Abstract
Background
The prognosis of patients with recurrent small cell lung cancer (SCLC) remains poor and treatment options are limited. We performed a multi-institution retrospective cohort study to evaluate the outcome of thoracic reirradiation, identify prognostic factors and assess treatment-related toxicity.
Methods
Data of 33 patients re-irradiated for recurrent SCLC at 4 international university hospitals, were analysed. Overall survival (OS) acute and late toxicities were evaluated and prognostic factors for reirradiation were identified.
Results
Reirradiation (Re-RT) was performed at a median interval time of 24 months after the first thoracic radiotherapy series. Median survival after reirradiation was 7 months (range, 1–54 months). The Re-RT dose in EQD2 ranged from 20 to 87.50 Gy with a median of 32.50 Gy. The 1- and 2-year OS were 33% and 17%, respectively. Patients with a good performance status (KPS >70%), absence of extrathoracic disease, reirradiation dose (EQD2) of >40 Gy and a cumulative dose of first plus second series of radiotherapy (EQD2) >90 Gy were associated with improved OS. Acute pulmonary Grade 1–2 toxicity from re-irradiation was recorded in 11 patients (33%) and grade 3 acute toxicity was encountered 1 patient (3%).
Conclusions
Reirradiation for locoregionally recurrent SCLC is safe and shows promising outcomes. Patients reirradiated with doses >40 Gy experienced more favourable survival rates. In contrast, patients with a poor performance status or extrathoracic disease have a poor prognosis and Re-RT should be considered only for symptom control in this group.
Keywords: Small cell lung cancer (SCLC), recurrence, reirradiation, outcome, prognostic factor
Introduction
Small cell lung cancer (SCLC) represents 10% to 25% of all lung cancers and is considered to be a highly aggressive malignancy with early local recurrences and distant metastases (1-3). In limited stage SCLC, a multimodal treatment including chemoradiotherapy followed by prophylactic cranial irradiation is the standard of care and shows high rates of complete and partial remissions (4,5).
However, loco-regional recurrence and distant failure are often seen during follow-up after primary treatment. Retreatment options such as cytotoxic or targeted agents and surgery are limited or frequently result in less favourable outcomes (2,6,7). European guidelines recommend re-administering of the first-line chemotherapy regimen (platinum/etoposide combinations) for relapse >6 months after completion of initial therapy (5). For relapse ≤6 months after initial therapy, sequential treatment with single agents is recommended (5). Despite many clinical trials using cytotoxic or targeted agents or immune checkpoint inhibitors, the prognosis of patients with recurrent SCLC is still poor with overall survival (OS) rates of <40% at 6 months and<10% at 12 months, respectively (8). Reirradiation has the potential to provide sufficient local control, and patients without extrathoracic disease may experience long-term survival. In the past, reirradiation was challenging due to concerns regarding toxicities and the inability to safely deliver sufficient doses during the second course of radiotherapy (9).
As a result of modern radiation techniques and image-guidance, reirradiation for thoracic malignancies has become safer and has shown promising outcomes in patients with recurrent non-small cell lung cancer (NSCLC) (10-12). In contrast, data for recurrent SCLC are scarce (3,11-16). We performed a multi-institution retrospective cohort study to evaluate the outcome of reirradiation, identify prognostic factors and assess treatment-related toxicity in this group of patients.
Methods
Patients
Data of 33 patients with a loco-regional recurrence of SCLC, who underwent reirradiation at 4 university hospitals in Germany, Japan, Turkey and USA between 2008 and 2015, were collected. This study received approval by each local Ethical Committee and was based on a retrospective analysis of patient records. Data regarding patient characteristics at initial irradiation and reirradiation, outcomes and treatment-related toxicity were evaluated.
Initial diagnosis and treatment
All patients were diagnosed with a histologically confirmed SCLC between 2004 and 2013. Initial stage was considered very limited disease in 14 patients, limited disease in 17 patients and extensive disease in 2 patients according to the classification of the Veterans Administration Lung Study (17). Initial treatment consisted of concurrently chemoradiotherapy (platinum-based chemotherapy (cisplatin/carboplatin) with etoposide) in 91% of all patients. Two patients with extensive stage disease received palliative radiation for superior vena cava syndrome with sequential chemotherapy (carboplatin and etoposide). Thoracic radiotherapy was given twice-daily with a single dose of 1.5 Gy according to Turrisi et al. (18) in 13 (39%) patients, normofractionated with an EQD2 ≥60 Gy in 7 (21%) patients (61%) or in palliative intention with a median EQD2 of 54 Gy (range, 13–58 Gy) in 13 (39%) patients (Table 1). Prophylactic cranial irradiation after definitive chemoradiotherapy was applied in 76% of the patients.
Table 1. patient characteristics at initial irradiation.
| Variables | Number of patients | Proportion (%) |
|---|---|---|
| Age, years | ||
| ≤65 | 22 | 67 |
| >65 | 11 | 33 |
| Gender | ||
| Female | 21 | 64 |
| Male | 12 | 36 |
| T stage | ||
| 1–2 | 10 | 30 |
| 3–4 | 13 | 39 |
| N stage | ||
| 0–1 | 5 | 15 |
| 2–3 | 20 | 61 |
| Stage | ||
| Very limited disease | 14 | 42 |
| Limited disease | 17 | 52 |
| Extensive disease | 2 | 6 |
| Concurrent chemotherapy | ||
| Yes | 30 | 91 |
| No | 3 | 9 |
| Radiation dose (EQD2), Gy | ||
| <60 | 26 | 79 |
| ≥60 | 7 | 21 |
| Prophylactic cranial irradiation | ||
| Yes | 25 | 76 |
| No | 7 | 21 |
Diagnosis and treatment at recurrence
The diagnosis of a thoracic recurrence was made by experienced radiologists based on computed tomography (CT) or PET-CT imaging, with or without pathologic confirmation.
Reirradiation was applied to the availability of each center with three-dimensional conformal radiotherapy (3D-CRT) or intensity modulated radiotherapy (IMRT). Different reirradiation doses and fractionations were employed in relation to the tumor volume, and the proximity of organs at risk (OARs) and previous exposure. In order to compare the administered radiation dose of all patients the equivalent dose in 2 Gy fractions (EQD2) using α/β ratio of 10 was calculated for all radiation courses (initial- and re-irradiation, cumulative dose). Reirradiation doses ranged from 20 to 87.50 Gy (median: 32.50 Gy). Concurrent chemotherapy was given in 3 patients (9%) using topotecan. Further systemic treatment after the end of reirradiation was given in 7 patients (21%).
Evaluation of prognostic factors
A total of nine potential prognostic factors were investigated including gender, age at reirradiation (≤65 versus >65 years), time interval between end of first and second radiotherapy series (≤6 versus >6 months), Karnofsky performance score (50–70% versus 80–100%, median 80%), extrathoracic disease (yes versus no), radiation dose (EQD2) at reirradiation (Re-RT) (20–40 versus >40 Gy), concurrent chemotherapy (yes versus no), cumulative dose of first radiotherapy plus Re-RT (EQD2) (≤90 versus >90 Gy, median: 90 Gy) and further systemic treatment (yes versus no) (Table 2).
Table 2. Patient characteristics at reirradiation and survival analysis.
| Variables | Number of patients [%] | Survival at 6 months (%) |
Survival at 12 months (%) |
P value (univariate analysis) | P value (multivariate analysis) |
|---|---|---|---|---|---|
| Age at Re-RT, years | |||||
| ≤65 | 18 [55] | 50 | 25 | ||
| >65 | 15 [45] | 58 | 43 | 0.497 | |
| Gender | |||||
| Female | 21 [64] | 67 | 48 | ||
| Male | 12 [36] | 45 | 25 | 0.2 | |
| Time interval between end of first RT and second RT, months | |||||
| ≤6 | 4 [12] | 50 | 0 | ||
| >6 | 29 [88] | 54 | 38 | 0.104 | |
| KPS at Re-RT | |||||
| 50–70% | 11 [33] | 21 | 21 | ||
| 80–100% | 19 [58] | 68 | 47 | 0.016 | 0.091 |
| Extrathoracic disease at Re-RT | |||||
| Yes | 6 [18] | 0 | 0 | ||
| No | 27 [82] | 66 | 41 | 0.001 | 0.003 |
| Radiation dose (EQD2) of Re-RT, Gy | |||||
| 20–40 | 24 [73] | 40 | 25 | ||
| >40 | 9 [27] | 89 | 56 | 0.019 | 0.287 |
| Concurrent chemotherapy | |||||
| Yes | 4 [12] | 100 | 100 | ||
| No | 29 [88] | 45 | 24 | 0.085 | |
| Cumulative dose of first RT plus Re-RT (EQD2), Gy | |||||
| ≤90 | 17 [52] | 41 | 23 | ||
| >90 | 16 [48] | 67 | 45 | 0.019 | 0.729 |
| Chemotherapy after Re-RT | |||||
| Yes | 7 [21] | 100 | 43 | ||
| No | 26 [79] | 40 | 27 | 0.187 | |
Evaluation of outcomes and toxicity
Follow up was conducted until death of the patient or a median follow up time of 20 months. Each patient was examined for hematologic and non-hematologic toxicity graded according to Common Terminology Criteria for Adverse Events (CTCAE version 4.0).
Statistical analysis
Standard descriptive statistics (mean standard deviation and cross tabulation analysis) were used to describe the evaluated data. For the analyses of potential associations with OS, the Kaplan-Meier method, and the log-rank test were used P values <0.05 were considered significant. OS was calculated from the end of Re-RT. Prognostic factors found to be significant in univariate analyses were additionally evaluated in a multivariate analysis using the Cox proportional hazards model.
Results
The median survival after reirradiation was 7 months (range, 1–54 months). The 1- and 2-year survival rates for the entire patient cohort were 33% and 17%, respectively. Patient characteristics at initial irradiation are shown in Table 1. The patient characteristics at reirradiation and the results of the analyses of survival are summarized in Table 2.
In univariate analysis, OS was positively associated with a Karnofsky performance score of ≥80% (P=0.016, Figure 1), absence of extrathoracic disease (P<0.001, Figure 2), radiation dose (EQD2) >40 Gy (P=0.019, Figure 3) and cumulative EQD2 of >90 Gy (P=0.019, Figure 4). On univariate analysis, we found a trend (P<0.10) towards improved survival for administering concurrent chemotherapy (P=0.085), a longer (>6 months) interval between first irradiation and Re-RT (P=0.10).
Figure 1.
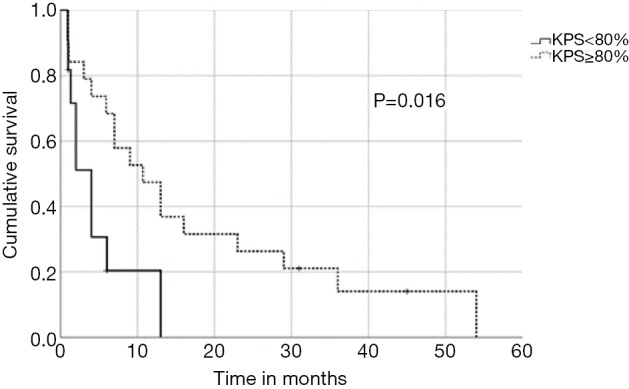
Kaplan-Meier curves according to Karnofsky performance status.
Figure 2.
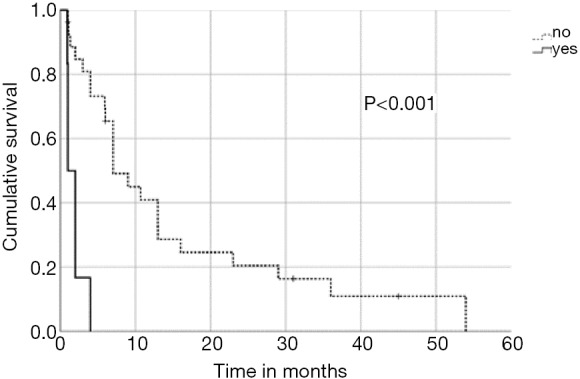
Kaplan-Meier curves according to extrathoracic disease.
Figure 3.
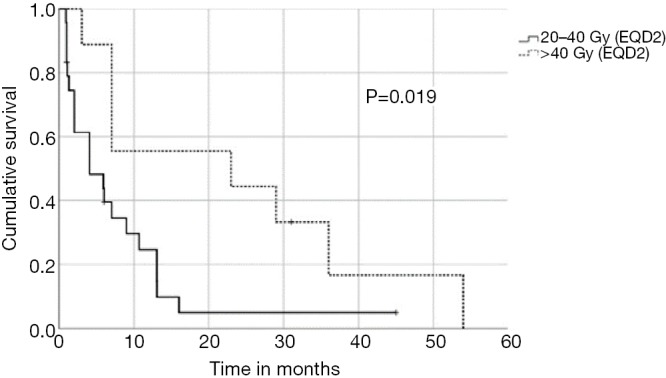
Kaplan-Meier curves according to the reirradiation dose (EQD2).
Figure 4.
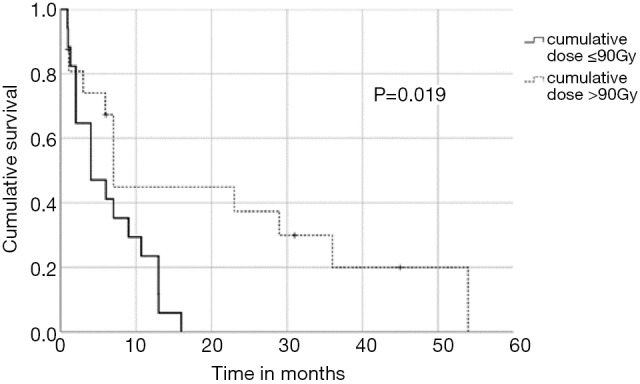
Kaplan-Meier curves according to cumulative radiation dose (EQD2).
On multivariate analysis, absence of extrathoracic disease achieved significance (P=0.003, HR: 6.568, 95%CI: 1.911–22.58). The results of multivariate analysis of OS are summarized in Table 2.
Acute esophageal toxicity grade 1–2 from reirradiation was found in 5 patients (15%); no grade 3 or 4 acute toxicity was observed. Late esophageal toxicity was not found at all centers. Acute pulmonary grade 1–2 toxicity from reirradiation was reported in 11 patients (33%), grade 3 toxicity in 1 patient (3%). Late pulmonary grade 1–2 toxicity from reirradiation was reported in 4 (12%) patients and no grade 3 or 4 late toxicity was observed. Acute hematological grade 2 toxicity occurred in 2 patients (6%). Late hematological toxicity wasn’t found at all centers.
Discussion
Despite an increasing incidence of lung cancer worldwide during the last 30 years, the treatment of SCLC and its high mortality rates of more than 75% have remained almost unchanged the last 30 years (4-8,19-21). The prognosis of patients with recurrent SCLC remains dismal with 6-month and 1-year OS rates of <40% and <10%, respectively, with established second-line treatment with topotecan (8). Moreover, several cytotoxic or targeted agents have been investigated also providing suboptimal outcomes (6).
In NSCLC, the introduction of immune checkpoint inhibition has been a great success and changed the prognosis of patients with advanced stage dramatically (22,23). Based on the result of the CheckMate 032 trial, PD-1 inhibition appears effective and well tolerated for recurrent SCLC. However, the 1-year OS rate of these patients was reported to be <30% (24), suggesting a modest effect of these therapies as salvage therapy for progressive disease.
After definitive chemoradiotherapy, recurrences often occur at the site of the original primary tumor or the initially affected lymph nodes (25). Therefore, reirradiation as a loco-regional treatment offers the chance of disease control as well as symptomatic relief in these patients.
In the treatment of NSCLC, reirradiation is a frequently utilized treatment option for palliative treatment and in patients with limited locoregionally recurrent disease. Studies examine Re-RT in NSCLC suggest that its utilization can result in improved OS with the chance of even long-term survival (9-15). Previous evidence regarding a role of reirradiation in recurrent SCLC is still limited, (3,11-16). Therefore, we performed a multi-institutional study to evaluate the outcome of reirradiation for recurrent SCLC, identify prognostic factors and assess treatment-related toxicity.
Our study revealed a median overall survival after reirradiation of 7 months (range, 1–54 months). For patients with a good performance status and a re-irradiation dose (EQD2) of >40 Gy resulted in a favourable 1- and 2-year overall survival rates of 89% and 56%, respectively. These results appear consistent with the reported outcomes of reirradiation in previous studies of patients with localized NSCLC (9,11,26,27). On the other hand, in the present study those patients with extrathoracic disease had an OS of only <6 months and extrathoracic disease was found to be an independent prognostic factor for patients with recurrent SCLC. As a result, reirradiation should be considered only as palliative treatment in order to provide symptomatic relief, and short fractionation schedules may be most appropriate.
However, many cancer centers are quite hesitant to administer reirradiation for recurrent thoracic malignancies. This could be explained by the potential damage to organs at risk. Potential side effects of reirradiation may include radiation-induced pneumonitis, lung fibrosis, pericarditis, myelopathy, esophagitis and fistula. In our study, we found grade 3 acute pulmonary toxicity only in 1 patient (3%). An earlier study of Ren et al. found that the mean lung dose (MLD) of the initial RT plan, V5 of the composite plans and overlap-V5/re-V5 were independent predictors for grade ≥3 radiation-induced pneumonitis and should be considered during planning of reirradiation (28).
Despite the relevance of the reirradiation dose, recovery of organs at risk is of importance for reirradiation. However, knowledge about recovery and tolerance of organs at risk such as lung and spinal cord for thoracic reirradiation is limited. Future studies are required to establish dose constraints in reirradiation setting to avoid high acute and late toxicity.
In order to identify patients benefiting from reirradiation, prognostic factors are essential and guide physicians for treatment decision-making. We demonstrated that a Karnofsky performance score of ≥80%, absence of extrathoracic disease, administered radiation dose (EQD2) >40 Gy at reirradiation and a cumulative radiation dose (EQD2) of >90 Gy were associated with improved OS. Based on our findings, it appears one may reasonably consider Re-RT with doses of >40 Gy in patients with good performance status. In contrast, reirradiation in patients with poor performance status or with extrathoracic disease should be limited to carefully selected symptomatic patients. The main limitation of this multicenter study is the retrospective design, which may have uncontrolled biases, and a relatively small sample size. Therefore, the recommendations must be considered with caution. However, the study represents the largest patient cohort reirradiated for recurrent SCLC.
Conclusions
Reirradiation for locoregionally recurrent SCLC appears safe and feasible if administered with modern radiation delivery techniques (3D-RT, IMRT). Patients reirradiated with doses >40Gy experienced favourable survival rates. In patients with poor performance status and extrathoracic disease, Re-RT may be considered only for selected symptomatic patients.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by institutional ethics committee of the University of Luebeck (No. 15-352A) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.01.19). HH reports personal fees from Astrazeneca, outside the submitted work; Other authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Pietanza MC, Byers LA, Minna JD, el al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244-55. 10.1158/1078-0432.CCR-14-2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Käsmann L, Janssen S, Schild SE, et al. Karnosky Performance Score and Radiation Dose Predict Survival of Patients Re-irradiated for a Locoregional Recurrence of Small Cell Lung Cancer. Anticancer Res 2016;36:803-5. [PubMed] [Google Scholar]
- 4.Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology endorsement of the American College of Chest Physicians guideline. J Clin Oncol 2015;33:4106-11. 10.1200/JCO.2015.63.7918 [DOI] [PubMed] [Google Scholar]
- 5.Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi99-vi105. 10.1093/annonc/mdt178 [DOI] [PubMed] [Google Scholar]
- 6.Gong J, Salgia R. Managing patients with relapsed small-cell lung cancer. J Oncol Pract 2018;14:359-66. 10.1200/JOP.18.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardizzoni A. Topotecan in the treatment of recurrent small cell lung cancer: an update. Oncologist 2004;9:4-13. 10.1634/theoncologist.9-90006-4 [DOI] [PubMed] [Google Scholar]
- 8.Horita N, Yamamoto M, Sato T, et al. Topotecan for Relapsed Small-cell Lung Cancer: Systematic Review and Meta-Analysis of 1347 Patients. Sci Rep 2015;5:15437. 10.1038/srep15437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyfhuis MA, Rice S, Remick J, et al. Reirradiation for locoregionally recurrent non-small cell lung cancer. J Thorac Dis 2018;10:S2522. 10.21037/jtd.2017.12.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 2014;90:819-27. 10.1016/j.ijrobp.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 11.Kruser TJ, McCabe BP, Mehta MP, et al. Reirradiation for Locoregionally Recurrent Lung Cancer Outcomes in Small Cell and Non-Small Cell Lung Carcinoma. Am J Clin Oncol 2014;37:70. 10.1097/COC.0b013e31826b9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlampp I, Rieber J, Adeberg S, et al. Re-irradiation in locally recurrent lung cancer patients. Strahlenther Onkol. 2019;195:725-33. 10.1007/s00066-019-01457-2 [DOI] [PubMed] [Google Scholar]
- 13.Griffioen GHMJ, Dahele M, de Haan PF, et al. High-dose, conventionally fractionated thoracic reirradiation for lung tumors. Lung Cancer 2014;83:356-62. 10.1016/j.lungcan.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 14.Sumita K, Harada H, Asakura H, et al. Re-irradiation for locoregionally recurrent tumors of the thorax: a single-institution, retrospective study. Radiat Oncol 2016;11:104. 10.1186/s13014-016-0673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aktan M, Kanyilmaz G, Koc M, et al. Thoracic re-irradiation for locally recurrent lung cancer. Asian Pac J Cancer Prev 2016;17:5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu KL, Jiang GL, Qian H, et al. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I-II clinical trial. Int J Radiat Oncol Biol Phys 2003;57:1345-50. 10.1016/S0360-3016(03)00768-5 [DOI] [PubMed] [Google Scholar]
- 17.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer 2002;37:271-6. 10.1016/S0169-5002(02)00072-7 [DOI] [PubMed] [Google Scholar]
- 18.Turrisi AT, Kim K, Blum R, et al. Twice-Daily Compared with Once-Daily Thoracic Radiotherapy in Limited Small-Cell Lung Cancer Treated Concurrently with Cisplatin and Etoposide. N Engl J Med 1999;340:265-71. 10.1056/NEJM199901283400403 [DOI] [PubMed] [Google Scholar]
- 19.Kelly K. Treatment of refractory and relapsed small cell lung cancer. Available online: https://www.uptodate.com/contents/treatment-of-refractory-and-relapsed-small-cell-lung-cancer (date: 02/02/2020).
- 20.Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 2017;7:1339. 10.1038/s41598-017-01571-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Didkowska J, Wojciechowska U, Mańczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 2016;4:150. 10.21037/atm.2016.03.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui H, Ma N, Wang Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res 2018;2018:6984948. 10.1155/2018/6984948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 24.Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol 2019;14:237-44. 10.1016/j.jtho.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bütof R, Gumina C, Valentini C, et al. Sites of recurrent disease and prognostic factors in SCLC patients treated with radiochemotherapy. Clin Transl Radiat Oncol 2017;7:36-42. 10.1016/j.ctro.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto Y, Murakami M, Yoden E, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys 2002;52:390-6. 10.1016/S0360-3016(01)02644-X [DOI] [PubMed] [Google Scholar]
- 27.Chao H-H, Berman AT, Simone CB, II, et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:281-92. 10.1016/j.jtho.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 28.Ren C, Ji T, Liu T, et al. The risk and predictors for severe radiation pneumonitis in lung cancer patients treated with thoracic reirradiation. Radiat Oncol 2018;13:69. 10.1186/s13014-018-1016-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


