Abstract
Introduction
Tissue factor (TF) and factor (F) VII, components of the extrinsic pathway of blood coagulation, are essential for hemostatic plug formation in response to injury; less clear are their roles in propagating thrombosis, as observational data in humans with congenital FVII deficiency suggests persistent thrombotic and bleeding risk even at significantly decreased FVII levels. We aimed to define the contribution of FVII to thrombus formation and hemostasis using a non-human primate model.
Methods
We treated baboons with a FVII antisense oligonucleotide (ASO) and measured platelet and fibrin deposition inside and distal to collagen- or TF-coated vascular grafts. We assessed hemostasis by measuring bleeding time (BT) and prothrombin time (PT). Enoxaparin and vehicle treatments served as controls.
Results
FVII–ASO treatment reduced FVII levels by 95% and significantly increased both the PT and BT. Lowering FVII levels did not decrease platelet deposition in collagen- or TF-coated grafts, in thrombi distal to the grafts, or fibrin content of either collagen- and TF-coated grafts. Lowering FVII levels were associated with a modest 25% reduction in platelet deposition at 60 min in the distal thrombus tail of TF-coated grafts only.
Conclusions
FVII inhibition by way of ASO is feasible yet significantly impairs hemostasis while only exhibiting antithrombotic effects when thrombosis is initiated by vessel wall surface-associated TF exposure.
Keywords: Blood platelet, Coagulation factor VII, Hemostasis, Shear flow, Thrombosis
Introduction
Vascular injury exposes flowing blood to subendothelial and extraluminal proteins including tissue factor (TF) and collagen which collectively trigger rapid platelet adhesion and activation of the coagulation system to incite thrombus formation. These processes facilitate the formation of a platelet plug (primary hemostasis) and thrombin generation, ultimately forming a stable fibrin clot (secondary hemostasis).20 TF, a transmembrane glycoprotein found on multiple extravascular cell types, forms a complex with circulating factor (F) VII, a vitamin K-dependent, trypsin-like serine protease produced in the liver and released into the circulation. The TF–FVIIa complex, in association with FVa, forms the extrinsic “X (ten)-ase” complex, catalyzing the production of activated FX (FXa), which then converts prothrombin to thrombin. Thrombin functions as both a catalyst for conversion of soluble fibrinogen to insoluble fibrin as well as an agonist to perpetuate platelet activation.
Within the classical concept of coagulation, TF and FVII comprise the “extrinsic” pathway of thrombin generation, which is essential for normal hemostasis. Congenital FVII deficiency is rarely encountered in humans, and while it most often presents with mucocutaneous bleeding and menorrhagia, the bleeding phenotype correlates poorly with measured FVII activity; thus a “safe” FVII threshold below which bleeding risk becomes clinically significant remains poorly defined.24 While a role for TF–FVII has been shown for the initiation of coagulation and thrombus formation under shear in vitro and in vivo, it remains unclear whether FVII plays a role in propagating thrombus formation.11
In vitro, exogenous thromboplastin reagents are used to activate FVII and the extrinsic pathway to ultimately generate a fibrin clot, forming the basis of the prothrombin time (PT) assay. In our in vivo baboon model of arterial-type thrombosis, thrombus formation can likewise be initiated by coating the surface of a large (4 mm i.d.) vascular graft with lipidated TF (Dade Innovin®) or collagen to incite platelet deposition and fibrin formation.12 Conversely, FVII or TF genetic knockout in animals results in an often lethal bleeding diathesis, highlighting the crucial role of these factors in hemostasis.3,5,23,25 Curiously, many scenarios exist in which the PT or factor VII activity levels correlate poorly with bleeding and thrombotic outcomes,10,19 and pathologic thrombosis can still be encountered in FVII-deficient humans.17,18 We, therefore, sought to determine whether FVII plays a role in thrombus propagation including pathologic venous thromboembolism (VTE) in our experimental thrombus propagation model in baboons.
This study was designed to determine whether lowering FVII levels exhibit antithrombotic effects prior to antihemostatic effects. We designed, created, optimized and used an antisense oligonucleotide (ASO) targeted to FVII to reduce FVII levels in vivo to below 5% of normal. Our results show that lowering FVII levels impaired hemostasis while only reducing platelet deposition in the tail of thrombi formed on TF, and having no effect on platelet deposition or fibrin formation on any part of collagen-coated grafts. These results would suggest that pharmacological reduction of FVII levels exhibits antihemostatic effects prior to antithrombotic effects, and that circulating FVII as low as 5% does not prevent thrombosis with either TF or collagen.
Materials and Methods
FVII Antisense Oligonucleotides
Single-stranded ASOs were designed to specifically target the mRNA transcript of baboon FVII. All oligonucleotides were 16 nucleotides in length, 3-10-3 cEt gapmers modified with a phosphorothioate backbone.30 The sequence of the FVII oligonucleotide (GCTAAACAACCGCCTT) is a perfect match for four species including baboon, cynomolgus monkey, rhesus monkey, and human and prevents transcription of the mRNA to cDNA resulting in a complete shutdown of FVII synthesis. Oligonucleotides were synthesized using a Perkin Elmer-Applied Biosystems 380B automated DNA synthesizer and purified as previously described.1 All ASOs were synthesized at Ionis Pharmaceuticals (Carlsbad, CA) and were formulated in sterile pyrogen-free phosphate-buffered saline (PBS, pH 7–7.4).
Animals
All studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Oregon Health & Science University. Animals were housed in a controlled environment at the Oregon National Primate Research Center (ONPRC). Twenty nonterminal thrombosis experiments were performed using three male baboons (Papio Anubis, 12–13 kg). Experiments were conducted on awake, slightly sedated animals to manage anxiety using low dose (< 2 mg/kg/h) ketamine. Animals were restrained in a chair for the duration of the thrombosis experiment. Each animal had a chronic exteriorized femoral arteriovenous shunt, as described below. All animals were followed up for > 80–110 days to monitor the clearance of ASO via serial measurements of FVII antigen/activity and PT. Hemostasis safety assessment (n = 62) was performed in six animals.
Antisense Oligonucleotide, Low-Molecular-Weight Heparin (LMWH) and Vehicle Treatment
To establish baseline values, each of the three shunted animals had a thrombosis experiment performed twice. Subsequently, each of the three shunted animals received a single intravenous injection of LMWH (enoxaparin, 1 mg/kg); two separate thrombosis experiments were performed in each baboon at 15 min and 120 min following administration of LMWH. Finally, after sufficient time to allow for LMWH clearance, these same animals were administered repeated subcutaneous doses of 50 mg/kg FVII-ASO up to four times per week. The maximum dosing period was 13 days, for a maximum of 7–8 doses and a cumulative dose of 350–400 mg/kg FVII–ASO.
Baboon Shunt Model of Thrombus Formation
A 2.0 cm-long vascular graft segment made of expanded polytetrafluoroethylene (ePTFE, 4.0 mm i.d.; WL Gore & Associates, Flagstaff, AZ) was coated with a solution of either equine type I collagen (1 mg/mL; Chronolog Corporation, Allentown, PA) or TF (Dade® Innovin®; Siemens Healthcare Diagnostics, Germany) as previously described.6,12,28 The collagen- or TF-coated grafts were then interposed between segments of silicon rubber tubing extending the surgically-placed chronic arteriovenous (AV) shunt of the baboon. Collagen- and TF-coated grafts were placed in the externalized shunt with blood allowed to flow through the graft and shunt in each thrombosis experiment. The flow rate through the graft was restricted to 100 mL/min (Transonics Systems flowmeter, Ithaca, NY) by clamping the distal shunt segment, thereby producing an initial mean wall shear rate of 265 s−1. Blood was allowed to flow continuously through the grafts for 60 min, during which time thrombus formation was quantified via gamma camera imaging of accumulated autologous 111Indium-labeled platelets. In addition, platelet accumulation was measured within a 10 cm-long “tail” segment of the arteriovenous shunt immediately distal to the interposed thrombogenic graft. After 60 min, the shunt segment was closed, the graft segment removed, rinsed with saline and stored for subsequent measurement of terminal 125Iodine-labeled fibrin accumulation. 111In-labeling of platelets and 125I-labeling of fibrin has been described previously.4,16
Blood Sample Collection
Prior to the start of experimental thrombosis studies, a blood sample was collected from the midstream of the shunt loop into a syringe prefilled with 3.2% sodium citrate, at a ratio of 9:1 blood:citrate. For follow-up samples, blood was collected by venous puncture of either the celiac or saphenous vein of the animal. All samples were immediately processed to platelet-poor plasma (3 min, 10,000×g) and used to determine the prothrombin time (PT) to assess animal eligibility for the thrombosis experiments. The remainder of each plasma sample was stored at – 80°C for later analysis of FVII protein levels and activity.
Coagulation PT Test
Prothrombin times (PT) were measured using a KC-4 coagulation analyzer (TCoag Ltd, Ireland). Initiation of PT assay was performed by adding Dade® Innovin® (Siemens Healthcare Diagnostics, Germany) according to the manufacturer’s protocol.
FVII Antigen Levels and FVII Activity
Factor VII antigen levels were determined using ELISA (FVII Elisa kit, Aniara, West Chester, OH). Plasma samples were diluted to either 1:10 or 1:20 (v/v) and incubated for 15 min in microwells that had been coated with an anti-FVII rabbit-polyclonal antibody. Plates were then incubated with an anti-(h)-FVII immunoconjugate for 1 h. Wells were washed five times, and the horseradish peroxidase (HRP) substrate was added (5 min). The reaction was stopped using 0.45 M sulfuric acid stop solution, and absorbance was measured at 450 nm. Factor VII activity was measured using a chromogenic assay (Biophen FVII kit, Aniara, West Chester, OH) according to the manufacturer’s protocol.
Hemostasis Assessment
Hemostasis was assessed using the standard template skin bleeding test (Surgicutt®, International Technidyne, Piscataway, NJ).13,15 In brief, a 40 mm Hg pressure cuff was applied to the upper arm of the animal, and a 5 mm long and 1 mm deep incision using the adult, Food and Drug Administration-approved Surgicutt device was made on the shaved volar surface of the animal’s forearm. Bleeding time (BT) was determined by collecting blood drops every 30 s onto a Whatman paper until the bleeding stopped. Bleeding volume was determined by dissolving the dried blood in Drabkin’s reagent (Sigma) and absorption was measured at 540 nm and compared to a standard curve of the animal’s blood taken on the same day.
Statistics
Hemostasis measurements were analyzed using Welch’s t test (Prism, GraphPad Software, Inc., La Jolla, CA) due to the unequal variances in the bleeding test groups. Real-time platelet deposition was analyzed using two-way ANOVA with Holm-Sidak posthoc analysis, which was chosen as this can be used for both pairwise comparisons and comparisons versus a control group; real-time platelet deposition rate was analyzed using one-way ANOVA (Sigma Stat, Systat Software, Inc., San Jose, CA). Data are reported as mean ± SEM.
Results
Effect of FVII–ASO on FVII Antigen Levels, Activity and Prothrombin Time
We generated antisense oligonucleotides based on baboon FVII mRNA. Baboons were subcutaneously administered the FVII ASO (50 mg/kg, 4 ×/week) for a period of 12–13 days. Blood samples were collected prior to and serially during ASO treatment to assess FVII antigen, activity and PT. Baboons were subcutaneously administered the FVII ASO for a period of 12–13 days. As shown in Fig. 1, by day 13, plasma FVII antigen levels decreased to approximately 5% of baseline (93.0–96.6% decrease); without additional doses of FVII–ASO, FVII antigen remained less than 10% of baseline at least until day 50 and did not return to baseline levels by study day 105. A concomitant decrease in FVII activity was observed by day 13 of FVII–ASO dosing, with a correlation value of R2 = 0.97 between FVII antigen and FVII activity (data not shown). Conversely, a twofold increase in PT was observed by day 13, with the peak PT occurring at day 28 at 2.7-fold over baseline. The PT also remained elevated compared to baseline (1.2-fold) at day 105.
Figure 1.
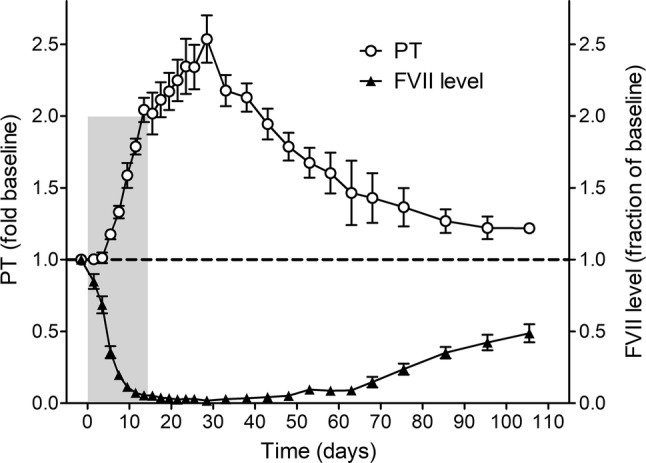
FVII antigen levels (black triangles, fraction of baseline) and prothrombin times (PT; fold-increase) over time. Each animal received up to 8 doses of FVII–ASO (50 mg/kg/dose) over a 2-week period (as indicated by the grey shaded box). Dosing of FVII–ASO was stopped once PT reached two-fold prolongation compared to baseline.
Effect of FVII ASO on Platelet Deposition and Fibrin Formation
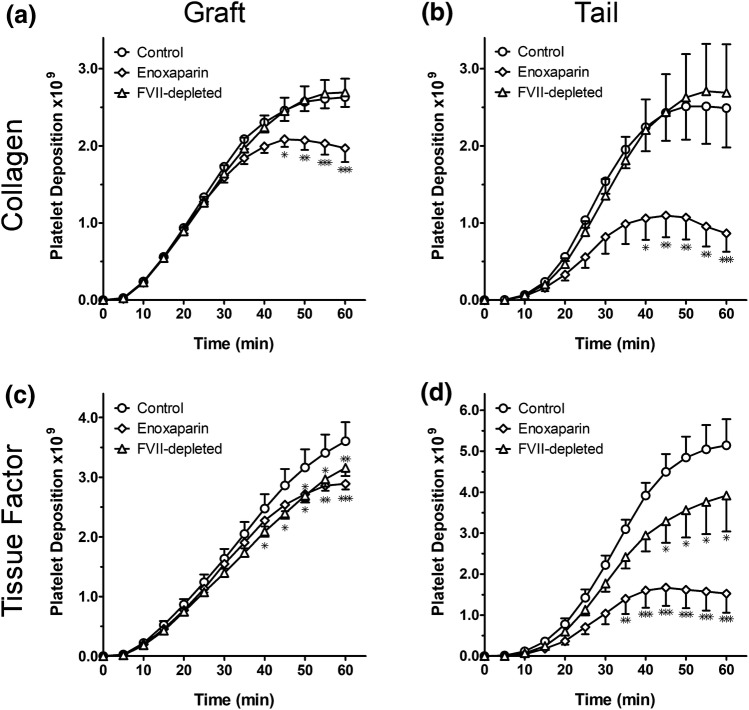
We next studied the effects of reducing FVII levels on the kinetics of platelet deposition and on fibrin formation on collagen- or TF-coated grafts placed in the AV shunt. Negative (no treatment) and positive (enoxaparin 1 mg/kg) control experiments (n = 6 for both) were performed in each animal prior to starting the FVII–ASO treatment regimen. Thrombosis experiments with collagen- and TF-coated grafts in FVII-depleted animals (n = 8) were performed 13–15 days post treatment initiation, which corresponds to the point at which the PT was increased to 20.2 ± 1.1 s (2.2 ± 0.1-fold over baseline) and FVII levels were reduced by > 93% compared to baseline. Thrombus formation in the grafts was monitored for 60 min using gamma camera imaging of radiolabeled platelets within the 2 cm graft and in the 10 cm “tail” segment distal to the graft. Endpoint measurement of radiolabeled fibrinogen was used to assess fibrin levels. All devices initiated thrombus formation in both untreated and treated animals.
Our results show that both TF- and collagen-coated grafts initiated platelet deposition within 10 min exposure to blood flow regardless of the treatment group (Table 1 and Fig. 2). The rate of platelet deposition in either TF- or collagen-coated devices continued to increase during the acceleration phase (10–20 min), then exhibited linear growth (20–40 min). Platelet deposition continued throughout the 60 min of observation on TF-coated grafts, while a trend toward a plateau in average platelet deposition rate was observed on collagen-coated grafts. Total platelet accumulation in the graft and tail was observed for TF-coated grafts as compared to collagen-coated grafts, suggesting a mechanistic or biophysical difference in thrombus formation based on whether the clot was initiated by TF or collagen, and that the plateau on collagen-coated grafts was not a spatial limitation.
Table 1.
Platelet deposition rate on collagen-coated and TF-coated grafts for the following times: 0–10 min, 10–20 min, 20–40 min, and 40–60 min.
| Graft surface | Time | Control (106 platelets/5 min) | Enoxaparin (106 platelets/5 min) | FVII-depleted (106 platelets/5 min) |
|---|---|---|---|---|
| Collagen | 0–10 min | 153 ± 39 | 139 ± 38 | 145 ± 33 |
| 10–20 min | 595 ± 41 | 485 ± 31 | 535 ± 34 | |
| 20–40 min | 763 ± 52 | 451 ± 48*** | 772 ± 48### | |
| 40–60 min | 144 ± 52 | − 55 ± 34** | 234 ± 49### | |
| Tissue factor | 0–10 min | 168 ± 44 | 123 ± 32 | 126 ± 31 |
| 10–20 min | 653 ± 73 | 446 ± 44* | 546 ± 44 | |
| 20–40 min | 1188 ± 59 | 684 ± 51*** | 923 ± 56**## | |
| 40–60 min | 589 ± 77 | 136 ± 40*** | 511 ± 85## |
*p < 0.05, **p < 0.01, ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. enoxaparin
Figure 2.
Effect of FVII depletion on platelet deposition during thrombus formation on tissue factor or collagen. Grafts were coated with either collagen [top row; (a, b)] or tissue factor [bottom row; (c, d)]. Platelet deposition was recorded within the graft [left column; (a, c)] as well as the downstream thrombus tail segment [right column; (b, d)]. The three treatment groups included control (circles, n = 6), enoxaparin (diamonds, n = 6), and FVII-depleted (ASO-treated) (triangles, n = 8). Graft experiments were performed 13–15 days after initiation of FVII–ASO treatment, when PT was prolonged ≥ 2.0 times the baseline, corresponding to 93–97% reduction in FVII antigen levels compared to baseline. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control.
FVII–ASO treatment caused a slight yet statistically significant reduction in platelet deposition by minute 60 on TF-coated grafts themselves; in the 10 cm tail segment of the TF graft, FVII depletion led to a modest 25% reduction in total platelet deposition that was observed by 45 min. In contrast, reduction of FVII levels with FVII–ASO had no effect on the initial or final rate of platelet deposition on collagen-coated grafts or in the 10 cm tail segments as compared to control animals. As expected, pretreatment of animals with the low molecular weight heparin enoxaparin resulted in a substantial reduction in platelet deposition in both collagen- and TF-coated grafts, with a greater than 50% reduction in platelet deposition in the tail segments observed for enoxaparin as compared to controls.
We next analyzed the degree of fibrin deposition within the graft and tail segments produced by either TF- or collagen-coated grafts. As shown in Fig. 3, we did not detect a reduction from baseline resulting from FVII to ASO treatments on either collagen- or TF-coated grafts.
Figure 3.
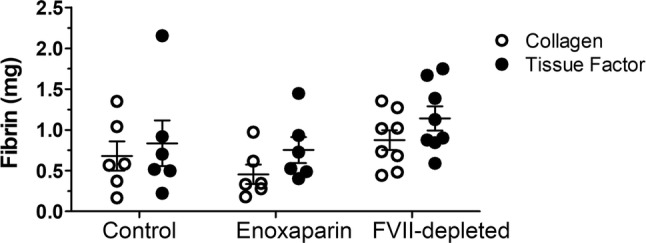
Fibrin deposition (mg) in grafts coated with either collagen (open circles) or tissue factor (black circles). Terminal fibrin deposition was quantified after 60 min of thrombus formation for animals treated with control (left, n = 6), enoxaparin (middle, n = 6) or FVII–ASO (right, n = 8).
Effect of FVII–ASO on Hemostasis
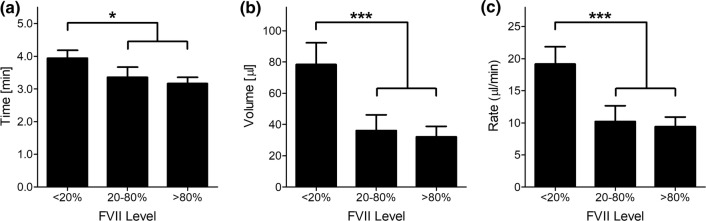
Lastly, we assessed the effect of reducing FVII levels on hemostasis and the FVII antigen level for each BT was quantified. Our results show that increased bleeding times, rates, and volumes above baseline were observed when the FVII antigen levels were reduced below 20% of normal (Fig. 4). Of note, based on the trend observed in pilot BT experiments, we did not reduce FVII levels to or below 2% as a precaution against causing lethal bleeding in the baboons. No differences in BT were observed in animals when FVII levels were above 20% of baseline.
Figure 4.
FVII antigen level versus measured bleeding time (a), volume (b) and rate (c). Measurements were grouped by FVII levels as follows: low FVII levels (< 20%, n = 33), midrange levels (20–80%, n = 14) and normal levels (> 80%, n = 15). *p < 0.05, ***p < 0.001 vs. < 20% FVII level group.
Discussion
We created and used an ASO platform to reduce FVII antigen levels in a non-human primate AV shunt thrombosis model in order to study the role of FVII in maintaining hemostasis and promoting and propagating thrombosis. We found that FVII depletion via ASO mildly reduced TF-mediated thrombosis and distal thrombus propagation while having no effect on collagen-mediated thrombosis nor distal thrombus propagation.
One-third of patients with FVII deficiency are asymptomatic and are only incidentally identified via a prolonged PT; bleeding risk is typically a concern only in individuals with FVII activity < 20% and a positive clinical history of abnormal bleeding.8,21 In our study, we similarly saw no increased incidence of abnormal bleeding when FVII activity was > 20%. Mild mucocutaneous bleeding is most common, though a minority of patients can experience soft tissue, gastrointestinal, uterine and central nervous system bleeding. Surgical bleeding is a common concern, though here too, the risk seems to correlate best with the nature of previous bleeding episodes rather than FVII activity levels.2,8
Interestingly, as many as 3–4% of cases of congenital FVII deficiency reported in the literature have also developed either arterial or venous thrombosis, even in the setting of FVII activity levels < 2%. While transient provoking risk factors have been identified in the majority of these cases, several were reportedly unprovoked.17,18 Our model of collagen- or tissue factor-initiated thrombus formation supports this concept, as even at the most profound degrees of FVII depletion, thrombus formation and propagation were either unaffected (collagen) or only mildly reduced (TF). We have previously shown that administration of a competitive FVIIa inhibitor (active site-inhibited FVIIa [FVIIai]) had no significant effect on the extent of thrombus formation in prosthetic vascular grafts despite increasing PT and BT in a primate model of arterial-type thrombosis.14 The current study examines FVII inhibition by decreasing the level of FVII antigen itself, allowing corroboration of our previous findings using a similar large vessel arterial-type thrombosis model.
The competing results of increased bleeding with retained potential for thrombosis despite lowered FVII levels seen both clinically and in our study require reconciliation. We propose several possible explanations. First, even at near-complete FVII reduction via ASO, the residual ~ 5% FVII antigen level may be sufficient to initiate thrombosis when exposed to sufficiently thrombogenic stimuli such as TF. Second, the intrinsic, or contact pathway of coagulation may play a relatively greater role in thrombus propagation in our model. We previously demonstrated in a baboon shunt thrombosis model similar to the currently described study that antibody-mediated FXI reduction almost completely abrogated platelet accumulation in a TF-coated graft, in contrast to the far milder reduction in platelet accumulation achieved with FVII–ASO in the current study.12 The relative importance of the contact pathway in thrombus propagation may also explain why the collagen-coated vascular graft triggered such robust platelet accumulation despite FVII–ASO treatment, as collagen is a known stimulus for both FXII auto-activation and platelet adhesion via von Willebrand factor.22 Even within TF-coated vascular grafts, the contact pathway could contribute to residual platelet accumulation despite FVII–ASO treatment, as the trace concentrations of pre-formed, circulating FVIIa or FVIIa/TF complexes could generate thrombin and amplify contact pathway activation via a positive feedback loop consisting of FVIII, FIX, and FXI.26,27 Independent platelet activation and short- polyphosphate release could also amplify FXII activation.29 In contrast, the FXa inhibitor enoxaparin stymied platelet accumulation to a greater degree than FVII–ASO in both TF- and collagen-coated vascular grafts, reflecting the importance of FXa in transmitting the procoagulant signals initiated by both the extrinsic and contact pathways.
Third, while our AV shunt model mimics arterial-type thrombosis, it must be acknowledged that this is an imperfect simulation. Therefore, the conflicting increase in BT and retained ability for graft thrombosis in our model despite lowered FVII levels may simply reflect differences between physiologic and artificial vasculature. In humans with severe FVII deficiency, bleeding may dominate, though a sufficiently prothrombotic stimulus exposing TF, collagen or other substances may still override this bleeding tendency and lead to pathologic thrombosis, as is seen clinically.
Fourth, it has been suggested that microparticle TF activity is inhibited until fusion with activated platelets, providing an explanation for localization of circulating TF-derived coagulation activity.7 TF-bearing microparticles were not measured, therefore, we are unable to conclude from our results whether circulating, microparticle-associated TF plays a role specifically in thrombus propagation.7 The lack of an effect of decreased FVII levels on thrombus formation on collagen in our model suggests that circulating TF does not play a major role in propagating thrombus formation in this model.
We acknowledge several limitations to our study. First, our model was applied to a limited number of baboons, and thus not powered to define efficacy. Second, as previously noted the thrombogenic grafts bonded exclusively with collagen or TF utilized in our model are simplified models of vascular injury; indeed, many additional sub-endothelial substances are also exposed to circulating blood in vivo at sites of vascular injury or in diseased blood vessels. Third, the BT assay, while widely used in both pre-clinical and clinical studies assessing hemostasis, is an imperfect surrogate for hemostatic capacity, most clearly demonstrated by the substantial variability in BT seen in humans with FVIII and FIX deficiencies (hemophilia A and B).9
In conclusion, our study shows that depletion of FVII via FVII–ASO treatment failed to reduce platelet deposition or fibrin formation on collagen-coated grafts; a reduction in platelet deposition was only observed in the thrombus tail on TF-coated grafts. We confirmed a requisite role of FVII in maintaining hemostasis, as evidenced by PT prolongation and, at very low FVII concentrations, increased bleeding. Collectively, these observations render the notion of pharmacologic inhibition of FVII activity for thrombosis prophylaxis untenable, though our findings do suggest important mechanisms by which pathologic thrombosis can continue to occur despite reduced FVII. Future studies by our group and others could further refine our thrombosis model to more closely mimic vascular endothelium and sub-endothelial layers, separately assess platelet and fibrin accumulation via a variety of exogenous antiplatelet agents, and more clearly define the contribution of the contact pathway activity.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (R01HL101972, R01GM116184, R01HL047014). This project has been sponsored in part by Ionis Pharmaceuticals.
Author Contributions
MW contributed to experimental design, performed experiments, analyzed and evaluated data, and wrote the manuscript. JC, SM, BM, and AG designed, developed and evaluated primate FVII–ASO-s. JJ and MH oversaw the primate studies. All other authors contributed to data interpretation and manuscript revisions.
Declaration of Competing Interest
MW, ET, and AG are paid employees of Aronora, Inc, and they as well as OHSU may have a financial interest in the results of this study. JC, SFM, and BM are paid employees of Ionis Pharmaceuticals. JJ Shatzel serves as a medical consultant for Aronora, Inc. This potential conflict of interest has been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee. The remaining authors declare no competing financial interests.
Human Studies
No human studies were carried out by the authors for this article.
Animal Studies
All animal studies were approved by the Oregon Health & Science University IACUC. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by our institutional committee.
Abbreviations
- ASO
Antisense oligonucleotide
- BT
Bleeding time
- FVa
Activated coagulation factor V
- FVII
Coagulation factor VII
- FVIIa
Activated coagulation factor VII
- FX
Coagulation factor X
- FXI
Coagulation factor XI
- i.d.
Inner diameter
- PT
Prothrombin time
- TF
Tissue factor
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michael Wallisch and Sven R. Olson are co-first authors.
References
- 1.Baker BF, et al. 2’-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 2.Benlakhal F, Mura T, Schved J-F, Giansily-Blaizot M, French Study Group of Factor VII Deficiency A retrospective analysis of 157 surgical procedures performed without replacement therapy in 83 unrelated factor VII-deficient patients. J. Thromb. Haemost. 2011;9:1149–1156. doi: 10.1111/j.1538-7836.2011.04291.x. [DOI] [PubMed] [Google Scholar]
- 3.Bugge TH, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc. Natl. Acad. Sci. USA. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadroy Y, Horbett TA, Hanson SR. Discrimination between platelet-mediated and coagulation-mediated mechanisms in a model of complex thrombus formation in vivo. J. Lab. Clin. Med. 1989;113:436–448. [PubMed] [Google Scholar]
- 5.Carmeliet P, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 6.Crosby JR, et al. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler. Thromb. Vasc. Biol. 2013;33:1670–1678. doi: 10.1161/ATVBAHA.113.301282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 8.Di Minno MN, Dolce A, Mariani G, STER Study Group Bleeding symptoms at disease presentation and prediction of ensuing bleeding in inherited FVII deficiency. Thromb. Haemost. 2013;109:1051–1059. doi: 10.1160/TH12-06-0431. [DOI] [PubMed] [Google Scholar]
- 9.Eyster ME, Gordon RA, Ballard JO. The bleeding time is longer than normal in hemophilia. Blood. 1981;58:719–723. doi: 10.1182/blood.V58.4.719.719. [DOI] [PubMed] [Google Scholar]
- 10.Green L, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br. J. Haematol. 2018;181:54–67. doi: 10.1111/bjh.15167. [DOI] [PubMed] [Google Scholar]
- 11.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018;38:709–725. doi: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 12.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 13.Gruber A, et al. Relative antithrombotic and antihemostatic effects of protein C activator versus low-molecular-weight heparin in primates. Blood. 2007;109:3733–3740. doi: 10.1182/blood-2006-07-035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber A, et al. Hemostatic effect of activated factor VII without promotion of thrombus growth in melagatran-anticoagulated primates. Thromb. Res. 2007;119:121–127. doi: 10.1016/j.thromres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Harker LA, et al. Clopidogrel inhibition of stent, graft, and vascular thrombogenesis with antithrombotic enhancement by aspirin in nonhuman primates. Circulation. 1998;98:2461–2469. doi: 10.1161/01.CIR.98.22.2461. [DOI] [PubMed] [Google Scholar]
- 16.Horbett TA, Cheng CM, Ratner BD, Hoffman AS, Hanson SR. The kinetics of baboon fibrinogen adsorption to polymers: in vitro and in vivo studies. J. Biomed. Mater. Res. 1986;20:739–772. doi: 10.1002/jbm.820200608. [DOI] [PubMed] [Google Scholar]
- 17.Mariani G, et al. Thrombosis in inherited factor VII deficiency. J. Thromb. Haemost. 2003;1:2153–2158. doi: 10.1046/j.1538-7836.2003.00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Marty S, et al. The paradoxical association between inherited factor VII deficiency and venous thrombosis. Haemophilia. 2008;14:564–570. doi: 10.1111/j.1365-2516.2007.01647.x. [DOI] [PubMed] [Google Scholar]
- 19.Menegatti M, Peyvandi F. Treatment of rare factor deficiencies other than hemophilia. Blood. 2019;133:415–424. doi: 10.1182/blood-2018-06-820738. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell JS, O’Sullivan JM, Preston RJS. Advances in understanding the molecular mechanisms that maintain normal haemostasis. Br. J. Haematol. 2019;186:24–36. doi: 10.1111/bjh.15872. [DOI] [PubMed] [Google Scholar]
- 21.Peyvandi F, et al. Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: Results from the European Network of Rare Bleeding Disorders. J. Thromb. Haemost. 2012;10:615–621. doi: 10.1111/j.1538-7836.2012.04653.x. [DOI] [PubMed] [Google Scholar]
- 22.Renne T, et al. In vivo roles of factor XII. Blood. 2012;120:4296–4303. doi: 10.1182/blood-2012-07-292094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen ED, et al. Mice lacking factor VII develop normally but suffer fatal perinatal bleeding. Nature. 1997;390:290–294. doi: 10.1038/36862. [DOI] [PubMed] [Google Scholar]
- 24.Sevenet P-O, Kaczor DA, Depasse F. Factor VII deficiency: from basics to clinical laboratory diagnosis and patient management. Clin. Appl. Thromb. Hemost. 2017;23:703–710. doi: 10.1177/1076029616670257. [DOI] [PubMed] [Google Scholar]
- 25.Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ. Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. doi: 10.1182/blood.V88.5.1583.1583. [DOI] [PubMed] [Google Scholar]
- 26.Tormoen GW, Khader A, Gruber A, McCarty OJT. Physiological levels of blood coagulation factors IX and X control coagulation kinetics in an in vitro model of circulating tissue factor. Phys. Biol. 2013;10:036003. doi: 10.1088/1478-3975/10/3/036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tormoen GW, Rugonyi S, Gruber A, McCarty OJT. The role of carrier number on the procoagulant activity of tissue factor in blood and plasma. Phys. Biol. 2011;8:066005. doi: 10.1088/1478-3975/8/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker EI, et al. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–944. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verhoef JJF, et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood. 2017;129:1707–1717. doi: 10.1182/blood-2016-08-734988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan WB, Seth PP. The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem. 2016;59:9645–9667. doi: 10.1021/acs.jmedchem.6b00551. [DOI] [PubMed] [Google Scholar]