Abstract
Background
Robotic anatomic segmentectomy (RATS) for early-stage lung cancer is being increasingly performed in spite of limited published evidence. To evaluate its safety and oncologic efficacy, we compared the outcomes of both RATS and video-assisted thoracoscopic (VATS) segmentectomy in patients with small-sized (<2 cm) peripheral stage IA lung cancer.
Methods
From November 2011 to January 2018, a total of 130 patients with resected stage IA non-small cell lung cancer (NSCLC) who underwent RATS (n=50) and VATS (n=80) pulmonary segmentectomy were included. Clinicopathologic data, recurrence rate, and survival were recorded.
Results
The demographics, pulmonary function, comorbidity, and tumor size were similar between RATS segmentectomy and VATS segmentectomy. The surgery time, intensive care unit stay, hospital stay, and blood loss were reduced in the RATS group compared to the VATS group. The number of totally dissected lymph nodes and postoperative complications were similar between the 2 groups. There was no operative mortality. The intensity of narcotic use during hospital stay and the time to return to routine daily activities were also reduced in the RATS group. There was no recurrence observed in the RATS group during the median 38-month follow-up period; meanwhile, during a median 85-month follow-up period in the VATS group, local recurrence and distant recurrence was observed in 2 patients (2.5%) and 3 patients (3.75%) respectively. There was no significant difference in the 5-year recurrence-free survival between the RATS and VATS groups (100% vs. 93.75%; P>0.05).
Conclusions
RATS can be performed safely and effectively in patients with early-stage NSCLC. The reduced narcotic use and earlier return to routine daily activities of RATS patients might reflect its less traumatic nature as compared to VATS. For stage IA disease with small tumors (<2 cm), segmentectomy performed by RATS has better oncologic efficacy when compared to VATS, although in this study, this difference did not reach statistical difference.
Keywords: Lung cancer, robotic surgery, video-assisted thoracoscopic surgery (VATS), segmentectomy, minimally invasive surgery, long-term survival
Introduction
Anatomic segmentectomy is the removal of a segment of the lobe in the lung. For many years, segmentectomy has been performed for the treatment of bronchiectasis. Since 1970, surgeons have increasingly applied segmentectomy for the treatment of non-small cell lung cancer (NSCLC). In 1995, a randomized controlled trial reported by the Lung Cancer Study Group (LCSG) demonstrated the advantages of lobectomy over sublobar resection in regards to local recurrence and long-term survival for patients with T1N0 NSCLC (1). However, this study did not specifically evaluate smaller lung tumors (<2 cm), which in recent years have been identified with greater frequency through the use of low-dose computed tomography (LDCT) screening (2-4). Secondly, the LCSG study indicated that the use of wedge resection without systemic lymph node dissection, in similar fashion to sublobar resection, leads to higher local recurrence compared to lobectomy. Therefore, the optimal treatment for small peripheral lung cancer still remains to be defined, and there is a pressing need to evaluate the benefits of anatomical segmentectomy for patients with small-sized (<2 cm) stage IA NSCLC (5).
Video-assisted thoracic surgery (VATS) is a minimally invasive thoracic surgery that has been adopted by many surgeons for small pulmonary lesion resection, with expected shorter hospital stay, reduced pain, and improved functional recovery in comparison to traditional open thoracotomy (6-8). In the past decade, thoracoscopic segmentectomy has gained popularity and has proven to be a safe procedure with comparable oncologic results for small peripheral NSCLC compared with lobectomy (8,9).
Meanwhile, the use of robotic lung resection has sharply increased in the past 5 years (10), but the application of robotic anatomical segmentectomy (RATS) has scarcely been reported on (11,12). After years of practice in an accredited academic center for minimally invasive thoracic surgery, we developed a robotic surgery program with the da Vinci Robotic System (Intuitive Surgical Inc., Mountain View, CA, USA) in 2009, and have been implementing this program ever since. In this study, we compared the safety and oncologic effectiveness of RATS to VATS segmentectomy for NSCLC patients with small-size (<2 cm) stage IA tumors. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-533).
Methods
This retrospective study analyzed 130 patients who were resected with stage IA (T1a-T1bN0M0) NSCLC at Shanghai Chest Hospital between November 2011 and January 2018. Patients were included if they had undergone RATS or VATS, in which the segmental veins, arteries, and bronchus were divided separately. Written informed consent was required from all patients before operation. The patients that were included were primarily stage IA NSCLC with T1a and T1b tumors (<2 cm). We excluded patients who had undergone induction therapy, with large tumors (>2 cm), central lesions, or multiple lesions. Patients with positive lymph nodes were also excluded. Clinical data, including age, sex, pulmonary function, comorbidity, perioperative outcomes, and mortality, were evaluated from a prospective database.
All patients underwent preoperative high resolution computed tomography (HRCT) and pulmonary function test with blood gas analysis. Cardiac function assessment was individualized. Abdominal ultrasound, magnetic resonance imaging (MRI) of the brain, and bone scintigraphy, were performed to exclude distant metastasis, and positron-emission tomography (PET) scan was performed when necessary. Video mediastinoscopy was not routinely used to evaluate stage IA patients preoperatively. The absence of radiographic nodal disease (hilar or mediastinal) was confirmed preoperatively.
Chest drainage tubes were removed when there was no obvious air leak and when liquid output was lower than 300 mL for a 24-hour period. Patient-controlled intravenous narcotics, lornoxicam injection, oral oxycodone, or a combination of the above, were used as a multimodal analgesia if the patient had a pain level greater than 3 of 10. Each dosage of oxycodone or lornoxicam was marked as 1 point. Patients were weaned from oral narcotics beginning the first week of discharge. Patients were instructed to go about their routine daily activity after discharge.
Statistical analyses were performed using SPSS 18.0 software (SPSS Inc, Chicago, IL, USA). All data were expressed as mean ± standard deviation. Differences between groups were tested by χ2 test or Fisher’s exact test. The Kaplan-Meier method and log-rank test were used to evaluate the overall survival and recurrence-free survival. The predictors of recurrence-free survival were evaluated by multivariate Cox regression analyses. A P value of lower than 0.05 was accepted as statistically significant.
Operative techniques
Docking and port selection of RATS
Patients were positioned on the lateral decubitus position similarly to VATS. The robot was placed to the posterior of the patient with a 30–45° angle between the vertebral column and the transverse axis of cart. Either 3 or 4 ports were used, and the fourth port was used to retract the lung if necessary. A 1 cm incision for the camera port was made in the seventh or eighth intercostal space on the posterior axillary line. We used a 12 mm robotic 0-degree camera. Robotic arm 1 and 2 were placed 10 cm from the camera port along the same rib. The 8 mm metal reusable da Vinci trocars were placed for robotic arm 1 and 2, and an 8 mm bipolar curved tip dissector and a Cadiere forceps were used respectively. Under direct vision, a utility port was made at the fourth or fifth intercostal space behind the pectoralis major, and a 12 mm plastic disposable port was placed. The fourth robotic arm, when used, was placed just anterior to the spinal process of the vertebral body below the major fissure. The camera port was placed more laterally on left side than the right side for the location of the heart.
Port selections of complete VATS
VATS segmentectomy was performed in the lateral decubitus position under general anesthesia with single-lung ventilation. The surgeon was standing at the abdominal side of the patients. Three or four incisions were made. The camera port was made at the sixth or seventh intercostal space on the anterior axillary line. The second port was at the fourth or fifth intercostal space behind the pectoralis major. The third port was usually at the sixth intercostal space on the posterior axillary line. A fourth port for assistance was made at the ninth intercostal space on the posterior axillary line if necessary. Extraction of the specimen was accomplished by extending the fourth or fifth intercostal space incision.
Segmentectomy technique
Segmentectomy was performed as reported previously, in which the vein, artery, and bronchus of 1 or more segments were divided separately (13,14). For both RATS and VATS, we used similar operative procedures. We first dissected the fissure, and removed the hilar lymph node around the segmental artery and bronchus. Segmental arteries or veins were clipped by hem-o-lok (Teleflex Medical, Research Triangle Park, NC, USA) and a titanium clipper, or stapled by endoscopic stapler. The segmental bronchus was routinely stapled. The intersegmental plane was stapled by endoscopic stapler after the bronchus was clamped and the remnant lung was reventilated. The surgical margin of the lung parenchyma was at least 2 cm. We did not use glue or sealant in any of the patients. A single 28-F chest tube was placed through the camera port.
Results
From November, 2011, to January, 2018, 50 patients underwent segmentectomies with RATS and 80 patients underwent segmentectomies with VATS for primary lung cancer (NSCLC) with stage IA tumors (<2 cm). The 2 groups were similar in age, sex, pulmonary function, and incidence of comorbidities. Patient characteristics are summarized in Table 1.
Table 1. Demographics and characteristics of patients.
| Characteristic | RATS (n=50) | VATS (n=80) | P value |
|---|---|---|---|
| Age, y ± SD | 54.7±10.3 | 57.7±9.7 | 0.097 |
| Gender, n | 0.765 | ||
| Female | 35 | 54 | |
| Male | 15 | 26 | |
| Smoke, n | 0.498 | ||
| Yes | 6 | 11 | |
| No | 44 | 69 | |
| FEV1%, mean ± SD | 93.1±16.5 | 89.4±17.9 | 0.263 |
| DLCO %, mean ± SD | 95.3±15.9 | 93.6±22.1 | 0.647 |
| Comorbidity, n (%) | 0.563 | ||
| COPD | 3 (6.0%) | 6 (7.5%) | |
| CAD | 2 (4.0%) | 0 (0.0%) | |
| DM | 2 (4.0%) | 4 (5.0%) | |
| History of malignancy, n (%) | 1 (2.0%) | 2 (2.5%) | |
| BMI, mean ± SD | 23.7±3.6 | 23.7±2.8 | 0.977 |
RATS, robotic anatomic segmentectomy; VATS, video-assisted thoracic surgery; SD, standard deviation.
The mean tumor size in RATS and VATS patients was 1.14 and 1.24 cm respectively (P=0.133). Data on tumor location, pathologic diagnosis, and resected segments in the RATS and VATS groups are presented in Table 2. Two pathologists had confirmed the diagnosis and staging according to the International Association for the Study of Lung Cancer 8th Tumor, Node, Metastasis, Staging System. The composition of histologic subtype was similar between the RATS group and VATS group (P=0.299).
Table 2. Pathology and distribution of segments.
| Variable | RATS (n=50) | VATS (n=80) | P value |
|---|---|---|---|
| Pathologic type, n (%) | 0.299 | ||
| Invasive adenocarcinoma | 34 (68.0%) | 45 (56.25%) | |
| Adenocarcinoma/lepidic pattern | 16 (32.0%) | 34 (42.5%) | |
| Large-cell neuroendocrine | 0 (0.0%) | 1 (1.25%) | |
| Segments, n | |||
| Left upper lobe | |||
| S1+2 | 4 | 10 | |
| S3 | 0 | 1 | |
| Lingula | 5 | 11 | |
| Upper division | 3 | 19 | |
| Left lower lobe | |||
| S6 | 10 | 8 | |
| Basilar | 1 | 0 | |
| Right upper lobe | |||
| S1 | 6 | 5 | |
| S2 | 11 | 9 | |
| S3 | 3 | 5 | |
| Right lower lobe | |||
| S6 | 6 | 12 | |
| Basilar | 1 | 0 |
RATS, robotic anatomic segmentectomy; VATS, video-assisted thoracic surgery.
The mean operative time (from incision to closure) in the RATS group was 89 minutes compared to 115 minutes in the VATS group (P=0.005). The blood loss was 50 mL in the RATS group compared to 80 mL in the VATS group (P=0.000). The number of dissected lymph nodes (N1 and N2) was similar between the RATS and VATS groups; however, the number of harvested N2 lymph nodes was slightly greater in the VATS group. The intensive care unit (ICU) stay and hospital stay were shorter in the RATS group with the difference being statistically significant (Table 3).
Table 3. Key clinical outcomes.
| Characteristic | RATS (n=50) | VATS (n=80) | P value |
|---|---|---|---|
| Tumor stage, n | 0.744 | ||
| IA1 | 22 | 30 | |
| IA2 | 27 | 49 | |
| IBa | 1 | 1 | |
| Tumor size, cm, mean ± SD | 1.14±0.35 | 1.24±0.39 | 0.133 |
| Surgery time, minutes (incision to closure), mean ± SD | 89.62±57.61 | 115.40±43.69 | 0.005 |
| Number of lymph nodes (N1 and N2), mean ± SD | 5.04±3.96 | 6.29±4.14 | 0.092 |
| Number of N2 stations, mean ± SD | 1.32±1.60 | 1.91±1.45 | 0.033 |
| Blood loss, mL, median [range] | 50 [50–100] | 80 [50–1000] | 0.000 |
| ICU stay, days, median [range] | 0 [0–3] | 1 [0–7] | 0.000 |
| Length of chest tube drainage, days, median [range] | 3 [1–7] | 3 [1–9] | 0.265 |
| Length of postoperative stay, days, median [range] | 4 [2–8] | 5 [2–12] | 0.001 |
| Follow-up time, months, median [range] | 38 [25–60] | 85 [43–99] | 0.000 |
a, patients of both groups were stage IB because of invasion of visceral pleura. RATS, robotic anatomic segmentectomy; VATS, video-assisted thoracic surgery; SD, standard deviation.
There were 6 (12%) minor morbidities in the RATS group compared with 11 (13.8%) in the VATS group (P=0.773). There were no operative, or 60-day or 90-day mortalities in either group. The data for the complications of the RATS and VATS groups are listed in Table 4; none of the patients had a major cardiopulmonary complication.
Table 4. Postoperative complications.
| Characteristic | RATS (n=50) | VATS (n=80) | P value |
|---|---|---|---|
| Pneumonia | 0 (0.0%) | 1 (1.3%) | 1.00 |
| Atrial fibrillation | 2 (4%) | 2 (2.5%) | 0.638 |
| Vocal cord paralysis | 0 (0.0%) | 1 (1.3%) | 1.00 |
| Postoperative hemorrhage | 1 (2%) | 1 (1.3%) | 1.00 |
| Air leak >5 days | 1 (2%) | 2 (2.5%) | 1.00 |
| Chylothorax | 1 (2%) | 2 (2.5%) | 1.00 |
| Wound infection | 1 (2%) | 2 (2.5%) | 1.00 |
| Total morbidity | 12% | 13.8% | 0.773 |
RATS, robotic anatomic segmentectomy; VATS, video-assisted thoracic surgery.
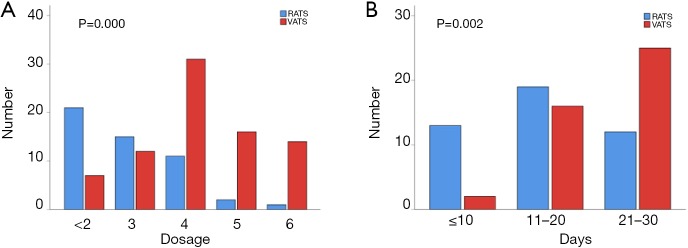
Patients undergoing RATS segmentectomy demonstrated a lower use of analgesics during the postoperative stay in hospital compared with the VATS patients (P=0.000) (Figure 1A). An earlier return to routine daily activities was also observed for the RATS group (P=0.002) (Figure 1B).
Figure 1.
Dosage of narcotic use and duration of return to routine daily activities. (A) Narcotic use was measured as the total number of oxycodone or lornoxicam used during the postoperative stay in hospital. (B) The number patients returning to routine daily activities is shown at set intervals.
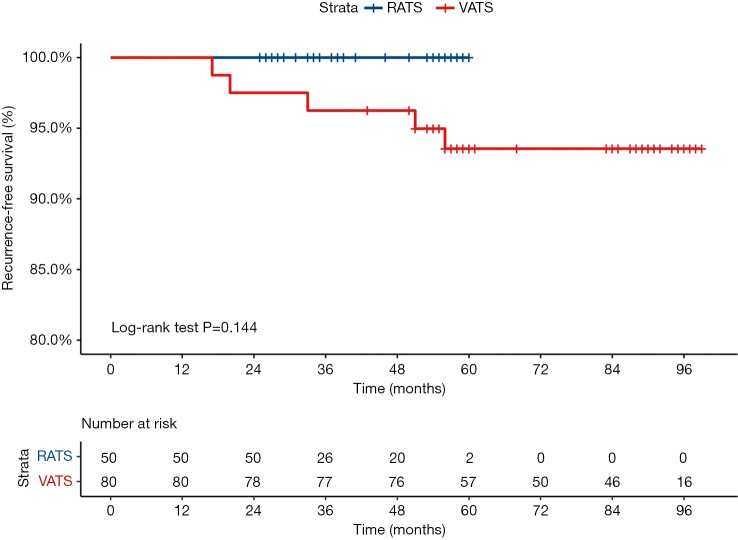
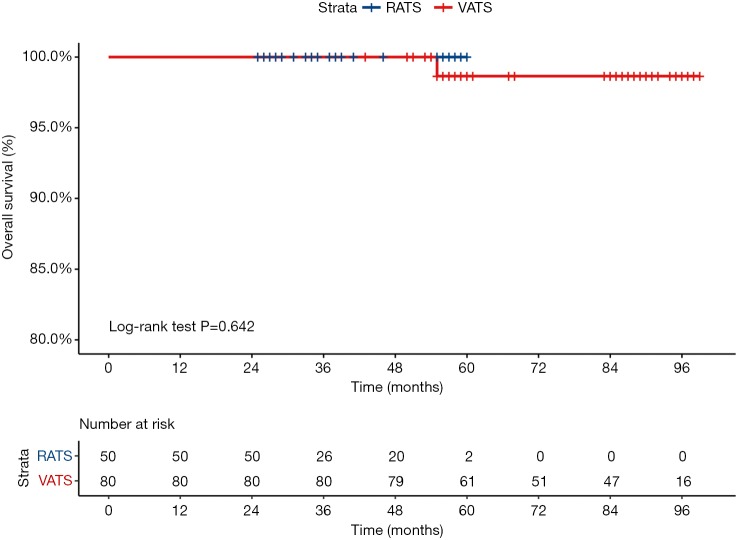
During a median 38-month follow-up period, there were no recurrences in the RATS group. During a median 85-month follow-up period in the VATS group, local recurrence was observed in 2 patients (2.5%), and distant recurrence was observed in 3 patients (3.75%). Five-year recurrence-free survival for stage IA (<2 cm) in the RATS group was 100%, while it was 93.75% in the VATS group (P=0.144) (Figure 2). Five-year overall survival for stage IA (<2 cm) in the RATS group was 100%, while it was 98.75% in the VATS group (P=0.642) (Figure 3).
Figure 2.
Probability of recurrence-free survival comparing stage IA (<2 cm) of RATS (blue line) versus VATS (red line) after anatomical segmentectomy for primary NSCLC. RATS, robotic anatomic segmentectomy; VATS, video-assisted thoracic surgery; NSCLC, non-small cell lung cancer.
Figure 3.
Probability of overall survival comparing stage IA (<2 cm) of RATS (blue line) versus VATS (red line) after anatomical segmentectomy for primary NSCLC. RATS, robotic anatomic segmentectomy; VATS, video-assisted thoracic surgery; NSCLC, non-small cell lung cancer.
Discussion
With the increase of experience in minimally invasive thoracoscopic surgery, more surgeons have become interesting in applying VATS in highly select patients with smaller NSCLC tumors, or with compromised cardiopulmonary function (15). Although VATS is considered to be a more complicated and time-consuming procedure, several studies have shown that VATS confers benefits of shorter length of chest tube duration, reduced length of stay, and lower rates of complications, while maintaining an equivalent survival rate compared to open segmentectomy (16-18). In a recent well-matched study, VATS also appeared to have similar operation time, rates of morbidity, local recurrence, and survival rate when compared to VATS lobectomy in stage IA NSCLC patients with small-size tumors (19).
Due to the growing knowledge of robotic lung resection techniques, the frequency of robotically performed lobectomy has tripled in the last 3 years (10). However, only 4 original reports that focus on robotic segmentectomy exist (11,13,20,21), and to our knowledge, the comparison between RATS and VATS for small (<2 cm) stage IA NSCLC has been understudied. Previous segmentectomy studies included patients with compromised cardiac or lung function, and thus might have underestimated the benefits of segmentectomy (12,15). In the current study, both the RATS and VATS groups mostly included patients with normal lung and cardiac function whose tumors were smaller than 2 cm.
We found that RATS segmentectomy could be performed safely in comparison to currently mature VATS procedures and could provide similar clinical outcomes of oncologic efficacy when applied in stage IA NSCLC patients with a tumor diameter smaller than 2 cm (21). Pardolesi et al.’ study reported a mean duration of surgery of 189 minutes, a mean tumor size of 1.11 cm, a postoperative complication rate of 17.6%, and a mean length of postoperative stay of 5 days. Meanwhile, Cerfolio et al.’s study reported a mean length of operation time of 88 minutes, a postoperative complication rate of 10%, and a mean postoperative stay of 3 days. Furthermore Demir et al.’s findings included a mean operation time of 76 minutes, a mean tumor size of 1.8 cm, a postoperative complication rate of 19%, and a mean length of postoperative stay of 4 days. In the present study, the surgery time of RATS segmentectomy was 89 minutes and blood loss was 50 mL with a mean tumor size of 1.14 cm, all of which were superior to those in the VATS group. Although we began performing RATS later than VATS, the surgeons performing RATS had acquired more than 10 years of advanced experience in performing VATS procedures. In fact, with an increase inexperience, the RATS segmentectomy group showed a sharper concurrent decrease in operation time than that for VATS (21). Because the robot allows the surgeon to dissect around smaller vessels, bronchi and surrounding lymph nodes more ably than VATS (20). It is believed that RATS is more suitable than VATS for complicated procedures such as segmentectomy. In this study, we demonstrated the statistically significant advantage that RATS holds over VATS in terms of length of ICU and hospital stay. These findings may indicate the less traumatic nature of RATS compared with VATS which may be related to the EndoWrist movements of the robotic instruments in the articulation within the hemithorax. Furthermore, our data also showed that a lower narcotic use and an earlier return to routine daily activities in the RATS group compared to the VATS group. Although the robotic system’s ports are larger and more numerous, the above advantages suggest that VATS may irritate the intercostal nerve via instrument torque thus resulting in increased pain. However, this conclusion needs be confirmed with further blinded pain assessment and trauma-related cytokine investigation (22).
Several factors influence the local recurrence in stage IA NSCLC segmentectomy, including tumor size, resection margin, lymph node dissection, and time of follow-up (22,23). In the current study, for stage IA NSCLC (<2 cm), no local recurrence or distant recurrence appeared in the RATS segmentectomy group within the median 38-month (25–60 months) follow-up time, while the local recurrence and distant recurrence rate in the VATS segmentectomy group was 2.5% and 3.75% respectively during a median follow-up time of 85 months (43–99 months) (P=0.144). The 5-year overall and recurrence-free survivals showed no statistical difference when the 2 groups were compared. Cerfolio et al. reported a local recurrence rate of 3.4% during a median 30-month follow-up for robotic segmentectomy with a median tumor size of 1.88 cm (13). Meanwhile, Zhong et al. reported a local recurrence rate of VATS segmentectomy of 5.1% with a mean tumor size of 1.1 cm during a 25-month mean follow-up period, which was comparable to VATS lobectomy for small-sized stage IA lung cancer (19). A retrospective review of 113 patients who underwent open segmentectomy at Mayo Clinic documented a 5% local recurrence rate and a 12.5% distant recurrence rate for patients with T1aN0 lesions during a median follow-up period of 24 months (24). Our results showed a survival advantage favoring RATS, although this did not reach statistical difference.
Our findings point to the similar capability of RATS and VATS in dissecting lymph nodes. This was somewhat unexpected, as our original impression was that the wrist-like action of the robotic instruments would confer superiority in this regard. The available evidence proves that VATS segmentectomy can harvest an equal numbers of lymph nodes compared to open segmentectomy (17,18). Previous reports comparing mediastinal lymph node dissection have also demonstrated the equivalence between RATS and open surgeries (25,26). The lack of difference in total dissected lymph nodes between the 2 groups supports performing a radical lymph node dissection in both the hilum and mediastinum via the VATS approach, given sufficient focus and effort.
Some limitations to this study should also be addressed. The retrospective nature of this research is a limitation which might have caused potential treatment-selection bias. There are two ongoing randomized controlled large-scale trials of sublobar resection for small-sized NSCLC: CALGB 14503 and JCOG 0802 /WJOG 4607L (9,27). Both trials might help to identify the role of segmentectomy in treating small stage IA NSCLC. However, neither trial has included operative approach (e.g., RATS vs. VATS, or VATS vs. open) as a primary research variable. As we know, the major critic to RATS is its additional cost. The initial purpose is to compare the perioperative outcomes and oncologic effect of the two avant de gard surgical techniques, though the difference of cost between two techniques cannot be neglected. Current study lacks a comparison of the costs of the two surgical methods, which is its limitation. Future prospective randomized studies on RATS, VATS, and open approaches are necessary to clarify the role of segmentectomy for small-sized stage IA NSCLC.
Conclusions
The increasingly frequent implementation of computed tomography (CT) in general medical examinations and in the screening of lung cancer will result in the greater detection of early stage lung cancers for which surgical resection will be indicated. Our research demonstrated that RATS is equally safe and provides excellent oncologic effectiveness when compared to VATS in the treatment of stage IA (<2 cm) NSCLC. Not only did RATS result in shorter operation time and postoperative stay, but also conferred less blood loss during the operation. The reduced narcotic use and earlier return to routine daily activities of RATS patients might reflect its less traumatic nature, however, this conclusion needs be confirmed with further blinded pain assessment and trauma-related cytokine investigation.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the following funding: National Nature Science Foundation (Grant No. 81772482); Shanghai Science and Technology Commission Foundation (Grant No. 18ZR1435100); Medical and Industrial Cross Scientific Research Foundation of Shanghai Jiao Tong University (Grant No. YG2017MS78).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Shanghai Chest Hospital. Written informed consent was required from all patients before operation.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-533.
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-533.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-533). The authors have no conflicts of interest to declare.
References
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 2.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. 10.1016/S0003-4975(00)02223-2 [DOI] [PubMed] [Google Scholar]
- 3.Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. 10.1016/j.ejcts.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Weber M, McWilliams A, Canfell K. Prospects for cost-effective lung cancer screening using risk calculators. Transl Cancer Res 2019;8:S141-4. 10.21037/tcr.2018.12.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer 2005;50:227-34. 10.1016/j.lungcan.2005.05.021 [DOI] [PubMed] [Google Scholar]
- 6.Zieliński M. Video-assisted thoracic surgery lobectomy oncologically is equal to the open thoracotomy one. Ann Transl Med 2019;7:S328. 10.21037/atm.2019.09.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park HS, Detterbeck FC, Boffa DJ, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg 2012;93:372-9. 10.1016/j.athoracsur.2011.06.054 [DOI] [PubMed] [Google Scholar]
- 8.Mei J, Guo C, Xia L, et al. Long-term survival outcomes of video-assisted thoracic surgery lobectomy for stage I-II non-small cell lung cancer are more favorable than thoracotomy: a propensity score-matched analysis from a high-volume center in China. Transl Lung Cancer Res 2019;8:155-66. 10.21037/tlcr.2018.12.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. 10.1093/jjco/hyp156 [DOI] [PubMed] [Google Scholar]
- 10.Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. 10.1016/j.athoracsur.2013.07.117 [DOI] [PubMed] [Google Scholar]
- 11.Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. 10.1016/j.athoracsur.2012.04.086 [DOI] [PubMed] [Google Scholar]
- 12.Yang CF, D'Amico TA. Open, thoracoscopic and robotic segmentectomy for lung cancer. Ann Cardiothorac Surg 2014;3:142-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; discussion 1095-6. 10.1016/j.athoracsur.2015.08.092 [DOI] [PubMed] [Google Scholar]
- 14.Pardolesi A, Veronesi G. Robot-assisted lung anatomic segmentectomy: technical aspects. Thorac Surg Clin 2014;24:163-8, vi. 10.1016/j.thorsurg.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 15.Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. 10.1016/j.athoracsur.2012.03.080 [DOI] [PubMed] [Google Scholar]
- 16.Atkins BZ, Harpole DH, Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. 10.1016/j.athoracsur.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 17.Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. 10.1016/j.jtcvs.2009.08.028 [DOI] [PubMed] [Google Scholar]
- 18.Leshnower BG, Miller DL, Fernandez FG, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg 2010;89:1571-6. 10.1016/j.athoracsur.2010.01.061 [DOI] [PubMed] [Google Scholar]
- 19.Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. 10.1016/j.athoracsur.2012.04.047 [DOI] [PubMed] [Google Scholar]
- 20.Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demir A, Ayalp K, Ozkan B, et al. Robotic and video-assisted thoracic surgery lung segmentectomy for malignant and benign lesions. Interact Cardiovasc Thorac Surg 2015;20:304-9. 10.1093/icvts/ivu399 [DOI] [PubMed] [Google Scholar]
- 22.Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 527-8. 10.1016/j.ejcts.2006.12.018 [DOI] [PubMed] [Google Scholar]
- 23.Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-7. 10.1016/j.athoracsur.2010.03.040 [DOI] [PubMed] [Google Scholar]
- 24.Donahue JM, Morse CR, Wigle DA, et al. Oncologic efficacy of anatomic segmentectomy in stage IA lung cancer patients with T1a tumors. Ann Thorac Surg 2012;93:381-7; discussion 387-8. 10.1016/j.athoracsur.2011.10.079 [DOI] [PubMed] [Google Scholar]
- 25.Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. 10.1016/j.jtcvs.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 26.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. 10.1016/j.jtcvs.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 27.Ohtaki Y, Shimizu K. Anatomical thoracoscopic segmentectomy for lung cancer. Gen Thorac Cardiovasc Surg 2014;62:586-93. 10.1007/s11748-014-0409-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as