Abstract
Dysfunction in a wide array of systems—including the immune, monoaminergic, and glutamatergic systems—is implicated in the pathophysiology of depression. One potential intersection point for these three systems is the kynurenine (KYN) pathway. This study explored the impact of the prototypic glutamatergic modulator ketamine on the endogenous KYN pathway in individuals with bipolar depression (BD), as well as the relationship between response to ketamine and depression-related behavioral and peripheral inflammatory markers. Thirty-nine participants with treatment-resistant BD (23F, ages 18–65) received a single ketamine infusion (0.5mg/kg) over 40 minutes. KYN pathway analytes—including plasma concentrations of indoleamine-2,3-dioxygenase (IDO), KYN, kynurenic acid (KynA), and quinolinic acid (QA)—were assessed at baseline (pre-infusion), 230 minutes, Day 1, and Day 3 post-ketamine. General linear models with restricted maximum likelihood estimation and robust sandwich variance estimators were implemented. A repeated effect of time was used to model the covariance of the residuals with an unstructured matrix. After controlling for age, sex, and body-mass index (BMI), post-ketamine IDO levels were significantly lower than baseline at all three time points. Conversely, ketamine treatment significantly increased KYN and KynA levels at Days 1 and 3 versus baseline. No change in QA levels was observed post-ketamine. A lower post-ketamine ratio of QA/KYN was observed at Day 1. In addition, baseline levels of pro-inflammatory cytokines and behavioral measures predicted KYN pathway changes post-ketamine. The results suggest that, in addition to having rapid and sustained antidepressant effects in BD participants, ketamine also impacts key components of the KYN pathway.
INTRODUCTION
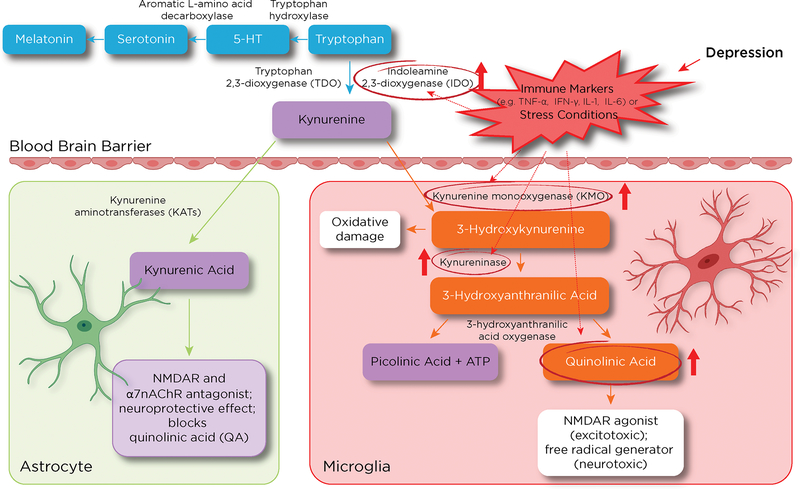
The etiology of bipolar depression (BD) remains unknown, in part due to both the heterogeneous nature of the disease and its complex underlying neuropathology (1). In addition, the lack of reliable biomarkers substantially complicates treatment of the disorder, worsens prognosis, and increases treatment-refractoriness. Recent studies have implicated both altered glutamatergic neurotransmission and excessive immune activation in the neurobiology of BD as well as treatment-resistance (2, 3). In particular, evidence from preclinical and clinical studies suggests that abnormal activation of the kynurenine (KYN) pathway may underlie BD (3, 4). Neuroactive byproducts of the KYN pathway are involved in the interface between inflammatory/immune response (5, 6) and serotoninergic neurotransmission via catabolism of tryptophan to kynurenine (7), ultimately altering downstream synaptic glutamatergic neurotransmission (8). The KYN pathway also facilitates inter-organ communication between the brain and the immune system by affecting neural afferents and circulating immune mediators that activate brain endothelial and innate immune cells (microglia) (9, 10). This communication is primarily achieved via two rate-limiting enzymes: tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase (IDO) (11, 12) (see Figure 1), both of which are implicated in the catabolism of tryptophan into KYN. Of the two enzymes, TDO is mostly intra-hepatic and regulated by glucocorticoid induction (13), whereas IDO is extra-hepatic, highly expressed in the brain, and tightly upregulated in response to proinflammatory mediators, glucocorticoids, and psychosocial stress (14).
Figure 1.
The impact of depression on the kynurenine (KYN) pathway in brain and periphery. The figure depicts KYN metabolites and their related effects on neuronal cells (inside boxes) and the enzymes that metabolize them (arrows). The impact of inflammation or stress-related conditions on key rate-limiting enzymes such as indoleamine 2,3-dioxygenase (IDO) shifts KYN metabolism towards microglial byproducts such as 3-hydroxykynurenine (3-HK) and quinolinic acid (QA). This metabolic change is associated with elevated oxidative stress (3-HK and QA) and glutamate excitotoxicity (QA) that could contribute to depressive symptoms (right panel). Conversely, during homeostasis, substantial amounts of KYN are converted to kynurenic acid (KynA), a process mediated by kynurenine aminotransferase II (KAT II) in astrocytes (left panel). At physiologic levels, KynA is an N-methyl-D-aspartate receptor (NMDAR) antagonist and contributes to the clearance of glutamate spillover in the brain.
Abbreviations: TNF-α: tumor necrosis factor alpha; IFN-gamma: interferon gamma; IL-6: interleukin-6; α7nAChR: alpha-7-nicotinic acetylcholine receptor.
In the brain, KYN can be differentially processed by either astrocytes or microglia to produce distinct neuroactive compounds. During homeostasis, kynurenine amino transferase converts KYN to kynurenic acid (KynA), a metabolite of the astrocytic process (15), which clears accumulated KYN in the central nervous system (CNS). KynA binds at the glycine co-agonist site of the NR2B N-methyl-D-aspartate (NMDA) receptor and also has antagonist properties at the α7-nicotinic acetylcholine receptor; both of these receptors are neuroprotective when activated physiologically (16, 17) and are closely linked to synaptic plasticity and cognitive processes (18), partly via their anti-inflammatory properties (19) and ability to clear glutamate spillover in the brain (2, 17).
Conversely, the microglial processing of KYN results in the formation of quinolinic acid (QA), a byproduct that exerts powerful excitotoxic effects and promotes neuronal apoptosis (3, 19, 20) (Figure 1). Evidence suggests that during depressive episodes, proinflammatory components of the KYN system in the brain are either directly or indirectly activated, altering the neuroprotective/neurotoxic balance of the KYN pathway and leading to the overproduction of neurotoxic microglial byproducts (such as QA) that potentiate NMDA activation (3, 5, 21–23). This increased activation of inflammatory circuitry within the brain, in turn, contributes to the pathological activation of the glutamatergic system (5, 24), which leads to decreased neurotrophic support, synaptic dysregulation, oxidative stress, excitotoxicity, and loss of glial tissue in multiple sites in the CNS (17, 25).
The KYN pathway is best known for regulating the interaction between the immune and stress pathways (see Figure 1) (26). Psychosocial stress stimulates inflammatory mediators in the periphery and brain via multiple mechanisms; for example, peripheral immune cells, including T-cells, can gain access to the brain (27), resulting in increased IDO production. This, in turn, triggers the activation of resident microglial cells that secrete the proinflammatory cytokine tumor necrosis factor alpha (TNF-α), with neuronal injury as the net result (see Figure 1). Postmortem human data support this result, with studies showing that proinflammatory cytokines (such as interleukin-6 (IL-6) and TNF-α) and other byproducts of microglial activation (mainly QA) were significantly upregulated in the frontal cortex of individuals with BD (28). Positive correlations have also been found between QA and specific proinflammatory immunologic variables, such as IL-6, in the CSF of individuals with previous suicide attempts (29). In contrast, KynA levels in the CSF were found to be inversely correlated with the severity of depressive symptoms (30), as well as significantly downregulated in suicidal individuals (29). Another study found that the QA/KynA ratio was significantly elevated in the CSF of suicide attempters compared to healthy participants (31), suggesting that net positive QA levels in the brain might result in detrimental structural and functional deficits, likely due to enhanced activation of microglial cells and a corresponding upsurge in NMDA receptor agonism (32, 33).
Previous studies found that a single infusion of ketamine leads to rapid (within hours), robust, and sustained antidepressant effects in individuals with BD (34, 35). Preclinical evidence also suggests that, within this time frame, ketamine reverses deficits in neuroplasticity and neurogenesis (36, 37). Ketamine has also been found to reduce levels of key proinflammatory mediators (38) as well as bone biomarkers and adipokines (39, 40) within hours to days following acute treatment. In addition, ketamine abolished lipopolysaccharide (LPS)-induced depressive-like behaviors in preclinical mouse models (41). Previous work from our laboratory also found that ketamine’s antidepressant effects may depend on its ability to affect KYN pathway metabolites (42).
This study explores the complex interactions between ketamine and relevant behavioral and biological immune/inflammatory markers that affect the KYN pathway in depression. The study had four main goals: 1) to explore the impact of a single dose of ketamine on specific proinflammatory KYN pathway metabolites in participants with treatment-resistant BD; 2) to assess whether baseline values of specific KYN pathway analytes predicted change in depressive symptom ratings post-ketamine; 3) to investigate whether baseline levels of inflammatory markers predicted change in KYN pathway analytes post-ketamine infusion; and 4) to determine whether inflammatory markers were longitudinally associated with KYN pathway analytes post-ketamine infusion.
MATERIALS AND METHODS
Study Design and Participants
The current data were drawn from the ketamine condition of a randomized, placebo (saline)-controlled, crossover study designed to assess the antidepressant efficacy of adjunctive ketamine administered intravenously at 0.5 mg/kg over 40 minutes in participants with BD; all participants were receiving adjunctive treatment with a mood stabilizer (either lithium or valproate). Details regarding study design have been previously published (34, 35). Clinical and demographic data are presented in Table 1, and additional information about the participant sample can be found in the Supplementary Materials.
TABLE 1.
Participant Demographics
| N (%) | Mean ± SD | |
|---|---|---|
| Age | 39 (100) | 45.92 ± 10.52 |
| Sex | ||
| Male | 16 (41) | |
| Female | 23 (59) | |
| Race | ||
| Caucasian | 32 (82) | |
| African-American | 4 (10) | |
| Other | 2 (5) | |
| Not reported | 1 (3) | |
| BMI | 29.84 ± 5.83 | |
| Age of illness onset | 17.51 ± 6.88 | |
| MADRS Total | 33.00 ± 4.39 | |
| Substance Use Disorder | 18 (46%) | |
| Alcohol Dependence | 14 (36%) | |
| Generalized Anxiety Disorder | 6 (15%) | |
| Mood stabilizer | ||
| Lithium Valproic Acid |
26 (67) 13 (33) |
Note. BMI data were missing for one participant; age of onset was missing for two participants; substance/alcohol abuse data were missing for one participant; and comorbid psychiatric diagnosis data were missing for five participants. BMI: body mass index; MADRS: Montgomery-Asberg Depression Rating Scale.
Psychiatric rating scales included the Beck Depression Inventory (BDI) (43), the Hamilton Depression Rating Scale (HAM-D) (44), the Montgomery-Asberg Depression Rating Scale (MADRS) (45), and the Snaith-Hamilton Pleasure Scale (SHAPS) (46). For the purposes of this analysis, empirically-derived unidimensional scores comprising items from all four scales were used (47). The three largest subscales—Depressed Mood, Negative Cognition, and Anhedonia—were selected for use, though results for all subscales are available upon request.
ELISA and multiplex immunoassay
Plasma samples were collected 60 minutes prior to ketamine infusion (baseline) and at 230 minutes, Day 1, and Day 3 post-ketamine infusion. Levels of human KYN, KynA, QA, and IDO were measured using specific ELISA kits. Circulating levels of several inflammatory cytokines (TNF-α, soluble tumor necrosis factor receptor 1 (sTNFR1), interferon gamma (IFN-γ), IL-2, IL-5, IL-6, IL-8, and IL-10) were measured in plasma using a high-sensitivity multiplex Luminex immunoassay. For additional details, see the Supplementary Materials.
Statistical Analysis
General linear models with restricted maximum likelihood estimation and robust sandwich variance estimators were implemented using PROC MIXED in SAS/STAT Version 9.3. The syntax for all models is provided in the Supplementary Materials. A repeated effect of time was used to model the covariance of the residuals with an unstructured matrix. Mood stabilizer (lithium or valproic acid), age, and body mass index (BMI) were entered as covariates in all analyses. The first research question—which sought to assess changes to the KYN pathway in response to ketamine—was investigated via pre-specified contrasts between baseline and follow-up points (230 minutes, Day 1, Day 3). The second research question—whether baseline KYN pathway values predicted change in depressive symptom ratings post-ketamine—was assessed by estimating the simple slopes for KYN pathway member-by-time interactions. The third research question—whether baseline levels of inflammatory markers predicted KYN pathway changes post-ketamine—was similarly assessed by estimating the simple slope for each baseline-to-follow-up contrast (i.e., simple slopes for the inflammatory marker-by-time interactions in predicting KYN pathway changes post-ketamine). Finally, the fourth research question—whether inflammatory markers were longitudinally associated with KYN pathway analytes post-ketamine infusion—was evaluated by determining whether the inflammatory markers changed over time (a mixed model predicting inflammatory marker from time and covariates); if so, whether that change correlated with changes in the KYN pathway was then assessed (Spearman correlations). Prior to the analyses, plasma data (for the KYN pathway and for inflammatory cytokines) were natural-log transformed after adding 1 to all values. Ratios (e.g., KYN/KynA) are the ratios of the transformed variables.
It should be noted that with regard to the third research question, high intercorrelations were observed among the pro- and anti-inflammatory circulating markers (i.e., TNF-α, sTNFR1, IFN-γ, IL-2, IL-5, IL-6, IL-8, and IL-10), and a principal components analysis was therefore implemented. Two components with eigenvalues greater than 1.0 explained 55% of the variance in the inflammatory markers (see Supplementary Table S1); the first component included mainly pro-inflammatory markers and represented TNF-α, sTNFR1, IFN-γ, IL-5, IL-6, and IL-8. The second component, which included mainly anti-inflammatory markers, represented IL-2, IL-8 (reverse loading), and IL-10. These pro- and anti-inflammatory component scores were used in the analysis rather than the values of the individual circulating inflammatory cytokines.
In addition, because this was a secondary analysis of participants from a clinical trial, an a priori power analysis was not performed. Given the exploratory nature of this study, we also did not adjust alpha or p-values for multiplicity. All parameter estimates with standard errors, as well as exact p-values, are shown in Supplementary Tables S1–S8.
RESULTS
Changes in KYN pathway metabolites post-ketamine infusion
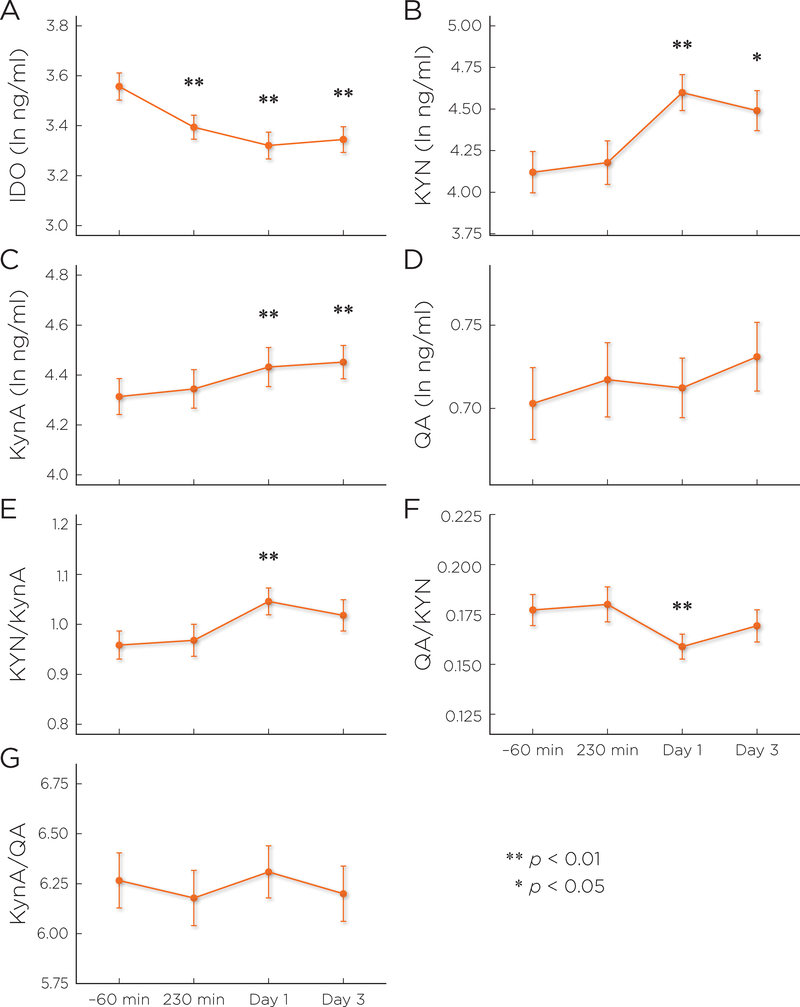
After controlling for type of mood stabilizer, age, and BMI, post-ketamine levels of IDO were significantly lower than baseline at all time points (Table 2, Figure 2). Both KYN and KynA levels were significantly higher at Days 1 and 3, as was their ratio (KYN/KynA). No changes in QA levels were observed post-ketamine administration, though the ratio of QA to KYN was significantly lower from baseline to Day 1. No post-ketamine difference was observed for KynA/QA ratio, a measure of NMDA agonist/antagonist balance. Model results appear in Table 2 and Supplementary Table S2.
TABLE 2.
Results of mixed models predicting post-ketamine change from baseline in the KYN pathway
| Dependent Variable | 230 minutes | Day 1 | Day 3 | |
|---|---|---|---|---|
| IDO (ln) | Change from baseline (95% CI) | −0.16 (−0.27 – −0.06) | −0.24 (−0.35 – −0.12) | −0.2 (−0.31 – −0.1) |
| Statistical Test | t(34) = −3.05, p = .004 | t(34) = −4.12, p = .0002 | t(34) = −3.91, p = .0004 | |
| KYN (ln) | Change from baseline (95% CI) | 0.07 (−0.11 – 0.26) | 0.5 (0.23 – 0.76) | 0.39 (0.08 – 0.7) |
| Statistical Test | t(34) = 0.77, p = .45 | t(34) = 3.69, p = .0008 | t(34) = 2.44, p = .02 | |
| KynA (ln) | Change from baseline (95% CI) | 0.03 (−0.04 – 0.1) | 0.12 (0.04 – 0.2) | 0.15 (0.06 – 0.23) |
| Statistical Test | t(34) = 0.82, p = .42 | t(34) = 2.85, p = .007 | t(34) = 3.36, p = .002 | |
| QA (ln) | Change from baseline (95% CI) | 0.01 (−0.01 – 0.04) | 0.01 (−0.01 – 0.04) | 0.03 (−0.02 – 0.08) |
| Statistical Test | t(34) = 1.28, p = .21 | t(34) = 0.94, p = .35 | t(34) = 1.26, p = .22 | |
| KYN/KynA | Change from baseline (95% CI) | 0.01 (−0.03 – 0.06) | 0.09 (0.03 – 0.15) | 0.07 (−0.01 – 0.14) |
| Statistical Test | t(34) = 0.59, p = .56 | t(34) = 2.91, p = .006 | t(34) = 1.76, p = .087 | |
| QA/KYN | Change from baseline (95% CI) | 0 (−0.01 – 0.01) | −0.02 (−0.03 – −0.01) | −0.01 (−0.02 – 0.01) |
| Statistical Test | t(34) = 0.24, p = .81 | t(34) = −3.31, p = .002 | t(34) = −0.91, p = .37 | |
| KynA/QA | Change from baseline (95% CI) | −0.09 (−0.32 – 0.13) | 0.03 (−0.18 – 0.25) | −0.12 (−0.42 – 0.19) |
| Statistical Test | t(34) = −0.82, p = .42 | t(34) = 0.29, p = .78 | t(34) = −0.75, p = .46 | |
IDO: indoleamine 2,3-dioxygenase; KYN: kynurenine; KynA: kynurenic acid; QA: quinolinic acid; ln: natural logarithm
Note: Results of a mixed model with empirical sandwich variance estimator and repeated effect of time. Type 3 tests are shown in Supplementary Table S2. Table shows specific contrast between estimated values at baseline and follow-up (illustrated in Figure 2). Age, body mass index (BMI), and type of mood stabilizer were included as covariates in all analyses.
Figure 2.
Results of mixed models: post-ketamine change in the kynurenine (KYN) pathway. Least square mean estimated scores (with standard error) are plotted by time point. Significance refers to change from baseline (−60 minutes). IDO: indoleamine 2,3-dioxygenase; KYN: kynurenine; KynA: kynurenic acid; QA: quinolinic acid. Results of the full analysis can be found in Supplementary Table S2.
Members of the KYN pathway as predictors of change in depressive symptoms post-ketamine infusion
Baseline concentrations of IDO, KYN, QA, and the KynA/QA ratio were evaluated as predictors of Depressed Mood, Negative Cognition, and Anhedonia subscale scores. Higher baseline IDO levels (t(33)=−3.84, p=.0005) and lower baseline QA levels (t(33)=2.35, p=.02) were associated with less severe baseline Depressed Mood scores, and lower QA/KYN ratio was associated with less severe baseline Negative Cognition scores (t(33)=2.58, p=.01) (see Supplementary Table S3 for slopes).
Baseline IDO levels were unrelated to antidepressant response to ketamine on any subscale, and lower baseline KYN levels were nominally related to improved Anhedonia scores at Day 3 (t(33)=2.09, p=.04) (Figure 3B, Supplementary Table S4). Higher baseline QA levels predicted improvement in Depressed Mood scores at 230 minutes (t(33)=−2.34, p=.03) and at Day 3 (t(33)=−3.21, p=.003) and improved Negative Cognition scores at Day 3 (t(33)=−2.79, p=.009) (Supplementary Table S4). A lower baseline ratio of KynA/QA (a measure of NMDA antagonist/agonist balance) predicted improvement in Depressed Mood score at 230 minutes (t(33)=2.53, p=.02) and in Anhedonia score at Day 3 (t(33)=2.29, p=.02) (Supplementary Table S4).
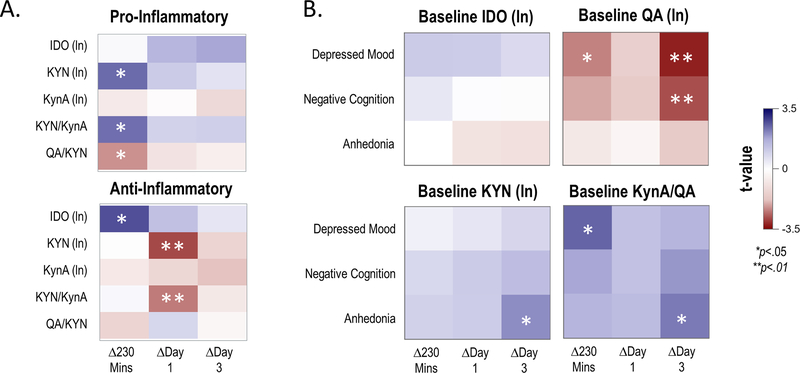
Figure 3.
Baseline inflammatory cytokine component scores as moderators of change in the kynurenine (KYN) pathway (Panel A), and baseline KYN levels as moderators of change in depressive symptom ratings (Panel B).
Panel A: Results of a mixed model with component scores of pro-inflammatory (top) or anti-inflammatory (bottom) cytokines entered as moderators of change in KYN pathway analytes (Y-axis) at three time points post-ketamine infusion (X-axis). T-values (all df=24) for the simple slope of the moderator for change at each time point are plotted; positive values indicate that higher cytokine component scores were associated with increases in respective KYN pathway analytes, and negative values indicate that higher cytokine component scores were associated with decreases in respective KYN pathway analytes.
Panel B: Results of a mixed model with baseline KYN pathway member entered as a moderator of change in depressive symptom ratings (Y-axis) post-ketamine infusion. T-values (all df=33) for the simple slope of the moderator for change at each time point are plotted; positive values indicate that higher baseline KYN pathway concentrations were associated with less improvement in depressive symptoms, and negative values indicate that higher baseline KYN pathway concentrations were associated with more improvement in depressive symptoms. In both models, age, body mass index (BMI), and type of mood stabilizer were included as covariates. Results of the full analysis can be found in Supplementary Table S5 and Supplementary Table S6.
IDO: indoleamine 2,3-dioxygenase; KYN: kynurenine; KynA: kynurenic acid; QA: quinolinic acid.
Baseline inflammatory markers as predictors of change in the KYN pathway post-ketamine infusion
Ten participants who were missing inflammatory marker concentrations were excluded from this analysis. The baseline values of the two inflammatory cytokine principal component scores (the pro-inflammatory cytokine component score, representing TNF-α, sTNFR1, IFN-γ, IL-5, IL-6, and IL-8, and the anti-inflammatory cytokine component score, representing IL-2, reverse loading IL-8, and IL-10) were entered as predictors of IDO, KYN, and KynA levels, as well as KYN/KynA and QA/KYN ratios. Baseline pro-inflammatory component score was not related to baseline KYN pathway values (Supplementary Table S5). Nominal trends were observed for higher baseline pro-inflammatory cytokine component levels to predict larger increases in KYN levels and in the KYN/KynA ratio. These were strongest at the 230-minute time point (t(24)=2.46, p=0.02 and t(24)=2.42, p=0.02, respectively) (Figure 3A, Supplementary Table S6), when neither KYN levels nor the KYN/KynA ratio had yet changed significantly from baseline.
In addition, higher baseline anti-inflammatory cytokine component scores were associated with higher baseline KYN levels (t(24)=2.94, p=.007) and higher baseline KYN/KynA ratio (t(24)=3.47, p=.002), but not with KynA levels or the QA/KynA ratio (Supplementary Table S5). Baseline anti-inflammatory component values predicted change in IDO levels at 230 minutes post-ketamine, such that higher anti-inflammatory levels were associated with less of a decrease in IDO levels (t(24)=2.74, p=0.01) (Figure 3A, Supplementary Table S6). Higher baseline anti-inflammatory levels were also associated with less of an increase in KYN levels (t(24)=−2.85, p=0.009) and in the KYN/KynA ratio (t(24)=−2.40, p=0.02) at Day 1 (Figure 3A, Supplementary Table S6).
The pro-inflammatory component did not change over time (Supplementary Table S7), but the anti-inflammatory component was increased at Day 3 (t(26)=2.87, p=.008). Change in the anti-inflammatory component was moderately and positively correlated with change in KynA levels at Day 3 (r=0.39, p=0.45) (see Supplementary Table S8).
DISCUSSION
This study is the first to explore the impact of ketamine on KYN pathway metabolites in individuals with treatment-resistant BD. A number of salient findings emerged. First, ketamine significantly lowered IDO levels at 230 minutes, Day 1, and Day 3 post-ketamine infusion. Surprisingly, participants who had higher baseline levels of anti-inflammatory cytokines experienced less of an immediate (230 minutes post-infusion) decrease in IDO levels. Second, and in contrast, plasma levels of KYN and KynA were significantly increased on Days 1 and 3 relative to baseline. Third, the relatively neuroprotective index ratio KYN/KynA was upregulated in response to ketamine at Day 1. Interestingly, increased baseline levels of anti-inflammatory cytokines were associated with less change at Day 1 for KYN and the KYN/KynA ratio, but not for KynA itself. Finally, a significant lower QA/KYN index ratio was observed, but only at Day 1 post-ketamine infusion, which coincides with ketamine’s peak antidepressant effects (34); given that this ratio is broadly accepted as an index of high inflammation and excitotoxicity, any decrease reflects a desirable outcome.
IDO is an intracellular enzyme mainly produced by immune cells and the brain and appears to be critical to some forms of immunologically-mediated depression (11, 21, 23, 29, 48). Preclinical studies also found that IDO is induced in both brain and periphery after a systemic inflammatory challenge (14, 48, 49). During depressive episodes, stress and immune activation enhance the conversion of tryptophan to KYN, mostly via IDO induction. In addition, numerous studies have shown that proinflammatory cytokines such as IFN-γ, IFN-α, IL-6, and TNF-α robustly induce IDO production via immune cells (50–52), which subsequently shifts tryptophan metabolism away from the liver (53). Furthermore, IDO levels are also affected by oxidative stress and LPS injection (5). During LPS-induced brain inflammation, most KYN and QA production is mediated via IDO induction (54). In humans, vulnerability to cytokine-induced depression was found to be enhanced by a polymorphism in the IDO gene, providing a possible explanation for the clinical heterogeneity surrounding the occurrence of depressive symptoms following immune system activation (55). In this context, genetic manipulation or pharmacological inhibition of IDO was found to abolish the LPS-induced depressive phenotype in mouse models independently of cytokine induction, suggesting that IDO itself is sufficient to trigger depressive-like behaviors in mice (56). Studies assessing indirect IDO activity (measured via the KYN/tryptophan ratio) found that increased plasma IDO levels mediated the link between inflammation and depressive symptomatology (57). The present data support the evidence that IDO is critical for shunting KYN towards the microglial pathway, but also suggest that ketamine’s robust ability to significantly lower IDO levels at 230 minutes, Day 1, and Day 3 may protect against this eventuality. Interestingly, we found that higher baseline IDO levels were associated with less severe depressive symptoms at baseline but were unrelated to degree of antidepressant response to ketamine.
In addition, our finding that KYN and KynA levels were significantly upregulated in individuals with BD compared to baseline in response to a single ketamine infusion at both Days 1 and 3 (Figure 2) broadly supports a number of recent results linking the KYN pathway and depression. For instance, a large, recent meta-analysis demonstrated that, compared to healthy volunteers, individuals with depression had lower overall plasma levels of KYN and KynA and higher levels of QA (58). Other studies found lower levels of KYN metabolites in depressed individuals (22) as well as in adolescents with melancholic depression (59). The severity of depressive symptoms in suicidal individuals has also been linked to increased plasma levels of QA and decreased plasma levels of KynA (20, 31). Another study found lower KYN levels and a higher QA/KYN ratio in participants with major depressive disorder (MDD) compared to healthy controls; notably, in that study, higher baseline KYN levels predicted remission in response to adjunctive treatment with celecoxib, suggesting that these metabolites were associated with better anti-inflammatory outcomes (60).
In this context, the present study found that higher baseline levels of KynA were associated with greater improvement in Depressed Mood score at 230 minutes and Day 3 and with Negative Cognition score at Day 3. In contrast, lower baseline KYN levels were linked to improved Anhedonia score at Day 3. Interestingly, higher baseline QA levels were associated with greater improvement in Depressed Mood score post-ketamine infusion at 230 minutes and Day 3, and with greater improvement in Negative Cognition score at Day 3.
With regard to QA in particular, selective serotonin reuptake inhibitor (SSRI) treatment was found to reduce QA levels in the rodent brain (61); in contrast, this study found that ketamine had no direct impact on QA levels in humans, though—as noted above—it did significantly affect other KYN byproduct metabolites, including IDO, KYN, and KynA. Interestingly, previous postmortem studies found elevated QA levels in the brains of severely depressed or suicidal individuals (33), suggesting an excess of QA’s precursor, KYN, which would thus be available for conversion to QA. There are two potential reasons why, under pathological conditions, the QA pathway appears to predominate over the neuroprotective KynA pathway (62). First, while microglia produce QA, astrocytes produce KynA; studies have shown that, in response to increased microglial activity, MDD participants typically have significant astrocytic dysfunction in critical areas such as the subgenual prefrontal cortex (63, 64). Second, QA binds with 10-fold greater affinity to NR2B NMDA glutamate receptor subunits, which are preferentially localized in the prefrontal cortex, amygdala, hippocampus, and ventral striatum (65). These regions thus bear a greater excitotoxic burden, which often reflects activation of the KYN pathway in the brain. KynA has no such preferential localization, though it should be noted that KynA inhibition can lead to inflammation and excitotoxicity. Previous studies found that a higher plasma KynA/QA ratio—a putative neuroprotective marker—was positively correlated with hippocampal and amygdalar volumes (66).
KYN readily crosses the blood-brain barrier. Overall, 40% of KYN in the CNS is produced in the brain, and 60% is supplied by TDO, which is present only in the liver (67). For this reason, it is almost certain that the increased plasma levels of KYN and KynA observed in the present study after ketamine administration derive from the liver. Interestingly, peripheral KYN possesses pronounced anti-inflammatory properties; it is known to block T-cell proliferation, induce T-cell death, and suppress the activity of natural killer cells and antigen-presenting cells (e.g., dendritic cells, monocytes, and macrophages) (29, 68). Thus, ketamine-induced increases in hepatic KYN production would be expected to counter the substantial peripheral proinflammatory state associated with depression. However, increased hepatic KYN production would also present a greater KYN load to the brain. While this might ordinarily increase production of toxic metabolites, the ketamine-induced suppression of IDO could protect against this potential eventuality.
Given the chronicity and long-lasting changes underlying BD, considerable evidence suggests that epigenetic modifications are involved in the pathophysiology of both depression (69) and glutamatergic dysregulation (70). Epigenetic mechanisms also help regulate kynurenine 3-monooxygenase (KMO), a critical enzyme in the KYN pathway that influences bioactive KYN pathway metabolites (71). Recent genetic, epigenetic, and pharmacological studies are targeting KMO as a way of impacting the bioactive byproducts of the KYN pathway, including KynA (72, 73). Interestingly, preclinical studies found that ketamine’s rapid-acting antidepressant actions are in part mediated epigenetically (74, 75). In this context, it is highly likely that ketamine’s effects on the KYN pathway may be mediated by epigenetic mechanisms.
One key strength of this study is that our participants were well-characterized and hospitalized for several weeks before and after ketamine administration. The study was also associated with several limitations, including the absence of a control group. In addition, 10 participants were missing inflammatory cytokine data. Because CSF measures were lacking, another potential limitation is that only peripheral measures of KYN pathway mediators were assessed. Finally, the lack of ethnic diversity in the cohort may limit the generalizability of the results.
Despite these limitations, this study is the first to demonstrate that ketamine, in addition to exerting rapid and sustained antidepressant effects, also impacts key components of the KYN pathway. These rate-limiting enzymes and analytes are highly impacted by inflammation and stress, underscoring ketamine’s acute anti-inflammatory effects.
Supplementary Material
Acknowledgements
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH) (ZIA-MH002857; NCT00088699; 04-M-0222); by a NARSAD Independent Investigator to Dr. Zarate; by a Brain & Behavior Mood Disorders Research Award to Dr. Zarate; and by the Intramural Research Program at the National Institute of Aging (Dr. Moaddel). The authors thank the 7SE research unit and staff for their support.
Footnotes
Conflict of Interest
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation. Drs. Zarate and Moaddel are listed as co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as co-inventors on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. They have assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
REFERENCES
- 1.Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS et al. The underlying neurobiology of bipolar disorder. World Psychiatry 2003; 2: 136–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry 2016; 21: 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birner A, Platzer M, Bengesser SA, Dalkner N, Fellendorf FT, Queissner R et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS One 2017; 12: e0172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev 2009; 33: 699–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr 2017: 1–16. [DOI] [PubMed] [Google Scholar]
- 6.Strasser B, Becker K, Fuchs D, Gostner JM. Kynurenine pathway metabolism and immune activation: Peripheral measurements in psychiatric and co-morbid conditions. Neuropharmacology 2017; 112: 286–296. [DOI] [PubMed] [Google Scholar]
- 7.Marazziti D, Baroni S, Picchetti M, Piccinni A, Silvestri S, Dell’Osso L. [New developments on the serotonin hypothesis of depression: shunt of tryptophan]. Riv Psichiatr 2013; 48: 23–34. [DOI] [PubMed] [Google Scholar]
- 8.Miller AH. Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology 2013; 38: 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarcz R, Stone TW. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017; 112: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andre C, O’Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N. Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J Neuroimmunol 2008; 200: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J Psychiatr Res 2015; 68: 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibney SM, Fagan EM, Waldron AM, O’Byrne J, Connor TJ, Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int J Neuropsychopharmacol 2014; 17: 917–928. [DOI] [PubMed] [Google Scholar]
- 14.Heisler JM, O’Connor JC. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun 2015; 50: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem 2001; 78: 842–853. [DOI] [PubMed] [Google Scholar]
- 16.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev 1993; 45: 309–379. [PubMed] [Google Scholar]
- 17.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 2013; 12: 64–82. [DOI] [PubMed] [Google Scholar]
- 18.Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 2010; 35: 1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganong AH, Cotman CW. Kynurenic acid and quinolinic acid act at N-methyl-D-aspartate receptors in the rat hippocampus. J Pharmacol Exp Ther 1986; 236: 293–299. [PubMed] [Google Scholar]
- 20.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992; 115: 1249–1273. [DOI] [PubMed] [Google Scholar]
- 21.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 2011; 36: 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 2007; 98: 143–151. [DOI] [PubMed] [Google Scholar]
- 23.Anderson G, Maes M. Bipolar disorder: role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr Psychiatry Rep 2015; 17: 8. [DOI] [PubMed] [Google Scholar]
- 24.Parrott JM, O’Connor JC. Kynurenine 3-monooxygenase: an influential mediator of neuropathology. Front Psychiatry 2015; 6: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savitz J The kynurenine pathway: a finger in every pie. Mol Psychiatry 2019; April 12 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci 2017; 20: 136–144. [DOI] [PubMed] [Google Scholar]
- 28.Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 2010; 15: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun 2015; 43: 110–117. [DOI] [PubMed] [Google Scholar]
- 30.Bryleva EY, Brundin L. Kynurenine pathway metabolites and suicidality. Neuropharmacology 2017; 112: 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2013; 38: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busse M, Busse S, Myint AM, Gos T, Dobrowolny H, Muller UJ et al. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? Eur Arch Psychiatry Clin Neurosci 2015; 265: 321–329. [DOI] [PubMed] [Google Scholar]
- 33.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 2011; 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 2012; 71: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010; 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry 2017; 7: e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadriu B, Gold PW, Luckenbaugh DA, Lener MS, Ballard ED, Niciu MJ et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013; 38: 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl) 2018; Aug 16 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 46.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995; 167: 99–103. [DOI] [PubMed] [Google Scholar]
- 47.Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N et al. Parsing the heterogeneity of depression: an exploratory factor analysis across commonly used depression rating scales. J Affect Disord 2018; 231: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobos N, de Vries EF, Kema IP, Patas K, Prins M, Nijholt IM et al. The role of indoleamine 2,3-dioxygenase in a mouse model of neuroinflammation-induced depression. J Alzheimers Dis 2012; 28: 905–915. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol 2009; 182: 3202–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids 2013; 45: 1319–1329. [DOI] [PubMed] [Google Scholar]
- 51.Hughes MM, Connor TJ, Harkin A. Stress-related immune markers in depression: implications for treatment. Int J Neuropsychopharmacol 2016; 19: pii: pyw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wigner P, Czarny P, Galecki P, Su KP, Sliwinski T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res 2018; 262: 566–574. [DOI] [PubMed] [Google Scholar]
- 53.Moffett JR, Blinder KL, Venkateshan CN, Namboodiri MA. Differential effects of kynurenine and tryptophan treatment on quinolinate immunoreactivity in rat lymphoid and non-lymphoid organs. Cell Tissue Res 1998; 293: 525–534. [DOI] [PubMed] [Google Scholar]
- 54.Kita T, Morrison PF, Heyes MP, Markey SP. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochem 2002; 82: 258–268. [DOI] [PubMed] [Google Scholar]
- 55.Smith AK, Simon JS, Gustafson EL, Noviello S, Cubells JF, Epstein MP et al. Association of a polymorphism in the indoleamine- 2,3-dioxygenase gene and interferon-alpha-induced depression in patients with chronic hepatitis C. Mol Psychiatry 2012; 17: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawson MA, Parrott JM, McCusker RH, Dantzer R, Kelley KW, O’Connor JC. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J Neuroinflammation 2013; 10: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA et al. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms?. Psychoneuroendocrinology 2014; 45: 202–210. [DOI] [PubMed] [Google Scholar]
- 58.Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa S, Omura Y et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci Biobehav Rev 2018; 90: 16–25. [DOI] [PubMed] [Google Scholar]
- 59.Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM et al. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry 2010; 51: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krause D, Myint AM, Schuett C, Musil R, Dehning S, Cerovecki A et al. High kynurenine (a tryptophan metabolite) predicts remission in patients with major depression to add-on treatment with celecoxib. Front Psychiatry 2017; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eskelund A, Li Y, Budac DP, Muller HK, Gulinello M, Sanchez C et al. Drugs with antidepressant properties affect tryptophan metabolites differently in rodent models with depression-like behavior. J Neurochem 2017; 142: 118–131. [DOI] [PubMed] [Google Scholar]
- 62.Muller N, Myint AM, Schwarz MJ. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders--relation to drug treatment. Dialogues Clin Neurosci 2009; 11: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A 1998; 95: 13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 2013; 14: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Carvalho LP, Bochet P, Rossier J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem Int 1996; 28: 445–452. [DOI] [PubMed] [Google Scholar]
- 66.Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015; 40: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 702–721. [DOI] [PubMed] [Google Scholar]
- 68.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 2006; 108: 4118–4125. [DOI] [PubMed] [Google Scholar]
- 69.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature 2008; 455: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veldic M, Millischer V, Port JD, Ho AM, Jia YF, Geske JR et al. Genetic variant in SLC1A2 is associated with elevated anterior cingulate cortex glutamate and lifetime history of rapid cycling. Transl Psychiatry 2019; 23: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giorgini F, Huang SY, Sathyasaikumar KV, Notarangelo FM, Thomas MA, Tararina M et al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem 2013; 288: 36554–36566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amaral M, Levy C, Heyes DJ, Lafite P, Outeiro TF, Giorgini F et al. Structural basis of kynurenine 3-monooxygenase inhibition. Nature 2013; 496: 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith JR, Jamie JF, Guillemin GJ. Kynurenine-3-monooxygenase: a review of structure, mechanism, and inhibitors. Drug Discov Today 2016; 21: 315–324. [DOI] [PubMed] [Google Scholar]
- 74.Choi M, Lee SH, Wang SE, Ko SY, Song M, Choi JS et al. Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats. Proc Natl Acad Sci U S A 2015; 112: 15755–15760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 2002; 17 (Suppl 3): 306–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.