Abstract
Comparative cytogenetic analyses are being increasingly used to collect information on species evolution, for example, diversification of closely related lineages and identification of morphologically indistinguishable species or lineages. Here, we have described the karyotype of the fungus-farming ant Mycetomoellerius iheringi Emery, 1888 and investigated its evolutionary relationships on the basis of molecular and cytogenetic data. The M. iheringi karyotype consists of 2n = 20 chromosomes (2K = 18M + 2SM). We also demonstrated that this species has the classical insect TTAGG telomere organization. Phylogenetic reconstruction showed that M. iheringi is phylogenetically closer to M. cirratus Mayhé-Nunes & Brandão, 2005 and M. kempfi Fowler, 1982. We compared M. iheringi with other congeneric species such as M. holmgreni Wheeler, 1925 and inferred that M. iheringi probably underwent a major pericentric inversion in one of its largest chromosomes, making it submetacentric. We discussed our results in the light of the phylogenetic relationships and chromosomal evolution.
Keywords: chromosomal evolution, FISH, fungus growing, karyomorphometry, TTAGG, Trachymyrmex
Introduction
Fungus-farming ants (Formicidae: Myrmicinae: Attini) are exclusive to the New World and occur mainly in the Neotropical region, with some species found in the Nearctic region (Weber 1966; Rabeling et al. 2007). The most recently diverged species include the well-known leafcutter ants (genera Atta Fabricius, 1804 and Acromyrmex Mayr, 1865) as well as the genera Xerolitor Sosa-Calvo et al., 2018, Sericomyrmex Mayr, 1865 and Trachymyrmex Forel, 1893. Previous phylogenetic analyses have shown that the genus Trachymyrmex is paraphyletic (e.g., Schultz and Brady 2008; Sosa-Calvo et al. 2018; Micolino et al. 2019a). However, this taxonomic complication was recently resolved by multilocus phylogenetic analyses with a comprehensive number of species (Solomon et al. 2019). Thus, a new systematic arrangement of three clades was proposed as follows: MycetomoelleriusSolomon et al. 2019 (former Iheringi group), Paratrachymyrmex Solomon et al., 2019 (former Intermedius group), and Trachymyrmex (based on the type species Trachymyrmex septentrionalis McCook, 1881). Nevertheless, Trachymyrmexsensu stricto, largely containing North American species, is still most prominently studied (e.g., Rabeling et al. 2007; Seal et al. 2015; Sánchez-Peña et al. 2017).
Cytogenetics encompasses the study of chromosomes that may have direct implications on species evolution, such as the identification of cryptic species and diversification of closely related lineages (White 1978; King 1993). In general, ants exhibit one of the largest chromosomal variability among organisms (reviewed by Lorite and Palomeque 2010), leading to the hypothesis that chromosomal rearrangements, i.e., Robertsonian fissions and fusions (known major rearrangements that can change the chromosomal number within lineages), actively contributed to the diversification of ants (Imai et al. 1988, 2001; Cardoso et al. 2018a). Despite the large number of species in the three genera formerly included into “Trachymyrmex” (about 60 species, see above), there is limited cytogenetic information on this ant group. To date, only seven species have been karyotyped, three of which have not been identified to the species level (see Table 1). On the basis of the available data, the described chromosomal numbers appear to be stable within the three genera, ranging from 2n = 12 to 2n = 22 and predominantly comprising metacentric chromosomes (reviewed by Cardoso et al. 2018a).
Table 1.
Former “Trachymyrmex” species with their described karyotypes. 2n: diploid chromosome number; (n): haploid chromosome number; 2K: karyotype formula; Locality: sampling site; M: metacentric chromosomes; SM: submetacentric chromosomes.
| Species | 2n (n) | 2K | Locality | References |
|---|---|---|---|---|
| Mycetomoellerius fuscus* | 18 (9) | 16M + 2SM | Minas Gerais State, Brazil | Barros et al. (2013a) |
| Mycetomoellerius holmgreni | 20 (10) | 20M | Minas Gerais State, Brazil | Barros et al. (2018) |
| Mycetomoellerius iheringi | 20 (10) | 18M + 2SM | Santa Catarina State, Brazil | Present study |
| Mycetomoellerius relictus | 20 (10) | 20M | Minas Gerais State, Brazil | Barros et al. (2013b) |
| Trachymyrmex septentrionalis | 20 (10) | 20M | Barro Colorado Island, Panama | Murakami et al. (1998) |
| “Trachymyrmex” sp. 1 | 12 (6) | 12M | Barro Colorado Island, Panama | Murakami et al. (1998) |
| “Trachymyrmex” sp. 2 | 18 (9) | 18M | Barro Colorado Island, Panama | Murakami et al. (1998) |
| “Trachymyrmex” sp. 3 | 22 (11) | 18M + 4SM | Minas Gerais State, Brazil | Barros et al. (2013b) |
* current junior synonym of M. urichii.
Mycetomoellerius iheringi Emery, 1888, the type species of the genus, is a species endemic to South America, and it occurs mainly in the southern regions. The exclusive characteristic of M. iheringi is the finely striated discal area of the mandibles, which sets it apart from the congeneric species Mycetomoellerius kempfi Fowler, 1982 (Mayhé-Nunes and Brandão 2005). A feature of M. iheringi biology that facilitates field identification is the subterranean nest in the sand with a slim opening (Mayhé-Nunes and Brandão 2005). Some groups have been identified by morphological similarities within the former “Trachymyrmex”, including the Iheringi group that also includes Mycetomoellerius holmgreni Wheeler, 1925 whose karyotype has been already described (Mayhé-Nunes and Brandão 2005; Barros et al. 2018). This fact allows cytogenetic comparisons with M. iheringi. However, the phylogenetic position of M. iheringi has not yet been described; only the relationship between its fungal cultivars has been reported (see Solomon et al. 2019).
Here, we have described the M. iheringi karyotype on the basis of karyomorphometric analysis and fluorescence in situ hybridization (FISH) with a telomeric probe. In addition, we identified the phylogenetic position of M. iheringi and examined its relationship with other species of the genus. We have discussed our results in the light of chromosomal evolution among fungus-farming ants.
Material and methods
Colony sampling
Colonies of M. iheringi were collected from the Restinga environment of the Brazilian Atlantic coast at Joaquina Beach, Florianópolis, Santa Catarina State, Brazil (27°37'44"S; 48°26'52"W). A total of five distantly spaced colonies were sampled. Such colonies were maintained in vivo at the Laboratório de Genética Evolutiva e de Populações, Universidade Federal de Ouro Preto, Brazil, according to the protocol established by Cardoso et al. (2011).
Chromosome preparation and FISH mapping
Metaphase chromosomes from the brain ganglia of pre-pupal larvae were obtained using the method of Imai et al. (1988). The ganglia were dissected under a stereomicroscope and incubated in hypotonic solution containing 1% sodium citrate and 0.005% colchicine for 60 min, and consecutively dissociated and fixed on stereoscopic microscope slides in acetic acid: ethanol: distilled water (3:3:4) and acetic acid: ethanol (1:1). Subsequently, the metaphase chromosomes were examined under a phase-contrast microscope and stained with 4% Giemsa stain dissolved in Sorensen’s buffer, pH 6.8, to determine the chromosome number and morphology. We classified the chromosomes according to the nomenclature proposed by Levan et al. (1964), which is based on the ratio of the chromosomal arms (r), given by centromere position. The chromosomes were classified into metacentric (r = 1.0–1.7), submetacentric (r = 1.7–3.0), subtelocentric (r = 3.0–7.0), and acrocentric (r > 7.0) categories, as modified by Crozier (1970). The metaphase chromosomes were measured using IMAGE-PRO PLUS software (Media Cybernetics, LP, USA), and the values were calibrated by the scale bar and transferred to EXCEL (Microsoft, Redmond, WA, USA). In addition, the degree of variation and karyotype measurement were validated using statistical tests, according to Cristiano et al. (2017).
FISH experiments were performed as previously described by Kubat et al. (2008), with detailed modifications for ants by Micolino et al. (2019a). For the hybridizations, we used the TTAGG(6) telomeric motif, which has fine conservation in most insects and the advantage of being able to detect chromosomal rearrangements such as telomere-related inversions and fusions. The TTAGG(6) probe was directly labeled with Cy3 at the 5' terminal during synthesis (Sigma, St. Louis, MO, USA). The summarized technique involves several saline washes, alcohol dehydration, and formamide denaturation, until hybridization with the probe. For visualization, the metaphase chromosomes were stained with 4',6-diamidino-2-phenylindole (DAPI Fluoroshield, Sigma-Aldrich) in an antifade solution. The metaphase chromosomes were analyzed under an OLYMPUS BX53 epifluorescence microscope with OLYMPUS CELLSENS IMAGING software (Olympus American, Inc., Center Valley, PA, USA), using WU (330–385 nm) and WG (510–550 nm) filters for DAPI and rhodamine, respectively. About 10–20 metaphases were analyzed in both cytogenetic analyses, and the images were edited with ADOBE PHOTOSHOP CC software.
DNA extraction, sequencing, and phylogenetic analysis
We extracted the DNA from M. iheringi ant workers, according to the standard CTAB/chloroform technique (Sambrook and Russell 2001). We sequenced the fragments of four nuclear genes, elongation factor 1-alpha-F1 (EF1α-F1), elongation factor 1-alpha-F2 (EF1α-F2), wingless (Wg), and long-wavelength rhodopsin (LW Rh), and one mitochondrial gene, cytochrome c oxidase I (COI) (GenBank accession numbers: MT174160–MT174169). The primers used to generate the sequence data are listed in Table 2. Polymerase chain reaction was performed using a final volume of 25 μL, according to the manufacturer’s instructions (Promega, Madison, WI, USA). The amplification conditions and sequencing were based on the methodology outlined in previous studies (see Schultz and Brady 2008, Cardoso et al. 2015a, b, Ward et al. 2015).
Table 2.
Primers used for sequencing four nuclear (EF1α-F1, EF1α-F2, Wg and LW Rh) and one mitochondrial (COI) gene fragments in the fungus-farming ant Mycetomoellerius iheringi.
| Gene region | Primer | Sequence 5' to 3' | Source |
|---|---|---|---|
| EF1α-F1 | 1424F | GCGCCKGCGGCTCTCACCACCGAGG | Brady et al. (2006) |
| 1829R | GGAAGGCCTCGACGCACATMGG | Brady et al. (2006) | |
| EF1α-F2 | 557F | GAACGTGAACGTGGTATYACSAT | Brady et al. (2006) |
| 1118R | TTACCTGAAGGGGAAGACGRAG | Brady et al. (2006) | |
| LW Rh | LR143F | GACAAAGTKCCACCRGARATGCT | Ward and Downie (2005) |
| LR639ER | YTTACCGRTTCCATCCRAACA | Ward and Downie (2005) | |
| Wg | wg578F | TGCACNGTGAARACYTGCTGGATGCG | Ward and Downie (2005) |
| wg1032R | ACYTCGCAGCACCARTGGAA | Abouheif and Wray (2002) | |
| COI | LCO1490 | GGTCAACAAATCATAAAGATATTGG | Folmer et al. (1994) |
| HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA | Folmer et al. (1994) |
The gene fragments were aligned and concatenated using MEGA7 software (Kumar et al. 2016) and incorporated into the dataset of Solomon et al. (2019). The phylogeny was inferred using the maximum likelihood criterion in RAxML (Stamatakis 2014) by using the simultaneous best-tree search and rapid bootstrapping analysis (1000 replicates) with the GTR + G model of evolution. The generated tree and branch labels were visualized using FIGTREE software (Rambaut 2009).
Results
Cytogenetic data
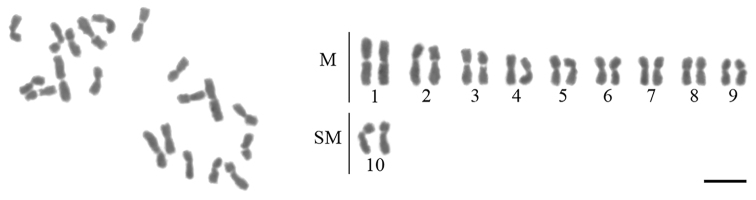
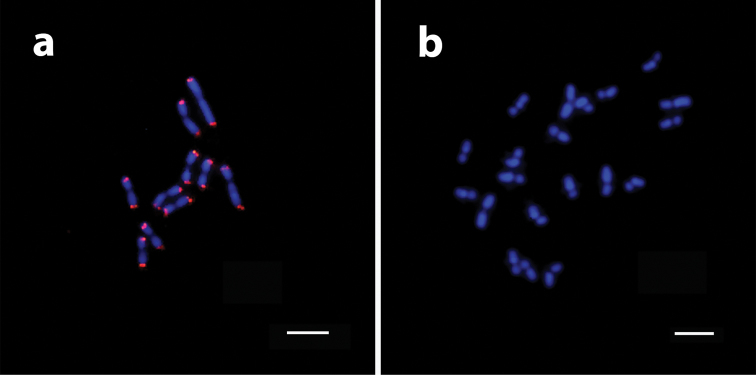
The karyotype of M. iheringi has 2n = 20 chromosomes (Fig. 1). Our karyomorphometric analysis revealed that this karyotype consists of nine metacentric pairs and one submetacentric pair; the karyotype formula is 2K = 18M + 2SM, and the fundamental number is FN = 40. The total average length of all chromosomes (i.e., of the diploid karyotype) was estimated to be 82.51 ± 0.52 μm. The average chromosome length ranged from 5.77 ± 0.91 μm to 3.37 ± 0.4 μm (Table 3). The telomere distribution of the TTAGG(6) motif was displayed at both ends of all M. iheringi chromosomes (Fig. 2a). No signals for interstitial telomeric sites (ITS) were detected using this probe. Moreover, DAPI staining revealed that both arms of all chromosomes were completely labeled, i.e., mostly A-T rich, whereas the centromeric region showed no labeling for this fluorochrome (Fig. 2b).
Figure 1.
Mitotic metaphase of Mycetomoellerius iheringi with 2n = 20 chromosomes and its karyotypic morphology. M: metacentric chromosomes; SM: submetacentric chromosomes. Scale bar: 5 μm.
Table 3.
Karyomorphometric analysis of the chromosomes of Mycetomoellerius iheringi. TL: total length; L: long arm length; S: short arm length; RL: relative length; r: arm ratio (= L/S); ∑: total average length of all chromosomes or Karyotype lenght (KL).
| Chromosome | TL | L | S | RL | r | Classification |
| 1 | 5.77±0.91 | 3.03±0.48 | 2.74±0.43 | 6.97±0.34 | 1.1±0.05 | Metacentric |
| 2 | 5.46±0.75 | 2.86±0.46 | 2.6±0.32 | 6.61±0.24 | 1.1±0.08 | Metacentric |
| 3 | 5.09±0.66 | 3.02±0.41 | 2.08±0.27 | 6.17±0.29 | 1.46±0.09 | Metacentric |
| 4 | 4.71±0.53 | 2.67±0.29 | 2.04±0.28 | 5.72±0.34 | 1.32±0.12 | Metacentric |
| 5 | 4.38±0.49 | 2.38±0.29 | 1.99±0.29 | 5.31±0.2 | 1.21±0.18 | Metacentric |
| 6 | 4.2±0.46 | 2.3±0.23 | 1.91±0.27 | 5.1±0.15 | 1.22±0.14 | Metacentric |
| 7 | 4.07±0.46 | 2.24±0.2 | 1.83±0.33 | 4.94±0.16 | 1.26±0.21 | Metacentric |
| 8 | 4.01±0.44 | 2.3±0.26 | 1.72±0.26 | 4.87±0.16 | 1.32±0.19 | Metacentric |
| 9 | 3.89±0.43 | 2.19±0.3 | 1.7±0.18 | 4.72±0.11 | 1.31±0.14 | Metacentric |
| 10 | 3.83±0.45 | 2.16±0.3 | 1.67±0.17 | 4.65±0.06 | 1.3±0.11 | Metacentric |
| 11 | 3.78±0.43 | 2.15±0.28 | 1.63±0.2 | 4.59±0.1 | 1.32±0.15 | Metacentric |
| 12 | 3.73±0.41 | 2.07±0.3 | 1.66±0.15 | 4.53±0.15 | 1.25±0.15 | Metacentric |
| 13 | 3.7±0.39 | 2.03±0.26 | 1.67±0.19 | 4.5±0.14 | 1.22±0.14 | Metacentric |
| 14 | 3.66±0.4 | 2.08±0.24 | 1.58±0.2 | 4.44±0.13 | 1.33±0.14 | Metacentric |
| 15 | 3.58±0.35 | 2.01±0.28 | 1.57±0.13 | 4.35±0.13 | 1.29±0.17 | Metacentric |
| 16 | 3.54±0.38 | 2.01±0.26 | 1.54±0.17 | 4.3±0.12 | 1.32±0.16 | Metacentric |
| 17 | 3.51±0.4 | 2.04±0.19 | 1.47±0.25 | 4.26±0.13 | 1.41±0.16 | Metacentric |
| 18 | 3.37±0.4 | 1.94±0.29 | 1.43±0.12 | 4.09±0.11 | 1.36±0.13 | Metacentric |
| 19 | 4.29±1.1 | 2.74±0.68 | 1.56±0.42 | 5.15±0.72 | 1.77±0.06 | Submetacentric |
| 20 | 3.94±0.59 | 2.51±0.37 | 1.43±0.22 | 4.76±0.25 | 1.76±0.03 | Submetacentric |
| ∑ | 82.51±0.52 |
Figure 2.
DAPI-stained Mycetomoellerius iheringi chromosomal metaphases aFISH mapping of the TTAGG(6) telomeric motif on haploid metaphase b chromosomes uniformly stained with DAPI fluorochrome, except for the centromeric region. Scale bar: 5 μm.
Molecular data
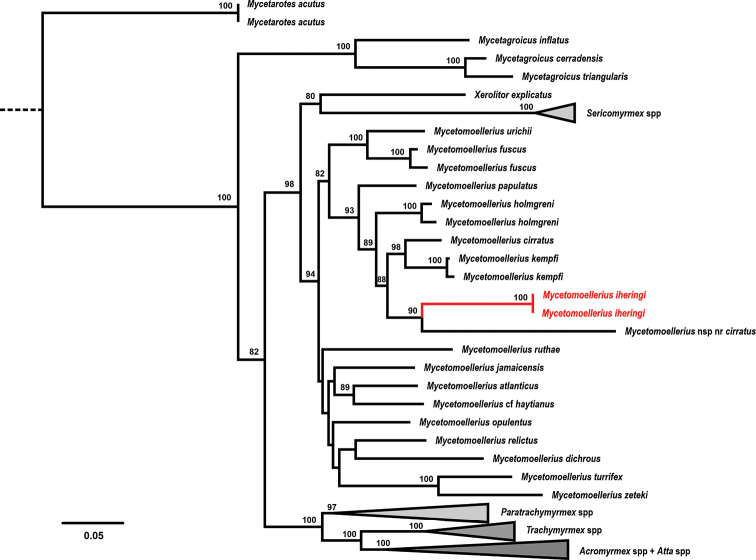
The maximum likelihood phylogeny showed M. iheringi as the sister species of a lineage defined as Mycetomoellerius n. sp. nr cirratus (see Solomon et al. 2019) (bootstrap value, PB = 90). The clade composed of M. cirratus Mayhé-Nunes & Brandão, 2005 + M. kempfi (PB = 98) forms the sister group of M. iheringi + M. n. sp. nr cirratus (PB = 88). The species M. holmgreni previously diverged from the aforementioned clades (PB = 89), and M. papulatus Santschi, 1922 was estimated to be the most basal of the “Iheringi group” (PB = 93) (Fig. 3).
Figure 3.
Maximum-likelihood phylogeny of “higher” fungus-farming ants generated in RAxML. Mycetomoellerius iheringi is indicated in red. Node numbers represent the bootstrapping values after 1000 replications; values < 80 are not shown. Scale bar indicates nucleotide substitutions per site.
Discussion
Here, we have provided the karyotypic description of the fungus-farming ant Mycetomoellerius iheringi, which has 2n = 20 chromosomes; we presented its phylogenetic position in the clade of the “Iheringi group”. Considering the cytogenetic data available from fungus-farming ants, we observed a numerical constancy among the karyotypes of the lineages that diverged most recently (i.e., leafcutter ants of the genera Atta and Acromyrmex), suggesting this karyotypic characteristic is shared by the relatively recent lineages. Trachymyrmex septentrionalis, a sister clade of leafcutter ants, has 2n = 20 metacentric chromosomes, equal to those of two Mycetomoellerius species, M. holmgreni and M. relictus Borgmeier, 1934 (see Table 1). All Atta species karyotyped to date have 2n = 22 chromosomes, and most Acromyrmex species have 2n = 38 (reviewed by Cardoso et al. 2018a). In other Hymenoptera species, such as stingless bees of the tribe Meliponini Lepeletier, 1836, this scenario can also be seen in the genera with a conserved chromosome number (Travenzoli et al. 2019).
In the new taxonomic status, Mycetomoellerius is composed of about 30 described species (Solomon et al. 2019), but only four have known karyotypes and, interestingly, a prevalence of metacentric chromosomes (see Table 1). The species M. iheringi and M. holmgreni are closely related morphologically (Mayhé-Nunes and Brandão 2005), and, as we have shown, M. holmgreni diverged previously from M. iheringi. Moreover, both species co-occur in southern Brazilian sand-dune habitats (Cardoso and Schoereder 2014). Importantly, the karyotypes of these two species are similar: they have analogous karyotype measurements and DAPI-staining pattern as well the chromosomal number 2n = 20, differing by only one pair of submetacentric chromosomes (Barros et al. 2018; Cardoso et al. 2018b). A likely, and the most parsimonious, scenario for explaining such cytogenetic differences would involve at least one major chromosomal rearrangement. Therefore, we suggest a pericentric inversion occurred in one of the larger M. iheringi chromosomes, resulting in the current karyotype morphology. Such chromosomal rearrangement could have occurred in any lineage of the clades underlying M. holmgreni; however, such lineages should be karyotyped to verify this hypothesis. The base chromosome number, defined as the haploid number present in the initial lineage of a monophyletic clade, may be directly related to the chromosomal variability within that clade (Guerra 2008). Thus, the assumption of this major inversion is attributable to the fact that M. holmgreni has a karyotype formed by only metacentric chromosomes, which becomes a putative ancestral characteristic of the underlying lineages, such as M. iheringi.
The application of classical and molecular cytogenetic techniques, such as chromosomal banding and FISH mapping, has increasingly contributed to comparative evolutionary studies. Because of new ant cytogenetic data, valuable information is being collected and correlated to their evolution and exceptional chromosomal diversity. For instance, fusion and fission rearrangements have been proposed to play a crucial role in the diversification of the fungus-farming ants of the genus Mycetophylax Emery, 1913 (Cardoso et al. 2014; Micolino et al. 2019b). Indeed, chromosomal changes may be directly related to the speciation process for a range of taxa (Rieseberg 2001; Faria and Navarro 2010). In particular, inversions are abundant in natural populations and can have several evolutionary implications, such as adaptation and divergence of lineages (Ayala and Coluzzi 2005; Wellenreuther and Bernatchez 2018). Inversion polymorphisms may contribute to speciation by reducing recombination and consequently protecting genomic regions from introgression (Hoffmann and Rieseberg 2008). Moreover, a model has predicted that closely related lineages that co-occur in a region could readily differ by one or more inversions because such lineages would persist longer in the face of gene flow than in the absence of these inversions (Noor et al. 2001). Our data support such a model, mainly because the species M. iheringi and M. holmgreni live sympatrically and are phylogenetically close.
The rich karyotypic diversity of ants deserves special attention. Inversion polymorphisms, for example, have been reported in many ant species. For example, intrapopulational polymorphism has been detected in the Iridomyrmex gracilis Lowne, 1865 complex. Such populations with the same chromosome number but distinct karyotype structures have led authors to propose that a pericentric inversion occurred in a metacentric chromosome, making it acrocentric (n = 6M + 1SM + 1A to n = 5M + 1SM + 2A) (Crozier 1968). The chromosome number and morphology of Pachycondyla Smith, 1858 are variable; their karyotypes show a predominance of submetacentric and acrocentric chromosomes, which allows the interpretation that fission and pericentric inversions (where metacentric chromosomes turn acrocentric or vice versa) would be the most frequent chromosomal rearrangements in the evolution of this genus and even contribute to the speciation processes (Mariano et al. 2012). The intraspecific chromosomal variability in social organization (monogyny vs. polygyny) found in the fire ant Solenopsis invicta Buren, 1972 can also be explained by at least one large inversion, which would account for a lack of recombination over more than half of the two heteromorphic “social chromosomes” (Wang et al. 2013).
Another interesting finding was reported in Mycetomoellerius fuscus Emery, 1894 (current junior synonym of M. urichii Forel, 1893, see Micolino et al. 2019a for discussion), a species with a geographic distribution similar to M. iheringi and M. holmgreni and found largely in southern South America (Brandão and Mayhé-Nunes 2007). They are phylogenetically closer than previously expected (Micolino et al. 2019a; Solomon et al. 2019). Mycetomoellerius fuscus has a chromosomal morphology of eight metacentric pairs and a submetacentric pair (2n = 18) (Barros et al. 2013a). As the submetacentric pair is the biggest chromosome of the karyotype, there could have been a Robertsonian fusion rearrangement, followed by a pericentric inversion, making it submetacentric. The other few species of “Trachymyrmex” with the described karyotype (see Table 1) do not allow us to picture a full scenario for the karyoevolution of the genera. Further, unidentified specimens vary relatively widely from 2n = 12 to 2n = 22. The karyotype 2n = 12 presented by Murakami et al. (1998) is quite intriguing, as this unidentified specimen could be a key piece to understanding the chromosomal evolution of the clade to which it belongs. We emphasize that specimens submitted for cytogenetic analysis should be taxonomically identified. The non-identification of a specific sample triggers a series of problems, such as in the comparison with sister groups and eventual karyoevolutionary trajectories.
Our karyomorphometric approach was used primarily to reveal the chromosomal morphology of M. iheringi. Besides, future karyomorphometric comparisons among populations or even closely related lineages may serve as a basis for a possible delimitation of incipient lineages. For example, populations of M. holmgreni distributed on a North/South continuum of its distribution area diverged significantly in the length of their chromosomes, and the results were supported by flow cytometry analyses of the genome size (Cardoso et al. 2018b). Further, those populations were later identified to differ in the proportion of repetitive DNA by using FISH with microsatellite probes (Micolino et al. 2019a) Thus, the authors demonstrated the importance of using a standardized karyomorphometric approach coupled with genome size estimation to identify hidden chromosomal variations (see Cardoso et al. 2018b).
Finally, we used a FISH probe of the highly conserved TTAGG telomeric sequence in most insects (reviewed by Kuznetsova et al. 2020) to test the assumption that the putative inversion rearrangement occurred in M. iheringi and involved the telomere. However, we did not observe any signal for the probe at the interstitial telomeric sites, which would denote inversion involving the telomere. Indeed, the TTAGG sequence also seems to be fairly conserved in ants (Lorite et al. 2002), including fungus-farming ants such as Acromyrmex striatus Roger, 1863 (Pereira et al. 2018), Mycetophylax spp. (Micolino et al. 2019b), and M. holmgreni (Micolino et al. 2019a). In conclusion, we have described another ant species with the TTAGG sequence conserved in its telomeres, and we suggest a significant chromosomal mechanism, a major pericentric inversion, most likely occurred in M. iheringi and could have been involved in its diversification process.
Acknowledgements
We are grateful to many people who made this work possible. We thank all our colleagues at the Lab and Research Group of Genetics and Evolution of Ants (GEF-UFOP) for their help with the data. We are also grateful for the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) – MPC fellowship 309579/2018-0, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná, and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). The sample collection was authorized by the “Instituto Chico Mendes de Conservação da Biodiversidade” – ICMBio (Special permit number 60019).
Citation
Micolino R, Cristiano MP, Cardoso DC (2020) Karyotype and putative chromosomal inversion suggested by integration of cytogenetic and molecular data of the fungus-farming ant Mycetomoellerius iheringi Emery, 1888. Comparative Cytogenetics 14(2): 197–210. https://doi.org/10.3897/CompCytogen.v14i2.49846
References
- Abouheif E, Wray GA. (2002) Evolution of the gene network underlying wing polyphenism in ants. Science 297: 249–252. 10.1126/science.1071468 [DOI] [PubMed] [Google Scholar]
- Ayala FJ, Coluzzi M. (2005) Chromosome speciation: humans, Drosophila, and mosquitoes. Proceedings of the National Academy of Sciences of the United States of America 102: 6535–6542. 10.1073/pnas.0501847102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LAC, Aguiar HJAC, Mariano CSF, Delabie JHC, Pompolo SG. (2013a) Cytogenetic characterization of the ant Trachymyrmex fuscus Emery, 1934 (Formicidae: Myrmicinae: Attini) with the description of a chromosomal polymorphism. Annales de la Société Entomologique de France 49: 367–373. 10.1080/00379271.2013.856201 [DOI] [Google Scholar]
- Barros LAC, Mariano CSF, Pompolo SG. (2013b) Cytogenetic studies of five taxa of the tribe Attini (Formicidae: Myrmicinae). Caryologia 66: 59–64. 10.1080/00087114.2013.780443 [DOI] [Google Scholar]
- Barros LAC, Teixeira GA, Aguiar HJAC, Lopes DM, Pompolo SG. (2018) Cytogenetic studies in Trachymyrmex holmgreni Wheeler, 1925 (Formicidae: Myrmicinae) by conventional and molecular methods. Sociobiology 65: 185–190. 10.13102/sociobiology.v65i2.2214 [DOI] [Google Scholar]
- Brady SG, Schultz TR, Fisher BL, Ward PS. (2006) Evaluating alternative hypotheses for the early evolution and diversification of ants. Proceedings of the National Academy of Sciences of the United States of America 103: 18172–18177. 10.1073/pnas.0605858103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão CRF, Mayhé-Nunes AJ. (2007) A phylogenetic hypothesis for the Trachymyrmex species groups, and the transition from fungus-growing to leaf-cutting in the Attini. Memoirs of the American Entomological Institute 80: 72–88. [Google Scholar]
- Cardoso DC, Cristiano MP, Tavares MG. (2011) Methodological remarks on rearing basal Attini ants in the laboratory for biological and evolutionary studies: overview of the genus Mycetophylax. Insectes Sociaux 58: 427–430. 10.1007/s00040-011-0160-3 [DOI] [Google Scholar]
- Cardoso DC, Santos HG, Cristiano MP. (2018a) The Ant Chromosome database – ACdb: an online resource for ant (Hymenoptera: Formicidae) chromosome researchers. Myrmecological News 27: 87–91. [Google Scholar]
- Cardoso DC, Heinze J, Moura MN, Cristiano MP. (2018b) Chromosomal variation among populations of a fungus-farming ant: implications for karyotype evolution and potential restriction to gene flow. BMC Evolutionary Biology 18: 1–146. 10.1186/s12862-018-1247-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso DC, Pompolo SG, Cristiano MP, Tavares MG. (2014) The role of fusion in ant chromosome evolution: insights from cytogenetic analysis using a molecular phylogenetic approach in the genus Mycetophylax PLoS ONE 9: e87473. 10.1371/journal.pone.0087473 [DOI] [PMC free article] [PubMed]
- Cardoso DC, Schoereder JH. (2014) Biotic and abiotic factors shaping ant (Hymenoptera: Formicidae) assemblages in Brazilian coastal sand dunes: the case of Restinga in Santa Catarina. Florida Entomologist 97: 1443–1450. 10.1653/024.097.0419 [DOI] [Google Scholar]
- Cardoso DC, Cristiano MP, Tavares MG, Schubart CD, Heinze J. (2015a) Phylogeography of the sand dune ant Mycetophylax simplex along the Brazilian Atlantic Forest coast: remarkably low mtDNA diversity and shallow population structure. BMC Evolutionary Biology 15: 1–106. 10.1186/s12862-015-0383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso DC, Cristiano MP, Tavares MG, Schubart CD, Heinze J. (2015b) Erratum to: Phylogeography of the sand dune ant Mycetophylax simplex along the Brazilian Atlantic Forest coast: remarkably low mtDNA diversity and shallow population structure. BMC Evolutionary Biology 15: 1–190. 10.1186/s12862-015-0444-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiano MP, Pereira TTP, Simões LP, Sandoval-Gómez VE, Cardoso DC. (2017) Reassessing the chromosome number and morphology of the turtle ant Cephalotes pusillus (Klug, 1824) using karyomorphometrical analysis and observations of new nesting behavior. Insects 8: 1–114. 10.3390/insects8040114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RH. (1968) Cytotaxonomic studies on some Australian dolichoderine ants (Hymenoptera: Formicidae). Caryologia 21: 241–259. 10.1080/00087114.1968.10796302 [DOI] [Google Scholar]
- Crozier RH. (1970) Karyotypes of twenty-one ant species (Hymenoptera: Formicidae), with reviews of the known ant karyotypes. Canadian Journal of Genetics and Cytology 12: 109–128. 10.1139/g70-018 [DOI] [PubMed] [Google Scholar]
- Faria R, Navarro A. (2010) Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends in Ecology and Evolution 25: 660–669. 10.1016/j.tree.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299. [PubMed] [Google Scholar]
- Guerra M. (2008) Chromosome numbers in plant cytotaxonomy: concepts and implications. Cytogenetic and Genome Research 120: 339–350. 10.1159/000121083 [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Rieseberg LH. (2008) Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation. Annual Review of Ecology, Evolution, and Systematics 39: 21–42. 10.1146/annurev.ecolsys.39.110707.173532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai HT, Taylor RW, Crosland MWJ, Crozier RH. (1988) Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. The Japanese Journal of Genetics 63: 159–185. 10.1266/jjg.63.159 [DOI] [PubMed] [Google Scholar]
- Imai HT, Satta Y, Takahata N. (2001) Integrative study on chromosome evolution of mammals, ants and wasps based on the minimum interaction theory. Journal of Theoretical Biology 210: 475–497. 10.1006/jtbi.2001.2327 [DOI] [PubMed] [Google Scholar]
- King M. (1993) Species evolution: the role of chromosome change. Cambridge University Press.
- Kubat Z, Hobza R, Vyskot B, Kejnovsky E. (2008) Microsatellite accumulation on the Y chromosome in Silene latifolia. Genome 51: 350–356. 10.1139/G08-024 [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K, Medicine E. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova V, Grozeva S, Gokhman V. (2020) Telomere structure in insects: a review. Journal of Zoological Systematics and Evolutionary Research 58: 127–158. 10.1111/jzs.12332 [DOI] [Google Scholar]
- Levan A, Fredga K, Sandberg AA. (1964) Nomeclature for centromeric position on chromosomes. Hereditas 52: 201–220. ttps:// 10.1111/j.1601-5223.1964.tb01953.x [DOI]
- Lorite P, Carrillo JA, Palomeque T. (2002) Conservation of (TTAGG)n telomeric sequences among ants (Hymenoptera, Formicidae). Journal of Heredity 93: 282–285. 10.1093/jhered/93.4.282 [DOI] [PubMed] [Google Scholar]
- Lorite P, Palomeque T. (2010) Karyotype evolution in ants (Hymenoptera: Formicidae), with a review of the known ant chromosome numbers. Myrmecological News 13: 89–102. [Google Scholar]
- Mariano CDSF, Pompolo SDG, Silva JG, Delabie JHC. (2012) Contribution of cytogenetics to the debate on the paraphyly of Pachycondyla spp. (Hymenoptera, Formicidae, Ponerinae). Psyche 973897: 1–9. 10.1155/2012/973897 [DOI] [Google Scholar]
- Mayhé-Nunes AJ, Brandão CRF. (2005) Revisionary studies on the attine ant genus Trachymyrmex Forel. Part 2: the Iheringi group (Hymenoptera: Formicidae). Sociobiology 45: 271–305. [Google Scholar]
- Micolino R, Cristiano MP, Cardoso DC. (2019a) Population-based cytogenetic banding analysis and phylogenetic relationships of the neotropical fungus-farming ant Trachymyrmex holmgreni Wheeler, 1925. Cytogenetic and Genome Research 159: 151–161. 10.1159/000503913 [DOI] [PubMed] [Google Scholar]
- Micolino R, Cristiano MP, Travenzoli NM, Lopes DM, Cardoso DC. (2019b) Chromosomal dynamics in space and time: evolutionary history of Mycetophylax ants across past climatic changes in the Brazilian Atlantic coast. Scientific Reports 9(18800): 1–13. 10.1038/s41598-019-55135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Fujiwara A, Yoshida MC. (1998) Cytogenetics of ten ant species of the tribe Attini (Hymenoptera, Formicidae) in Barro Colorado Island, Panama. Chromosome Science 2: 135–139. [Google Scholar]
- Noor MAF, Grams KL, Bertucci LA, Reiland J. (2001) Chromosomal inversions and the reproductive isolation of species. Proceedings of the National Academy of Sciences of the United States of America 98: 12084–12088. 10.1073/pnas.221274498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira TTP, Reis ACCC, Cardoso DC, Cristiano MP. (2018) Molecular phylogenetic reconstruction and localization of the (TTAGG)n telomeric repeats in the chromosomes of Acromyrmex striatus (Roger, 1863) suggests a lower ancestral karyotype for leafcutter ants (Hymenoptera). Comparative Cytogenetics 12: 13–26. 10.3897/CompCytogen.v12i1.21799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabeling C, Cover SP, Johnson RA, Mueller UG. (2007) A review of the North American species of the fungus-gardening ant genus Trachymyrmex (Hymenoptera: Formicidae). Zootaxa 1664: 1–54. 10.5281/zenodo.180014 [DOI] [Google Scholar]
- Rambaut A. (2009) FigTree v1.4.3. http://tree.bio.ed.ac.uk/software/figtree/ [accessed on 10 January 2020]
- Rieseberg LH. (2001) Chromosomal rearrangements and speciation. Trends in Ecology and Evolution 16: 351–358. 10.1016/S0169-5347(01)02187-5 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York.
- Sánchez-Peña SR, Chacón-Cardosa MC, Canales-del-Castillo R, Ward L, Resendez-Pérez D. (2017) A new species of Trachymyrmex (Hymenoptera, Formicidae) fungus-growing ant from the Sierra Madre Oriental of northeastern Mexico. ZooKeys 706: 73–94. 10.3897/zookeys.706.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz TR, Brady SG. (2008) Major evolutionary transitions in ant agriculture. Proceedings of the National Academy of Sciences of the United States of America 105: 5435–5440. 10.1073/pnas.0711024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal J, Brown L, Ontiveros C, Thiebaud J, Mueller UG. (2015) Gone to Texas: phylogeography of two Trachymyrmex (Hymenoptera: Formicidae) species along the southeastern coastal plain of North America. Biological Journal of the Linnean Society 114: 689–698. 10.1111/bij.12426 [DOI] [Google Scholar]
- Solomon SE, Rabeling C, Sosa-Calvo J, Lopes CT, Rodrigues A, Vasconcelos HL, Bacci M, Mueller UG, Schultz TR. (2019) The molecular phylogenetics of Trachymyrmex Forel ants and their fungal cultivars provide insights into the origin and coevolutionary history of ‘higher-attine’ ant agriculture. Systematic Entomology 44: 939–956. 10.1111/syen.12370 [DOI] [Google Scholar]
- Sosa-Calvo J, Schultz TR, Ješovnik A, Dahan RA, Rabeling C. (2018) Evolution, systematics, and natural history of a new genus of cryptobiotic fungus-growing ants. Systematic Entomology 43: 549–567. 10.1111/syen.12289 [DOI] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travenzoli NM, Lima BA, Cardoso DC, Dergam JA, Fernandes-Salomão TM, Lopes DM. (2019) Cytogenetic analysis and chromosomal mapping of repetitive DNA in Melipona species (Hymenoptera, Meliponini). Cytogenetic and Genome Research 158: 213–224. 10.1159/000501754 [DOI] [PubMed] [Google Scholar]
- Wang J, Wurm Y, Nipitwattanaphon M, Riba-Grognuz O, Huang YC, Shoemaker D, Keller L. (2013) A Y-like social chromosome causes alternative colony organization in fire ants. Nature 493: 664–668. 10.1038/nature11832 [DOI] [PubMed] [Google Scholar]
- Ward PS, Downie D. (2005) The ant subfamily Pseudomyrmecinae (Hymenoptera: Formicidae): phylogeny and evolution of big-eyed arboreal ants. Systematic Entomology 30: 310–335. 10.1111/j.1365-3113.2004.00281.x [DOI] [Google Scholar]
- Ward PS, Brady SG, Fisher BL, Schultz TR. (2015) The evolution of Myrmicine ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Systematic Entomology 40: 61–81. 10.1111/syen.12090 [DOI] [Google Scholar]
- Weber NA. (1966) Fungus-growing ants. Science 153: 587–604. 10.1126/science.153.3736.587 [DOI] [PubMed] [Google Scholar]
- Wellenreuther M, Bernatchez L. (2018) Eco-evolutionary genomics of chromosomal inversions. Trends in Ecology and Evolution 33: 427–440. 10.1016/j.tree.2018.04.002 [DOI] [PubMed] [Google Scholar]
- White MJD. (1978) Modes of speciation. W.H. Freeman.