Abstract
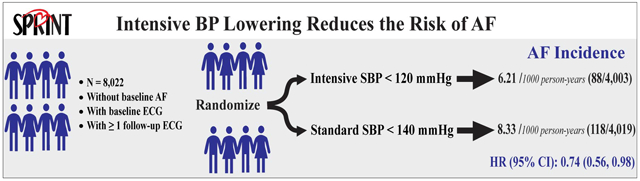
It remains uncertain whether intensive control of blood pressure (BP) results in a lower risk of atrial fibrillation (AF) in patients with hypertension. Using data from the Systolic Blood Pressure Intervention Trial (SPRINT), which enrolled participants with hypertension at increased risk of cardiovascular disease (CVD), we examined whether intensive BP lowering (target systolic BP (SBP) <120 mm Hg), compared with standard BP lowering (target SBP<140 mm Hg), results in a lower risk of AF. This analysis included 8,022 participants (4,003 randomized to the intensive arm and 4,019 to standard BP arm) who were free of AF at the time of enrollment and with available baseline and follow-up electrocardiographic data. AF was ascertained from standard 12-lead electrocardiograms recorded at biannual study examinations and an exit visit. During up to 5.2 years of follow-up and a total of 28,322 person-years, 206 incident AF cases occurred; 88 in the intensive BP lowering arm and 118 in the standard BP lowering arm. Intensive BP lowering was associated with a 26% lower risk of developing new AF (hazard ratio= 0.74; 95% confidence interval, 0.56 to 0.98; p-value =0.037). This effect was consistent among pre-specified subgroups of SPRINT participants stratified by age, sex, race, SBP tertiles, prior CVD, and prior chronic kidney disease when interactions between treatment effect and these subgroups were assessed using Hommel-adjusted p-values. In conclusion, intensive treatment to a target of SBP <120 mm Hg in patients with hypertension at high risk of CVD has the potential to reduce the risk of AF.
Clinical Trial Registration:
Keywords: Blood Pressure, Intensive Lowering, Hypertension, Atrial Fibrillation, SPRINT Trial
Graphical Abstract
INTRODUCTION
Hypertension is the most common modifiable risk factor for atrial fibrillation (AF). More than one-fifth of the new AF cases are due to hypertension (1), and each 20 mm Hg increase in systolic blood pressure (SBP) is associated with a 21% higher risk of AF (2). Because of the close relationship between high SBP and AF, hypertension has been one of the key components in all AF risk prediction scores (3-5). The coexistence of hypertension with AF in about 60-80% of patients with permanent AF led to the emerging argument that AF should be considered as another manifestation of hypertensive target organ damage (6).
Several epidemiologic studies have shown that systolic BP (SBP) at levels previously considered as pre-hypertension or high normal (130–139 mm Hg) are also associated with increased risk of AF, compared with normal SBP (<120 mm Hg) (7-10). These findings suggest that intensive control of BP targeting SBP <120 mm Hg may result in further lowering in AF risk among patients with hypertension. However, evidence is yet to be established from randomized controlled trials to support this suggestion. In the ACCORD BP trial (Action to Control Cardiovascular Risk in Diabetes BP trial) study, the association between intensive BP lowering (targeting SBP <120 mm Hg) and incident AF failed to reach statistical significance in patients with hypertension and diabetes mellitus (11). On the other hand, in the Cardio-Sis trial (Controllo della Pressione Arteriosa Sistolica trial) lowering SBP to a target of <130 mm Hg (versus a target of <140 mm Hg) resulted in a significant reduction in AF incidence in patients with hypertension without diabetes (12). Therefore, it remains uncertain whether intensive BP lowering to a target of SBP <120 mm Hg results in further lowering of the risk of AF in patients with hypertension. We utilized data from the SPRINT trial (the Systolic Blood Pressure Intervention Trial) to fill this gap in knowledge.
METHODS
The electrocardiographic data that support the findings of this study are available from the corresponding author upon reasonable request. Investigators interested in obtaining other SPRINT data may utilize the NHLBI BioLINCC repository.
Study Population and Design
The design, processes, and rationale of the SPRINT trial have been published previously (13, 14). Briefly, SPRINT was conducted at 102 clinical sites organized into five clinical center networks in the United States. Inclusion criteria included 50 years of age or older, a SBP between 130 and 180 mm Hg at the screening visit and an increased risk of cardiovascular disease events defined as history of clinical or subclinical cardiovascular disease, chronic kidney disease, a 10-year cardiovascular disease risk ≥15% estimated by the Framingham general cardiovascular disease risk score, or an age ≥75 years. Participants with type 2 diabetes mellitus, symptomatic heart failure within 6 months of enrollment or ejection fraction <35%, or prior stroke were excluded. Details about the inclusion and exclusion criteria applied in the SPRINT trial have been published previously (13).
A total of 9,361 participants were enrolled in SPRINT between November 2010 and March 2013, of whom 4,683 were randomized to an SBP target of <140 mm Hg (standard treatment arm), and 4,678 participants were randomized to <120 mm Hg (intensive treatment arm). The standard treatment arm protocol intended to achieve SBP in the range of 135 to139 mm Hg by downward titration of medication if the SBP was less than 135 mm Hg. Randomization was stratified by clinical site. The SPRINT intervention was stopped early after a median follow up of 3.26 years because of a 25% reduction in the primary composite cardiovascular disease endpoint and a 27% reduction in all-cause mortality in the intensive treatment group. For the purpose of this analysis, we excluded participants with missing or uninterpretable baseline ECG data, with baseline AF, or without follow-up ECG. After all exclusions, 8,022 participants remained and included in the analysis (Figure 1). The study was approved by the institutional review board at each participating site, and written informed consent was obtained from all participants.
Figure 1:
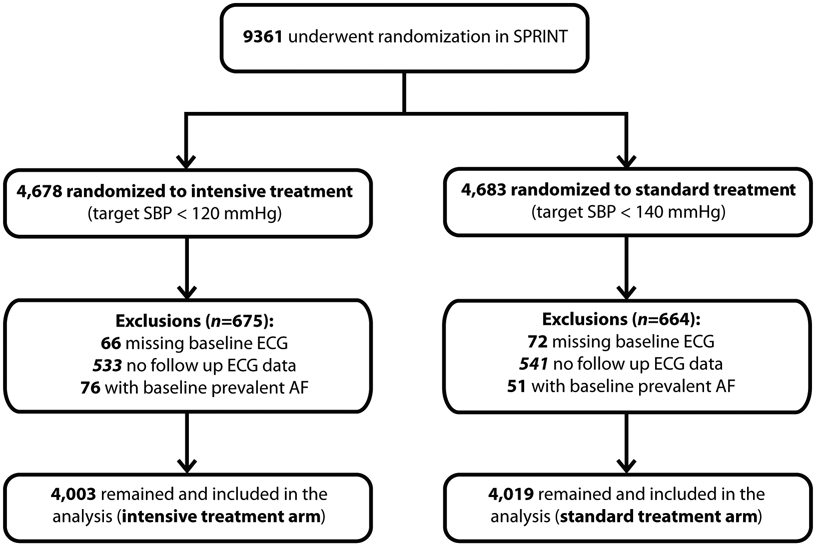
Inclusions and exclusions of the participants included in the analysis
ECG= electrocardiogram; AF= atrial fibrillation
Ascertainment of AF
AF was ascertained from standard 12-lead ECG tracings obtained at baseline, year 2, year 4, and at a close-out visit. Digital ECG data were recorded using a GE MAC 1200 electrocardiograph (GE, Milwaukee, Wisconsin) at 10 mm/mV calibration and a speed of 25 mm/s. ECG reading was performed centrally at the Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine, Winston-Salem, North Carolina by staff blinded to the treatment assignment. All ECG tracings were initially inspected visually for technical errors and inadequate quality before being automatically processed using GE 12-SL Marquette version 2001 (GE, Milwaukee, Wisconsin). ECG abnormalities, including AF, were classified using the standards of the Minnesota ECG Classification (15). For the purpose of this analysis, all ECGs which were automatically classified as AF were visually confirmed by the SPRINT ECG Reading Center staff. Also, all ECGs with Minnesota ECG Classification codes that suppress the detection of AF were reviewed to ensure the inclusion of all AF cases. That is to say, ECGs with Minnesota ECG codes for conditions such as ventricular pacemaker that take priority in reporting according to the rules of the Minnesota ECG classification and subsequently can mask detection of AF were reviewed to ensure the inclusion of all AF cases.
Other Study Measurements
Demographic data were collected at baseline before randomization. Details of the assessment of BP, the adjustment of medication doses, and antihypertensive drug regimens during the trial were published previously (14). Briefly, at each visit, trained clinical staff used an automated BP device (Omron-HEM-907 XL) to measure BP. Standardized BP procedures included measuring BP early in the visit and not following stressful exam components such as blood draws, proper positioning of the participant in a chair with back support, and adequate cuff size determination.
Statistical Analyses
We used Cox proportional hazards regression to compare the time to the first occurrence of AF in those without baseline AF. The clinical site at randomization was used as a stratification factor as for the primary SPRINT results (13). Follow-up time was censored at the date of the last ECG or death. Interactions between treatment effect and SPRINT pre-specified subgroups (age (<75 vs. ≥75 years), sex, race (black vs. non-black), SBP tertiles (≤132, >132 to <145, ≥145 mm Hg), prior cardiovascular disease, and prior chronic kidney disease (defined as an estimated glomerular filtration rate of less than 60 ml per minute per 1.73 m2 of body-surface area) were assessed with a likelihood-ratio test for the interaction with the use of Hommel-adjusted (16) p values. There was no evidence that the proportional hazards assumption was violated. All p-values reported were 2-sided, and statistical significance threshold was chosen as 5%. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 8,022 participants (mean age 68 (±9) years, 35.5% women, and 31.6% black) were included in the analysis; 4,003 in the intensive BP lowering arm and 4,019 in the standard BP lowering arm (Figure 1). The baseline characteristics are presented in Table 1. There were no significant differences between the treatment arms.
Table 1.
Baseline characteristics
| Characteristics* |
Total (n=8,022) |
Intensive Arm (n=4,003) |
Standard Arm (n=4,019) |
|---|---|---|---|
| Age (years) | 67.7±9.2 | 67.7±9.2 | 67.7±9.3 |
| Age ≥75 years | 2170 (27.1) | 1084 (27.1) | 1086 (27.0) |
| Sex (women) | 2845 (35.5) | 1436 (35.9) | 1409 (35.1) |
| Black race† | 2533 (31.6) | 1242 (31.0) | 1291 (32.1) |
| Smoking | |||
| Former smoker | 3396 (42.3) | 1684 (42.1) | 1712 (42.6) |
| Current smoker | 1043 (13.0) | 534(13.3) | 509 (12.7) |
| Body mass index (kg/m2) | 29.9 (5.7) | 29.95(5.8) | 29.8 (5.7) |
| Systolic BP (mm Hg) | 139.6±15.5 | 139.5±15.7 | 139.6±15.3 |
| Diastolic BP (mm Hg) | 78.1±11.8 | 78.2±11.8 | 78.1±78.1 |
| Systolic BP tertiles | |||
| ≤132 mm Hg | 2685 (33.5) | 1366 (34.1) | 1319 (32.8) |
| >132 to <145 mm Hg | 2634 (32.8) | 1281 (32.0) | 1353 (33.7) |
| ≥145 mm Hg | 2703 (33.7) | 1356 (33.8) | 1347 (33.5) |
| Number of BP medications | 1.8 (1.0) | 1.84 (1.0) | 1.81 (1.0) |
| Not using BP medications | 775 (9.7) | 374 (9.3) | 401 (10.0) |
| Serum creatinine (mg/dL) | 1.07±0.34 | 1.07±0.34 | 1.07±0.33 |
| Urine albumin/creatinine (mg/g) | 37.2±125.4 | 38.3±132.4 | 36.1±118.1 |
| Chronic kidney disease†† | 2192 (27.3) | 1123 (28.1) | 1069 (26.6) |
| Total cholesterol (mg/dL) | 190.1±41.2 | 190.7±41.7 | 190.4±40.8 |
| HDL- cholesterol (mg/dL) | 52.9±14.4 | 52.9±14.3 | 52.9±14.5 |
| Triglycerides (mg/dL) | 126.4±91.5 | 126.3±88.3 | 126.4±93.7 |
| Fasting plasma glucose (mg/dL) | 98.8±13.4 | 98.9±13.6 | 98.7±13.1 |
| Prior cardiovascular disease | 1564 (19.5) | 779 (19.5) | 785 (19.5) |
BP= blood pressure; e-GFR= estimated glomerular filtration rate (ml/min/1.73m2); HDL= high density lipoprotein
Data are presented as number (%) or mean ± standard deviation. No significant differences (P<0.05) between the treatment arms
Black race includes Hispanic black and black as part of a multiracial identification. Race and ethnic group were self-reported
Defined as baseline estimated glomerular filtration rate <60 ml/min/1.73m2
SPRINT participants were followed for up to 5.2 years (median, 3.8 years), contributing to 28,322 person-years of follow-up. A total of 206 incident AF cases occurred during follow up. Among those who had more than one follow-up ECG after developing AF (n=77), 55 (71.4%) showed AF in all subsequent follow-up ECGs while the rest (n=22; (28.6%)) converted to sinus rhythm. In the intensive BP arm there were less incident AF cases than the standard arm; 88 incident AF cases compared with 118 in the standard arm (hazard ratio=0.74, 95% confidence interval: 0.56 to 0.98, P=0.037) (Table 2). This effect was consistent among SPRINT pre-specified subgroups when interactions between treatment effect and these subgroups were assessed using Hommel-adjusted p-values. Figure 2 shows the unadjusted comparison of the effect of intensive vs. standard BP lowering on the risk of incident AF in each of the pre-specified subgroups.
Table 2.
Effect of intensive versus standard BP lowering on incident AF in SPRINT
| Treatment arm | Participants (n) |
Events (n) |
Event rate (1000 person-years) |
HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Intensive BP lowering | 4003 | 88 | 6.21 | 0.74 (0.56,0.98) | 0.037 |
| Standard BP lowering | 4019 | 118 | 8.33 |
Clinical site at randomization was used as a stratification factor
AF= atrial fibrillation; BP= blood pressure; HR (95% CI) = hazard ratio (95% confidence interval)
Figure 2:
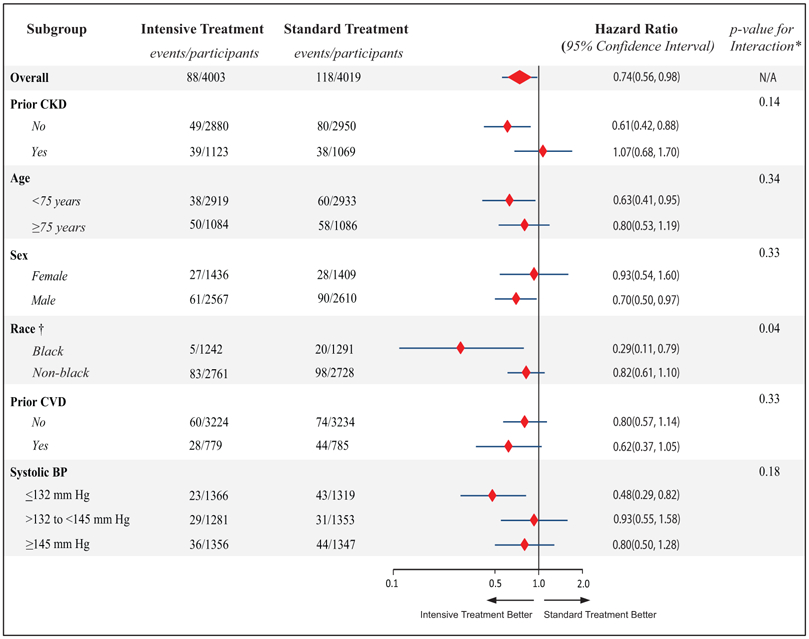
Effect of intensive BP lowering on the risk of incident AF in pre-specified subgroups
BP= blood pressure; AF= atrial fibrillation; CKD= chronic kidney disease, CVD= cardiovascular disease
*p-values listed are unadjusted.
†Black race includes Hispanic black and black as part of a multiracial identification. Hommel-adjusted p-value for race is 0.24
DISCUSSION
In this analysis from the SPRINT trial, which recruited participants who were at increased risk of cardiovascular disease, we showed that intensive BP control targeting SBP <120 mm Hg resulted in a lower risk of AF. About 46% of the United States adults have high BP, and up to 6.1 million suffer from AF (17). These high rates and the strong relationship between high BP and AF highlight the importance of AF prevention in patients with hypertension. In the absence of specific drug therapies as primary AF prevention in patients with hypertension, management of risk factors is of utmost importance. Hypertension is not only the most common, but also the most important modifiable AF risk factor given the magnitude of AF risk associated with high BP (1, 2, 18, 19). This underscores the relevant clinical and public health implications of our findings.
In the main SPRINT results, intensive BP lowering was associated with a 25% reduction in composite cardiovascular events and a 27% reduction in all-cause mortality (13). These results formed the basis for the current hypertension guidelines which suggested lower levels of BP for the definition of normal BP and hypertension compared to prior guidelines (20). Our study showing a 26% lower risk of AF rates in the SPRINT participants randomized to the intensive arm adds further evidence to the benefits of BP lowering beyond the previous standards of <140 mm Hg (20). Further studies may be needed, however, to examine whether the favorable impact of intensive BP lowering on the risk of AF also reduces the downstream complications of AF such as stroke, heart failure, and myocardial infarction (21-23). Also, a bidirectional relationship between AF with heart failure has been reported (24). Examining the impact of the temporal relation between heart failure and AF on the observed treatment effect on AF incidence may be warranted. However, due to the relatively small number of individual cardiovascular outcomes in SPRINT, we could not address these questions.
In our subgroup analysis, we observed a more favorable effect of intensive BP on AF risk in blacks than in non-blacks using unadjusted p-value for interaction, which became non-significant after using Hommel adjusted p-value for interaction. We realize that subgroup analyses should be interpreted with caution, and we cannot rule out that the observed differences by race could be due to chance only. However, given the established racial differences in both hypertension (25) and AF (26), this finding may warrant further investigation.
The pathophysiological basis for AF in hypertension is complex and not entirely clear. Hypothesized mechanisms involve several hemodynamic and neurohormonal factors interacting with each other. Left ventricular stress (LV) stress due to pressure overload, and higher levels of angiotensin II, aldosterone, norepinephrine, and inflammation are believed to play key roles in promoting retrograde left atrial stretching and structural remodeling which serves as a substrate for AF development (18). In contrast, normal levels of BP are associated with less strain on the LV and better LV emptying, and thus prevent functional and structural left atrial abnormalities, and potentially lower the risk of AF (19). These observations may explain the increased risk of AF in individuals with SBP levels higher than 120 mm Hg (7-10), and the benefits of intensive control of SBP we observed in our study.
It is possible that the improvement in AF risk among SPRINT participants randomized to the intensive BP arm in our study was mediated by a favorable impact of the intensive BP lowering on atrial remodeling. BP control before the occurrence of atrial remodeling is needed to prevent hypertension-related AF (6). Also, in a prior report from the ACCORD BP trial we showed that intensive BP control resulted in a lower risk of development of new ECG markers of left atrial abnormities indicating a favorable impact on atrial remodeling (11). These markers of atrial remodeling were strongly associated with AF in the ARIC (Atherosclerosis Risk in Communities) study (27).
To our knowledge, our current analysis is the first evidence from a randomized controlled trial in support of the benefit in reduction of AF risk as a result of aggressive BP lowering <120 mm Hg, compared to standard BP lowering <140 mm Hg. The two previous trials that tried to address this question did not have enough statistical power (11) or used higher SBP levels (12). In the ACCORD BP trial, which has a sample size of less than half of the SPRINT and included participants with both hypertension and diabetes, the effect of intensive BP lowering on AF risk did not reach statistical significance due to lack of statistical power (11). On the other hand, in the Cardio-Sis trial, which included 1111 patients with hypertension without diabetes mellitus, a lower risk of AF was observed in the intensive BP arm (Hazard ratio=0.46, 95% confidence interval 0.22 to 0.98; p-value 0.04). However, the comparison was between a higher target of SBP lowering (SBP<130 mm Hg) to a standard BP lowering (SBP goal <140 mm Hg) (12). Other reports addressing this question were from previously collected randomized clinical trials that did not randomize patients to different SBP control groups (8, 28).
Limitations
Our study has limitations. SPRINT was a treatment strategy trial; it examined the effect of different levels of SBP rather than the effect of individual drugs. Therefore, we could not examine the impact of individual BP medications or the address the potential for higher doses in the intensive arm. It is possible that some paroxysmal and intermittent AF cases were not detected in SPRINT since we did not use long-term ECG recording. However, any misclassification should have impacted both arms equally. Our findings may not be applicable to hypertension patients not included in SPRINT such as those with diabetes mellitus or prior stroke, and those at lower risk for cardiovascular disease. Our study has several strengths that are worth mentioning as well, including the clinical trial design with random treatment assignment, large sample size, racially diverse population, representation of women, a large proportion of participants over 75 years old, achievement and maintenance of the intended differences in SBP between arms throughout the study, standardized data collection, and centralized, blinded ECG reading
Perspectives
Our results suggest that intensive treatment to a target SBP of <120 mm Hg, compared to a target SBP of <140 mm Hg, in patients with hypertension has the potential to reduce the risk of AF. Nevertheless, further studies are needed to examine whether this favorable impact of intensive BP lowering on the risk of AF also reduces the downstream complications of AF such as stroke, heart failure, myocardial infarction. Examining the long-term effect of intensive BP strategy on AF risk may also be needed since it is unclear whether intensive BP prevented AF or just delayed its onset.
Novelty and Significance:
What Is New
This is the first evidence from a randomized controlled trial in support of the benefit in the reduction of AF risk as a result of aggressive blood pressure lowering <120 mm Hg, compared to standard blood pressure lowering <140 mm Hg.
What Is Relevant?
These results add further evidence to the benefits of intensive blood pressure lowering shown in the results of the main SPRINT trial which formed the basis for the current hypertension guidelines.
Summary.
In this analysis from the SPRINT randomized-controlled clinical trial, we found that a strategy targeting a systolic blood pressure lowering to less than 120 mm Hg resulted in a 26% lower risk of incident atrial fibrillation, compared to a strategy targeting a systolic blood pressure of less than 140 mm Hg.
Acknowledgments
Sources of Funding
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgment list: https://www.sprinttrial.org/public/dspScience.cfm.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services or of the Veterans Administration.
Footnotes
Conflict of Interest: None
REFERENCES
- 1.-.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.-.Emdin CA, Anderson SG, Salimi-Khorshidi G, Woodward M, MacMahon S, Dwyer T, Rahimi K. Usual blood pressure, atrial fibrillation and vascular risk: evidence from 4.3 million adults. Int J Epidemiol. 2017;46:162–172. doi: 10.1093/ije/dyw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 -.Li YG, Pastori D, Farcomeni A, Yang PS, Jang E, Joung B, Wang YT, Guo YT, Lip GYH. . A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian Subjects: Derivation in 471,446 Chinese Subjects, With Internal Validation and External Application in 451,199 Korean Subjects. Chest. 2019;155:510–518. doi: 10.1016/j.chest.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 -.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ . Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5 -.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 -.Dzeshka MS, Shahid F, Shantsila A, Lip GY. Atrial fibrillation and hypertension. Hypertension. 2017;70:854–861. doi: 10.1161/HYPERTENSIONAHA.117.08934 [DOI] [PubMed] [Google Scholar]
- 7 -.O’Neal WT, Soliman EZ, Qureshi W, Alonso A, Heckbert SR, Herrington D. Sustained pre-hypertensive blood pressure and incident atrial fibrillation: the Multi-ethnic Study of Atherosclerosis. J Am Soc Hypertens. 2015;9:191–196. doi: 10.1016/j.jash.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 -.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009; 119:2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9 -.Grundvold I, Skretteberg PT, Liestøl K, rikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertension. 2012;59:198–204. doi: 10.1161/HYPERTENSIONAHA.111.179713 [DOI] [PubMed] [Google Scholar]
- 10 -.Thomas MC, Dublin S, Kaplan RC, Glazer NL, Lumley T, Longstreth WT Jr, Smith NL, Psaty BM, Siscovick DS, Heckbert SR. Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens. 2008;21:1111–1116. doi: 10.1038/ajh.2008.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11 -.Chen LY, Bigger JT, Hickey KT, Chen H, Lopez-Jimenez C, Banerji MA, Evans G, Fleg JL, Papademetriou V, Thomas A, Woo V, Seaquist ER, Soliman EZ. Effect of Intensive Blood Pressure Lowering on Incident Atrial Fibrillation and P-Wave Indices in the ACCORD Blood Pressure Trial. Am J Hypertens. 2016;29:1276–1282. doi: 10.1093/ajh/hpv172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12 -.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G; Cardio-Sis Investigators. Usual versus tight control of systolic blood pressure in nondiabetic patients with hypertension (Cardio-Sis): an open-label randomized trial. Lancet. 2009;374:525–533. doi: 10.1016/S0140-6736(09)61340-4 [DOI] [PubMed] [Google Scholar]
- 13 -.SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahmsan M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14 -.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr, Whelton PK; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15 -.Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings (Second edition). Published by Springer-London, 2009 [Google Scholar]
- 16 -.Hommel G A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988;75:383–386 [Google Scholar]
- 17 -.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019. ;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 18 -.Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation: Doubts and certainties from basic and clinical Studies. Circ Res. 2018;122:352–368. doi: 10.1161/CIRCRESAHA.117.311402. [DOI] [PubMed] [Google Scholar]
- 19 -.Dzeshka MS, Shahid F, Shantsila A, Lip GY. Hypertension and atrial fibrillation: an intimate association of epidemiology, pathophysiology, and outcomes. Am J Hypertens. 2017;30:733–755. doi: 10.1093/ajh/hpx013 [DOI] [PubMed] [Google Scholar]
- 20 -.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018. ;138:e426–e483. doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 21 -.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 22 -.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M . Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. doi: 10.1001/jamainternmed.2013.11912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 -.Lubitz SA, Benjamin EJ, Ellinor PT. Atrial fibrillation in congestive heart failure. Heart Fail Clin. 2010;6:187–200. doi: 10.1016/j.hfc.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24 -.O’Neal WT, Qureshi W, Zhang ZM, Soliman EZ. Bidirectional association between atrial fibrillation and congestive heart failure in the elderly. J Cardiovasc Med (Hagerstown). 2016. ;17:181–6. doi: 10.2459/JCM.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 25 -.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348:135–138. doi: 10.1097/MAJ.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26 -.Soliman EZ, Prineas RJ. The paradox of atrial fibrillation in African Americans. J Electrocardiol. 2014;47:804–808. doi: 10.1016/j.jelectrocard.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 27 -.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28 -.Okin PM, Hille DA, Larstorp AC, Wachtell K, Kjeldsen SE, Dahlöf B, Devereux RB. Effect of lower on-treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients. Hypertension. 2015;66:368–373. doi: 10.1161/HYPERTENSIONAHA.115.05728 [DOI] [PubMed] [Google Scholar]


