Abstract
Background
Exercise has been examined as an adjunctive treatment for substance use disorders (SUDs), yet few exercise interventions have been conducted among patients undergoing intensive outpatient (IOP) treatment, who may be the most vulnerable to relapse and for whom exercise could provide the most benefits. This study examined the effects of aerobic exercise, in addition to IOP treatment, on psychological variables and endocannabinoids in individuals with SUDs.
Methods
Twenty-one SUD patients (mean age 35 years) were recruited from local IOPs. Participants were randomized to either treatment-as-usual (TAU, at their outpatient clinic) or TAU plus aerobic exercise training (EX). EX participants engaged in supervised, moderate-intensity exercise for 30 minutes, 3 times/week for 6 weeks. TAU participants came into the laboratory once per week for assessments and a 30-minute quiet rest session. Participants provided blood samples and completed questionnaires evaluating substance use, mood states, depression, anxiety, perceived stress, self-efficacy to abstain from substance use, and craving. Data were analyzed with Mann-Whitney U tests or mixed model ANOVAs to determine group differences in outcomes acutely and over 6 weeks.
Results
Over 6 weeks, there were reductions in perceived stress (p<0.01) and craving (p<0.05) for both groups. There were no group differences in abstinence rates or changes from baseline in self-efficacy, depression, or anxiety (p>0.05). Acutely, both exercise and quiet rest sessions led to reductions in craving, tension, depression, anger, confusion, and total mood disturbance (all ps<0.05). In addition, the EX group experienced acute increases in vigor and circulating concentrations of the endocannabinoid, anandamide (p<0.01).
Conclusions
An adjunctive aerobic exercise program during SUD treatment was associated with similar reductions in perceived stress and drug craving as standard care. Thirty minutes of exercise or quiet rest led to acute improvements in mood, but exercise produced the additional benefit of increases in vigor and circulating anandamide.
Keywords: AEA, 2-AG, vigor, drug, treatment
Introduction
In 2017, about 20 million people (7% of individuals ≥12 years old) were diagnosed with an alcohol- or illicit drug-related substance use disorder (SUD) in the past year,1 costing an estimated $600 billion.2 While early and successful treatment can help lower costs, only 4 million of the 20 million individuals with SUDs (20%) received any treatment.1 Unfortunately, 40%−60% of those who receive treatment will relapse within one year.3 In order to be effective, treatment must be readily accessible, attend to multiple needs of the individual, address comorbid psychological concerns (e.g., stress reactivity, depression, and anxiety), and be multi-faceted, incorporating group and individual therapy in combination with other approaches such as pharmacotherapy.2 Because it is low cost, readily accessible, and does not come with a surrounding stigma or adverse side effects, exercise has been proposed as an adjunctive treatment to promote sustained abstinence among SUD patients.4
There is a well-documented inverse relationship between physical activity and illicit drug use or diagnosed SUDs in the general population;5,6 however, there have been mixed results on the effects of exercise programs in clinical samples. In general, exercise training can improve mood, lower craving, and yield abstinence rates that are superior to or at least comparable with other treatment strategies (e.g., cognitive behavioral therapy).7,8 There are many sources of heterogeneity across studies (e.g., exercise dose or duration, SUD type); however, the timing of exercise program initiation during the recovery process could greatly influence the results. For instance, several interventions have been conducted in tightly controlled inpatient settings during the first few weeks of initial abstinence but have not examined the effects of exercise after patients have re-entered their previous drug-related environments.9–11 Conversely, other exercise trials have been conducted in samples comprised of patients who are in different stages of their treatment (e.g., from 24-hour inpatient care, to partial hospitalization, to 12-step programs, to individual outpatient counseling),12,13 making it difficult to interpret the effects of exercise on specific stages of treatment. Intensive Outpatient Programs (IOPs) are the second most common treatment approach after regular outpatient therapy.14 IOPs are intensive group and individual therapy programs (9–25 hours per week) for patients who do not need medical detoxification or 24-hour care and supervision; however, there is limited research on the effects of exercise at this focused level of care. Examining the effects of exercise in an IOP-specific sample may better represent the therapeutic potential of exercise in a real world setting among individuals at high-risk for relapse and for whom exercise may provide the most benefits.
SUDs have a well-characterized neurobiological progression,15 yet potential biological mechanisms underlying the effects of exercise on treatment outcomes remain relatively unexplored. The persistence of drug-seeking behaviors is thought to be a combination of a decline in normal reward functioning, leading to depression and anhedonia, and an increase in aversive “anti-reward” processes, leading to stress, anxiety, and physical symptoms.16 There are likely several interacting factors responsible for the effects of exercise on drug-related behaviors;17 however, the activation of the endocannabinoid (eCB) system that occurs with exercise is promising due to its ability to influence neurobiological systems (e.g., dopaminergic) involved in addiction, reward, and relapse processes.18,19
The eCB system is a widely-distributed neuromodulatory network that regulates synaptic excitability and neurotransmitter release. The system’s two primary endogenous ligands, called “endocannabinoids” (eCBs) N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), are heavily represented in regions of the brain associated with reward processing, decision-making and impulse control, and stress reactivity (for extensive review, see Hillard20). Recent studies have found that the eCB system is downregulated in SUD patients,21,22 which may contribute to increased mood disturbance and heightened stress reactivity.23,24 Pharmacologically targeting the eCB system has led to improved SUD treatment outcomes in both preclinical and clinical studies.25–27 Correspondingly, exercise may be a non-pharmacological method of augmenting the eCB system, as it has been found to reliably increase circulating levels of eCBs in heathy individuals.28–30 It is unknown if exercise can activate the eCB system in SUD patients; however, if eCB activation occurs, it could offer insight into the mood improvements, better stress management, or attenuated withdrawal symptoms observed in previous exercise trials.25,27
Therefore, the purpose of this study was to determine the effects of acute (single session) and chronic (6 weeks) aerobic exercise, as an adjunctive to IOP treatment-as-usual, on a variety of psychological outcomes and eCBs. Specifically, our primary hypothesis was that the addition of 6 weeks of aerobic exercise to standard IOP treatment would improve continuous abstinence rates, depression, anxiety, self-efficacy, and perceived stress compared to IOP treatment alone (treatment-as-usual control group). As a secondary objective, we further hypothesized that 30 minutes of exercise would cause acute improvements in mood states, reductions in craving, and elevations in circulating AEA and 2-AG compared to a quiet rest session.
Materials and Methods
Participants
Participants were recruited from IOPs at five local agencies. The IOPs in this study ranged from 10–16 hours/week for 4–8 weeks and focused primarily on skill building, goal setting, problem solving, and stress management. Inclusion criteria for the study included: a) ability to read and write in English sufficient to provide consent and complete study questionnaires, b) diagnosis of any SUD meeting IOP level of care, and c) currently physically inactive, not participating in any formal/structured exercise program. Exclusion criteria included: a) severe depression, psychotic, or bipolar disorders, b) cardiac disease or other medical condition making exercise unsafe, c) cancer, autoimmune, or other chronic conditions, d) pregnancy or plans to become pregnant, and e) concurrent methadone treatment. Participants provided written informed consent, and this study was approved by the local institutional review board. The trial is registered on ClinicalTrials.gov ( NCT02570360).
Procedures
During a baseline study visit, participants completed questionnaires on demographics, substance use history (Timeline Follow Back),31 and severity (Severity of Dependence Scale).32 Participants also provided information on depression (Patient Health Questionnaire-9)33 and anxiety (Generalized Anxiety Disorder-7 item),34 self-efficacy to abstain (Situational Confidence Questionnaire),35 and stress (Perceived Stress Scale)36 both at baseline and 6 weeks. At the end of the baseline visit, they were randomized to either 6 weeks of treatment-as-usual (TAU; control) or TAU plus exercise (EX). Six weeks was selected as the duration from both a practical and translational perspective since 6 weeks was the average length of the five local IOP treatment programs, and all participants were concurrently attending their IOP during their study participation. The randomization sequence was generated by the study coordinator using a free online service. Randomization followed a stratified block design using sequences of permuted blocks of equal length containing the treatment assignments. The pre-defined strata were based on the participant’s treatment clinic (e.g., 1 of the 5 local IOPs).
Treatment-As-Usual Protocol (TAU; control group)
After their baseline visit, participants attended their IOP treatment as usual but also came into the laboratory for once-weekly, 60-minute sessions for 6 weeks to complete questionnaires. Study visits were scheduled so that they occurred at the same time of day. At the beginning of each visit, they reported their substance use over the past 7 days. They also completed questionnaires related to substance-specific cravings37–40 and mood (Profile of Mood States)41 before and after 30 minutes of quiet rest each week in order to examine acute changes in these outcomes. Quiet rest sessions took place in a sound-dampened chamber. Participants were not allowed to use electronic or mobile devices but were provided standard reading materials (e.g., magazines on current events and pop culture) to enhance adherence to the study protocol.
Exercise Protocol (EX)
Like the TAU group, the EX group attended their IOP treatment-as-usual and attended three adjunctive, supervised exercise sessions per week (18 sessions) after their baseline visit. Exercise sessions were scheduled at the same time of day whenever possible to promote adherence to the protocol and minimize time-of-day effects on the outcomes. Once per week at the beginning of their visit, they reported their substance use over the past 7 days. They also completed questionnaires related to cravings and mood states before and after exercise once per week in order to examine the acute effects of exercise on these outcomes. They did not complete mood or cravings questionnaires the other 2 visits per week.
Exercise was predominantly incline walking performed on a private treadmill in the laboratory and consisted of a 5-minute warm-up followed by 30 minutes at a moderate-to-vigorous intensity (70–75% age-predicted maximum heart rate; 12–15 Borg’s rating of perceived exertion [RPE]42) followed by a 5-minute cool down. Exercise intensity was increased gradually over the first three weeks from 65%−70% heart rate max (12–14 RPE) to 75% heart rate max (RPE 14–15) the final three weeks. This exercise intensity was selected as it has been shown to reliably activate the eCB system. 29,30 All sessions were monitored by bachelors or masters-level kinesiology research assistants who were trained in the study protocol and supervised by the study coordinator. Social interaction was minimized during exercise except during warm-up and cool-down times.
Quantification of Endocannabinoids
Using standard procedures, blood was collected from a superficial arm vein from all participants within 5 minutes before and after exercise or quiet rest on the first and last study visits (i.e., baseline and week 6). AEA and 2-AG were quantified using selective ion monitoring, isotope-dilution, liquid chromatography/mass spectrometry (LC/MS/MS) as described previously.43
Baseline Measures
Timeline Follow Back
The Timeline Follow Back is a calendar-based, self-report survey used to recall previous days of substance use. It has demonstrated high test-retest reliability (Intraclass correlation coefficients (ICCs) = 0.70–0.94) and has been validated against objective measures of substance use (e.g., urine metabolite screen).31 It was administered during the baseline assessment and was used to assess substance use over the past 3 months.
Severity of Dependence Scale (SDS)
Substance use severity at baseline was determined using the SDS.44 It is a 5-item, Likert-type scale asking participants about their perceptions, patterns, and emotions surrounding their substance use in the month prior to entering treatment. It demonstrates high test-retest reliability for a variety of drugs (all ICCs ≥0.73), and scores on the SDS have been strongly correlated with drug use in the past month (all ps<0.001). 32
Primary outcomes: Effects of the 6-week intervention on continuous abstinence, depression, anxiety, self-efficacy, and perceived stress
Continuous Abstinence
Self-report of substance use was completed on a weekly basis prior to exercise or quiet rest. Participants were asked whether they used any substance (e.g., tobacco, alcohol, cannabis/synthetic cannabis, cocaine, methamphetamine, ecstasy, psychedelics [LSD, psilocybin mushrooms], heroin, opiates/pain killers, benzodiazepines, other) during the past week. If they indicated use, they were asked how many days out of the previous 7 days they used a particular substance. The number of participants in each group who were continuously abstinent or not since the start of the study were used in analyses (since all participants were supposed to completely abstain from substance use as part of IOP treatment).
Patient Health Questionnaire (PHQ-9) and Generalize Anxiety Disorder Survey (GAD-7)
The PHQ-9 and GAD-7 were used to severity of depression and anxiety and baseline and 6-week follow-up. Scores of 0–4 indicate no anxiety/depression, 5–9 indicate mild, 10–14 indicate moderate, and above 15 on each scale indicates severe depression or anxiety symptoms.33,34
Situational Confidence Questionnaire (SCQ)
The SCQ was completed at baseline and 6-week follow-up to assess participants’ self-efficacy in their ability to avoid drinking heavily and/or using drugs in a variety of situations. The SCQ was developed to assess an individual’s perceived self-efficacy in abstaining from substance use across 8 categories of high-risk situations. Scores on the SCQ have been positively associated with successful treatment outcomes.35
Perceived Stress Scale (PSS)
The PSS was completed at baseline and 6 weeks. The PSS is a widely used, general stress questionnaire that has been applied in a variety of study populations. The PSS assesses feelings of unpredictability or uncontrollability in life over the past month. It demonstrates high test-retest reliability (ICC=0.85) and has been correlated with other similar measures.45
Secondary outcomes: Within-session changes in craving and mood
Craving Questionnaires-Short Forms
The craving questionnaires are 10–14 item, validated measures that assess substance craving across four dimensions: 1) compulsivity—lack of control using substances, 2) emotionality—using substances to control mood outcomes, 3) expectancy—anticipation of positive results from substances, and 4) purposefulness—using substances for intended outcomes. The four dimensions have demonstrated good inter-item correlation (Cronbach’s α = 0.61–0.90) and have been associated with current craving intensity as measured by visual analog scales (all ps<0.01).37–40 The questionnaires for each class of substance are structured similarly to be used for comparison purposes. The study included craving questionnaires for marijuana,39 alcohol,37 stimulants,40 and heroin/opiates.38 Participants completed questionnaires that referred to their specific substance(s) of abuse. For participants with multiple SUDs, the substance with the highest craving scores was used in analyses. Craving was assessed before and after EX/TAU sessions once per week.
Profile of Mood States (POMS)
The POMS is a 65-item questionnaire that was administered in order to examine the mood states of the participants before and after EX/TAU sessions once per week. Six mood states are evaluated using the POMS: tension, depression, anger, vigor, fatigue, and confusion, with internal consistencies of each mood state ranging from α = 0.84–0.95. The POMS has been shown repeatedly to be a valid and sensitive measure of general mood.41
Statistical Analyses
Independent samples t-tests were used to assess group differences in baseline characteristics between the EX and TAU groups. A chi-square analysis was used to determine whether there were group differences in the number of participants in each group who were continuously abstinent from week 1 to week 6. A series of 2 (group) X 2 (time: baseline or 6-weeks) mixed models analysis of variance (ANOVA) (or Mann-Whitney U for nonparametric alternative) were used for the primary outcomes of depression, anxiety, stress, and self-efficacy. A series of 2 (group) X 2 (time: pre- or post-exercise or quiet rest) ANOVAs were used for the secondary outcomes of mood, craving, AEA, and 2-AG. Homogeneity of variances (i.e., Levene’s test) and normality (i.e., Shapiro-Wilk test) of the data were assessed for each ANOVA. Non-normally distributed data were first log-transformed prior to parametric tests. Data that remained non-normal after log-transformation were assessed non-parametrically using the Mann-Whitney U test with group (EX or TAU) as the independent variable and the change score of the outcome as the dependent variable. Cohen’s effect sizes r are approximated for Mann-Whitney U tests, where r=0.1 is considered a small effect, r=0.3 a medium effect, and r=0.5 a large effect.46
Intention-to-treat analyses were used for the baseline and 6-week follow-up outcomes (e.g., depression, anxiety, stress, and self-efficacy). For participants who did not complete 6-week follow-up testing, their baseline observations were carried forward. Where possible, intention-to-treat was also used for the secondary within-session analyses for those who had within-session data available (i.e., we did not impute data for participants who had no within-session data since they dropped out right after their baseline visit), and for any participants who dropped out during the study, their last within-session observations were carried forward. After imputing missing data, the main analyses (i.e., Mann-Whitney U or ANOVA) were conducted. If the main analyses indicated significant results for any outcome, analyses were re-run omitting all participants with missing data to determine the sensitivity of the tests. If the results changed between the analyses using imputed data and the analyses that omitted participants with missing data (i.e., going from significant to not significant), both results were reported. Overall significance was defined as α=0.05, and simple effects were calculated after observing significant interactions, and were adjusted for multiple comparisons using the Bonferroni method. Mean change values (M), 95% confidence intervals, p-values, and effect sizes (partial η2) are reported, where an effect size of η2=0.01 is considered a small effect; 0.06 a medium effect, and 0.14 a large effect.46 Analyses were conducted using IBM SPSS Version 25.0 (IBM, Armonk, New York).
Results
Participant Characteristics
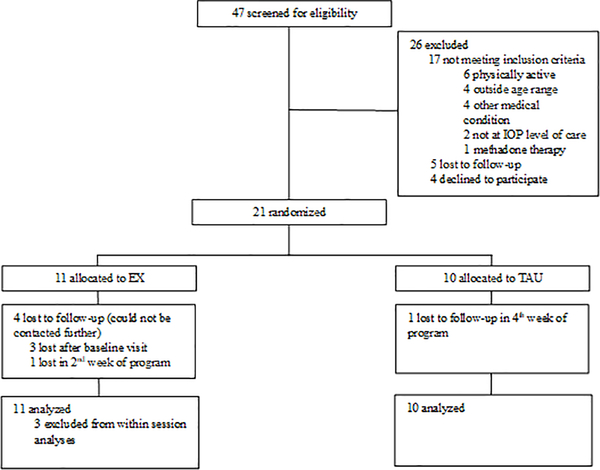
A total of 47 individuals were screened for eligibility. Of these, 26 were excluded, and 21 participants provided informed consent and were randomized to treatment arms. Three individuals, all assigned to adjunctive EX, did not attend more than their baseline visit and were not included in within-session (i.e., acute change) analyses since they had no baseline within-session exercise data. See Figure 1 for the CONSORT diagram.
Figure 1: CONSORT Diagram.
EX=exercise group; TAU= treatment-as-usual group; IOP=Intensive Outpatient Program
Table 1 lists the baseline sample characteristics for each study group. Participants were mostly white and college-educated young adults. There were no group differences in type, severity of, polysubstance use, or in any other measures at baseline. There were also no significant differences in substance use history and severity, self-efficacy, perceived stress, depression, or anxiety between the three individuals who did not return after the baseline session compared with the other 18 study participants.
Table 1:
Baseline characteristics of the EX and TAU groups
| EX (n = 11) | TAU (n = 10) | |
|---|---|---|
| Sex (male, female) | 6m, 5f | 6m, 4f |
| Age (years) | 35.1 (10.2) | 35.0 (7.1) |
| Body mass index (kg/m2) | 30.2 (5.9) | 29.1 (5.7) |
| Race/ethnicity (n) | ||
| White | 7 | 9 |
| Black or African American | 2 | 0 |
| Hispanic or Latino | 1 | 0 |
| More than one race/ethnicity | 1 | 1 |
| Marital Status (n) | ||
| Single | 9 | 7 |
| Married | 2 | 2 |
| Divorced | 0 | 1 |
| Education (n) | ||
| ≤12 years | 1 | 2 |
| >12–16 years | 8 | 7 |
| >16 years | 2 | 1 |
| Alcohol use disorder (n) | 8 | 8 |
| SDS score | 9 (4.2) | 8.0 (4.2) |
| Days used in past 90 days | 43.4 (19.1) | 40.3 (27.4) |
| Cannabis use disorder (n) | 6 | 4 |
| SDS score | 4.0 (2.8) | 6.8 (6.0) |
| Days used in past 90 days | 44.5 (25.9) | 42.5 (22.5) |
| Stimulant use disorder (n) | 5 | 3 |
| SDS score | 8.8 (3.0) | 9.3 (0.6) |
| Days used in past 90 days | 24.2 (8.6) | 29.7 (12.9) |
| Opioid use disorder (n) | 2 | 2 |
| SDS score | 12.0 (0.0) | 6.5 (6.4) |
| Days used in past 90 days | 35.5 (7.8) | 9.0 (9.9) |
| Polysubstance Use Disorder | 6 | 6 |
| Psychiatric Medication Use (n) | ||
| No Medications | 4 | 3 |
| Antidepressants | 5 | 6 |
| Anxiolytics | 3 | 3 |
| Alcohol Inhibitors | 2 | 0 |
| Opioid Partial Agonists | 1 | 0 |
Data are presented as mean (standard deviation) unless otherwise noted. There were no significant baseline differences between EX and TAU groups. SDS=Severity of Dependence Scale.
Retention and Adherence
Participants assigned to the TAU completed on average 5 out of 6 sessions (83%) of their scheduled visits. One participant in the TAU group was lost to follow-up in the 4th week. The eight participants who began exercise completed on average 15 out of 18 sessions (83%) of their scheduled exercise visits. Other than the three participants randomized to EX who attended only the baseline session, only one participant in the EX group was lost to follow-up in the second week of exercise. EX participants exercised within the moderate-to-vigorous intensity range as assessed by both percent of age-predicted maximum heart rate (M=71.9, SD=10) and ratings of perceived exertion (M=13.2, SD=0.9). Exercise performance data can been found in supplementary table 1.
Substance Use
Two individuals in the EX group and four in the TAU group reported abstaining from substance use the entire 6 weeks, and continuous abstinence rates were not different between groups (X2=1.22; p=0.27). The other six individuals in the EX group reported having used alcohol, cannabis, stimulants, or opioids an average of 31%, 31%, 27%, and 0% of days, respectively, over the 6 weeks, compared to baseline use rates of 48%, 50%, 60%, and 40% of days, respectively, over the past 3 months (from Timeline Follow Back). The other six individuals in the TAU group reported having used alcohol, cannabis, stimulant, or opioids an average of 31%, 14%, 17%, and 0% of days, respectively, over the 6 weeks, compared to baseline use rates of 44%, 48%, 33%, and 10% of days, respectively, over the past 3 months.
Changes in Self-Efficacy, Stress, Depression, and Anxiety Over 6 Weeks
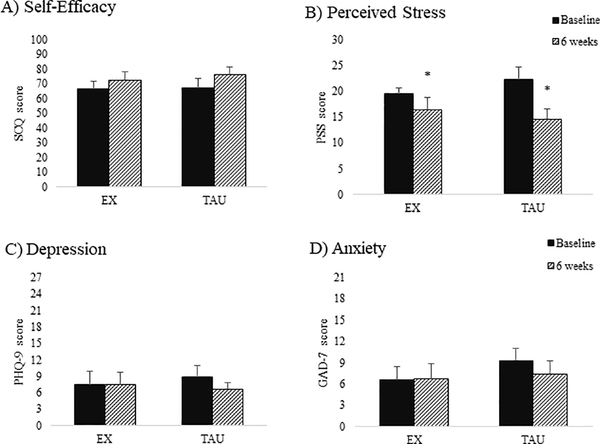
Due to not meeting the normality assumption, changes in self-efficacy, depression, and anxiety from week 1 to week 6 were assessed with Mann-Whitney U tests. There were no group differences for changes in self-efficacy to abstain from drug use (ρ=0.35, r=0.21), depression (ρ=0.56, r=−0.14), or anxiety (ρ=0.81, r=0.06). Changes in perceived stress from week 1 to week 6 were assessed with ANOVA. There was a decrease in perceived stress (M=−5.60 [−8.47,−2.79], p=0.001, η2=0.48) but no group by time interaction. These results remained significant after removing those with missing data (M=−5.92 [−9.15,−2.69], p=0.002, η2=0.53). Results for psychological outcomes are depicted in Figure 2.
Figure 2: Means and standard errors for psychological outcomes at baseline and 6 weeks.
A) Average self-efficacy to abstain from drug or alcohol use scores from the Situational Confidence Questionnaire (SCQ; range 0–100); B) Average perceived stress scores from the Perceived Stress Scale (PSS; range 0–30). C) Average depression scores from the Patient Health Questionnaire-9 (PHQ-9; range 0–27); D) Average anxiety scores from the Generalized Anxiety Disorder-7 item (GAD-7; range 0–21); *indicates significant main effect for time (p < 0.001); EX=exercise group; TAU= treatment-as-usual group
Acute Effects of Exercise or Quiet Rest on Mood and Craving
Within-session mood and craving outcomes were assessed with a 2 (group) x 2 time (pre- or post-exercise or quiet rest) ANOVA, averaging the pre- and post-session data across the 6 weeks. Thirty minutes of exercise (EX) or quiet rest (TAU) led to acute decreases in tension, depression, anger, confusion, and total mood disturbance, and these changes remained significant after excluding those with missing data (all ps<0.01, Table 2). There was a group by time interaction for vigor (p<0.0001, η2=0.62), and simple effects indicated that vigor increased acutely in the EX group but not the TAU group (Table 2). The interaction remained significant when those with missing data were excluded from the analysis (MEX= 6.07 [3.93, 8.21]; MTAU=−0.79 [−2.67, 1.11], p<0.001, η2=0.53 for interaction).
Table 2:
Mood states and craving change scores from before and after 30 minutes of exercise or quiet rest
| Mean change (95% CI) | p-value for acute time effect | η2 for acute time effect | |
|---|---|---|---|
| Tension | |||
| EX | −2.81 (−5.66, 0.03) | 0.001 | 0.54 |
| TAU | −5.00 (−7.54, −1.21) | ||
| Depression | |||
| EX | −3.38 (−7.39, 0.64) | 0.005 | 0.39 |
| TAU | −4.8 (−8.39, −1.21) | ||
| Anger | |||
| EX | −2.06 (−4.76, 0.64) | 0.009 | 0.36 |
| TAU | −3.05 (−5.47, −0.63) | ||
| Vigor * | |||
| EX | 5.69 (3.75, 7.63) | 0.001 | 0.51 |
| TAU | −0.65 (−2.39, 1.09) | ||
| Fatigue | |||
| EX | −1.06 (−3.48, 1.36) | 0.107 | 0.15 |
| TAU | −1.55 (−3.71, 0.61) | ||
| Confusion | |||
| EX | −2.75 (−4.98, −0.52) | 0.005 | 0.40 |
| TAU | −1.85 (−3.84, 0.14) | ||
| Total Mood Disturbance | |||
| EX | −17.75 (−31.36, −4.14) | 0.001 | 0.48 |
| TAU | −15.60 (−27.77, −3.43) | ||
| Craving | |||
| EX | −0.34 (−0.71, −0.02) | 0.030 | 0.26 |
| TAU | −0.15 (−0.46, 0.16) | ||
Data presented are mean change scores (95% confidence intervals) from pre- to post- exercise (EX) or quiet rest (TAU) averaged across measurement times. Effect sizes (partial η2) are reported, where an effect size of η2=0.01 is considered a small effect; 0.06 a medium effect, and 0.14 a large effect. Mood states are the score of each subscale from the Profile of Mood States. Total mood disturbance was calculated by summing the scores from the negative mood states, subtracting the vigor score, and adding 100 to account for negative values. Craving is the average craving score (range 1–7) for their specific substance of abuse. For participants with polysubstance use disorders, the substance with the highest craving score was used.
indicates a significant group by time interaction (p < 0.0001) for vigor. There were no other significant interactions.
There was a main effect for time indicated decreased craving scores acutely (Table 2); however, when participants with missing data were excluded from analysis, the results were no longer significant (M=−0.25[−0.52, 0.01], p=0.06, η2=0.23). Pre-session craving scores were also assessed, comparing pre-session craving in week 1 to week 6, to determine the effects of the intervention on craving over time. Craving decreased with no difference between groups from week 1 to week 6 (M=−0.74 [−1.45, −0.02], p=0.04, η2=0.23), but this became non-significant after removing those with missing data (M=−0.76 [−1.57, 0.05], p=0.06, η2=0.23),
Endocannabinoids
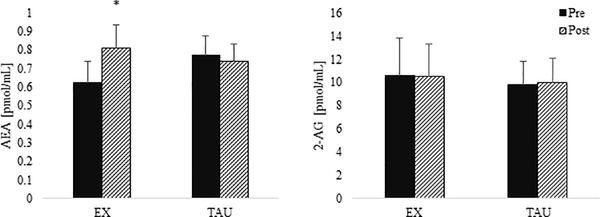
AEA and 2-AG data were log-transformed prior to 2 (group) by 2 (pre- or post-session) ANOVA analyses. There was a group by time interaction for AEA (p=0.002, Figure 3), and simple effects indicated that AEA increased acutely after exercise (MEX(log)=0.24 [0.12, 0.37]) but not quiet rest (MTAU(log)=−0.04 [−0.15, 0.07]). The interaction remained significant after those with missing data were excluded from the analysis (MEX(log)=0.23 [0.76, 0.38], MTAU(log)=−0.04 [−0.19, 0.11], p=0.02, η2=0.38 for interaction). There were no group differences or acute changes in 2-AG (Figure 3).
Figure 3: AEA and 2-AG before and after 30 minutes of exercise or quiet rest.
Means and standard error bars for circulating anandamide (AEA) and 2-arachidonoylglycerol (2-AG) concentrations in pmol/mL before (pre) and after (post) exercise (for EX group) or quiet rest (for TAU group) averaged across baseline and 6 weeks. Data were log-transformed prior to analyses although raw data are presented here. *indicates a significant increase in AEA after exercise (p < 0.001)
Discussion
Substance Use
There were no group differences in continuous abstinence rates over the 6 weeks. Frequency of use during the intervention compared to baseline use appeared to be lower in both groups, although baseline and intervention substance use were not statistically assessed due to the difference in measurement tool and time frame (i.e., over the past 90 days at baseline with the Timeline Follow Back compared to every 7 days during the intervention). The lower rates of substance use in both groups may suggest that the severity of their SUD decreased as a result of IOP treatment, regardless of adjunctive exercise. Similar exercise interventions (3 times per week for 4–8 weeks) in small samples of SUD outpatients (N = 17–24) and have also not found significant reductions in substance use over time.47,48 Longer and larger exercise trials (12 weeks, N=50–302) have also found no differences in substance use either over time or between supervised exercise and comparison programs.49,50 Conversely, others have shown reductions in substance use and improved abstinence with exercise,13,51 though both of these studies lacked control groups. Despite this, exercise appears to impart some enduring benefits on substance use. A meta-analysis of abstinence rates among SUD populations (mostly tobacco) found that those in exercise groups had increased odds of remaining abstinent within a 3-month follow-up period compared to controls.7 Therefore, it is possible that the effects of exercise may produce long-term effects not observed in the current study.
Psychological Outcomes
Self-efficacy is the belief in one’s ability to succeed (abstain in this case) in specific situations.52 In general, self-efficacy has been found to be significantly associated with reduced substance use and increased abstinence durations.53,54 Because most participants were not successfully abstaining throughout the intervention, it is not surprising that there were minimal improvements in self-efficacy in both groups. There were also no changes in depression or anxiety scores over the 6 weeks. This could be a floor effect since baseline values for these measures for both groups were relatively mild, and the majority of participants were already taking antidepressants or anxiolytics.
Perceived stress scores at baseline were more than a standard deviation higher than published normative values.45 This aligns with the “anti-reward” theory of drug withdrawal and relapse, which states that withdrawal symptoms such as anhedonia, craving, and heightened stress derive from a combination of attenuated reward processing and exacerbated hypothalamic-pituitary-adrenal axis activity, which together promote an aversive state.55 Fortunately, 6-week stress scores (M=15.5, SD=6.9) were comparable to age-based normative values (M=13.0, SD=6.2) at the end of the intervention for both groups,45 suggesting that IOP treatment may help alleviate stress and that an adjunctive exercise program does not contribute to stress for SUD patients.
In general, indices of negative mood states (e.g., tension, depression, etc.) in this sample were slightly greater than, but not significantly different from, adult normative values.56 However, the current sample had considerably lower vigor scores (M=8.4, SD=4.2) compared to adult normative values (M=19.4, SD=6.7).56 Therefore, the finding of increased vigor after exercise but not after quiet rest could be particularly important for promoting enhanced mental well-being among SUD patients. Another study has noted large effect sizes for enhancements in positive mood after exercise in polysubstance use disorder inpatients with some of the effects lasting several hours after exercise.57 Acute reductions in tension, depression, anger, confusion, and total mood disturbance occurred after both exercise and quiet rest. Other studies have found similar improvements in mood states after both moderate-intensity exercise and reading.58 Though the duration of mood improvements after exercise or quiet rest was not assessed in this study, other investigations have found that mood improvements after exercise tend to last longer than those after quiet rest.59
Craving
There were significant decreases in craving over the course of the intervention, although these results should be interpreted cautiously since they became non-significant after excluding those with missing data. These results suggest that IOP care, with or without an added exercise component, reduces craving over time. These results are in agreement with the broader treatment literature showing there are significant decreases in craving during the first several weeks of recovery, whether or not adjunctive therapies are utilized (e.g., pharmacotherapies).60,61 The addition of adjunctive treatments tends to reduce craving more quickly and provides longer-lasting craving reductions than outpatient therapy with a placebo treatment.25,61 Thirty minutes of aerobic exercise or quiet rest also led to acute reductions in craving. Acute exercise has been shown to reduce craving for many substances including tobacco,62 cannabis,51 and alcohol,63 but other studies have noted negligible, or in some cases, increases in craving after acute exercise for polysubstance use.57 Interestingly, thirty minutes of quiet rest also led to acute reductions in craving, which is contrary to other studies.47,62 However, most investigations utilized a single session of passive sitting without reading materials, mobile devices, or any other distractions.64 Another study investigated the effects of repeated quiet rest sessions compared with exercise training, and they found that there were no changes in cocaine craving among control participants who sat quietly without reading materials, mobile devices, or other distractions for 30 minutes, 3 times per week for 4 weeks.47 In the present study, TAU participants were not allowed to use electronic devices, but they were provided with standardized reading materials. There is evidence indicating that reading can lead to mood improvements that are similar to moderate-intensity exercise,58 so it is possible that the simple act of reading or not being connected to technology may have led to acute improvements in mood and craving. Though providing reading materials could be considered a limitation, it was thought that not giving TAU participants access to reading materials over 6 weeks would not only lead to poorer adherence but would also be less comparable to the EX group wherein participants were also allowed to listen to music. There are currently few studies with mixed findings on whether listening to music during exercise has additional, positive effects on mood,65,66 but it is thought that music may lead to greater exercise adherence.67
Endocannabinoids
There were acute increases in circulating AEA concentrations following exercise. These results indicate that the eCB system can be activated by exercise in both SUD patients and healthy adults.28,29,68 In healthy adults, increased AEA after exercise has been associated with a variety of beneficial psychological outcomes including positive affect and vigor and could be one indicator of appropriate adaptation to an acute physical stressor.30,69 Activation of AEA by exercise may also be an important step to restoring the eCB system, which is often downregulated with chronic heavy drug use, although much research is needed.21,22 A blunted AEA response may be an indicator of an insufficient or maladaptive stress response. For instance, circulating AEA concentrations were inversely associated with motion sickness severity during a stress-evoking parabolic flight protocol.70 Other research has found that reductions in circulating AEA align with worsening psychological outcomes during a period of exercise deprivation in participants characterized as “exercise dependent.”71 Conversely, exercise had no effect on circulating 2-AG concentrations. In response to a physical and psychological stress, 2-AG typically increases, suggesting it is part of the adaptive response to stress.70,72,73 Therefore, lack of an acute 2-AG response to exercise could indicate dysfunction in the eCB system among SUD patients and may contribute to aberrant acute stress responses in SUD patients.24 Supporting this idea, Crombie et al74 also found that 2-AG increased after 30 minutes of exercise in healthy controls but not in patients with post-traumatic stress disorder, which is another patient population with inadequate stress adaptation and noted eCB dysfunction.
Limitations
The main limitation of this study is the small sample size, which could have contributed to non-significant outcomes. Furthermore, the small sample size precluded subgroup analyses by demographic variables that have been shown to affect exercise-related SUD treatment outcomes such as sex or racial and ethnic background.75,76 In light of the small sample, effect sizes for the treatment effects of EX and TAU were calculated for the main outcomes. Large effect sizes (η2≥0.14) were observed for those outcomes that already demonstrated significant results (e.g., changes in stress, acute changes in mood states, craving, and AEA). Despite being a small sample, the characteristics of the participants in the current study were representative of the larger SUD outpatient population. For instance, according to an analysis of 269,783 SUD outpatients, the primary substances of abuse were alcohol (48%), cannabis (26%), stimulants (13%), or opioids (13%); and approximately half of outpatients (47%) reported using at least two or more substances.77 In the present study, the majority of participants in this study were in treatment for alcohol use, followed by cannabis use, and then stimulant or opioid use; and 57% (12 participants) reported receiving treatment for two or more substances. The overall continuous abstinence rate of 29% (6 participants) is also similar to the conservative estimate produced by a meta-analysis of 21 SUD treatment trials, which found that approximately 32% of individuals who receive treatment achieve complete abstinence (follow-up periods ranged from 6 months to >5 years). Retention in this study, 71% (15 participants), was higher than the typical 52% completion rate for outpatient treatment.77 This could indicate that participation in an additional intervention led to improved treatment retention or that highly motivated individuals were more likely to volunteer for a research study alongside IOP treatment. In addition, 57% (12 participants) were taking prescription medications for comorbid depression and/or anxiety, which is similar to epidemiological reports indicating a 53% psychiatric comorbidity rate among those with SUDs.78 Thus, even though the sample was small, the results are generalizable and useful to estimate sample sizes for future trials.
Other limitations of this study include only having one post-session assessment time, which occurred immediately after exercise or quiet rest. It is possible that the effects of exercise on mood or craving may have lasted longer than the effects of quiet rest, which could have implications for SUD patients as they return to their normal daily routines and previous drug cues after an exercise session. For example, Ellingsen et al. (2018) reported improvements in mood and positive affect up to 4 hours post-exercise in a small sample of polysubstance use disorder patients, but depending on the exercise modality, they also observed increases in craving several hours later as well.57 Another limitation is that extra-intervention physical activity was not measured objectively before or during the study. Only 5% of Americans meet minimum physical activity recommendations when assessed objectively,79 and physical activity among SUD patients tends to be lower than what is observed in the general public.6 The screening criteria for this study included no current participation in a structured exercise program. Correspondingly, the “physically active” category (Figure 1) contained the majority of excluded individuals, meaning many individuals were honest about their current activity levels and were perhaps uncomfortable with the prospect of being randomized to the TAU (no exercise) group. Thus, it is likely that our enrolled participants were not physically active prior to the study. Physical activity or sedentary behavior throughout the 6-week study was also not assessed, so it is possible that a change in these behaviors (e.g., less extended screen time) also affected certain outcomes.80,81 There was no assessment of diet in this study, and food intake patterns may change dramatically during initial SUD treatment and could potentially affect mood or craving outcomes. 82
In conclusion, the results from this study add to the growing body of literature indicating that adjunctive exercise may provide benefits to individuals currently in treatment for SUDs. Patients enrolled in an IOP were able to adhere to an exercise program on top of their time-intensive treatment programs. Importantly, adding exercise to standard care did not negatively influence the positive effects of IOP on mood, stress, and craving. In addition to its other well-established health benefits, exercise provided the further benefit of increasing vigor and AEA, both of which tend to be lower among SUD patients.22 Given the complex issues surrounding recruitment and retention of this clinical population, large, multisite, and highly collaborative clinical exercise trials are needed to determine the efficacy of exercise and its potential underlying mechanisms across a variety of SUD populations and disease severities.
Supplementary Material
Acknowledgments
Funding: This work was supported by NIH under grant R36DA040140, the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin, and the University of Wisconsin Virginia Horne Henry Fund. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declarations of interest: CJH is a member of the Scientific Advisory Board of Phytecs, Inc. AGB, KMC, RTB, and KFK have no disclosures.
RCT#: NCT02570360
References
- 1.Bose J, Hedden S, Lipari R, Park-Lee E. 2017 NSDUH Annual National Report | CBHSQ. https://www.samhsa.gov/data/report/2017-nsduh-annual-national-report.Published 2018. Accessed May 6, 2019.
- 2.NIDA. Principles of Effective Treatment | National Institute on Drug Abuse (NIDA). https://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/principles-effective-treatment. Published 2018. Accessed May 6, 2019.
- 3.Fleury M-J, Djouini A, Huỳnh C, et al. Remission from substance use disorders: A systematic review and meta-analysis. Drug Alcohol Depend. 2016;168:293–306. doi: 10.1016/j.drugalcdep.2016.08.625 [DOI] [PubMed] [Google Scholar]
- 4.Abrantes AM, Blevins CE. Exercise in the Context of Substance Use Treatment: Key Issues and Future Directions. Curr Opin Psychol. April2019. doi: 10.1016/J.COPSYC.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 5.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37(8):1622–1644. doi: 10.1016/j.neubiorev.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brellenthin AG, Lee D-C. Physical Activity and the Development of Substance Use Disorders: Current Knowledge and Future Directions. Prog Prev Med (New York, NY). 2018;3(3). doi: 10.1097/pp9.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Wang Y, Wang Y, Li R, Zhou C. Impact of physical exercise on substance use disorders: a meta-analysis. PLoS One. 2014;9(10):e110728. doi: 10.1371/journal.pone.0110728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallgren M, Vancampfort D, Giesen ES, Lundin A, Stubbs B. Exercise as treatment for alcohol use disorders: systematic review and meta-analysis. Br J Sports Med. 2017; 51(14):1058–1064. doi: 10.1136/bjsports-2016-096814 [DOI] [PubMed] [Google Scholar]
- 9.Flemmen G, Unhjem R, Wang E. High-intensity interval training in patients with substance use disorder. Biomed Res Int. 2014;2014:616935. doi: 10.1155/2014/616935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ermalinski R, Hanson PG, Lubin B, Thornby JI, Nahormek PA. Impact of a body-mind treatment component on alcoholic inpatients. J Psychosoc Nurs Ment Health Serv. 1997;35(7):39–45. [DOI] [PubMed] [Google Scholar]
- 11.Rawson RA, Chudzynski J, Mooney L, et al. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug Alcohol Depend. 2015;156:21–28. doi: 10.1016/j.drugalcdep.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown RA, Abrantes AM, Read JP, et al. Aerobic exercise for alcohol recovery: rationale, program description, and preliminary findings. Behav Modif. 2009;33(2):220–249. doi: 10.1177/0145445508329112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RA, Abrantes AM, Read JP, et al. A Pilot Study of Aerobic Exercise as an Adjunctive Treatment for Drug Dependence. Ment Health Phys Act. 2010;3(1):27–34. doi: 10.1016/j.mhpa.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarty D, Braude L, Lyman DR, et al. Substance abuse intensive outpatient programs: assessing the evidence. Psychiatr Serv. 2014;65(6):718–726. doi: 10.1176/appi.ps.201300249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442 [DOI] [PubMed] [Google Scholar]
- 17.Greer TL, Ring KM, Warden D, et al. Rationale for Using Exercise in the Treatment of Stimulant Use Disorders. J Glob drug policy Pract. 2012;6(1). [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sport Med. 2004;38(5):536–541. doi: 10.1136/bjsm.2004.011718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tantimonaco M, Ceci R, Sabatini S, et al. Physical activity and the endocannabinoid system: an overview. Cell Mol Life Sci. 2014;71(14):2681–2698. doi: 10.1007/s00018-014-1575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillard CJ. Chapter One – The Endocannabinoid Signaling System in the CNS: A Primer. In: International Review of Neurobiology. Vol 125.; 2015:1–47. doi: 10.1016/bs.irn.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccarini, Hompes T, Verhaeghen A, et al. Changes in Cerebral CB1 Receptor Availability after Acute and Chronic Alcohol Abuse and Monitored Abstinence. J Neurosci. 2014;34(8):2822–2831. doi: 10.1523/JNEUROSCI.0849-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan, Page E, Schaefer C, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. 2013;202:381–382. doi: 10.1192/bjp.bp.112.121178 [DOI] [PubMed] [Google Scholar]
- 23.Hill Patel. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol Mood Anxiety Disord. 2013;3(1):19. doi: 10.1186/2045-5380-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha R New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Curr Psychiatry Rep 2011;13(5):398–405. doi: 10.1007/s11920-011-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allsop DJ, Copeland J, Lintzeris N, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA psychiatry. 2014;71(3):281–291. doi: 10.1001/jamapsychiatry.2013.3947 [DOI] [PubMed] [Google Scholar]
- 26.Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone Decreases Marijuana Withdrawal and a Laboratory Measure of Marijuana Relapse. Neuropsychopharmacology. 2013;38(8):1557–1565. doi: 10.1038/npp.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justinova Z, Panlilio LV, Moreno-Sanz G, et al. Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse. Neuropsychopharmacology March2015. doi: 10.1038/npp.2015.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heyman E, Gamelin F-X, Goekint M, et al. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–851. doi: 10.1016/j.psyneuen.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 29.Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2013;113(4):869–875. doi: 10.1007/s00421-012-2495-5 [DOI] [PubMed] [Google Scholar]
- 30.Brellenthin AG, Crombie KM, Hillard CJ, Koltyn KF. Endocannabinoid and Mood Responses to Exercise in Adults with Varying Activity Levels. Med Sci Sport Exerc. 2017;49(8):1688–1696. doi: 10.1249/MSS.0000000000001276 [DOI] [PubMed] [Google Scholar]
- 31.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68(1):134–144. http://www.ncbi.nlm.nih.gov/pubmed/10710848.Accessed January 22, 2017. [DOI] [PubMed] [Google Scholar]
- 32.Ferri C, Marsden J, de Araujo M, Laranjeira R, Gossop M. Validity and reliability of the Severity of Dependence Scale (SDS) in a Brazilian sample of drug users. Drug Alcohol Rev. 2000;19(4):451–455. doi: 10.1080/713659418 [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A Brief Measure for Assessing Generalized Anxiety Disorder. Arch Intern Med. 2006;166(10):1092. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 35.Annis H, Graham JM. Situational Confidence Questionnaire (SCQ) : User’s Guide. Toronto: Addiction Research Foundation of Ontario; 1988. [Google Scholar]
- 36.Levenstein S, Prantera C, Varvo V, Scribano ML. Development of the Perceived Stress Questionnaire: A new tool for psychosomatic research. J Psychosom Res. 1993;37(1):19–32. doi: 10.1016/0022-3999(93)90120-5 [DOI] [PubMed] [Google Scholar]
- 37.Singleton EG, Heishman SJ, Henningfield JE. Cognitive features of human drug craving. Problems of Drug Dependence. In: Proceedings of the 58th Annual Meeting, The College on Problems of Drug Dependence, Inc. Rockville, MD: National Institute on Drug Abuse; 1996:185. [Google Scholar]
- 38.Heinz AJ, Epstein DH, Schroeder JR, Singleton EG, Heishman SJ, Preston KL. Heroin and cocaine craving and use during treatment: measurement validation and potential relationships. J Subst Abuse Treat. 2006;31(4):355–364. doi: 10.1016/j.jsat.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 39.Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102(1–3):35–40. doi: 10.1016/j.drugalcdep.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83(3):233–237. doi: 10.1016/j.drugalcdep.2005.11.022 [DOI] [PubMed] [Google Scholar]
- 41.McNair D, Lorr M, & Droppleman L Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 42.Borg G Borg’s Perceived Exertion and Pain Scales. Champaign, Illinois: Human Kinetics; 1998. [Google Scholar]
- 43.Crombie KM, Brellenthin AG, Hillard CJ, Koltyn KF. Psychobiological Responses to Aerobic Exercise in Individuals With Posttraumatic Stress Disorder. J Trauma Stress. 2018;31(1). doi: 10.1002/jts.22253 [DOI] [PubMed] [Google Scholar]
- 44.Gossop M, Darke S, Griffiths P, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction. 1995;90(5):607–614. [DOI] [PubMed] [Google Scholar]
- 45.Cohen S, Williamson G. Perceived Stress in a Probability Sample of the United States. (Spacapan S, Oskamp S, eds.). Newbury Park, CA: Sage Publications; 1988. [Google Scholar]
- 46.Fritz CO, Morris PE, Richler JJ. Effect size estimates: Current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi: 10.1037/a0024338 [DOI] [PubMed] [Google Scholar]
- 47.De La Garza R, Yoon JH, Thompson-Lake DGY, et al. Treadmill Exercise Improves Fitness and Reduces Craving and Use of Cocaine in Individuals with Concurrent Cocaine and Tobacco-use Disorder. Psychiatry Res. 2016;245:133–140. doi: 10.1016/j.psychres.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roessler KK. Exercise treatment for drug abuse--a Danish pilot study. Scand J Public Health. 2010;38(6):664–669. doi: 10.1177/1403494810371249 [DOI] [PubMed] [Google Scholar]
- 49.Trivedi MH, Greer TL, Rethorst CD, et al. Randomized Controlled Trial Comparing Exercise to Health Education for Stimulant Use Disorder. J Clin Psychiatry. February2017. doi: 10.4088/JCP.15m10591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colledge F, Vogel M, Dürsteler-Macfarland K, et al. A pilot randomized trial of exercise as adjunct therapy in a heroin-assisted treatment setting. J Subst Abuse Treat. January2017. doi: 10.1016/j.jsat.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 51.Buchowski MS, Meade NN, Charboneau E, et al. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011;6(3):e17465. doi: 10.1371/journal.pone.0017465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bandura A Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 53.Greenfield SF, Hufford MR, Vagge LM, Muenz LR, Costello ME, Weiss RD. The relationship of self-efficacy expectancies to relapse among alcohol dependent men and [DOI] [PubMed]
- 54.Connor JP, Gullo MJ, Feeney GFX, Kavanagh DJ, Young RM. The relationship between cannabis outcome expectancies and cannabis refusal self-efficacy in a treatment population. Addiction. 2014;109(1):111–119. doi: 10.1111/add.12366 [DOI] [PubMed] [Google Scholar]
- 55.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- 56.Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55(1):79–86. [DOI] [PubMed] [Google Scholar]
- 57.Ellingsen MM, Johannesen SL, Martinsen EW, Hallgren M. Effects of acute exercise on drug craving, self-esteem, mood and affect in adults with poly-substance dependence: Feasibility and preliminary findings. Drug Alcohol Rev. 2018;37(6):789–793. doi: 10.1111/dar.12818 [DOI] [PubMed] [Google Scholar]
- 58.Jin P Efficacy of Tai Chi, brisk walking, meditation, and reading in reducing mental and emotional stress. J Psychosom Res. 1992;36(4):361–370. doi: 10.1016/0022-3999(92)90072-A [DOI] [PubMed] [Google Scholar]
- 59.Raglin JS, Morgan WP. Influence of exercise and quiet rest on state anxiety and blood pressure. Med Sci Sports Exerc. 1987;19(5):456–463. [PubMed] [Google Scholar]
- 60.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. [DOI] [PubMed] [Google Scholar]
- 61.Chick J, Anton R, Checinski K, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35(6):587–593. [DOI] [PubMed] [Google Scholar]
- 62.Janse Van Rensburg K, Taylor A, Hodgson T, Benattayallah A. Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: an fMRI study. Psychopharmacology (Berl). 2009;203(3):589–598. doi: 10.1007/s00213-008-1405-3 [DOI] [PubMed] [Google Scholar]
- 63.Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99(12):1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x [DOI] [PubMed] [Google Scholar]
- 64.Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology (Berl). 2012;222(1):1–15. doi: 10.1007/s00213-012-2731-z [DOI] [PubMed] [Google Scholar]
- 65.Hayakawa Y, Miki H, Takada K, Tanaka K. EFFECTS OF MUSIC ON MOOD DURING BENCH STEPPING EXERCISE. Percept Mot Skills. 2000;90(1):307–311. doi: 10.2466/pms.2000.90.1.307 [DOI] [PubMed] [Google Scholar]
- 66.Macone D, Baldari C, Zelli A, Guidetti L. Music and Physical Activity in Psychological Well-Being. Percept Mot Skills. 2006;103(1):285–295. doi: 10.2466/pms.103.1.285-295 [DOI] [PubMed] [Google Scholar]
- 67.Karageorghis CI, Priest D-L. Music in the exercise domain: a review and synthesis (Part II). Int Rev Sport Exerc Psychol. 2012;5(1):67–84. doi: 10.1080/1750984X.2011.631027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14(17):2209–2211. doi: 10.1097/01.wnr.0000097048.56589.47 [DOI] [PubMed] [Google Scholar]
- 69.Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner’s high”. J Exp Biol. 2012;215(Pt 8):1331–1336. doi: 10.1242/jeb.063677 [DOI] [PubMed] [Google Scholar]
- 70.Choukèr A, Kaufmann I, Kreth S, et al. Motion sickness, stress and the endocannabinoid system. PLoS One. 2010;5(5):e10752. doi: 10.1371/journal.pone.0010752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antunes HKM, Leite GSF, Lee KS, et al. Exercise deprivation increases negative mood in exercise-addicted subjects and modifies their biochemical markers. Physiol Behav. 2016;156:182–190. doi: 10.1016/j.physbeh.2016.01.028 [DOI] [PubMed] [Google Scholar]
- 72.Hill MN, McLaughlin RJ, Bingham B, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 2010;107(20):9406–9411. doi: 10.1073/pnas.0914661107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34(8):1257–1262. doi: 10.1016/j.psyneuen.2009.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crombie KM, Leitzelar BN, Brellenthin AG, Hillard CJ, Koltyn KF. Loss of exercise- and stress-induced increases in circulating 2-arachidonoylglycerol concentrations in adults with chronic PTSD. Biol Psychol. 2019;145:1–7. doi: 10.1016/j.biopsycho.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 75.Sanchez K, Greer TL, Walker R, Carmody T, Rethorst CD, Trivedi MH. Racial and ethnic differences in treatment outcomes among adults with stimulant use disorders after a dosed exercise intervention. J Ethn Subst Abuse. 2017;16(4):495–510. doi: 10.1080/15332640.2017.1317310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lynch WJ, Abel J, Robinson AM, Smith MA. Exercise as a Sex-Specific Treatment for Substance Use Disorder. Curr Addict Reports. 2017:1–15. doi: 10.1007/s40429-017-0177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stahler GJ, Mennis J, DuCette JP. Residential and outpatient treatment completion for substance use disorders in the U.S.: Moderation analysis by demographics and drug of choice. Addict Behav. 2016;58:129–135. doi: 10.1016/j.addbeh.2016.02.030 [DOI] [PubMed] [Google Scholar]
- 78.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 79.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical Activity in the United States Measured by Accelerometer. Med Sci Sport Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 80.Hallgren M, Owen N, Stubbs B, et al. Passive and mentally-active sedentary behaviors and incident major depressive disorder: A 13-year cohort study. J Affect Disord. 2018;241:579–585. doi: 10.1016/j.jad.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 81.Tull MT, Lee AA, Geers AL, Gratz KL. Exploring the role of sedentary behavior and physical activity in depression and anxiety symptom severity among patients with substance use disorders. Ment Health Phys Act. March2018. doi: 10.1016/j.mhpa.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hodgkins C, Frost-Pineda K, Gold MS. Weight Gain During Substance Abuse Treatment. J Addict Dis. 2007;26(sup1):41–50. doi: 10.1300/J069v26S01_05 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.