar]
- Ukwaja KN, Alobu I, Lgwenyi C, & Hopewell PC (2013). The high cost of free tuberculosis services: patient and household costs associated with tuberculosis care in Ebonyi State, Nigeria. PloS one, 8(8), e73134. doi: 10.1371/journal.pone.0073134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar NA, Fordham R, Abubakar I, & Bachmann M (2012). The indirect cost due to pulmonary Tuberculosis in patients receiving treatment in Bauchi State—Nigeria. Cost Effectiveness and Resource Allocation, 10(1), 6. doi: 10.1186/1478-7547-10-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassall A, Seme A, Compernolle P, & Meheus F (2010). Patient costs of accessing collaborative tuberculosis and human immunodeficiency virus interventions in Ethiopia. Int J Tuberc Lung Dis, 14(5), 604–610. [PubMed] [Google Scholar]
- Wingfield T, Boccia D, Tovar M, Gavino A, Zevallos K, Montoya R,… Evans CA (2014). Defining Catastrophic Costs and Comparing Their Importance for Adverse Tuberculosis Outcome with Multi-Drug Resistance: A Prospective Cohort Study, Peru. PLoS medicine, 11(7), e1001675. doi: 10.1371/journal.pmed.1001675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield T, Tovar MA, Huff D, Boccia D, Montoya R, Ramos E,… Evans CA (2016). The economic effects of supporting tuberculosis-affected households in Peru. Eur Respir J, 48(5), 1396–1410. doi: 10.1183/13993003.00066-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). WHO End TB Strategy. Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- World Health Organization. (2017). Global tuberculosis report 2017.
- XE Currency Converter. Currency exchange rates. Retrieved July 2017, from http://www.xe.com/currencyconverter/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.