Abstract
目的
探索丝氨酸羟甲基转移酶2 (SHMT2)在小鼠肝脏缺血再灌注损伤的表达及作用。
方法
SPF级C57BL/6小鼠60只,分为正常组(sham)、生理盐水对照组(NS)、空载腺相关病毒组(AVV-GFP)、及腺相关病毒沉默组(AAV-SHMT2),每组15只。建模前2周尾静脉给予注射腺相关病毒及生理盐水,建立小鼠肝脏70%缺血再灌注模型。收集缺血再灌注模型小鼠的血清及肝组织。采用qPCR、Western blot、免疫荧光和免疫组化检测各组AST/ALT的浓度、SHMT2、JNK、NF-κB、Caspase-3及下游炎症因子表达水平的变化。并利用HE染色观察各组肝组织病理损伤,TUNEL法检测细胞凋亡。
结果
肝脏缺血再灌注后SHMT2表达随时间升高,在24 h达最高(相对表达量为1.5,P < 0.05)。肝脏缺血再灌注24 h后,AAV-SHMT2组AST/ALT水平含量(588/416 U/L)均明显高于对照组(416/345 U/L),空载组(387/321 U/L)(P < 0.05)。AAV-SHMT2组与对照组和空载组相比,SHMT2水平明显降低(相对表达量为0.24,P < 0.05),p-JNK,p-p65水平明显升高(相对表达量为0.80,0.97,P < 0.05),炎症因子水平TNF-α、IL-1β与其保持一致升高(相对表达量为1.6,1.2,P < 0.05)。空载组和对照组比较无统计性差异(P>0.05)。
结论
SHMT2在肝脏缺血再灌注中有可能通过抑制JNK通路激活来缓减肝细胞的凋亡以及抑制NF-κB通路过度激活来减轻肝脏缺血再灌注的损伤。
Keywords: 肝脏缺血再灌注, 丝氨酸羟甲基转移酶, JNK, NF-κB
Abstract
Objective
To investigate the protective effect of serine hydroxymethyl transferase 2 (SHMT2) against hepatic ischemia-reperfusion injury in mice.
Methods
Sixty C57BL/6 mice were divided equally into sham-operated group, saline adeno-associated virus group (AVV-GFP), and adeno-associated virus silencing group (AAV-SHMT2). The adeno-associated virus and normal saline were injected into the tail vein of the mice 2 weeks before establishment of a 70% ischemia-reperfusion model in the liver. qPCR, Western blotting, immunofluorescence and immunohistochemistry were used to detect the changes of AST/ALT concentration, SHMT2, JNK, NF-κB, caspase-3 and downstream inflammatory factors in the mice, and HE staining was used to observe the pathological damage of the liver tissue in each group; the cell apoptosis in the liver was detected using TUNEL assay.
Results
The expression of SHMT2 increased with time after hepatic ischemia-reperfusion and reached the highest level at 24 h (the relative expression was 1.5, P < 0.05). At 24 h after hepatic ischemia-reperfusion, the levels of AST/ALT in AAV-SHMT2 group (588/416 U/L) were significantly higher than those in the control group (416/345 U/L) and the empty vector group (387/321 U/L) (P < 0.05). Compared with those in the control group and the empty vector group, the level of SHMT2 was significantly decreased in AAV-SHMT2 group (with a relative expression of 0.24, P < 0.05), the levels of p-JNK and p-p65 were significantly increased (relative expression of 0.80 and 0.97, respectively, P < 0.05), and the levels TNF-α and IL-1β were consistently elevated (relative expression levels of 1.6 and 1.2, respectively, P < 0.05). No significant differences were found in these parameters between the empty vector group and the control group (P>0.05).
Conclusion
SHMT2 may alleviate liver cell apoptosis in mice with hepatic ischemia-reperfusion injury by inhibiting the activation of JNK pathway and excessive activation of NF-κB pathway to reduce hepatic damage.
Keywords: hepatic ischemia-reperfusion, serine hydroxymethyl transferase, JNK, nuclear factor-κB
缺血-再灌注损伤(IRI)是肝移植术后的急性和慢性排斥反应的一种重要风险因素,是目前肝移植方面最具挑战性的的问题[1-3]。缺血再灌注将导致一系列复杂的事件,包括活性氧家族和危险信号的释放,信号转导级联的激活,炎性细胞因子和趋化因子的产生以及粘附分子的上调对肝脏造成明显损害,导致肝细胞坏死,凋亡[4-5]。丝氨酸羟甲基转移酶2 (SHMT2)能将丝氨酸转化为甘氨酸,是细胞的线粒体中甘氨酸的重要来源[6-7]。研究证明SHMT2在一部分胶质瘤细胞中升高,促进新陈代谢的变化,使细胞在缺血性肿瘤微环境中存活[8]。c-Jun氨基末端激酶(JNK)是MAPK家族重要成员之一,由于它可以调控细胞内外不同的应激反应,因此也被称为应激激活蛋白激酶。激活后的JNK信号通路可通过磷酸化不同的底物,如核蛋白和非核蛋白,对细胞的生存、活动和其他信号通路进行调控,从而发挥促进凋亡及促进炎症损伤的作用[9-10]。丝氨酸羟甲基转移酶2 (SHMT2)是调节丝氨酸分解代谢的中心酶,其代谢的甘氨酸在线粒体功能中起着重要作用。有研究表明,在缺氧条件下,体内的SHMT2通过抑制丙酮酸激酶(PKM2)和减少氧消耗来保证机体重要的功能细胞发挥作用[11-12]。目前绝大多数研究主要以研究SHMT2在肿瘤发生发展及侵袭转移的作用。SHMT2在很多肿瘤中经研究证实可以看作是一个有意义的及独立的预后因素,在肝癌,胆管癌,胃癌等被看作癌症的一个潜在的治疗靶点[13-14]。也有近年来关于SHMT2的研究不局限于肿瘤,渐渐地在代谢,免疫方面有陆续的研究报道[15-16]。目前在SHMT2在缺血再灌注中的作用研究较少,而对SHMT2在肝脏缺血再灌注损伤的研究未见报道。本文研究以腺相关病毒为载体沉默肝脏内SHMT2基因,观察其下游JNK、p-JNK、p65、p-P65的影响以及对肝脏缺血再灌注损伤的保护作用,发现随着时间的增加,SHMT2表达逐渐升高。我们推测在缺血缺氧环境下SHMT2的表达可能存在一些变化,并发挥着重要作用,其中诱导原因可能包含缺氧和缺血,并进一步验证SHMT2可以通过抑制JNK通路来抑制凋亡并抑制NF-κB通路的激活炎症的发生。
1. 材料和方法
1.1. 动物实验及主要试剂
SPF级雄性C57BL/6小鼠60只,5~6周龄,体质量18~21 g,由重庆医科大学动物中心提供。SHMT2 (Cat: ab180786)、P65、p-P65、GAPDH(Cat:ab8245)抗体(Abcam),JNK、p-JNK (Cat:9255)抗体(CST)。SHMT2基因引物:上游:5'-TGGCAAGAGATACTACGGAGG-3',下游:5'-GCAGGTCCAACCCCATGAT-3'。β-actin基因上游引物:5'-CCTGGCACCCAGCACAAT-3',下游引物:5'- GCCGATCCACACGGAGTACT- 3('生工)。使用RNeasy 96 kit(TaKaRa)提取小鼠肝组织总RNA,并将mRNA逆转为cDNA,qPCR试剂盒(DBI)。逆转录试剂盒和SYBR Green PCR Master MIXPCR试剂盒,含有针对AAV-SHMT2的纯化滴度为1×1012 PFU腺相关病毒(ABM)。TUNEL检测试剂盒(罗氏)。
1.2. 实验分组及动物模型的建立
采用抽签法随机分为4组:(1)正常组:共15只小鼠,不做任何处理建立缺血再灌注模型;(2)生理盐水对照组:共15只小鼠,肝缺血再灌注术前14 d尾静脉注射100 μL的生理盐水作为对照;(3) AAV-SHMT2组:共15只小鼠,肝缺血再灌注术前14 d尾静脉注射100 μL 1× 1012 PFU腺相关病毒;(4)空载组:肝缺血再灌注术前14 d尾静脉注射100 μL 1×1012PFU空载腺相关病毒。术后4、8、16、20、24 h,处死模型动物,收集肝脏、血清标本。
实验分组及模型制备[7]:戊巴比妥钠(60 mg/kg)腹腔注射麻醉后,取腹部正中切口,分离肝脏左叶、中叶肝蒂,以无损伤血管夹阻断肝脏左叶及中叶的血流,造成70%的肝脏缺血。肝脏缺血期间用4-0丝线单层连续缝合关闭腹腔,并皮下注射0.4 mL 0.9%得NaC1溶液以补充术中体液丢失。缺血75 min后再次开腹取出血管夹形成再灌注,双层连续缝合关闭腹腔。假手术组仅行麻醉、开腹和关腹,不阻断肝脏血流。
1.3. 肝功能检测
丙氨酸转氨酶(ALT)和谷草转氨酶(AST)各组取血800 μL,离心力1200 g,离心10 min,分离上层血清,自行检测ALT和AST。
1.4. qPCR检测
采用RNEASY 96 KIT分别提取4组肝组织的总RNA,反转录制备cDNA,按照说明书步骤操作。取样本cDNA,加入SYB R GreenⅠ进行定量qPCR反应。反应条件:预变性95 ℃ 5 min,95 ℃ 30 s,60 ℃ 30 s循环40次。95 ℃ 15 s,60 ℃ 30 s,95 ℃ 15 s。
1.5. Western blot检测
收集4组小鼠缺血再灌注肝组织,加入PIPA裂解液裂解组织,匀浆器研磨。加入样本进行SDS-PAGE电泳,转膜,封闭,再加入一抗过夜。第2天TBST洗涤3次后,后加入二抗常温孵育1 h。TBST清洗后,加入显影液拍照。
1.6. 免疫荧光检测
取标本冰冻切片,PBS洗片3×5 min。消除非特异性背景染色:滴加正常山羊血清封闭液(PBS稀释)室温20 min后甩去液体,PBS洗片3×5 min。滴加一抗:4 ℃过夜,之后37 ℃复温30 min或37 ℃孵育1 h,PBS洗片3次,每次5 min。滴加荧光素标记的二抗37 ℃孵育60 min。PBS洗片3次,每次5 min。抗荧光淬灭剂封片,镜检。
1.7. TUNEL法检测肝细胞凋亡水平
按照说明书指导进行TUNEL实验,检测各组肝组织中肝细胞凋亡程度。细胞固定:(1)用新制备的4%多聚甲醛溶液固定,室温30 min。(2)细胞的通透:PBS洗片,与通透液(0.1% Triton® X-100溶于0.1%枸橼酸钠溶液中)在冰浴中孵育2 min;(3)标记:PBS冲洗2次,擦干样品周围的水,滴加50 μL的TUNEL反应混合溶液,在湿盒中37 ℃孵育60 min。PBS冲洗3次。(4)信号转化和分析:擦干样品周围的水分,加入50 μL转化剂- POD,在湿盒中37 ℃孵育30 min。PBS冲洗3次,加入50~100 μL DAB底物溶液,室温孵育10 min,PBS冲洗3次。封片,在荧光镜下分析结果。在显微镜下,观察各组肝细min染色的情况,以细胞核呈棕色为TUNEL阳性细胞,统计高倍镜下阳性细胞数目,评估各组肝细胞凋亡水平。
1.8. 统计学方法
结果以均数±标准差表示实验所得数据,采用SPSS20.0统计软件进行分析,组间比较行方差分析检验。P < 0.05认为差异具有统计学意义。
2. 结果
2.1. Western blot、免疫荧光和组化检测肝脏缺血再灌注后SHMT2随时间的表达变化
取正常组肝脏组织应用Western blot检测肝脏在缺血再灌注第4、8、16、20、24 h时SHMT2的表达。发现SHMT2在肝缺血再灌注后明显升高,并在24 h达到最大值。(P < 0.01,图 1A、B)我们取肝脏缺血再灌注24 h后及对照组标本切片进行SHMT2的免疫荧光检测,发现在24 h SHMT2荧光表达最为明显。(图 1C)在相应时段采用qPCR检测SHMT2的表达,发现同样的变化,第24小时时SHMT2的mRNA表达量较假手术组明显升高(P < 0.01,图 1D)。进一步验证之前的结果。实验结果显示肝脏缺血再灌注24 h后SHMT2明显被激活,因此在后续实验中我们采用缺血再灌注24 h为实验时间点。
1.
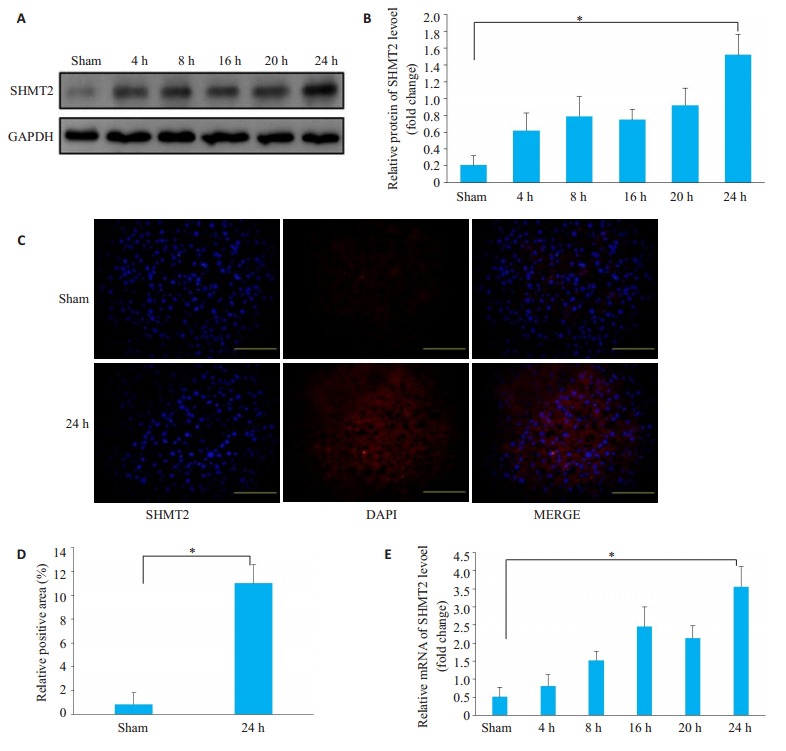
小鼠肝脏缺血再灌注后SHMT2的表达水平变化
Changes in SHMT2 expression after liver ischemia-reperfusion in mice. A, B: Western blotting; C, D: Immunofluorescence assay (Original magnification: ×100), SHMT2: Red fluorescence (cytoplasm); DAPI: Blue fluorescence (nucleus); Merge: Superimposed; E: qPCR detection of mRNA expression level. *P < 0.01.
2.2. 注射SHMT2沉默腺相关病毒后SHMT2表达变化
小鼠尾静脉注射腺相关病毒后14 d,取各组假手术小鼠的肝组织,通过Western blot验证病毒干扰的效果。实验结果发现通过腺相关病毒干扰后SHMT2在肝组织内表达明显降低。Western blot检测发现AAV-SHMT2组SHMT2的表达明显降低(P < 0.01,图 2A、B);qPCR检测SHMT2的表达,发现同样的变化,沉默组SHMT2的mRNA表达量较对照组组明显降低(P < 0.05,图 2C)。
2.
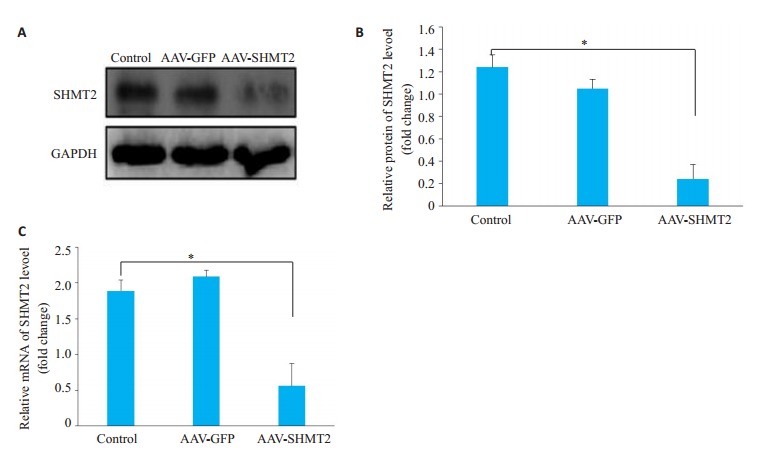
腺相关病毒沉默后SHMT2的蛋白水平变化
Changes in SHMT2 protein levels after adenoassociated virus silencing. *P < 0.05.
2.3. 肝脏缺血再灌注后3、6、12、24 h小鼠血清ALT、AST水平变化
之前的实验结果证明在24 h时SHMT2表达最高,因此我们进行观察各组3、6、12、24 h时AST/ALT的水平。结果显示每个时间点腺相关病毒沉默组AST/ALT水平明显高于对照组和空载组(P < 0.01,图 3A、B)。
3.
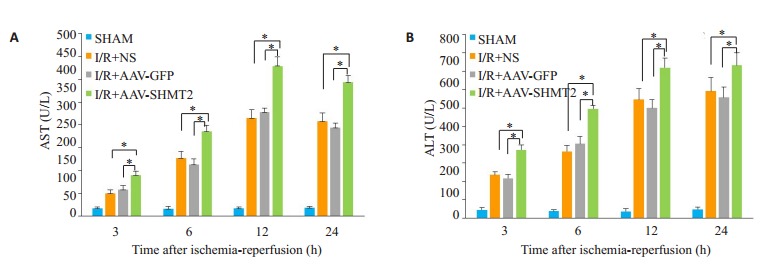
各组AST/ALT水平变化
Changes in AST/ALT levels in each group. A: The level of ALT in each group; B: The level of AST in each group. *P < 0.01.
2.4. HE染色肝脏组织病理变化
检测各组24 h各组的肝脏组织病理变化。光镜下观察到腺相关病毒沉默组出现肝细胞水肿、肝窦淤血、炎症细胞浸润等,与对照组及空载组相比,腺相关病毒组的肝脏损伤更严重(图 4A)。以Suzuki评分体系量化肝组织损伤程度,腺相关病毒组的肝脏损伤明显高于空载组和对照组(P < 0.01,图 4B)。
4.
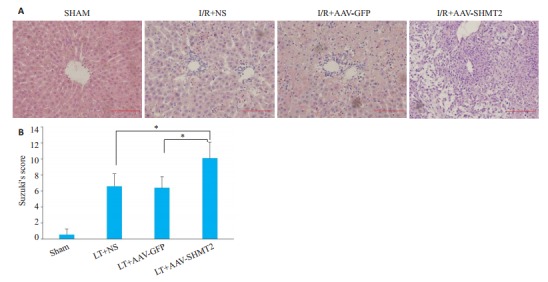
各组病理组织学的变化HE(×200)及Suzuki评分
Histopathological changes of each group (× 200) and Suzuki score. A: HE staining results in control group, saline group, no-load group, and silent group; B: Suzuki score results. *P < 0.01.
2.5. TUNEL染色及Western blot检测Caspse-3的激活表达
取缺血再灌注24 h的肝脏进行比较,TUNEL结果显示AAV-SHMT2组比对照组、空载组中可见更多的棕黄色染色的凋亡细胞。(P < 0.01,图 4A、B) Western blot结果显示AAV-SHMT2组中cleaved-casepse3比对照组和空载组明显升高(P < 0.01,图 4C、D)。
2.6. Western blot检测检测SHMT2、JNK、P65、TNF-α、IL-1表达
检测各组肝脏中SHMT2、JNK、p- JNK、P65、pP65、TNF-α、IL-1的表达水平。结果显示在肝脏缺血再灌注24 h的腺相关病毒沉默组的p-JNK、p-P65明显高于对照组、空载组(P < 0.01)。继续检测下游的炎症因子发现,腺相关病毒沉默组TNF-α、IL-1β表达明显高于对照组(P < 0.01,图 5)。且空载组和对照组比较无统计学差异。
5.
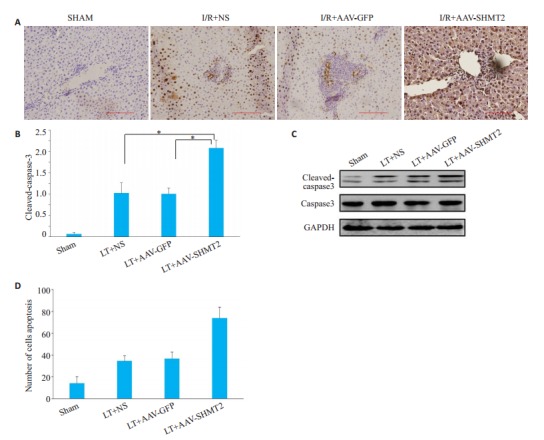
各组在24 h的Suzuki评分,caspase-3的蛋白表达变化及肝组织凋亡形态学
Suzuki score, caspase-3 protein expression and liver tissue apoptosis morphology (TUNEL × 200). A, B: TUNEL staining to observe liver cell apoptosis (× 200) and count at 24 hours after operation in each group; C, D: The protein level of Cleaved-aspase-3. *P < 0.01.
6.
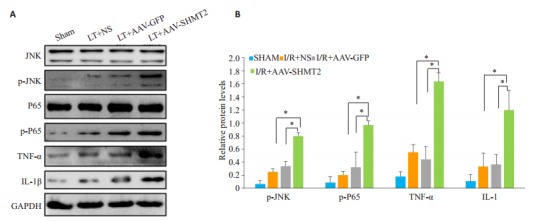
各组的Western blot检测结果
Western blot results of each group. A-E: Western blot results of SHMT2, JNK pathway, NF-κB pathway and downstream inflammatory factors in each group at 24 hours. *P < 0.01.
3. 讨论
肝脏缺血再灌注损伤是肝胆外科手术中经常遇到的病理生理过程,尤其是在肝移植手术中不可避免,造成早期的肝脏功能缺失,严重影响手术疗效和患者预后。但由于肝脏缺血再灌注发生发展的复杂性,目前治疗手段尚未取得理想的效果。研究发现SHMT2通过对线粒体内的丝氨酸,甘氨酸的调节作用来影响下游凋亡及炎症通路来缓解肝脏缺血再灌注[8, 17]。在本实验中的正常组中我们发现随着缺血再灌注的时间加长,SHMT2的表达也在明显升高,并在24 h升到最高。Ye等[18]证明SHMT2在低氧条件下表达会升高,以产生甘氨酸和ME-THF,从而增加了NADPH的合成,在缺血缺氧环境下有利于适应对抗氧化应激。Marina等[19]证明SHMT2的缺失能够在细胞中诱发强烈的凋亡信号。Bethany等[20]证明甘氨酸在抑制细胞凋亡中发挥着重要的作用,其中SHMT2起着主要调节作用。因此本研究探究了SHMT2的缺失与JNK通路的激活的关系, 并发现在SHMT2沉默组中的p-JNK表达明显升高,腺相关病毒沉默组与对照组和空载组相比,肝功能损伤更严重,AST/ALT明显高于对照组。蛋白水平的检测发现caspase3在腺相关病毒沉默组明显升高,TUNEL检测发现腺相关病毒沉默组有着更高的凋亡。,表明SHMT2被干扰后,肝脏在缺血再灌注后凋亡明显被激活。这说明SHMT2对保护肝脏缺血再灌注后的凋亡有着不可或缺的作用。
先前的研究表明NF-κB的激活是促进炎症进一步发展扩大的关键因子,在肝脏缺血再灌注中起着重要作用[21-23]。研究表明[24-25]肝脏缺血再灌早期JNK磷酸化水平升高,JNK在肝脏缺血再灌注中发挥有害作用,而JNK抑制剂可降低损害[26]。JNK短时间活化可促进细胞生存,但是JNK长时间处于活化状态引起促凋亡和抗凋亡蛋白表达改变,激活细胞死亡通路[27]。Hyo-Yeon等[28]发现缺血/再灌可显著增加肝细胞内JNK1和JNK2的表达,引起肝细胞凋亡,通过抑制JNK活性,则可以减少肝细胞凋亡发挥保护作用。Yun-Chen等[29]证明在JNK被激活的情况下的情况下可导致NF-κB活性增强加重炎症反应。本实验中我们发现在相同24 h的缺血再灌注的肝脏组织中的AAV- SHMT2组中,随着SHMT2被沉默,JNK出现明显激活,并伴随着NF-κB的蛋白水平表达增高,p-JNK、p-P65明显表达高于空载组及对照组。从而增加了损伤性炎症因子的释放。实验中我们还观察到,在AAV-SHMT2组中缺血再灌注24 h后p-P65的表达随着p-JNK表达升高也随之升高,继续检测其下游的炎症因子,发现TNF-α、IL-1β也随之升高,形成炎症因子的级联放大体系,导致缺血再灌注的肝脏肝细胞水肿、肝窦淤血、炎症细胞浸润等急性再灌注损伤。本实验通过构建肝脏缺血再灌注模型,并通过沉默SHMT2的方法说明在肝脏缺血再灌注中SHMT2是对肝脏起保护作用。在沉默SHMT2的情况下肝脏缺血再灌注后明显激活了JNK通路,JNK活性增强,使得下游的caspase-3进一步激活,从而促进了凋亡。通过对组织切片的观察我们发现沉默SHMT2后炎症细胞浸润明显增加。Western blot实验验证发现SHMT2的缺失介导了NF-κB的激活,增加了其下游损伤性炎症因子TNF-α、IL-1β的激活,使得肝脏缺血再灌注损伤加重。
综上所述,本实验初步探究了SHMT2在JNK通路和NF-κB通路介导的肝脏缺血再灌注损伤中的不可或缺的作用。本实验沉默SHMT2基因后会导致JNK通路的过度激活,进一步介导NF-κB通路激活,促进了肝脏缺血再灌注后的凋亡及损伤性炎症因子的释放。证明了在肝脏缺血再关注中SHMT2的存在是不可或缺的。这可能为肝脏缺血再灌注的早期损伤带来新的基因治疗靶点。
Funding Statement
西南医科大学应用基础研究基金(16XYJC0161)
References
- 1.de Oliveira THC, Marques PE, Poosti F, et al. Intravital microscopic evaluation of the effects of a CXCR2 antagonist in a model of liver ischemia reperfusion injury in mice. http://cn.bing.com/academic/profile?id=6f13c6b135c574d80c3de3b1f3523084&encoded=0&v=paper_preview&mkt=zh-cn. Front Immunol. 2018;8:1917. doi: 10.3389/fimmu.2017.01917. [de Oliveira THC, Marques PE, Poosti F, et al. Intravital microscopic evaluation of the effects of a CXCR2 antagonist in a model of liver ischemia reperfusion injury in mice[J]. Front Immunol, 2018, 8: 1917.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokota S, Yoshida O, Yoshihiro O, et al. Liver transplantation in the mouse: Insights into liver immunobiology, tissue injury, and allograft tolerance. http://cn.bing.com/academic/profile?id=83f1913276e9b830cc8c1c98061d3454&encoded=0&v=paper_preview&mkt=zh-cn. Liver Transplant. 2016;22(4):536–46. doi: 10.1002/lt.24394. [Yokota S, Yoshida O, Yoshihiro O, et al. Liver transplantation in the mouse: Insights into liver immunobiology, tissue injury, and allograft tolerance[J]. Liver Transplant, 2016, 22(4): 536-46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickkholgh A, Maluf D. Emerging graft protective strategies in clinical liver transplantation. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1080/17474124.2017.1322901. Expert Rev Gastroenterol Hepatol. 2017;11(7):623–31. doi: 10.1080/17474124.2017.1322901. [Nickkholgh A, Maluf D. Emerging graft protective strategies in clinical liver transplantation[J]. Expert Rev Gastroenterol Hepatol, 2017, 11(7): 623-31.] [DOI] [PubMed] [Google Scholar]
- 4.Rebecca AS, Zarrinpar A, Rossetti M, et al. Early cytokine signatures of ischemia/reperfusion injury in human orthotopic liver transplantation. http://cn.bing.com/academic/profile?id=de729198564be9b29503093545d2e95a&encoded=0&v=paper_preview&mkt=zh-cn. JCI Insight. 2016;1(20):e89679. doi: 10.1172/jci.insight.89679. [Rebecca AS, Zarrinpar A, Rossetti M, et al. Early cytokine signatures of ischemia/reperfusion injury in human orthotopic liver transplantation[J]. JCI Insight, 2016, 1(20): e89679.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu XS, Wang MH, Li JZ, et al. Tauroursodeoxycholic acid alleviates hepatic ischemia reperfusion injury by suppressing the function of Kupffer cells in mice. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6644aa524987db47b609ef4fbdae3d55. Biomed Pharmacother. 2018;106:1271–81. doi: 10.1016/j.biopha.2018.06.046. [Xu XS, Wang MH, Li JZ, et al. Tauroursodeoxycholic acid alleviates hepatic ischemia reperfusion injury by suppressing the function of Kupffer cells in mice[J]. Biomed Pharmacother, 2018, 106: 1271-81.] [DOI] [PubMed] [Google Scholar]
- 6.Konno M, Asai A, Kawamoto K, et al. The one-carbon metabolism pathway highlights therapeutic targets for gastrointestinal cancer (Review) http://cn.bing.com/academic/profile?id=a3e11c47d7be74061e81f9fcbd3960a0&encoded=0&v=paper_preview&mkt=zh-cn. Int J Oncol. 2017;50(4):1057–63. doi: 10.3892/ijo.2017.3885. [Konno M, Asai A, Kawamoto K, et al. The one-carbon metabolism pathway highlights therapeutic targets for gastrointestinal cancer (Review)[J]. Int J Oncol, 2017, 50(4): 1057-63.] [DOI] [PubMed] [Google Scholar]
- 7.沈崇灵.法理学[M].北京出版社, 1994: 51-2.
- 8.Kim D, Fiske BP, Birsoy K, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=65a6188d7c713a7b1c7678d43f194533. Nature. 2015;520(7547):363–7. doi: 10.1038/nature14363. [Kim D, Fiske BP, Birsoy K, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance[J]. Nature, 2015, 520(7547): 363-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhanasekaran DN, Reddy EP. JNK-signaling: A multiplexing hub in programmed cell death. http://d.old.wanfangdata.com.cn/Periodical/zgxdyxzz200707011. Genes Cancer. 2017;8(9/10):682–94. doi: 10.18632/genesandcancer.155. [Dhanasekaran DN, Reddy EP. JNK-signaling: A multiplexing hub in programmed cell death[J]. Genes Cancer, 2017, 8(9/10): 682-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulut G, Kurdoglu Z, Dönmez YB, et al. Effects of jnk inhibitor on inflammation and fibrosis in the ovary tissue of a rat model of polycystic ovary syndrome. http://cn.bing.com/academic/profile?id=d196681537f6ced1baf09ec8050f62b0&encoded=0&v=paper_preview&mkt=zh-cn. Int J Clin Exp Pathol. 2015;8(8):8774–85. [Bulut G, Kurdoglu Z, Dönmez YB, et al. Effects of jnk inhibitor on inflammation and fibrosis in the ovary tissue of a rat model of polycystic ovary syndrome[J]. Int J Clin Exp Pathol, 2015, 8(8): 8774-85.] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee GY, Haverty PM, Li L, et al. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6c4237726d0418c098b3f57c11f5f9b2. Cancer Res. 2014;74(11):3114–26. doi: 10.1158/0008-5472.CAN-13-2683. [Lee GY, Haverty PM, Li L, et al. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes[J]. Cancer Res, 2014, 74(11): 3114-26.] [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Lou CY, Wang WY. STIM1 deficiency protects the liver from ischemia/reperfusion injury in mice. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e96844882539338284ef00c5f705c4cf. Biochem Biophys Res Commun. 2018;496(2):422–8. doi: 10.1016/j.bbrc.2018.01.006. [Li Y, Lou CY, Wang WY. STIM1 deficiency protects the liver from ischemia/reperfusion injury in mice[J]. Biochem Biophys Res Commun, 2018, 496(2): 422-8.] [DOI] [PubMed] [Google Scholar]
- 13.Ning S, Ma S, Saleh AQ, et al. SHMT2 overexpression predicts poor prognosis in intrahepatic cholangiocarcinoma. http://cn.bing.com/academic/profile?id=294d7a7084274d7461d465f3e44ab7bf&encoded=0&v=paper_preview&mkt=zh-cn. Gastroenterol Res Pract. 2018:4369253. doi: 10.1155/2018/4369253. [Ning S, Ma S, Saleh AQ, et al. SHMT2 overexpression predicts poor prognosis in intrahepatic cholangiocarcinoma[J]. Gastroenterol Res Pract, 2018: 4369253.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chern CW, Way CC, Xing QT, et al. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma. http://cn.bing.com/academic/profile?id=f6d0ab9fe8d072d97e70b8729734896b&encoded=0&v=paper_preview&mkt=zh-cn. Oncotarget. 2016;7(33):53005–17. doi: 10.18632/oncotarget.10415. [Chern CW, Way CC, Xing QT, et al. Downregulating serine hydroxymethyltransferase 2 (SHMT2) suppresses tumorigenesis in human hepatocellular carcinoma[J]. Oncotarget, 2016, 7(33): 53005- 17.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang MH, Yuan FC, He B, et al. SHMT2 promotes liver regeneration through glycine-activated Akt/mTOR pathway. http://cn.bing.com/academic/profile?id=c3fe6f1e3dcdf8e4ce360dda256d8d87&encoded=0&v=paper_preview&mkt=zh-cn. Transplantation. 2019;103(7):e188–97. doi: 10.1097/TP.0000000000002747. [Wang MH, Yuan FC, He B, et al. SHMT2 promotes liver regeneration through glycine-activated Akt/mTOR pathway[J]. Transplantation, 2019, 103(7): e188-97.] [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Liu Y, Gao H, et al. Serine hydroxymethyl transferase 2 regulates the AMPK/mTOR pathway and induces autophagy to promote chemotherapy resistance in colon cancer cells. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xbyfzmyxzz201904010. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35(4):344–50, 356. [Liu F, Liu Y, Gao H, et al. Serine hydroxymethyl transferase 2 regulates the AMPK/mTOR pathway and induces autophagy to promote chemotherapy resistance in colon cancer cells[J]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 2019, 35(4): 344-50, 356.] [PubMed] [Google Scholar]
- 17.Ming W, Siyi W, Li XJ, et al. Overexpression of mitochondrial serine hydroxyl-methyltransferase 2 is associated with poor prognosis and promotes cell proliferation and invasion in gliomas. http://cn.bing.com/academic/profile?id=901e98e78522cff58e5b09624fa7d832&encoded=0&v=paper_preview&mkt=zh-cn. Onco Targets Ther. 2017;10:3781–8. doi: 10.2147/OTT.S130409. [Ming W, Siyi W, Li XJ, et al. Overexpression of mitochondrial serine hydroxyl-methyltransferase 2 is associated with poor prognosis and promotes cell proliferation and invasion in gliomas[J]. Onco Targets Ther, 2017, 10: 3781-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J, Fan J, Venneti S, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8606cf0a725f8040f42f08b4985c9df1. Cancer Discov. 2014;4(12):1406–17. doi: 10.1158/2159-8290.CD-14-0250. [Ye J, Fan J, Venneti S, et al. Serine catabolism regulates mitochondrial redox control during hypoxia[J]. Cancer Discov, 2014, 4(12): 1406- 17.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marani M, Paone A, Alessio F, et al. A pyrazolopyran derivative preferentially inhibits the activity of human cytosolic serine hydroxymethyltransferase and induces cell death in lung cancer cells. http://cn.bing.com/academic/profile?id=843aaf292110abeea8eb60c2e9a9a3c7&encoded=0&v=paper_preview&mkt=zh-cn. Oncotarget. 2016;7(4):4570–83. doi: 10.18632/oncotarget.6726. [Marani M, Paone A, Alessio F, et al. A pyrazolopyran derivative preferentially inhibits the activity of human cytosolic serine hydroxymethyltransferase and induces cell death in lung cancer cells[J]. Oncotarget, 2016, 7(4): 4570-83.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethany KR, Spate LD, Lee K, et al. Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not Lead to live births. http://cn.bing.com/academic/profile?id=f21fb89efbe893183bc58d377cd3a05b&encoded=0&v=paper_preview&mkt=zh-cn. Mol Reprod Dev. 2016;83(3):246–58. doi: 10.1002/mrd.22618. [Bethany KR, Spate LD, Lee K, et al. Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not Lead to live births[J]. Mol Reprod Dev, 2016, 83(3): 246-58.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto-Moure B, Lloris-Carsi JM, Barrios-Pitarque C, et al. Pharmacology of Ischemia-Reperfusion. Translational research considerations. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9904ab8fb4812d1786df2b6bdb6992e7. J Invest Surg. 2016;29(4):234–49. doi: 10.3109/08941939.2015.1119219. [Prieto-Moure B, Lloris-Carsi JM, Barrios-Pitarque C, et al. Pharmacology of Ischemia-Reperfusion. Translational research considerations[J]. J Invest Surg, 2016, 29(4): 234-49.] [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_1069550. Oncotarget. 2018;9(6):7204–18. doi: 10.18632/oncotarget.23208. [Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs[J]. Oncotarget, 2018, 9 (6): 7204-18.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanno E, Kondylis V, Heinrichsdorff J, et al. Hepatocyte IKK2 protects Mdr2-/- mice from chronic liver failure. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Open J-Gate000003874574. PLoS One. 2011;6(10):e25942. doi: 10.1371/journal.pone.0025942. [Hanno E, Kondylis V, Heinrichsdorff J, et al. Hepatocyte IKK2 protects Mdr2-/- mice from chronic liver failure[J]. PLoS One, 2011, 6(10): e25942.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang N, Gan H, He Q, et al. Dysfunction of cholesterol sensor SCAP promotes inflammation activation in THP-1 macrophages. http://d.old.wanfangdata.com.cn/Periodical/dsjydxxb201713003. Exp Cell Res. 2018;367(2):162–9. doi: 10.1016/j.yexcr.2018.03.032. [Ouyang N, Gan H, He Q, et al. Dysfunction of cholesterol sensor SCAP promotes inflammation activation in THP-1 macrophages[J]. Exp Cell Res, 2018, 367(2): 162-9.] [DOI] [PubMed] [Google Scholar]
- 25.Luo SY, Rui L, Le ZY, et al. Anfibatide protects against rat cerebral ischemia/reperfusion injury via TLR4/JNK/caspase-3 pathway. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=07390c1f1a1c38aa97860f3bf4308f7c. Eur J Pharmacol. 2017;807:127–37. doi: 10.1016/j.ejphar.2017.04.002. [Luo SY, Rui L, Le ZY, et al. Anfibatide protects against rat cerebral ischemia/reperfusion injury via TLR4/JNK/caspase-3 pathway[J]. Eur J Pharmacol, 2017, 807: 127-37.] [DOI] [PubMed] [Google Scholar]
- 26.Theruvath TP, Snoddy MC, Zhi Z, et al. Mitochondrial permeability transition in liver ischemia and reperfusion: role of c-Jun NTerminal kinase 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2656605/ Transplantation. 2008;85(10):1500–4. doi: 10.1097/TP.0b013e31816fefb5. [Theruvath TP, Snoddy MC, Zhi Z, et al. Mitochondrial permeability transition in liver ischemia and reperfusion: role of c-Jun NTerminal kinase 2[J]. Transplantation, 2008, 85(10): 1500-4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-κB and JNK activation in hepatocytes. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a4aa6d1de95b748e15a128614c1559bd. Biochem Pharmacol. 2006;72(9):1090–101. doi: 10.1016/j.bcp.2006.07.003. [Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-κB and JNK activation in hepatocytes[J]. Biochem Pharmacol, 2006, 72(9): 1090-101.] [DOI] [PubMed] [Google Scholar]
- 28.Hyo-Yeon K, Lee SM. Ferulic acid attenuates ischemia/reperfusioninduced hepatocyte apoptosis via inhibition of JNK activation. https://www.sciencedirect.com/science/article/pii/S092809871200053X. Eur J Pharm Sci. 2012;45(5):708–15. doi: 10.1016/j.ejps.2012.01.010. [Hyo-Yeon K, Lee SM. Ferulic acid attenuates ischemia/reperfusioninduced hepatocyte apoptosis via inhibition of JNK activation[J]. Eur J Pharm Sci, 2012, 45(5): 708-15.] [DOI] [PubMed] [Google Scholar]
- 29.Yun-Chen T, Lin JY, Chao-Hung L, et al. Carthamus tinctorius L. prevents LPS-induced TNFα signaling activation and cell apoptosis through JNK1/2-NFκB pathway inhibition in H9c2 cardiomyoblast cells. J Ethnopharmacol. 2010;130(3):505–13. doi: 10.1016/j.jep.2010.05.038. [Yun-Chen T, Lin JY, Chao-Hung L, et al. Carthamus tinctorius L. prevents LPS-induced TNFα signaling activation and cell apoptosis through JNK1/2-NFκB pathway inhibition in H9c2 cardiomyoblast cells[J]. J Ethnopharmacol, 2010, 130(3): 505-13.] [DOI] [PubMed] [Google Scholar]


