Abstract
Objective:
We examined the association between sleep and odds of developing knee pain, and whether this relationship varied by status of widespread pain (WSP).
Design:
At the 60-month visit of the Multicenter Osteoarthritis Study, sleep quality and restless sleep were each assessed by using a single item from two validated questionnaires; each sleep measure was categorized into three levels with poor/most restless sleep as the reference. WSP was defined as pain above and below the waist on both sides of the body and axially using a standard homunculus, based on the American College of Rheumatology criteria. Outcomes from 60-84 months included 1) knee pain worsening (KPW, defined as minimal clinically important difference in WOMAC pain], 2) prevalent and 3) incident consistent frequent knee pain. We applied Generalized Estimating Equations in multivariable logistic regression models.
Results:
We studied 2329 participants (4658 knees) [67.9y, BMI: 30.9], We found that WSP modified the relationship between sleep quality and KPW (p<0.01 for interaction). Among persons with WSP, odds ratio (95% confidence interval) for KPW was 0.54 (0.35, 0.78) for those with very good sleep quality (p-trend<0.001); additionally, we found the strongest association of sleep quality in persons with >8 painful joint sites (p-trend<0.01), but not in those with ≤ 2 painful joint sites. Similar results were observed using restless sleep, in the presence of WSP. Cross-sectional relationship was suggested significant between sleep and prevalence of consistent frequent knee pain.
Conclusion:
Better sleep was related to less knee pain worsening with coexisting widespread pain.
Keywords: Sleep, widespread pain, knee osteoarthritis pain
Introduction
Knee osteoarthritis (OA) is the most common cause of chronic knee pain in middle aged and older adults (1, 2). With OA as one of the 10 leading causes of function loss and disability worldwide (3), pain in knee OA drives many patients to seek medical help and treatment. Hence, pain management remains a major goal of OA treatment. Because the prevalence of widespread pain and poor sleep are high in persons with knee OA (4–6), understanding these relationships altogether may provide new insights of pain management through sleep.
Sleep disturbance is more prevalent in patients with knee OA than those without OA (4), with about 50-80% of persons with knee pain or knee OA experiencing sleep problems (5–8). Poor sleep quality, such as insomnia and short sleep duration is related to increased systemic inflammatory cytokines (9), such as the C-reactive protein (10). Emerging evidence has also suggested the role of low-grade systemic inflammation and inflammatory synovium in the development and progression of pain symptoms and cartilage damage in osteoarthritis (10, 11). Together, this evidence implies a plausible link between sleep and painful knee OA.
Historically, a reciprocal relationship has been noted between sleep and chronic pain, especially for those with widespread pain (8, 12, 13). A recent review of longitudinal studies and clinical trials concluded that disordered sleep is more likely to precede pain than vice versa (14). Additionally, greater numbers of painful joint sites predicted worsened pain outcomes even after knee or hip arthroplasty (15); and widespread pain was associated with higher odds of knee pain worsening, even though the relationship was not statistically significant (6). This evidence suggests that chronic multi-joint pain could further affect worsened knee pain.
Although the tools or instruments vary regarding sleep measures, sleep has been suggested as a predictor of the development of widespread pain (16–18). However, data on sleep in relation to knee pain per se are limited (19, 20), and often did not account for coexisting widespread pain in this relationship. In this study, we examined the association of two different sleep measures, sleep quality and restless sleep with 1) knee pain worsening and 2) consistent frequent knee pain, stratified by baseline widespread pain status (absence and presence). Given the literature cited above documenting an association of sleep with widespread pain, and poor sleep as one of the characteristic symptoms in chronic central pain (21), we hypothesized that better sleep quality or less frequent restless sleep might improve knee pain to a greater degree among those with widespread pain than those without it. We further examined whether the number of painful joint sites affected the sleep and knee pain relationship.
Methods
The Multicenter Osteoarthritis Study (MOST) is a prospective study, focusing on symptomatic knee OA in a community-based sample of adults with or at high risk of knee OA, based on the presence of knee symptoms, history of knee injury or surgery or being overweight. At the beginning in 2003, 3,026 individuals (60.1% women) aged 50-79 years were enrolled from Birmingham, Alabama and Iowa City, Iowa. Those who had rheumatoid arthritis or other forms of inflammatory arthritis were not eligible for the study. Those with fibromyalgia were neither identified nor excluded in MOST. The details of MOST have been described elsewhere (22). After enrolment, participants were followed for up to 84 months. The institutional review boards [University of California, San Francisco (10-00500), Boston University (H-32956); University of Alabama at Birmingham (IRB-000329007) and University of Iowa (200003064 MOST-R)] granted approval for the MOST study. All participants provided written informed consent for study participation.
Sample of the study participants
We included 2,329 participants (4,658 knees) with valid values for sleep variables at 60 months (baseline of the study) regardless of their radiographic knee OA status in the analyses for knee pain worsening. For the outcome of prevalent consistent frequent knee pain, 2,320 persons (4,640 knees) were included for the cross-sectional analysis; after excluding those with prevalent consistent frequent knee pain at baseline, 1,323 persons (2,646 knees) were included in the longitudinal analyses for incident consistent frequent knee pain.
Baseline assessment for sleep quality
Self-reported sleep quality [(a single item drawn from the Pittsburgh Sleep Quality Index (PSQI)] was assessed: “During the past 7 days, how would you rate your sleep quality overall” on a four-point Likert scale of “very good,” “fairly good,” “fairly bad,” and “very bad.” We also used frequency of restless sleep [a single item drawn from the Center for Epidemiologic Studies-Depression Scale (CES-D)] in the past week as a second sleep measure: “During the past week, my sleep was restless” on a four-point Likert scale or frequency: “Rarely or none of the time (less than 1 day),” “Some or little of the time (1-2 days),” “Occasionally or a moderate amount of the time (3-4 days),” and “Most or all of the time (5-7 days).” Because those who reported “very bad” sleep quality in the PSQI and those who reported restless sleep “Most or all of the time” in the CES-D included less than 10% of the sample, we combined the lowest two categories of sleep response as “poor sleep quality” for the item in the PSQI (combining “very bad” and “fairly bad”). For restless sleep in the CES-D, we combined the two most frequent restless categories to one group.
Measurements of knee pain
At each clinic visit, the participants were asked about knee pain twice using the following question: “During the past 30 days, have you had pain, aching, or stiffness in your right/left knee on most days?” first at a telephone screening and then again at the clinic visit on average 30 days later. A positive response to this question at both time points defined presence of consistent frequent knee pain. We defined incident consistent frequent knee pain as occurring, when participants free of this condition at baseline developed new consistent frequent knee pain 2 years after baseline.
For the outcome of knee pain worsening, The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score ranges from 0 (no pain) to 20 (most pain) points. Knee pain worsening was calculated based on the difference of knee-specific WOMAC pain score surveyed at the baseline and the 2-year follow-up visits. Based on the published estimates for the minimal clinically important difference (MCID) in WOMAC (23), worsened knee pain was defined as an increase in WOMAC pain score of ≥14%; or, if WOMAC knee pain score of 0 at baseline increased by ≥ 2 at follow-up.
Other covariates
At the 60-month visit, the following information was collected: demographics, medication use, and lifestyle factors including tobacco use (pack-years), general health assessments, including fatigue [range from 0 (least fatigue) to 10 (most fatigue)], depression indicator by CES-D (cut-off score is 16 to define low and high depression (22), and the Charlson’s comorbidity index (range 0 to 9). Body weight (in kg) and standing height (in meters) were measured by clinic staff. All subjects obtained a fixed flexion posterior-anterior radiograph which was read centrally for Kellgren and Lawrence (KL) grade. A KL grade ≥2 was defined as radiographic knee OA. Using a standard homunculus which including circles over the following joint regions: shoulders, elbows, wrists, hands, hips, knees, ankles, feet, neck and lower back, subjects identified body sites with pain on most of the past 30 days. Widespread pain was defined according to the American College of Rheumatology criteria, based upon pain being present above the waist, below the waist, on both sides of the body, and in the axial region (24). Also, the number of painful joint sites (including knee joints) was counted and ranged from 0 to 21. We further examined whether the association between sleep quality or restless sleep and knee pain differed by the number of painful joint sites according to the status of widespread pain.
Statistical analysis
We first assessed whether widespread pain modified the association between sleep quality or restless sleep and knee pain, by including a cross product term for each sleep variable and widespread pain status (absence or presence) in a separate regression model. Because this analysis only suggested the association between sleep and worsening knee pain differed by widespread pain status (p for interaction <0.01) for both sleep measures, no stratification was done for the analyses with frequent consistent knee pain (cross-sectional analysis for prevalence and longitudinal analysis for incidence). For knee pain worsening, we conducted longitudinal analysis to assess the relationships with knee pain worsening 2 years after baseline. Further, we pre-specified additional analysis to be conducted, if there was a significant association noted between a sleep measure and a knee pain outcome, stratified by the median number of painful joint sites in those with and without widespread pain.
In all analyses, we estimated the odds ratio (OR) and its 95% confidence interval (CI) using poor sleep quality, or restless sleep at least 3-4 days in the past week as the referent group. For sleep quality, we compared very good and fairly good sleep quality as two separate categories. A similar approach was taken for restless sleep in the CES-D. We used logistic regression with Generalized Estimating Equations to account for the correlations between two knees within an individual to estimate odds ratios. We adjusted for the following potential confounders: age (years), sex (men versus women), race (white versus non-white), study site, BMI (kg/m2), education level (college versus below college), tobacco smoking (pack-years), feeling of fatigue in the past seven days (Likert scale of 0-10), Charlson’s comorbidity index (range 0-9), high (CES-D score ≥16) vs low (CES-D<16) number of depressive symptoms [the cut-off was defined previously (25)], and prescription use of NSAIDs (yes, no). We used CES-D as a dichotomous variable due to collinearity between restless sleep (single item included in the CES-D) and the full range score (0-50) in the CES-D variable.
Because not all participants had the same assessment for physical activity, we conducted sensitivity analysis among those whose step counts were assessed by accelerometry (76% of the analytic sample), as the rest of the subjects either had no assessment for physical activity (19%) or were assessed using the Physical Activity Scale for the Elderly (5%).
All statistical analysis was conducted using SAS version 9.3. A two-sided p value less than 0.05 was considered statistically significant.
Results
We included 2,329 participants (4,658 knees), of whom 60.5% were female and 84.3% were white. The mean age was 67.9 (standard deviation, SD 7.8) years and mean BMI was 30.9 (SD: 6.1) kg/m2. There were 414 persons (18%) who reported poor sleep quality and 526 (23%) who experienced restless sleep at least 3-4 days in the past week. The correlation between these two single items from validated questionnaires for sleep measures is moderate to high (r=0.69, p<0.001).
The baseline characteristics across three categories of sleep quality in the past week are described in Table 1. Persons who reported better sleep quality tended to have less fatigue, less frequent use of prescription NSAIDs, have less severe knee pain reflected by a lower WOMAC score at baseline, and have less pain in other body sites, reflected by a lower proportion of having widespread pain at baseline. Similarly, those with better sleep quality had a lower Charlson’s comorbidity index and were less likely to have high depressive symptoms.
Table 1.
Baseline characteristics of participants (n=2329) according to sleep quality, a single item in the Pittsburgh Sleep Quality Index (PSQI) in the past 7 days
| Sleep quality in past week in the PSQI | |||
|---|---|---|---|
| Baseline characteristics | 0,1=Poor | 2=Fairly good | 3 =Very good |
| Number of participants, n (%) | 414 (17.8) | 1296 (55.6) | 619 (26.6) |
| Age (years), mean (SD) | 60.2 (7.7) | 62.3 (7.9) | 63.0 (7.6) |
| BMI (kg/m2), mean (SD) | 31.9 (7.0) | 30.8 (5.9) | 30.5 (5.9) |
| White, n (%) | 345 (83.3) | 1103 (85.1) | 515 (83.2) |
| Women, n (%) | 289 (69.8) | 776 (59.9) | 344 (55.6) |
| Education level (College and above), n (%) | 165 (39.9) | 588 (45.4) | 319 (51.5) |
| Currently work for pay, n (%) | 178 (43) | 583 (45) | 286 (46) |
| Tobacco smoking pack-years, mean (SD) | 8.8 (18.2) | 8.6 (16.9) | 9.4 (18.5) |
| Level of fatigue, median (interquartile range) | 5 (3,7) | 3 (2,5) | 2 (1, 3) |
| Charlson comorbidity index, mean (SD) | 0.7 (1.3) | 0.5 (1.0) | 0.4 (0.8) |
| High depressive symptoms1 (yes), n (%) | 127 (30.7) | 121 (9.3) | 12 (3.4) |
| WOMAC2 pain score, median (interquartile range) | 3 (1, 7) | 2 (0, 5) | 1 (0, 3) |
| Prescription use of NSAID3s, n (%) | 76 (18.5) | 168 (13.0) | 73 (11.8) |
| Widespread pain (presence), n (%) | 235 (56.8) | 558 (43.1) | 163 (26.3) |
| Prevalence of radiographic knee OA4 (yes), n (%) | 444 (56.4) | 1414 (56.2) | 636 (52.8) |
High depressive symptom was defined based on the CES-D ≥16 (reference 22)
The Western Ontario and McMaster Universities Osteoarthritis Index
Nonsteroidal anti-inflammatory drugs
Radiographic knee OA was defined as a Kallgren Lawrence grade ≥2 present in a knee.
The multivariable adjusted ORs (95% CIs) for the associations between sleep quality and knee pain outcomes are presented in Table 2. We found a significant inverse relationship between sleep quality and consistent frequent knee pain cross-sectionally but not longitudinally. For knee pain worsening, the status of widespread pain (absence or presence) modified the relationship with knee pain worsening with sleep quality (p for interaction=0.002); this relationship was stronger when widespread pain was present (p-trend<0.01) than when it was absent (p-trend<0.04). Among those with widespread pain, compared with those with poor sleep quality, persons with very good sleep quality had 47% lower odds (OR: 0.53; 95% CI: 0.35, 0.78) of developing worsened knee pain, and those with fairly good sleep quality had 28% lower odds (OR: 0.72; 95% CI: 0.54, 0.96).
Table 2.
Odds ratio (OR) [95% confidence interval (CI)] for the association of sleep quality, a single item from the Pittsburgh Sleep Quality Index (PSQI) with knee pain outcomes stratified by baseline widespread pain status
| Sleep quality in past week in the PSQI | ||||
|---|---|---|---|---|
| Knee pain outcomes | 0,1=Poor (Referent group) | 2=Fairly good | 3 =Very good | p-trend |
| Consistent frequent knee pain | ||||
| Cross-sectional association for prevalent consistent frequent knee pain (N=4640) | ||||
| Knee (n/N)1 | 376/824 (45.6%) | 887/2582 (34.4%) | 342/1234 (27.7%) | |
| OR (95% CI)2 | 1.0 (ref) | 0.84 (0.68, 1.03) | 0.69 (0.54, 0.90) | 0.005 |
| Longitudinal association for incident consistent frequent knee pain (N=2646) | ||||
| Knee (n/N) | 88/385 (17.6%) | 309/1503 (61.7%) | 104/758 (20.8%) | |
| OR (95% CI) | 1.0 (ref) | 1.07 (0.84, 1.36) | 0.80 (0.60, 1.07) | 0.08 |
| Knee pain worsening (N=4658) | ||||
| Widespread pain absence (N=2746) | ||||
| Knee (n/N) | 87/358 (24.3%) | 304/1476 (20.6%) | 159/912 (17.4%) | |
| OR (95% CI) | 1.0 (ref) | 0.86 (0.62, 1.21) | 0.70 (0.48, 1.02) | 0.037 |
| Widespread pain presence (N=1912) | ||||
| Knee (n/N) | 176/470 (37.4%) | 359/1116 (32.1%) | 87/326 (26.7%) | |
| OR (95% CI) | 1.0 (ref) | 0.72 (0.54, 0.96) | 0.53 (0.35, 0.78) | 0.01 |
Number of knees: n (knees with pain worsening or incident joint pain) / N (total number of knees)
Model adjusted for age (years), sex (men vs. women), race (white vs. non-white), study site, BMI (kg/m2), education level (college and above vs. below college), tobacco use (pack-years), Charlson’s comorbidity index (range 0-9), fatigue (10-point scale), CES-D depression indicator (yes, no), and prescription use of NSAIDs (yes, no).
We examined the relationship of restless sleep with each knee pain outcome using the same approach (Table 3). Similar to the results for sleep quality, there was an inverse association between restless sleep and the prevalence of consistent frequent knee pain but not with the incidence. Widespread pain status modified the relationship between restless sleep and knee pain worsening (p for interaction <0.003). Compared to those with the most frequent restless sleep, persons who reported less frequent restless sleep (1-2 d/wk or <1 d/wk) had lower odds of developing knee pain worsening when widespread pain was present (p-trend=0.05). But this protective association was not observed when widespread pain was absent.
Table 3.
Odds ratio (OR) [95% confidence interval (CI)] for the association of restless sleep (a single item) using the Center for Epidemiology Study of Depression (CES-D) with knee pain outcomes stratified by baseline widespread pain status in the Multicenter Osteoarthritis Study
| Restless sleep in past week in the CES-D | ||||
|---|---|---|---|---|
| Knee pain outcomes | More than 3-4 days (Referent group) | 1-2 days | < 1 day | p-trend |
| Consistent frequent knee pain | ||||
| Cross-sectional association for prevalent consistent frequent knee pain (N=4640) | ||||
| Knee (n/N) | 475/1050 (29.6%) | 626/1948 (39.0%) | 504/1642 (31.4%) | |
| OR (95% CI) | 1.0 (ref) | 0.73 (0.60, 0.89) | 0.76 (0.61, 0.94) | 0.03 |
| Longitudinal association for incident consistent frequent knee pain (N=2646) | ||||
| Knee (n/N) | 122/497 (24.4%) | 221/1178 (44.1%) | 158/971 (31.5%) | |
| OR (95% CI) | 1.0 (ref) | 0.91 (0.60, 1.37) | 1.14 (0.70, 1.84) | 0.35 |
| Knee pain worsening (N=4658) | ||||
| Widespread pain absence (N=2746) | ||||
| Knee (n/N) | 81/434 (18.7%) | 253/1174 (21.6%) | 216/1138 (19.0%) | |
| OR (95% CI) | 1.0 (ref) | 1.33 (0.93, 1.91) | 1.11 (0.75, 1.63) | 0.85 |
| Widespread pain presence (N=1912) | ||||
| Knee (n/N) | 222/618 (35.9%) | 243/780 (31.1%) | 157/514 (30.5%) | |
| OR (95% CI) | 1.0 (ref) | 0.78 (0.59 1.02) | 0.73 (0.53, 1.00) | 0.05 |
Number of knees: n (knees with pain worsening or incident joint pain) / N (total number of knees)
Model adjusted for age (years), sex (men vs. women), race (white vs. non-white), study site, BMI (kg/m2), education level (college and above vs. below college), tobacco use (pack-years), Charlson’s comorbidity index (range 0-9), fatigue (10-point scale), CES-D depression indicator (yes, no), and prescription use of NSAIDs (yes, no).
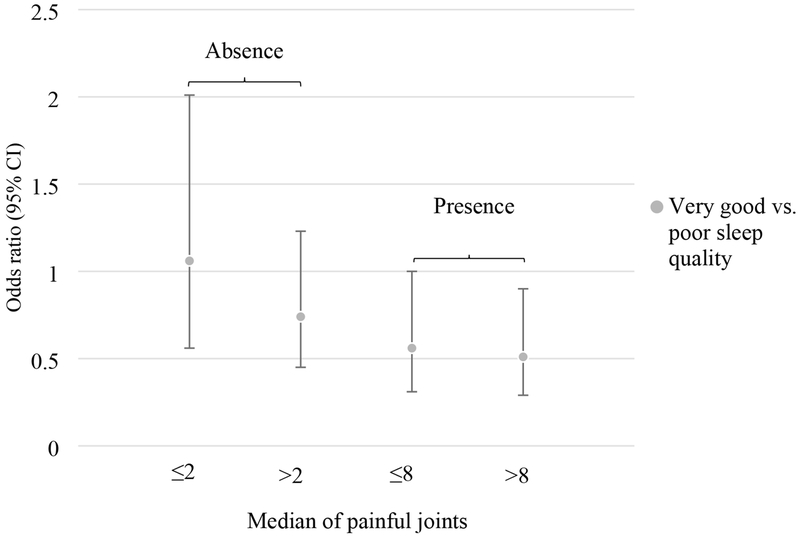
We further examined whether the number of painful joint sites within each stratum of widespread pain influenced the association between sleep and knee pain worsening. The median for painful joint sites was 8 for those with widespread pain and 2 for those without widespread pain. We found that the strength of the relationship between sleep quality and knee pain worsening was greater among those with more painful joint sites in the presence of widespread pain (p-trend =0.01), whilst none of the results reached statistical significance in the absence of widespread pain (Figure 1). In a similar analysis for restless sleep, we found corroborative results, although no significant association was found. These stratified results by number of painful joint sites are presented in Supplemental Table 1.
Figure 1.
Odds ratio (95% confidence interval, CI) of knee pain worsening in those with very good sleep quality compared with those with poor sleep quality (a single item assessed by the Pittsburgh Sleep Quality Index) stratified by the median number of painful joint sites by widespread pain status (absence or presence)
In this study sample, 55.3% of knees had prevalent radiographic knee OA, whose distribution was similar among all categories of sleep quality (p=0.11). Further adjusting for baseline radiographic knee OA did not change the results. In sensitivity analysis among those whose physical activity was assessed by accelerometry (76% of the analytic sample), further adjustment for steps per day in the models produced similar results. For example, compared with those who had poor sleep quality, those who reported a very good sleep quality had 44% low odds of developing knee pain worsening [OR (95% CI): 0.66 (0.42, 1.03)] when widespread pain was present (p for trend =0.01). When widespread pain was absent, although the inverse relationship between sleep quality and knee pain worsening suggested a significant trend (p=0.046), none of the results reached statistical significance. Similarly, compared with those whose restless sleep >3-4 days/week, the OR was 0.73 (95% CI: 0.48, 1.02) among those who experienced <1day/week restless sleep (p for trend =0.06), and no relationship was found in absence of widespread pain (p for trend =0.75).
Discussion
In a sample of individuals with or at high risk of knee OA, we found that better sleep quality (use of single item from the PSQI) was associated with lower odds of developing knee pain worsening over two years, especially in those with widespread pain. The more painful sites present, the more likely knee pain was related to sleep quality. Using a single question to assess restless sleep in the CES-D, we found consistent results for knee pain worsening among persons with coexisting widespread pain at baseline. Although sleep quality or restless sleep lowered the odds of prevalent consistent frequent knee pain, the relationship did not hold longitudinally 2 years later. Our findings highlight the importance of improving sleep as a potential strategy to relieve pain severity in knee OA, particularly in persons with widespread pain; our results also suggest that this effort is more likely to be successful in those with multiple painful joint sites.
Few studies have examined the temporal association of sleep to knee pain or other chronic pain in previous studies (26–28). In one clinical trial, improved sleep continuance is suggested to reduce WOMAC knee pain in a 6-month trial (29). Here, we additionally addressed co-existing musculoskeletal painful joint sites and widespread pain in our analyses. Our results raise the question as to whether, in those with knee pain, improvement in sleep quality or restless sleep might particularly benefit those with widespread pain. These research questions and findings have important clinical relevance, as widespread pain, knee pain and poor sleep are highly prevalent in persons with knee OA.
In our study, among those with WSP, 32.5% of the subjects had worsening of knee pain two years later; this is in contrast with 20.0% of those without widespread pain. The larger effect estimate in those with widespread pain than those without it in our study may be explained by a phenomenon of chronic maladaptive pain (17), whereby persons with widespread pain at baseline may have increased abnormal pain facilitation and deficits in pain inhibition; and therefore widespread pain may increase their risk of developing pain at other joint sites (17). This phenomenon might explain, in part, our observed effects of sleep quality or restless sleep in reducing the odds of knee pain worsening in the presence of widespread pain.
Upon stratification of the status of widespread pain at baseline, we further divided persons based on the median of painful joint sites in each widespread pain stratum, and found a stronger association between sleep quality and knee pain worsening among those with more painful joint sites. Widespread pain is perceived as “centralized” pain and predominantly controlled by the central nervous system (21, 30, 31). This concept has been further extended to pain in OA. Because centralized pain is closely related to disturbances of neurotransmitters in which sleep, for example, can play a role (21); and because the number of painful body regions is significantly related to WOMAC pain (15,32), these could explain the larger effect size of sleep quality in reducing knee pain severity among those with more painful joint sites (see Figure 1).
In this study, we did not find significant associations of sleep quality with incident consistent frequent knee pain, while the significant cross-sectional associations were consistent with previous studies (5, 26). In one study, persistence of knee pain was associated with an increased risk of total knee replacement independent of WOMAC knee pain (33), suggesting consistent frequent knee pain may reflect pain persistence. Hence, the dimensions of WOMAC knee pain severity and consistent frequent knee pain are likely different. Moreover, the sleep assessments used in this study were focused on sleep quality or restless sleep in the past 7 days, a time frame that is consistent with that over which WOMAC pain was assessed, but it is different from the assessment of consistent frequent knee pain (prior 30 days). This may explain the discrepancy of sleep in relation to WOMAC knee pain worsening versus consistent frequent knee pain longitudinally, but the cross-sectional relationship between sleep and consistent frequent knee pain can be bi-directional.
Biological mechanisms by which sleep may affect pain have been proposed (34). Poor sleep quality or lack of sleep continuance is linked with increase systemic inflammation. Accompanying this finding, shorter sleep duration was associated with higher levels of CRP (11, 35, 36), and CRP has been consistently associated with painful knee OA (5). Further, as sleep disturbance may interact directly with central pain processing (16, 37), and growing body of evidence has suggested that environmental disruption of circadian rhythms may predispose to osteoarthritic-like damage to the joints, supported by the clock genes such as the brain and muscle Arnt-like protein 1 (Bmall) found in the chondrocyte of articular cartilage (38, 39). This evidence may further support the link between poor or disrupted sleep and knee OA pain.
Strengths of this study include using multiple sleep measures and knee pain outcomes to assess the associations in a well-characterized prospective cohort while simultaneously considering the potential impact of body pain at other sites. We additionally performed longitudinal analyses that enable inferences about directionality of these associations that prior cross-sectional studies could not consider. A major limitation is that we used a single questionnaire item for sleep quality from the PSQI or restless sleep in the CES-D instead of an entire sleep quality questionnaire or objective polysomnography. This is because a full PSQI was not available, nor was duration of sleep measured in MOST. The correlation between the two sleep measures was moderately high (r=0.69), suggesting that there are some overlapping characteristics between these two sleep constructs, which may partly explain similar results. Future studies with long-term comprehensive and objective sleep assessments are needed to verify these results to elucidate the effect of sleep on knee pain when other body pain comorbidities exist.
Conclusion
In individuals with or at risk of knee osteoarthritis, those who had better sleep quality or less frequent restless sleep had lower odds of developing worsening knee pain over two years. This association was more apparent in those with widespread pain and more painful joint sites. However, neither sleep measure was associated with consistent frequent knee pain longitudinally. Because poor sleep and chronic painful joints commonly co-exist in adults with knee osteoarthritis, our study adds to other studies to support sleep improvement as a potential means of lessening knee pain severity, particularly in persons with widespread pain.
Supplementary Material
Acknowledgement
Other contributors: none.
Funding sources: NIH grants: T32 AR7598 (Dai Z), AR070892 (Neogi, T), AR477785, U01 AG18820 (Felson DT), U01 AG19069 (Nevitt, M), U01 AG18832 (Torner J), and U01 AG18947 (Lewis CE). Dr. Felson was also supported by the NIHR Manchester BRC.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
All authors had no conflicts of interest to disclose.
Funding: This study was supported by NIH grants: T32 AR7598 (Dai Z), AR477785 (Felson DT), U01 AG19069 (Nevitt, M), U01 AG18832 (Torner J), U01 AG18947 (Lewis CE), U01 AG18820 (Felson DT) and AR070892 (Neogi, T).
Footnotes
Disclosure: All authors state that they have no conflict of interest.
References
- 1.Felson DT, Niu J, Clancy M, Aliabadi P, Sack B, Guermazi A, et al. Low levels of vitamin d and worsening of knee osteoarthritis: Results of two longitudinal studies. Arthritis Rheum 2007;56:129–36. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26:355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leveille SG, Zhang Y, McMullen W, Kelly-Hayes M, Felson DT. Sex differences in musculoskeletal pain in older adults. Pain 2005;116:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddle DL, Stratford PW. Knee pain during daily tasks, knee osteoarthritis severity, and widespread pain. Phys Ther 2014;94:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlesso LC, Niu J, Segal NA, Frey-Law LA, Lewis CE, Nevitt MC, et al. The effect of widespread pain on knee pain worsening, incident knee osteoarthritis (oa), and incident knee pain: The multicenter oa (most) study. J Rheumatol 2017;44:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc 2000;48:1241–51. [DOI] [PubMed] [Google Scholar]
- 8.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004;8:119–32. [DOI] [PubMed] [Google Scholar]
- 9.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016;80:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: A prospective cohort study. Ann Rheum Dis 2013;72:535–40. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- 12.Jansson-Frojmark M, B K Bidirectionality between pain and insomnia symptoms: A prospective study. Br J Health Psychol 2012;17:420–31. [DOI] [PubMed] [Google Scholar]
- 13.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: A multilevel daily process study. Sleep 2012;35:675–87A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain 2013;14:1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: Longitudinal data on an adult female population in norway. Arthritis Rheum 2012;64:281–4. [DOI] [PubMed] [Google Scholar]
- 17.Choy EH. The role of sleep in pain and fibromyalgia. Br J Health Psychol 2015;11:513–20. [DOI] [PubMed] [Google Scholar]
- 18.McBeth J, Lacey RJ, Wilkie R. Predictors of new-onset widespread pain in older adults: Results from a population-based prospective cohort study in the uk. Arthritis Rheumatol 2014;66:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: Linkages with pain, disability, and depressive symptoms. Arthritis Care Res (Hoboken) 2015;67:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salwen JK, Smith MT, Finan PH. Mid-treatment sleep duration predicts clinically significant knee osteoarthritis pain reduction at 6 months: Effects from a behavioral sleep medicine clinical trial. Sleep 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin Exp Rheumatol 2017;35 Suppl 107:79–84. [PubMed] [Google Scholar]
- 22.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The multicenter osteoarthritis study: Opportunities for rehabilitation research. PM R 2013;5:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol 2002;29:131–8. [PubMed] [Google Scholar]
- 24.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The american college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 25.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for epidemiologic studies depression scale (ces-d) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997;12:277–87. [DOI] [PubMed] [Google Scholar]
- 26.Chen CJ, McHugh G, Campbell M, Luker K. Subjective and objective sleep quality in individuals with osteoarthritis in taiwan. Musculoskeletal Care 2014. [DOI] [PubMed] [Google Scholar]
- 27.Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum 2005;53:911–9. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Hayman LL, Shmerling RH, Bean JF, Leveille SG. Characteristics of chronic pain associated with sleep difficulty in older adults: The maintenance of balance, independent living, intellect, and zest in the elderly (mobilize) boston study. J Am Geriatr Soc 2011;59:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: A randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol 2015;67:1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackey S The “continuum of pain” and the american academy of pain medicine. Pain Med 2015;16:413–5. [DOI] [PubMed] [Google Scholar]
- 31.Jones D, Leslie K. The pain continuum: Towards prevention and better management of pain. Anaesth Intensive Care 2011;39:163–5. [DOI] [PubMed] [Google Scholar]
- 32.Dave AJ, Selzer F, Losina E, Klara KM, Collins JE, Usiskin I, et al. Is there an association between whole-body pain with osteoarthritis-related knee pain, pain catastrophizing, and mental health? Clin Orthop Relat Res 2015;473:3894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise BL, Felson DT, Clancy M, Niu J, Neogi T, Lane NE, et al. Consistency of knee pain and risk of knee replacement: The multicenter osteoarthritis study. J Rheumatol 2011;38:1390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: A conceptual model. Curr Pain Headache Rep 2009;13:447–54. [DOI] [PubMed] [Google Scholar]
- 35.Huang WY, Huang CC, Chang CC, Kor CT, Chen TY, Wu HM. Associations of self-reported sleep quality with circulating interferon gamma-inducible protein 10, interleukin 6, and high-sensitivity c-reactive protein in healthy menopausal women. PLoS One 2017;12:e0169216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Mendoza JBJ, Vgontzas AN, Gaines J, Liao D, Bixler EO. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun 2017;61:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum 2013;65:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudek M, Gossan N, Yang N, Im HJ, Ruckshanthi JP, Yoshitane H, et al. The chondrocyte clock gene bmal1 controls cartilage homeostasis and integrity. J Clin Invest 2016;126:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kc R, Li X, Voigt RM, Ellman MB, Summa KC, Vitaterna MH, et al. Environmental disruption of circadian rhythm predisposes mice to osteoarthritis-like changes in knee joint. J Cell Physiol 2015;230:2174–83. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.