Abstract
Interleukin (IL)-4 is known as a cytokine mainly involved in allergy and inflammation, but recent studies have suggested that IL-4 plays a part in the differentiation process of various cells. Since the effect of IL-4 on intestinal epithelial cells, particularly cryptic cells including stem cells, is poorly understood, we investigated IL-4-induced changes in intestinal epithelial cells using mouse jejunal organoids called enteroids. IL-4 treatment decreased cell proliferation, the expression of the stem cell markers leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) and olfactomedin 4 (Olfm4), and Lgr5-positive cells in enteroids. Among the differentiation markers, IL-4 significantly decreased the gene expression levels of the Paneth cell markers lysozyme 1 (Lyz1) and regenerating islet-derived protein 3 gamma (Reg3γ). A fluorescent immunostaining showed that IL-4 attenuated the emission and fluorescence intensity derived from lysozyme, which is enriched in Paneth cells. These results suggest that functional changes in Paneth cells caused by IL-4 may contribute to the reduction in Lgr5-positive cells and proliferative activity. IL-4 may affects gut function by altering the proliferation and the gene expression in enteroids.
Electronic supplementary material
The online version of this article (10.1007/s10616-020-00395-7) contains supplementary material, which is available to authorized users.
Keywords: Enteroids, IL-4, Lgr5, Stem cells, Paneth cells, Proliferation
Introduction
The epithelium covering the internal surface of the small intestines is composed of multiple cell types, including undifferentiated stem cells, transit amplifying cells (TA cells, which are one-step differentiated stem cells), and functionally mature epithelial cells such as enterocytes, Paneth cells, goblet cells, tuft cells, and enteroendocrine cells (Sato et al. 2009). Differentiation to these functional cells is regulated by numerous signaling pathways (van Neerven and Vermeulen 2019), growth factors (Fujii et al. 2018), and cytokines (Andrews et al. 2018). Among the cytokines, interleukin (IL)-13 is known to promote differentiation and proliferation of tuft and goblet cells, and IL-33 also increases goblet and Paneth cell numbers (Andrews et al. 2018).
IL-4 is a cytokine with well documented functions in allergy and inflammation, promoting differentiation of naïve T cells into Th2 cells and acting as a switch factor for IgE synthesis in activated B cells (Saggini et al. 2011; Schmidt-Weber 2012; Junttila 2018). Overproduction of IL-4 is therefore strongly associated with allergic and inflammatory diseases. However, several recent studies have demonstrated that IL-4 is involved in the differentiation of multiple non-immune cells such as myoblasts (Possidonio et al. 2011) and oligodendrocytes (Zhang et al. 2019). Considering that the intestinal tissue will be exposed to higher concentrations of IL-4 under allergic and inflammatory conditions (Bartuzi et al. 2000; Kordulewska et al. 2018), it is important to assess the effects of IL-4 on the growth, differentiation, and function of specific intestinal epithelial cell types.
von Moltke et al. (2016) reported that IL-4 contributes to the prevention of parasitic infections in the intestines, possibly by promoting the Type 2 immunity of epithelial tuft cells (Howitt et al. 2016). Indeed, IL-4 promotes differentiation of intestinal stem cells into tuft cells and goblet cells (Gerbe et al. 2016). However, the effect of IL-4 on the differentiation of stem cells into other functional epithelial cells is not understood. Studying the effects of IL-4 on the proliferation process of intestinal stem cells is also important to know the interaction between IL-4 and intestinal epithelium, but no information is available about it.
The functions of differentiated epithelial cells have been extensively investigated using cell lines such as Caco-2, HT-29, and T84 (Shimizu 2010), but it had been difficult to examine intestinal stem cells until Sato et al. (2009) established the organoid culture method. Therefore, we used small intestinal organoids called enteroids to evaluate the effects of IL-4 on the proliferation and gene expression in enteroids.
Materials and methods
Animals
Wild-type C57BL/6J mice were obtained from CLEA Japan, Inc. (Tokyo, Japan) and Lgr5-enhanced green fluorescent protein (EGFP)-IRES-creERT2 (Lgr5-EGFP) mice from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in standard plastic cages in an animal room maintained at 23–25 °C and 50–56% humidity under a 12 h light/12 h dark cycle (lights on 8:00–20:00) with free access to tap water and standard laboratory rodent feed (Oriental Yeast Co., Ltd., Tokyo, Japan). Animal experiments were conducted in accordance with the guidelines for the maintenance and handling of experimental animals established by the Tokyo University of Agriculture Ethics Committee.
Enteroid culture
Enteroids were isolated from fresh jejunal crypts obtained from wild-type and Lgr5-EGFP mice as previously described (Saito et al. 2017). In brief, the jejunum was collected from animals euthanized by cervical dislocation, washed with ice-cold Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+ and Mg2+, and cut vertically. The luminal side was placed on ice, and the villi were scraped with a scalpel. The tissue was agitated in 10 mL of 30 mM ethylenediaminetetraacetic acid (EDTA)-3Na/Hanks’ balanced salt solution (HBSS) for 10 min at room temperature, placed in a tube with 10 mL of ice-cold HBSS, and shaken 20 times to separate the crypts. The jejunum was then transferred to a second tube with HBSS and shaken 50 times. This process was repeated a third and fourth time with shaking 50 and 100 times, respectively. Each crypt/villi fraction was centrifuged at 400×g at 4 °C for 4 min. The supernatant was removed and pellets individually suspended in 500 μL of 10 μM Y-27632/HBSS for counting of crypts and villi under light microscopy. The % crypt purity was calculated as [crypts/(villi + crypts)] × 100. Tubes with crypt purity > 80% were centrifuged at 400×g at 4 °C for 4 min and the supernatant was removed. Crypts were then plated on 24-well plates (Nippon Gene Co., Ltd., Tokyo, Japan) at 200 per well and embedded in 40 μL/well of 60% Matrigel® (BD Biosciences, San Jose, CA). The Matrigel® suspension was allowed to polymerize at 37 °C for 10 min before supplementation of fresh medium (Advanced Dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12) supplemented with 1 × B-27 supplement (Thermo Fisher Scientific, Inc., Waltham, MA), 1 × penicillin/streptomycin, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 × l-Alanyl-l-glutamine (all from NACALAI TESQUE, Inc., Kyoto, Japan), 10% R-spondin conditioned medium (CM) (kindly supplied by Dr. Eitaro Aihara, University of Cincinnati College of Medicine), 5% Noggin CM (kindly supplied by Dr. Hans Clevers, Utrecht University), 1 mM N-Acetyl-l-cysteine (SIGMA-ALDRICH, Co., St. Louis, MO), and 50 ng/mL recombinant murine epidermal growth factor (EGF) (Funakoshi Co., Ltd., Tokyo, Japan)). This combined medium was used for all cultures and is defined as ‘basic medium’. The basic medium was replaced every 2–3 days. For passage, enteroids from wild-type mice were collected in ice-cold DPBS, mechanically disrupted by passage through a syringe with a 27G needle (Terumo corporation, Tokyo, Japan), and subsequently transferred to fresh 60% Matrigel®. The enteroids were passaged every 4–6 days. Enteroids from Lgr5-EGFP mice were freshly prepared before each experiment because fluorescence intensity gradually diminished with repeated passage.
Experimental design
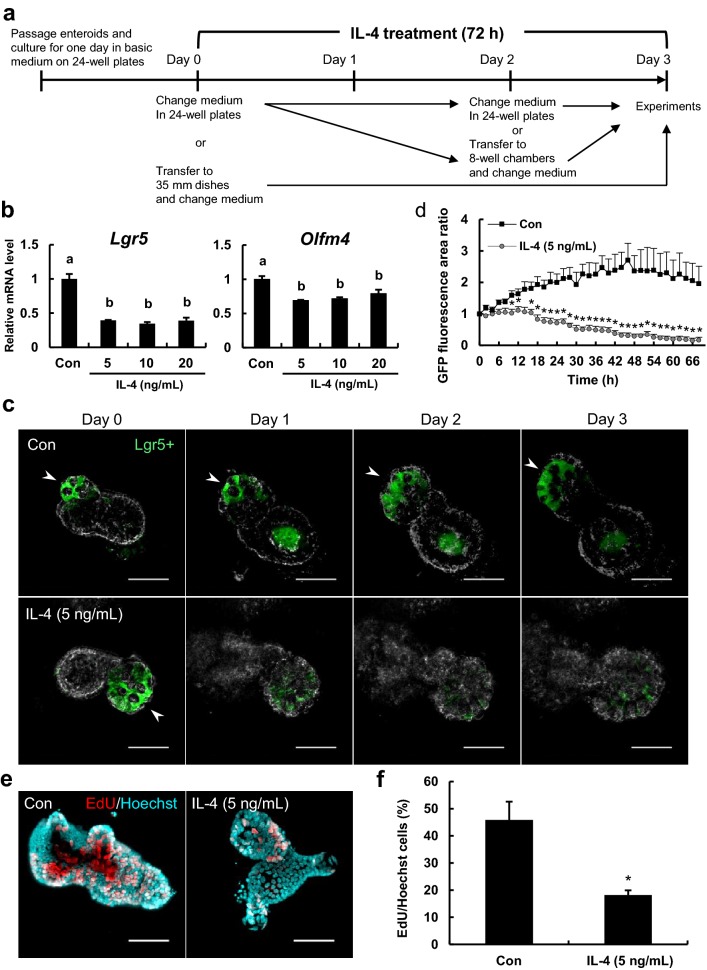
Figure 1a shows the experimental design. For measurement of mRNA expression by real-time reverse transcription polymerase chain reaction (qPCR), enteroids were passaged and cultured in the basic medium for 1 day on 24-well culture plates. On day 0, control group enteroids were cultured in the basic medium for 48 h while IL-4-treated group enteroids were cultured in the basic medium supplemented with 5, 10, and 20 ng/mL recombinant murine IL-4 (Funakoshi Co., Ltd., Tokyo, Japan) for the same period. After medium change on day 2, both groups were cultured for an additional 24 h and harvested on day 3. For 5-ethynyl-2-deoxyuridine (EdU) assay and immunofluorescence staining, enteroids treated as described until day 2 were transferred to Nunc™ Lab-Tek™ II 8-well glass bottom chambers (Thermo Fisher Scientific Inc.) and suspended in 5 μL/well of 60% Matrigel® (about 20 organoids/well). After 10 min polymerization, the enteroids were cultured in 200 μL of the basic medium with or without 5 ng/mL IL-4 for an additional 24 h. For measuring changes in EGFP signals from Lgr5-positive cells, the enteroids from Lgr5-EGFP mice were cultured in 24-well cell culture plates. On day 0, the enteroids were transferred to 35 mm dishes (Eppendorf North America, Inc., Hauppauge, NY) and cultured in the basic medium with or without 5 ng/mL IL-4 for 72 h at 37 °C under 5% CO2. Specific measurement protocols are detailed below.
Fig. 1.
IL-4 reduces stem cell proliferation and renewal in murine enteroids. a Experimental design. Enteroids were treated with IL-4 for 72 h starting 1 day after passage. b Lgr5 and Olfm4 mRNA levels in enteroids as measured by qPCR. Enteroids were cultured in medium containing 0, 5, 10, or 20 ng/mL IL-4. Expression levels of Lgr5 and Olfm4 are relative to beta-2 microglobulin (B2m). The bar represents mean ± SE (n = 3). Groups without a common letter differ significantly (p < 0.05). c Confocal images of Lgr5-EGFP enteroids treated with IL-4. Lgr5-positive cells are green. Arrowheads indicate areas with Lgr5-positive cells. Scale bars: 50 μm. d Changes in the fluorescence area ratio of enteroids treated with IL-4. The areas emitting Lgr5-EGFP fluorescence were quantified using ImageJ. The fluorescence ratio was calculated as the fluorescence area measured at each time point divided by the fluorescence area at 0 h. The bar represents the mean ± SE (n = 4). Asterisk indicates a significant difference (p < 0.05). e Proliferative response of enteroids treated with IL-4. Enteroids were cultured in medium with or without IL-4 (5 ng/mL) for 72 h. Proliferating cells were detected using EdU (red) and total cell nuclei using Hoechst (blue). Scale bars: 50 μm. f Proportion of EdU-positive cells in enteroids. The number of proliferating cells (red) and total nuclei (blue) were counted using ImageJ. The bar represents the mean ± SE (n = 4). Asterisk indicates a significant difference (p < 0.05). (Color figure online)
qPCR
Total RNA was isolated from enteroids using ISOGEN II (Nippon Gene Co., Ltd.). cDNA was synthesized using a PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara Bio Inc., Shiga, Japan) according to the manufacturer’s protocol. qPCR was performed as described previously (Saito et al. 2017) using THUNDERBIRD® SYBR® Green (Toyobo Co., Ltd., Osaka, Japan) and primer sets listed in Online Resource 1 and the following thermocycle protocol: 1 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 30 s at 60 °C, and 1 min at 72 °C.
Monitoring changes in EGFP signals from Lgr5-positive cells
During enteroid culture, the changes in EGFP signals from Lgr5-positive cells were monitored using a confocal laser scanning microscope (FLUOVIEW FV10i; Olympus Corporation, Tokyo, Japan). The area of cells emitting Lgr5-EGFP fluorescence was measured using ImageJ 1.52 k software (National Institutes of Health (NIH), Bethesda, MD).
EdU assay
On culture day 3 (Fig. 1a), EdU solution was added to the medium for 3 h. Enteroids were then fixed in 3.7% formaldehyde solution at room temperature for 15 min and stained using a Click-iT® EdU Cell Proliferation Assay kit (Thermo Fisher Scientific Inc.) according to the manufacturer’s protocol. After staining, enteroids were cleared using the water-based optical clearing agent SeeDB (Ke et al. 2013) as described previously (Saito et al. 2017) and examined under a confocal laser scanning microscope (FLUOVIEW FV10i). The proportion of EdU-positive cells was calculated as the number of red-stained (proliferating) cells relative to the total number of nuclei (blue).
Immunofluorescence staining
On day 3 (Fig. 1a), enteroids were fixed in 200 μL of 4% paraformaldehyde/PBS for 20 min at room temperature, washed three times for 20 min with 200 μL PBS, blocked in 200 μL blocking buffer (PBS containing 2% donkey serum and 0.3% Triton X-100) overnight at 4 °C, then incubated with 200 μL anti-lysozyme antibody solution (1:400, kindly supplied by Dr. Tokiyoshi Ayabe, Hokkaido University) at 4 °C overnight. After three 30-min washes with 200 μL PBS, the samples were incubated with 200 μL donkey anti-rabbit IgG conjugated to Alexa Fluor® 488 (Thermo Fisher Scientific Inc.) (1:1000) at 4 °C overnight under darkness. Nuclei were counterstained with 200 μL Hoechst 33342 (Thermo Fisher Scientific Inc.) (1:2000) at room temperature for 30 min. Enteroids were washed twice with 200 μL PBS, cleared with SeeDB, and examined using a confocal laser scanning microscope (FLUOVIEW FV10i). Fluorescence emission intensity was measured using ImageJ (NIH).
Statistics
Data are expressed as mean ± standard error (SE) or proportion (%). Paired means and multiple means were compared by Student’s t-tests and by ANOVA with post hoc Tukey’s multiple comparison tests, respectively. All statistical analyses were conducted using SPSS 21.0 J. A p < 0.05 was considered significant for all tests.
Results
IL-4 reduced the expression of the stem cell markers Lgr5 and Olfm4 in enteroids
The effects of IL-4 on intestinal epithelial stem cells were first examined by monitoring expression of the stem cell markers Lgr5 and Olfm4 in cultured jejunal enteroids derived from wild-type mice. The expression levels of Lgr5 and Olfm4 mRNA in enteroids were significantly reduced by IL-4 (Fig. 1b). Enteroids were prepared from Lgr5-EGFP mice and changes in the fluorescence area ratio of EGFP-positive cells were monitored for 3 days in the presence and absence of IL-4 (Fig. 1c, d). Unlike results in the control group, Lgr5-EGFP fluorescence area markedly decreased starting 1 day after IL-4 addition (Fig. 1c). The area of cells emitting Lgr5-EGFP fluorescence gradually increased in the control (untreated) enteroids during culture for 48 h, suggesting expansion of the stem cell population, whereas this area significantly decreased after 10 h of IL-4 treatment (Fig. 1d). The decreasing effect of IL-4 on stem cells quite disappeared after removing IL-4 from the culture using Lgr5-EGFP enteroids (Online Resource 2a). The IL-4 group significantly decreased the fluorescence area ratio of EGFP-positive cells after 24 h of culture with IL-4, compared with the control group, whereas the recovery group significantly suppressed the decrease after 36 h of culture without IL-4 (Online Resource 2b).
IL-4 decreased cell proliferation in enteroids
IL-4 treatment also significantly reduced the amount of EdU incorporation in enteroids (Fig. 1e) and the number of EdU-positive cells (Fig. 1f), consistent with reduced numbers of proliferative cells.
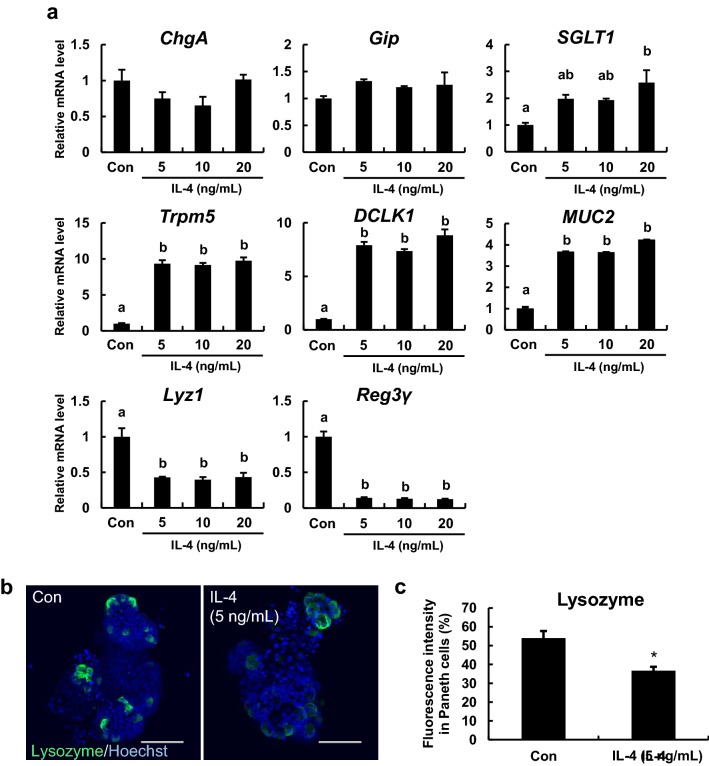
IL-4 altered the expression levels of multiple differentiation markers in enteroids
The expression levels of the endocrine cell marker chromogranin A (ChgA) and the K cell marker glucose-dependent insulinotropic polypeptide (Gip) were not changed at any IL-4 concentration, while the expression level of the enterocyte marker sodium/glucose cotransporter member 1 (SGLT1) tended to increase at concentrations of IL-4 (5 and 10 ng/mL) and was significantly increased at the concentrations of IL-4 (20 ng/mL). In addition, IL-4 significantly increased the mRNA levels of the tuft cell markers transient receptor potential cation channel subfamily M member 5 (Trpm5) and doublecortin like kinase 1 (DCLK1) and the goblet cell marker mucin 2 (MUC2), and significantly reduced mRNA levels of the Paneth cell markers Lyz1 and Reg3γ (Fig. 2a). Moreover, visualization of lysozyme, which is enriched in Paneth cells, by fluorescent immunostaining also confirmed that IL-4 clearly attenuated the emission and fluorescence intensity derived from lysozyme (Fig. 2b, c).
Fig. 2.
Effects of IL-4 on differentiated cell markers in enteroids. a Effects of IL-4 treatment on ChgA, Gip, SGLT1, Trpm5, DCLK1, MUC2, Lyz1, and Reg3γ mRNA expression levels in enteroids. Enteroids were cultured in medium containing 0, 5, 10, or 20 ng/mL IL-4. The mRNA expression levels of the indicated markers were measured using qPCR and normalized to B2m expression. The bar represents the mean ± SE (n = 3). Groups without a common letter differ significantly (p < 0.05). b Immunofluorescence staining of lysozyme (a Paneth cell marker) in enteroids cultured with or without 5 ng/mL IL-4 for 72 h. Paneth cells are green and nuclei are blue. Scale bars: 50 μm. c Fluorescence intensity derived from lysozyme. The bar represents the mean ± SE (n = 7–8). Asterisks indicate a significant difference (p < 0.05). The fluorescence intensity was quantified using ImageJ. (Color figure online)
IL-4 increased the expression level of transcription factor atonal homolog 1 (Atoh1)
Figure 3 shows the effect of IL-4 on gene expression of hairy and enhancer of split 1 (HES-1) and Atoh1. IL-4 differentially modulated the expression of the differentiation-associated transcriptions factors HES-1 and Atoh1. While the mRNA expression of the former was not affected, IL-4 significantly increased Atoh1 mRNA level in enteroids.
Fig. 3.
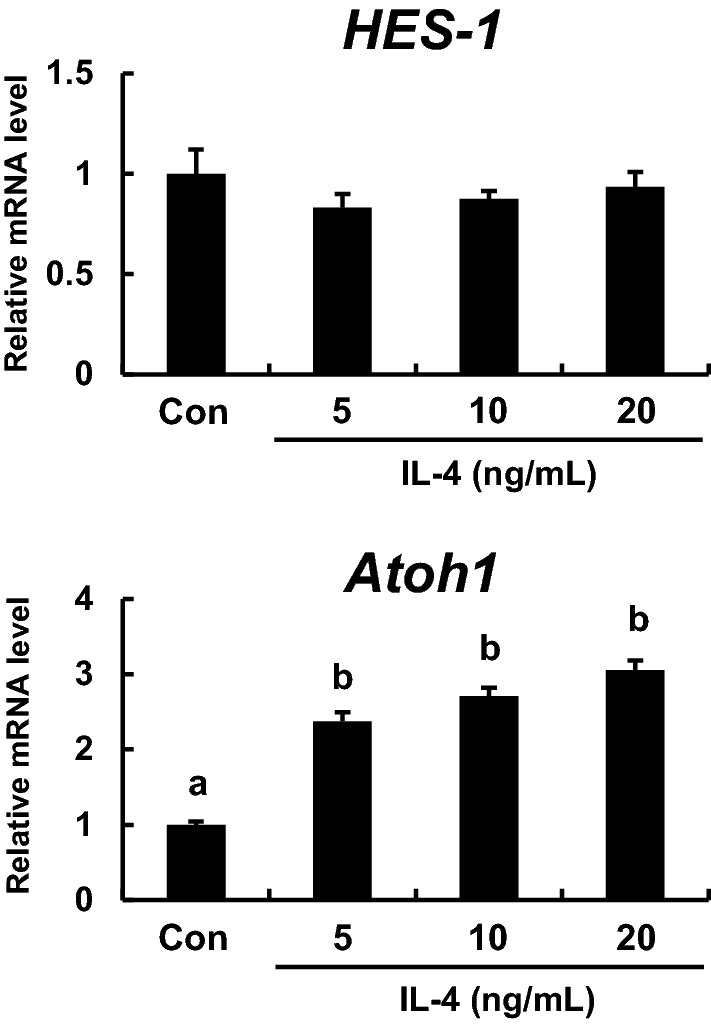
Effects of IL-4 on HES-1 and Atoh1 mRNA levels. Enteroids were cultured in medium containing 0, 5, 10, or 20 ng/mL IL-4. The mRNA expression levels of transcription factors involved in differentiation (HES-1 and Atoh1) were measured using qPCR and normalized to B2m expression. The bar represents the mean ± SE (n = 3). Groups without a common letter differ significantly (p < 0.05)
Discussion
We demonstrate that IL-4 can suppress cell stemness and selectively alter the function of mature cell types in murine enteroids. Decreased expression of the stem cell marker Lgr5 was observed at both the mRNA expression level as measured by qPCR (Fig. 1b) and the protein level as estimated by fluorescence intensity measurements of Lgr5-EGFP (Fig. 1c, d). The Wnt signaling pathway member Lgr5 is an established stem cell marker protein (Barker et al. 2007), thus the reduced Lgr5 expression and Lgr5-positive cells suggests that IL-4 disrupts both proliferation and self-renewal capacity. This is also supported by the finding that IL-4 significantly reduced the mRNA expression level of Olfm4 (Fig. 1b), another stem cell marker. Further, IL-4 reduced proliferative cell numbers as evidenced by EdU assay (Fig. 1e, f) and stem cells are the predominant proliferative cells in the intestinal epithelium. Therefore, IL-4 may seriously impair self-renewal capacity of the intestinal epithelium.
The effects of IL-4 on intestinal epithelial cell differentiation were then assessed by examining the expression levels of 8 cell type-specific markers (Fig. 2a). The mRNA level of the enterocyte marker SGLT1 was significantly increased at the concentrations of IL-4 (20 ng/mL). This increase may be associated with protecting enterocytes from injury by elevating glucose uptake via SGLT1 (Yu et al. 2005). As already reported (Gerbe et al. 2016; von Moltke et al. 2016; Vila et al. 2018), expression levels of the tuft cell markers Trpm5 and DCLK1 and the goblet cell marker MUC2 were markedly increased in enteroids by IL-4, while IL-4 did not detectably alter the mRNA levels of enteroendocrine cell markers (Fig. 2a) despite these cells having similar secretory phenotypes. Surprisingly, the mRNA expression level of Lyz1, a Paneth cell marker, was clearly decreased by IL-4. Also, fluorescence immunostaining revealed that IL-4 significantly reduced lysozyme production in enteroids (Fig. 2b). Moreover, the mRNA expression level of Reg3γ, an anti-bacterial peptide produced in Paneth cells, was significantly reduced by IL-4. Collectively, these results suggest that IL-4 impairs Paneth cell function. It is also possible that IL-4 regulates differentiation into Paneth cells. Paneth cells are located next to stem cells in the intestinal crypt and express signaling molecules essential for stem cell maintenance (Sato et al. 2011), thus IL-4-induced functional changes to Paneth cells may contribute to the loss of Lgr5-positive cells and reduced stemness.
Crosstalk between the Notch and Wnt signaling pathways is important for regulating stem cell proliferation and regeneration (Nakamura et al. 2007). Therefore, reduced Lgr5-positive stem cells in IL-4-treated enteroids may stem from changes in Notch/Wnt signaling. Notch signaling is known to activate HES-1 and suppress Atoh1. The former is a Notch effector and transcription factor involved in differentiation into absorptive cells while the latter is a transcription factor associated with differentiation of secretory cells in the gut (Demitrack and Samuelson 2016; Kay et al. 2017). In the present study, IL-4 treatment significantly increased Atoh1 expression in enteroids without changing HES-1 expression (Fig. 3), suggesting that IL-4 does not affect the differentiation into absorptive cells. However, IL-4 increased the mRNA level of SGLT1, an absorptive cell marker (Fig. 2a). This contradiction may reflect that IL-4 simply elevated the level of SGLT1 mRNA rather than promoting differentiation into absorptive cells. Further immunofluorescence experiment to determine whether IL-4 increases the number of absorptive cells will be needed. Demitrack and Samuelson (2016) reported that Atoh1 is expressed in secretory progenitors as well as differentiated secretory cells. In the present study, IL-4 elevated the mRNA expression levels of markers for tuft cells and goblet cells, secretory cells (Fig. 2a). In addition, IL-4 has been reported to increase the numbers of tuft cells and goblet cells (Gerbe et al. 2016; von Moltke et al. 2016; Vila et al. 2018). Therefore, the increased Atoh1 expression induced by IL-4 may be associated with the increase in such secretory cells as tuft cells and goblet cells. Further, Atoh1 is also a suppressive factor for Lgr5 expression (van Es et al. 2012). Hence, the increased Atoh1 expression induced by IL-4 could negatively affect Lgr5 stem cell maintenance. Han et al. (2018) found that celastrol enhanced Atoh1 expression in inner ear stem cells. A similar increase in intestinal stem cells could account for the observed decrease in Lgr5 expression (Fig. 1b).
It is likely that the effects of IL-4 on stem and Paneth cells are not direct as single-cell RNA-sequencing transcriptome analysis performed by Sato et al. (2011) found that little IL-4 receptor was expressed either on Lgr5-positive cells or on Paneth cells in enteroids. Rather, the IL-4 signal is likely first received by other IL-4 receptor-expressing cell(s) such as tuft cells or goblet cells (Gerbe et al. 2016; Haber et al. 2017), which in turn release factors that act on cryptic cells. Further study on the detailed mechanisms underlying these actions of IL-4 are required. Exposure to food allergens causes inflammatory cytokine release and promotes the development of Th2 inflammatory responses in the intestines. Th2-associated inflammatory conditions in the intestines recruit eosinophils that release of IL-4 and IL-13, which trigger the production of food antigen-specific IgE (Rothenberg et al. 2001). Progressive inflammatory reactions in the intestines are often accompanied by epithelial barrier disruption (Lechuga and Ivanov 2017). Mochizuki et al. (2009) reported that IL-4 increased tight junction permeability and disrupted barrier function in human intestinal T-84 cell monolayers. Our study suggests that deleterious changes in crypt cells caused by IL-4, such as deterioration of stem cells and loss of intestinal epithelial cell homeostasis, may also contribute to disruption of intestinal epithelial barrier function under allergic and inflammatory conditions.
In conclusion, the present study demonstrates for the first time that IL-4 can reduce stem cells and proliferative activity, and alter the functions of Paneth cells in enteroids. These findings have given a new insight into modulating the functions of the intestinal epithelial cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our gratitude to Dr. Hans Clevers (Hubrecht Institute) for the kind gift of the Noggin-secreting cell line, Dr. Eitaro Aihara (Cincinnati University) for kindly donating the R-spondin-secreting cell line, and Dr. Tokiyoshi Ayabe (Hokkaido University) for kind gift of anti-lysozyme antibody.
Author contributions
YS, KI, MT, Makoto Shimizu, and KK designed the experiments. YS, Mika Sato, and AI performed the experiments. YS, KI, Makoto Shimizu, and KK wrote the paper.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for JSPS fellows Grant Number JP18J22466.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andrews C, McLean MH, Durum SK. Cytokine tuning of intestinal epithelial function. Front Immunol. 2018;9:1–15. doi: 10.3389/fimmu.2018.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bartuzi Z, Zbikowska-Gotz M, Romański B, Sinkiewicz W. Evaluating the profile of selected cytokines in patients with food allergy and chronic gastritis. Med Sci Monit. 2000;6:1128–1135. [PubMed] [Google Scholar]
- Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol. 2016;594:4791–4803. doi: 10.1113/JP271667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, Sugimoto S, Sato T. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. 2018;23:787–793. doi: 10.1016/j.stem.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Ronzenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Gu YY, Cong N, Ma R, Chi FL. Celastrol enhances Atoh1 expression in inner ear stem cells and promotes their differentiation into functional auditory neuronal-like cells. Organogenesis. 2018;14:82–93. doi: 10.1080/15476278.2018.1462433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, Garrett WS. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. 2018;9:1–6. doi: 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SK, Harrington HA, Shepherd S, Brennan K, Dale T, Osborne JM, Gavaghan DJ, Byrne HM. The role of the Hes1 crosstalk hub in Notch-Wnt interactions of the intestinal crypt. PLoS Comput Biol. 2017;13:1–28. doi: 10.1371/journal.pcbi.1005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke MT, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci. 2013;16:1154–1161. doi: 10.1038/nn.3447. [DOI] [PubMed] [Google Scholar]
- Kordulewska NK, Cieślińska A, Fiedorowicz E, Jarmolowska B, Piskorz-Ogórek K, Kostyra E. Cytokines concentrations in serum samples from allergic children-multiple analysis to define biomarkers for better diagnosis of allergic inflammatory process. Immunobiology. 2018;223:648–657. doi: 10.1016/j.imbio.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Lechuga S, Ivanov AI. Disruption of the epithelial barrier during intestinal inflammation: quest for new molecules and mechanisms. Biochim Biophys Acta Mol Cell Res. 2017;1864:1183–1194. doi: 10.1016/j.bbamcr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Satsu H, Totsuka M, Shimizu M. Transepithelial transport of macromolecular substances in IL-4 treated human intestinal T84 cell monolayers. Biosci Biotechnol Biochem. 2009;73:2422–2426. doi: 10.1271/bbb.90383. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- Possidonio ACB, Senna ML, Portilho DM, Soares CP, Sampaio LS, Eincker-Lamas M, Branco MTLC, Costa ML, Mermelstein C. α-cyclodextrin enhances myoblast fusion and muscle differentiation by the release of IL-4. Cytokine. 2011;55:280–287. doi: 10.1016/j.cyto.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139–155. doi: 10.1034/j.1600-065X.2001.790114.x. [DOI] [PubMed] [Google Scholar]
- Saggini A, Maccauro G, Tripodi D, De Lutils MA, Conti F, Felaco P, Fulcheri M, Galzio R, Caraffa A, Antinolfi P, Felaco M, Pandolfi F, Sabatino G, Neri G, Shaik-Dasthagirisaheb YB. Allergic inflammation: role of cytokines with special emphasis on IL-4. Int J Immunopathol Pharmacol. 2011;24:305–311. doi: 10.1177/039463201102400204. [DOI] [PubMed] [Google Scholar]
- Saito Y, Iwatsuki K, Hanyu H, Maruyama N, Aihara E, Tadaishi M, Shimizu M, Kobayashi-Hattori K. Effect of essential amino acids on enteroids: methionine deprivation suppresses proliferation and affects differentiation in enteroid stem cells. Biochem Biophys Res Commun. 2017;488:171–176. doi: 10.1016/j.bbrc.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Weber CB. Anti-IL-4 as a new strategy in allergy. Chem Immunol Allergy. 2012;96:120–125. doi: 10.1159/000332235. [DOI] [PubMed] [Google Scholar]
- Shimizu M. Interaction between food substances and the intestinal epithelium. Biosci Biotechnol Biochem. 2010;74:232–241. doi: 10.1271/bbb.90730. [DOI] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Gregorieff A, Zeinstra L, van den Born M, Korving J, Martens ACM, van den Oudenaarden A, Clevers H. Dll1 marks early secretory progenitors in gut crypts that can revert to stem cells upon tissue damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Neerven SM, Vermeulen L. The interplay between intrinsic and extrinsic Wnt signaling in controlling intestinal transformation. Differentiation. 2019;108:17–23. doi: 10.1016/j.diff.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila MF, Trudeau MP, Hung YT, Zeng Z, Urriola PE, Shurson GC, Saqui-Salces M. Dietary fiber sources and non-starch polysaccharide-degrading enzymes modify mucin expression and the immune profile of the swine ileum. PLoS ONE. 2018;13:1–16. doi: 10.1371/journal.pone.0207196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LCH, Glynn AN, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects intestinal epithelial cells against LPS-induced apoptosis and barrier defects: a novel cellular rescue mechanism? FASEB J. 2005;19:1822–1835. doi: 10.1096/fj.05-4226com. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhu W, Xu F, Dai X, Shi L, Cai W, Mu H, Hitchens TK, Foley LM, Liu X, Yu F, Chen J, Shi Y, Leak RK, Gao Y, Chen J, Hu X. The interleukin-4/PPARγ signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 2019;17:1–33. doi: 10.1371/journal.pbio.3000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.