Abstract
目的
探讨丙泊酚抑制巨噬细胞发生焦亡的分子机制。
方法
提取小鼠骨髓来源巨噬细胞,分为对照组、脂多糖(LPS)+三磷酸腺苷(ATP)刺激组以及丙泊酚+LPS+ATP组。对照组不给予任何处理;LPS+ATP组先给予脂多糖(LPS) 1 μg/mL刺激4 h,再加三磷酸腺苷(ATP)4 mmol/L刺激1 h;丙泊酚+LPS+ATP组先同时给予50 μmmol/L丙泊酚+LPS 1 μg/mL刺激4 h,再加ATP刺激1 h。处理完毕则收集细胞培养上清液和细胞。分别通过CCK8、流式分析检测细胞活力,酶联免疫吸附测定法法检测细胞上清炎症因子IL-1β、IL-18含量;用免疫印迹检测细胞caspase-1蛋白表达及细胞膜Toll样受体-4(TLR4)表达;流式分析及免疫组化荧光检测细胞焦亡情况。
结果
LPS+ATP可导致小鼠骨髓来源巨噬细胞(BMDM)活力显著降低(P < 0.05);炎症因子IL-1β、IL-18升高(P < 0.05);活化的caspase-1蛋白增加,细胞膜表面TLR-4表达升高(P < 0.05);予丙泊酚处理后,可改善LPS+ATP诱导的上述指标变化。
结论
LPS+ATP可诱导BMDM发生焦亡,丙泊酚有效抑制这种细胞死亡,提示丙泊酚麻醉有益于临床脓毒症患者的手术,有效调节患者免疫状态。
Keywords: 内毒素血症, 异丙酚, 焦亡, 巨噬细胞
Abstract
Objective
To investigate the molecular mechanism underlying the inhibitory effect of propofol on pyroptosis of macrophages.
Methods
Macrophages derived from bone marrow were extracted and divided into three groups: control group, LPS+ATP group and propofol+LPS+ATP group. The control group was not given any treatment; LPS+ATP group was given LPS 1 μg/mL stimulation for 4 h, then ATP 4 mM stimulation for 1 h; Propofol+LPS+ATP group was given propofol+LPS 1 μg/mL stimulation for 4 h, then ATP stimulation for 1 h. After treatment, the supernatant and cells of cell culture were collected. the cell activity was detected by CCK8 and flow cytometry. The inflammatory cytokines IL-1βand IL-18 were detected by Elisa. Western blot was used to detect the expression of caspase-1 protein and TLR4 on cell membran Immunohistochemical fluorescence was used to detect apoptosis of cells.
Results
LPS+ATP significantly decreased the viability of the macrophages and increased the cellular production of IL-1β and IL-18, activation of caspase-1 protein and the expression of TLR-4 on the cell membrane (P < 0.05). Treatment with propofol obviously reversed the changes induced by LPS+ATP.
Conclusion
LPS+ATP can induce pyroptosis of mouse bone marrow-derived macrophages, and propofol effectively inhibits such cell death, suggesting that propofol anesthesia is beneficial during operation and helps to regulate the immune function of in patients with sepsis.
Keywords: endotoxemia, propofol, pyrotopsis, macrophages
丙泊酚是一种新型的快效、短效静脉麻醉药。除了催眠、镇静与遗忘作用外,丙泊酚还具有降低动脉血压,抑制炎症反应的功能[1-2]。近年来,关于丙泊酚的非麻醉效能研究集中在其抗炎、抗氧化效应上[3-5]。相关研究表明丙泊酚具抗氧化,减轻细胞内钙离子超载,抑制细胞凋亡,减轻中性粒细胞和内皮细胞粘附,调节炎性细胞因子的平衡,直接改善细胞能量代谢障碍等作用[6-7]。丙泊酚可以通过抑制内毒素血症中的TLR-4的表达,从而抑制CD14基因的表达及IkB的磷酸化,减少NF-kB向细胞核内的转移,进而减少血浆和组织中的细胞因子如IL-1、TNF-a、IL-8等[8-10]。
当细胞发生焦亡时,由于细胞膜破裂导致内容物的释放,会激活强烈的炎症反应。Caspase-1作为焦亡过程的重要因子,在焦亡的发生过程中发生激活,从而使非选择性阳离子通道开放,诱发细胞焦亡[11-12]。
目前关于丙泊酚对炎症中细胞死亡方面的研究虽然很多,但都集中于凋亡[13-15]。有关丙泊酚抑制内毒素血中巨噬细胞发生焦亡的研究,目前暂时没有报道。在此背景之下,我们通过体外培养BMDM细胞,使用LPS加ATP刺激诱导BMDM发生焦亡,再予丙泊酚干预,探讨丙泊酚对脓毒血症中巨噬细胞发生焦亡的影响。
1. 材料和方法
1.1. 动物
C57BL/6J小鼠,购自广州中医药大学实验动物中心,雄性,清洁级,8周龄,体质量22±2 g,合格证号:[SCXK (粤) 2018-0034]。
1.2. 试剂及仪器
2,6-双异丙基酚(MERYER);LPS(Santa Cruz);ATP (Biorbyt);CCK8试剂盒(BestBio);DMEM培养基(Hyclone);胎牛血清(ScienCell);β-actin、GAPDH抗体(碧云天);鼠二抗(Thermo Fisher);Pro-caspase1抗体、P20-caspase1抗体、P-10caspase1抗体(Abcam);原位细胞死亡检测试剂(Roche);化学发光成像仪(Tanon);蛋白印迹系统(Bio-RAD);倒置荧光显微镜(Olympus)。
1.3. 小鼠骨髓来源巨噬细胞培养和处理(BMDM)
本实验操作均按照“医学实验动物护理和使用指南”进行,并经广东省人民医院实验动物管理及伦理委员会批准。参照相关文献进行提取和培养BMDM[16]。取6~8周龄小鼠1只,脱颈椎处死,酒精浸泡。小鼠腹部朝上,镊子夹起大腿根部剪开皮肤后,剥下大腿皮肤至足踝处,完整取下大腿。将股骨和胫骨连接处的肌腱剪断,剪去两骨的骨骺端,暴露骨髓腔。将开放了骨髓腔的骨头泡在PBS或者培养基内,以保持细胞活性。使用1 mL注射器的针头连接5 mL的注射器以便冲洗骨髓腔,冲洗液可用培养基或者PBS溶液。可从骨头的两端分别冲洗,反复多次,直至骨头发白为止。冲洗完全的液体呈淡粉色,混浊,可见许多细胞团块(一般用50 mL离心管收集冲洗液体)。300~350 g离心5 min。弃上清,加入2~3 mL红细胞裂解液,去除骨髓细胞中的红细胞,裂解完成后以约1: 2的体积加入PBS或培养基终止反应。离心弃上清,加3~5 mL完全培养基[DMEM高糖培养基+10%胎牛血清+1%双抗+10 ng/mL GM-CSF (Sigma-Aldrich, SRP3201)]重悬,将细胞种入75细胞培养瓶中,37 ℃二氧化碳孵箱孵育夜。第2天将上清吸出置于50 mL离心管中,贴壁细胞与培养瓶弃之。将上清中细胞计数,并重新种入培养瓶或者孔板中(25细胞培养瓶一般种3~3.5×106细胞,3~5 mL完全培养基培养,6孔板一般种1.5~2×106细胞,2~3 mL培养基培养),置于37 ℃二氧化碳孵箱孵育进行培养(第1天)。中途可观察细胞1次,至第4或者第5天进行换液(观察细胞若第4天已长满培养瓶或孔板底部可进行换液,若没长满可推迟到第5天换液),换液是将旧的培养基吸出弃之,加入新的完全培养基。培养至第7天或者第8天(具体看细胞状态如何)可得到成熟的BMDM细胞,可用于相应的细胞实验。
1.4. 实验分组及给药方案
将步骤1.3所得成熟BMDM根据实验需要分成3组:①对照l组;②LPS+ATP组;③丙泊酚+LPS+ATP组。对照组不予处理;LPS+ATP组先给予脂多糖(LPS) 1 μg/mL刺激4 h,再加三磷酸腺苷(ATP) 4 mmol/L刺激1 h;丙泊酚+LPS+ATP组先同时给予50 μmol/L丙泊酚+LPS 1 ug/mL刺激4 h,再加ATP刺激1 h。处理完毕则收集细胞培养上清液和细胞,检测相关指标。
1.5. 细胞焦亡模型的建立
细胞换液后,用4 μg/mL LPS刺激细胞4 h后,再用4 mmol/L ATP刺激细胞1 h,收集细胞及细胞培养上清液,CCK8及流式分析法检测细胞活力;ELISA法检测细胞因子介素-1 (IL-1)、介素-18 (IL-18)水平;Western blot法检测caspase1蛋白表达的水平;流式分析细胞焦亡情况。
1.6. 细胞活力检测
取HUVEC细胞悬液加入96孔板(每孔约1×104细胞),加入不同浓度的大黄素溶液(0,20,40,60,80,100 μmol/L),持续培养24 h,换液后,每个孔分别加入10%的CCK-8溶液100 μL,在培养箱中孵育1 h,用酶标仪检测450 nm出的吸光度,计算细胞存活率。另外,取对照组、LPS+ATP组、丙泊酚+LPS+ATP组细胞悬液,用上述方法分别检测各组细胞活力,细胞活力(%) =[A加药 -A空白]/[A未加药-A空白] ×100%。
1.7. 乳酸脱氢酶(LDH)检测
取各组经过处理的细胞上清液,按照检测试剂盒说明书操作步骤,用酶标仪在450 nm处检测各组样本的吸光度A450 nm,计算各组乳酸脱氢酶的活性。
1.8. Western blot法检测蛋白表达
取各组培养的细胞,去除培养基后,经PBS洗两次后加入裂解液裂解细胞,收集蛋白。采用BCA法测定蛋白浓度,取20 μg上样,经SDS-PAGE电泳、转膜。用5%脱脂牛奶封闭1 h。分别用抗体TLR-4(1:500)、Pro-caspase1 (1: 500)、P20-caspase1 (1: 500)进行4 ℃孵育过夜。用TBST洗膜3次,每次10 min,加入对应荧光二抗(1 :10 000),室温孵育1 h。洗膜3次,每次10 min,使用Odyssey双色红外激光扫描显影仪扫描荧光蛋白条带,用Odyssey系统软件进行结果分析,通过目的蛋白条带灰度值与内参β-actin/GADPH条带灰度值的比值表示目的蛋白的表达水平。
1.9. ELISA法检测细胞培养上清液中IL-1β、IL-18的水平
将试剂盒中的标准品稀释成5、10、20、40、80 ng/L,按照说明书设置空白孔,标准品孔,待测样品孔,盖上封板膜,混匀,37 ℃温育60 min,之后弃去液体,甩干,洗涤5次,加入显色剂A液和B液,加入终止液,空白孔调零,读出A值,计算出标准曲线的回归方程后代入样本A值计算出IL-1β及IL-18的浓度。
1.10. 流式细胞术检测细胞焦亡比例
将经过上述处理的细胞与Alexa Fluor 488-偶联caspase-1 FLICA在37 ℃共同孵育1 h。用4%多聚甲醛固定,再用TMR红标原位细胞死亡检测试剂对细胞进行染色。使用流式细胞术分析细胞,使用FACScalibur细胞仪和flowjoe-v10软件(Tree Star)采集30 000个事件进行分析。
1.11. 免疫荧光检测
为了检查细胞死亡形态,细胞被处理为在六孔板中显示的图像捕获。用DAPI (5 ng/mL)对细胞核(蓝色)进行染色,在荧光显微镜下观察细胞(Leica DMi8,德国)。使用FAM-FLICA Caspase-1检测试剂盒检测巨噬细胞中活化的Caspase-1。该试剂盒采用荧光抑制剂探针FAM-YVAD-FMK (FLICA)标记活细胞中原位激活的caspase-1酶,然后利用荧光显微镜和荧光平板阅读器分析荧光信号。用DAPI进行核染色。根据制造商的说明,在荧光显微镜(Leica DMi8,德国)下观察热裂解细胞,在490~495 nm的最佳激发范围内成像,在515~525 nm的最佳发射范围内成像。图像处理使用ImageJ软件(NIH, Bethesda, MD, USA)。利用Image Pro Plus 6.0软件(Media Cybernetics, Silver Spring, MD, USA)对裂解后的caspase-1荧光强度进行定量分析。
1.12. 统计学分析
使用SPSS 19.0统计学软件进行统计学分析,使用单因素方差分析进行组间比较,数据用均数±标准误表示,P < 0.05被认为差异有统计学意义。图片的采集和处理使用flowjoe-v10软件、GraphPad Prism7.0软件及ImageJ软件。
2. 结果
2.1. 各组细胞活力及胞质酶LDH含量的比较
CCK-8检测细胞活力及胞质酶LDH释放试验显示:与Control组相比,LPS+ATP组细胞活力明显降低(图 1A)(P < 0.05);乳酸脱氢酶LDH释放增加(图 1B) (P < 0.05),提示细胞糖酵解增高。加入丙泊酚处理后,可有效恢复细胞活力及抑制LDH释放。
1.
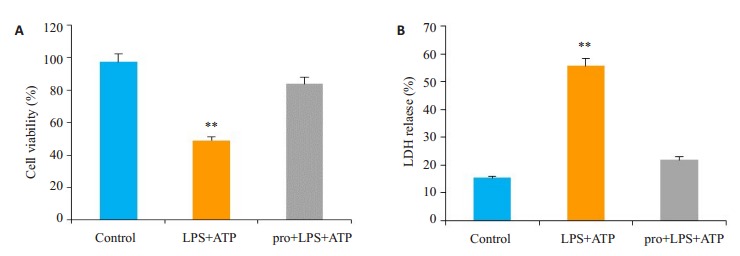
细胞计数Kit-8 (CCK-8)和LDH释放试验用于评定细胞活力
Cell count kit-8 (CCK-8) and LDH release test for detecting cell viability. **P < 0.05 vs control. A: Cell count Kit-8(CCK-8) was used to detect cell viability in each group. B: LDH release test to detect LDH release of each group.
2.2. 各组细胞上层清液中促炎症因子IL-1β和IL-18的变化情况
结果表明,LPS+ATP刺激引起BMDMs细胞上层清液中促炎症因子IL-1β含量均值由24.98 pg/mL升高至58.94 pg/mL,IL-18含量均值由301.8 pg/mL升高至424.6 pg/mL(P < 0.05),予丙泊酚处理后IL-1β和IL-18均显著下降,与对照组相比,差异无统计学意义(P>0.05)(图 2)。
2.
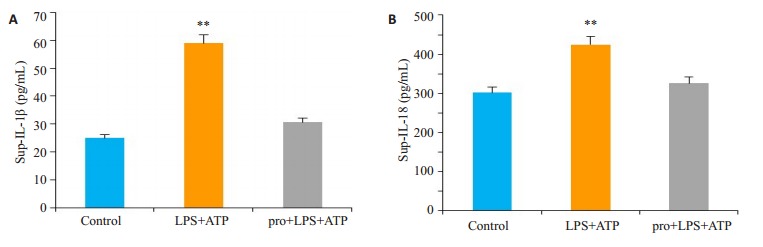
BMDMs细胞上层清液中促炎症因子IL-1β和IL-18的含量变化
Changes of IL-1 β and IL-18 in the supernatant of the cells. **P < 0.05 vs control. A: The content of proinflammatory factor IL-1 β in the supernatant of MDMs cells in each group. B: The content of pro-inflammatory factor L-18 in the supernatant of MDMs cells in each group.
2.3. 流式检测各组细胞发生焦亡的情况
与Control组相比,LPS+ATP组细胞焦亡比例明显增加(由1.02%上升至11.6%),而丙泊酚处理组的焦亡细胞比例仅为4.85% (图 3)。
3.
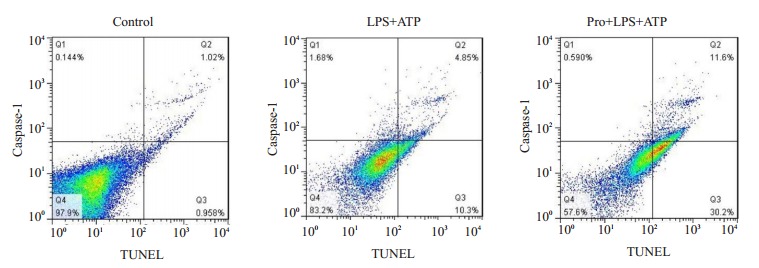
流式分析表明,LPS+ATP组细胞焦亡比例明显增加,丙泊酚可减少细胞的焦亡。
Flow cytometry analysis showing significantly increased pyrolytic cells in LPS+ATP group and propofol reduced pyrolytic cells.
2.4. 各组细胞膜上TLR4的表达及膜TLR4与细胞死亡比例的关系
为了进一步研究丙泊酚减少LPS + ATP诱导的BMDM焦亡的机制,我们检测了细胞膜表面的TLR-4表达。结果表明,LPS+ATP刺激后,膜表面TLR4蛋白的表达增加,而丙泊酚可以减少TLR4的膜表达(图 4A)。说明丙泊酚可能通过减少TLR4膜表面的表达,从而抑制LPS+ATP诱导的BMDM焦亡。流式分析结果进一步验证膜表面TLR4在各组中的变化,并表明膜表面TLR4的量与细胞死亡比例成正比(图 4B)。
4.
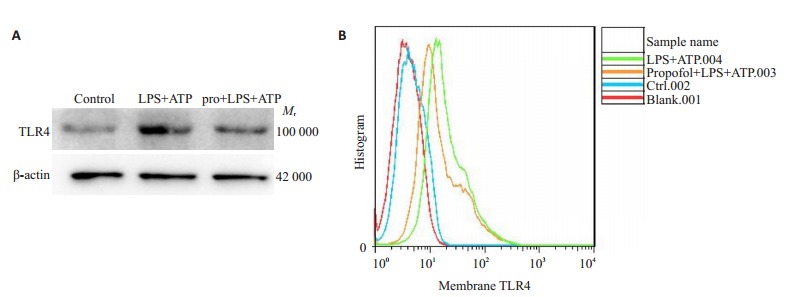
细胞膜上TLR4的表达及膜TLR4与细胞死亡比例的关系
Expression of TLR4 on cell membrane and its correlation with cell death percentage. A: Expression of TLR4 Protein in cell membrane of each group. B: The relationship between the amount of TLR4 on membrane surface and cell death.
2.5. 丙泊酚减少LPS和ATP诱导骨髓源性巨噬细胞的焦亡,依赖于减少caspase-1的活化
激活的Caspase-1是诱导焦亡的关键分子,我们用免疫印迹分析发现,在LPS+ATP刺激1 h后,活性caspase-1蛋白表达增加,而丙泊酚处理则可减少活性caspase-1的含量(图 5)。免疫荧光结果表明,LPS+ATP可诱导caspase-1的活化,丙泊酚减少caspase-1的活化。
5.
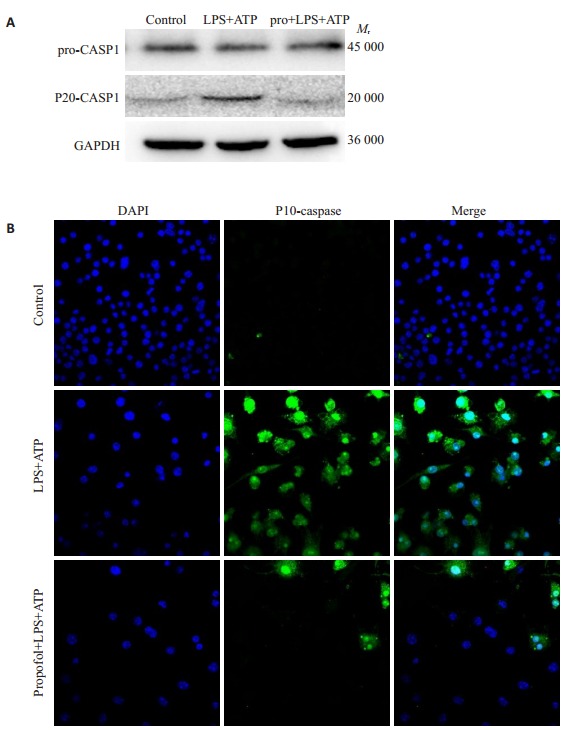
caspase1的表达情况
Expression of caspase 1 in the cells (Original magnitication: ×600). A: Expression of Caspase-1 protein in each group. B: Expression of Caspase-1 by immunofluorescence in each group.
3. 讨论
当机体发生脓毒症时,免疫失调作为关键因素,表现为前期促炎因子大量释放、后期免疫功能瘫痪等免疫失调,从而造成机体损伤甚至多器官功能衰竭[17-18]。近年来,越来越多的研究致力于内毒素血症发生发展与宿主细胞死亡机制及其与免疫炎性失调的关联上,试图从细胞死亡机制寻求更多治疗靶点[19-20]。我们的研究发现,LPS+ATP诱导了BMDM的焦亡,进一步证实了内毒素血中发生细胞焦亡。
作为内毒素血症或脓毒血症的源头调控分子,TLR4在细胞膜表面的数量一直是被严格控制的[21-23]。鉴于TLR4受体是外源微生物及体DAMPs的重要识别受体,在炎症反应中起到了关键性的促发作用,减少TLR4在细胞膜表面的表达就意味着能够在源头以及整体上减轻炎性相关蛋白的激活以及炎症因子的释放,并最终减轻重度炎症反应给机体带来的损伤[24-27]。我们前期结果也提示,在内毒素血症中,细胞膜表面TLR4的量与细胞死亡比例成正相关,丙泊酚通过有效减少TLR4的表达来减少内毒素血中细胞的死亡。
Caspase-1参与调解多种炎症因子的产生与释放,尤其是促进IL-1β的释放,加重炎症反应[28]。在脓毒症小鼠模型上进行caspase-1基因敲除,发现小鼠生存率提高,提示caspase-1基因敲除具有保护作用[29-30]。细胞内LPS激活非经典NLRP3炎症小体通路能使炎症因子的释放增多[31]。我们揭示了丙泊酚减少内毒素血中中骨髓源性巨噬细胞的焦亡,可能归因于丙泊酚可以减少caspase-1的活化。
关于TLR4与caspase-1在脓毒症中细胞焦亡的相互作用鲜有报道,本研究首次揭示丙泊酚可能通过作用于TLR4受体抑制LPS+ATP诱导的焦亡的作用,减少促炎因子IL-1β、IL-18的释放以及活化的caspase-1的产生,从而减轻细胞损伤。提示脓毒症中细胞发生焦亡可能受到TLR4-caspase1通路的调节,关于这一通路的具体机制未来还需更多研究去证实。
综上所述,本研究深入细胞水平证实丙泊酚抑制巨噬细胞发生焦亡的分子机制,将不仅为丙泊酚在临床抗炎中的应用提供更加有利的理论依据,也进一步加深了对内毒素血症病理生理机制的认识。
Biography
纪雪霞,主治医师,本科,E-mail: jxx_1413@163.com
Funding Statement
广东省医学科学技术研究基金(A2018277);广东省中医药局科研项目(20191006)
Contributor Information
纪 雪霞 (Xuexia JI), Email: jxx_1413@163.com.
周 国斌 (Guobin ZHOU), Email: zhougb_1414@sina.com.
References
- 1.Tang J, Hu JJ, Lu C, et al. Propofol inhibits lipopolysaccharideinduced tumor necrosis factor-alpha expression and myocardial depression through decreasing the generation of superoxide anion in cardiomyocytes. https://www.hindawi.com/journals/omcl/2014/157376/ Oxid Med Cell Longev. 2014:157376. doi: 10.1155/2014/157376. [Tang J, Hu JJ, Lu C, et al. Propofol inhibits lipopolysaccharideinduced tumor necrosis factor-alpha expression and myocardial depression through decreasing the generation of superoxide anion in cardiomyocytes[J]. Oxid Med Cell Longev, 2014: 157376.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nie Y, Song R, Chen W, et al. Effects of stellate ganglion block on cerebrovascular vasodilation in elderly patients and patients with subarachnoid haemorrhage. Br J Anaesth. 2016;117(1):131–2. doi: 10.1093/bja/aew157. [Nie Y, Song R, Chen W, et al. Effects of stellate ganglion block on cerebrovascular vasodilation in elderly patients and patients with subarachnoid haemorrhage[J]. Br J Anaesth, 2016, 117(1): 131-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng T, Yu J, Lei Z, et al. Propofol reduces lipopolysaccharideinduced, NADPH oxidase (NOX 2) mediated TNF-alpha and IL-6 production in macrophages. http://or.nsfc.gov.cn/handle/00001903-5/384314. Clin Dev Immunol. 2013;2013:325481. doi: 10.1155/2013/325481. [Meng T, Yu J, Lei Z, et al. Propofol reduces lipopolysaccharideinduced, NADPH oxidase (NOX 2) mediated TNF-alpha and IL-6 production in macrophages[J]. Clin Dev Immunol, 2013, 2013: 325481.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corcoran TB, Barden AE, Mas E, et al. Hemoglobin attenuates the effects of inspired oxygen on plasma isofurans in humans during upper-limb surgery. Free Radic Biol Med. 2011;51(6):1235–9. doi: 10.1016/j.freeradbiomed.2011.06.026. [Corcoran TB, Barden AE, Mas E, et al. Hemoglobin attenuates the effects of inspired oxygen on plasma isofurans in humans during upper-limb surgery[J]. Free Radic Biol Med, 2011, 51(6): 1235-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong HY, Zheng F, Zhang C, et al. Propofol protects hippocampal neurons from apoptosis in ischemic brain injury by increasing GLT- 1 expression and inhibiting the activation of NMDAR via the JNK/ Akt signaling pathway. Int J Mol Med. 2016;38(3):943–50. doi: 10.3892/ijmm.2016.2663. [Gong HY, Zheng F, Zhang C, et al. Propofol protects hippocampal neurons from apoptosis in ischemic brain injury by increasing GLT- 1 expression and inhibiting the activation of NMDAR via the JNK/ Akt signaling pathway[J]. Int J Mol Med, 2016, 38(3): 943-50.] [DOI] [PubMed] [Google Scholar]
- 6.Tang J, Jiang Y, Tang Y, et al. Effects of propofol on damage of rat intestinal epithelial cells induced by heat stress and lipopolysaccharides. Braz J Med Biol Res. 2013;46(6):507–12. doi: 10.1590/1414-431X20132785. [Tang J, Jiang Y, Tang Y, et al. Effects of propofol on damage of rat intestinal epithelial cells induced by heat stress and lipopolysaccharides[J]. Braz J Med Biol Res, 2013, 46(6): 507-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao T, Zhao Z, Hao L, et al. Propofol promotes proliferation of cultured adult rat hippocampal neural stem cells. J Neurosurg Anesthesiol. 2013;25(3):299–305. doi: 10.1097/ANA.0b013e31828baa93. [Tao T, Zhao Z, Hao L, et al. Propofol promotes proliferation of cultured adult rat hippocampal neural stem cells[J]. J Neurosurg Anesthesiol, 2013, 25(3): 299-305.] [DOI] [PubMed] [Google Scholar]
- 8.Pihlgren M, Silva AB, Madani R, et al. TLR4- and TRIF-dependent stimulation of B lymphocytes by peptide liposomes enables T cellindependent isotype switch in mice. Blood. 2013;121(1):85–94. doi: 10.1182/blood-2012-02-413831. [Pihlgren M, Silva AB, Madani R, et al. TLR4- and TRIF-dependent stimulation of B lymphocytes by peptide liposomes enables T cellindependent isotype switch in mice[J]. Blood, 2013, 121(1): 85-94.] [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Sun Y, Wu WK, et al. Propofol lowers serum PF4 level and partially corrects hypercoagulopathy in endotoxemic rats. Biochim Biophys Acta. 2010;1804(9):1895–901. doi: 10.1016/j.bbapap.2010.06.018. [Tang J, Sun Y, Wu WK, et al. Propofol lowers serum PF4 level and partially corrects hypercoagulopathy in endotoxemic rats[J]. Biochim Biophys Acta, 2010, 1804(9): 1895-901.] [DOI] [PubMed] [Google Scholar]
- 10.Farag E, Kot M, Podolyak A, et al. The relative effects of dexmedetomidine and propofol on cerebral blood flow velocity and regional brain oxygenation: A randomised noninferiority trial. Eur J Anaesthesiol. 2017;34(11):732–39. doi: 10.1097/EJA.0000000000000662. [Farag E, Kot M, Podolyak A, et al. The relative effects of dexmedetomidine and propofol on cerebral blood flow velocity and regional brain oxygenation: A randomised noninferiority trial[J]. Eur J Anaesthesiol, 2017, 34(11): 732-39.] [DOI] [PubMed] [Google Scholar]
- 11.Weigt SS, Palchevskiy V, Belperio JA. Inflammasomes and IL-1 biology in the Pathogenesis of allograft dysfunction. J Clin Invest. 2017;127(6):2022–9. doi: 10.1172/JCI93537. [Weigt SS, Palchevskiy V, Belperio JA. Inflammasomes and IL-1 biology in the Pathogenesis of allograft dysfunction[J]. J Clin Invest, 2017, 127(6): 2022-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, Jiao Y, Zhang T, et al. Longitudinal changes in plasma Caspase-1 and Caspase-3 during the first 2 years of HIV-1 infection in CD4Low and CD4High patient groups. PLoS One. 2015;10(3):e0121011. doi: 10.1371/journal.pone.0121011. [Song J, Jiao Y, Zhang T, et al. Longitudinal changes in plasma Caspase-1 and Caspase-3 during the first 2 years of HIV-1 infection in CD4Low and CD4High patient groups[J]. PLoS One, 2015, 10 (3): e0121011.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng F, Wang S, Zhang L, et al. Propofol through upregulating caveolin-3 attenuates post-hypoxic mitochondrial damage and cell death in H9C2 cardiomyocytes during hyperglycemia. http://cn.bing.com/academic/profile?id=b0aa38ef194ed73fe3d0a92de7d30be0&encoded=0&v=paper_preview&mkt=zh-cn. Cell Physiol Biochem. 2017;44(1):279–92. doi: 10.1159/000484680. [Deng F, Wang S, Zhang L, et al. Propofol through upregulating caveolin-3 attenuates post-hypoxic mitochondrial damage and cell death in H9C2 cardiomyocytes during hyperglycemia[J]. Cell Physiol Biochem, 2017, 44(1): 279-92.] [DOI] [PubMed] [Google Scholar]
- 14.Sumi C, Okamoto A, Tanaka H, et al. Propofol induces a metabolic switch to glycolysis and cell death in a mitochondrial electron transport chain-dependent manner. PLoS One. 2018;13(2):e0192796. doi: 10.1371/journal.pone.0192796. [Sumi C, Okamoto A, Tanaka H, et al. Propofol induces a metabolic switch to glycolysis and cell death in a mitochondrial electron transport chain-dependent manner[J]. PLoS One, 2018, 13(2): e0192796.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning HJ, Yuan HB, Xu HT, et al. Propofol reduces hypoxia-induced autophagic cell death through downregulating HIF 1α in alveolar epithelial type Ⅱ cells of rats. Mol Med Rep. 2017;16(2):1509–15. doi: 10.3892/mmr.2017.6697. [Ning HJ, Yuan HB, Xu HT, et al. Propofol reduces hypoxia-induced autophagic cell death through downregulating HIF 1α in alveolar epithelial type Ⅱ cells of rats[J]. Mol Med Rep, 2017, 16(2): 1509-15.] [DOI] [PubMed] [Google Scholar]
- 16.Kiefer K, Josefina C, Roberto G, et al. Ceramide imbalance and impaired TLR4 mediated autophagy in BMDM of an ORMDL3- overexpressing mouse model. Int J Mol Sci. 2019;20(6):pii: E1391. doi: 10.3390/ijms20061391. [Kiefer K, Josefina C, Roberto G, et al. Ceramide imbalance and impaired TLR4 mediated autophagy in BMDM of an ORMDL3- overexpressing mouse model[J]. Int J Mol Sci, 2019, 20(6): pii: E1391.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobo L A, Benjamim CF, Oliveira AC. The interplay between microbiota and inflammation: lessons from peritonitis and sepsis. Clin Transl Immunology. 2016;5(7):e90. doi: 10.1038/cti.2016.32. [Lobo L A, Benjamim CF, Oliveira AC. The interplay between microbiota and inflammation: lessons from peritonitis and sepsis[J]. Clin Transl Immunology, 2016, 5(7): e90.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessaro FHG, Ayala TS, Nolasco EL, et al. Insulin influences LPSinduced TNF-α and IL-6 release through distinct pathways in mouse macrophages from different compartments. Cell Physiol Biochem. 2017;42(5):2093–104. doi: 10.1159/000479904. [Tessaro FHG, Ayala TS, Nolasco EL, et al. Insulin influences LPSinduced TNF-α and IL-6 release through distinct pathways in mouse macrophages from different compartments[J]. Cell Physiol Biochem, 2017, 42(5): 2093-104.] [DOI] [PubMed] [Google Scholar]
- 19.D Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–92. doi: 10.1002/cbin.11137. [D Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy[J]. Cell Biol Int, 2019, 43(6): 582-92.] [DOI] [PubMed] [Google Scholar]
- 20.Zou L, Chen HH, Li D, et al. Imaging lymphoid cell death in vivo during polymicrobial sepsis. Crit Care Med. 2015;43(11):2303–12. doi: 10.1097/CCM.0000000000001254. [Zou L, Chen HH, Li D, et al. Imaging lymphoid cell death in vivo during polymicrobial sepsis[J]. Crit Care Med, 2015, 43(11): 2303- 12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang K, Zhang T, Yin N, et al. Geraniol alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis. Oncotarget. 2017;8(41):71038–53. doi: 10.18632/oncotarget.20298. [Jiang K, Zhang T, Yin N, et al. Geraniol alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis[J]. Oncotarget, 2017, 8(41): 71038-53.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YL, Xu G, Liang X, et al. Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury. http://cn.bing.com/academic/profile?id=37c42fa819f178cefbb41d2f4e17849c&encoded=0&v=paper_preview&mkt=zh-cn. Am J Transl Res. 2016;8(12):5685–95. [Chen YL, Xu G, Liang X, et al. Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury[J]. Am J Transl Res, 2016, 8(12): 5685-95.] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Ferro MC, Serrano DC, Sánchez-Pernaute O. Cell membrane bound TLR2 and TLR4: potential predictors of active systemic lupus erythematosus and lupus nephritis. J Rheumatol. 2016;43(7):1444–5. doi: 10.3899/jrheum.151386. [Pérez-Ferro MC, Serrano DC, Sánchez-Pernaute O. Cell membrane bound TLR2 and TLR4: potential predictors of active systemic lupus erythematosus and lupus nephritis[J]. J Rheumatol, 2016, 43 (7): 1444-5.] [DOI] [PubMed] [Google Scholar]
- 24.Shao L, Liu X, Zhu S, et al. The role of smurf1 in neuronal necroptosis after lipopolysaccharide induced neuroinflammation. Cell Mol Neurobiol. 2018;38(4):809–16. doi: 10.1007/s10571-017-0553-6. [Shao L, Liu X, Zhu S, et al. The role of smurf1 in neuronal necroptosis after lipopolysaccharide induced neuroinflammation[J]. Cell Mol Neurobiol, 2018, 38(4): 809-16.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jafari M, Nasiri M R, Sanaei R, et al. The NRAMP1, VDR, TNF-α, ICAM1, TLR2 and TLR4 gene polymorphisms in iranian patients with pulmonary tuberculosis: A case-control study. Infect Genet Evol. 2016;39:92–8. doi: 10.1016/j.meegid.2016.01.013. [Jafari M, Nasiri M R, Sanaei R, et al. The NRAMP1, VDR, TNF-α, ICAM1, TLR2 and TLR4 gene polymorphisms in iranian patients with pulmonary tuberculosis: A case-control study[J]. Infect Genet Evol, 2016, 39: 92-8.] [DOI] [PubMed] [Google Scholar]
- 26.Liaunardy-Jopeace A, Bryant CE, N J Gay. The COP Ⅱ adaptor protein TMED7 is required To initiate and mediate the delivery of TLR4 to the plasma membrane. Sci Signal. 2014;7(336):ra70. doi: 10.1126/scisignal.2005275. [Liaunardy-Jopeace A, Bryant CE, N J Gay. The COP Ⅱ adaptor protein TMED7 is required To initiate and mediate the delivery of TLR4 to the plasma membrane[J]. Sci Signal, 2014, 7(336): ra70.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Lou J, Ouyang C, et al. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc Natl Acad Sci USA. 2010;107(31):13806–11. doi: 10.1073/pnas.1009428107. [Wang D, Lou J, Ouyang C, et al. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane[J]. Proc Natl Acad Sci USA, 2010, 107 (31): 13806-11.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mezzasoma LC, Antognelli, Talesa VN. A novel role for brain natriuretic peptide: inhibition of IL-1β secretion via downregulation of NF-kB/Erk 1/2 and NALP3/ASC/Caspase-1 activation in human THP-1 monocyte. Mediators Inflamm. 2017;2017:5858315. doi: 10.1155/2017/5858315. [Mezzasoma LC, Antognelli, Talesa VN. A novel role for brain natriuretic peptide: inhibition of IL-1β secretion via downregulation of NF-kB/Erk 1/2 and NALP3/ASC/Caspase-1 activation in human THP-1 monocyte[J]. Mediators Inflamm, 2017, 2017: 5858315.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar A, Hall MW, Exline M, et al. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin- 1beta and interleukin-18. Am J Respir Crit Care Med. 2006;174(9):1003–10. doi: 10.1164/rccm.200604-546OC. [Sarkar A, Hall MW, Exline M, et al. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin- 1beta and interleukin-18[J]. Am J Respir Crit Care Med, 2006, 174 (9): 1003-10.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacey CA, Mitchell WJ, Dadelahi AS, et al. Caspase-1 and Caspase- 11 mediate pyroptosis, inflammation, and control of brucella joint infection. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e1bef22957af1cf41b39118cf1133e78. Infect Immun. 2018;86(9) doi: 10.1128/IAI.00361-18. [Lacey CA, Mitchell WJ, Dadelahi AS, et al. Caspase-1 and Caspase- 11 mediate pyroptosis, inflammation, and control of brucella joint infection[J]. Infect Immun, 2018. 86(9).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan J, Najafov A, Py BF. Roles of Caspases in Necrotic Cell Death. Cell. 2016;167(7):1693–704. doi: 10.1016/j.cell.2016.11.047. [Yuan J, Najafov A, Py BF. Roles of Caspases in Necrotic Cell Death[J]. Cell, 2016, 167(7): 1693-704.] [DOI] [PMC free article] [PubMed] [Google Scholar]


