Abstract
Squamous cell carcinoma (SCC) is one of the most common malignant tumors of the oral cavity. Probiotics have often been considered as effective anti-tumoral candidates. This study aimed to investigate the role of Pichia fermentans YSH secretion metabolites on the induction of apoptosis in SCC. Cytotoxicity, apoptotic effects, and visualization DNA damage were evaluated by MTT, flow cytometry, and DAPI staining assays, respectively. Real-time PCR was employed for evaluation of the mechanism of cellular apoptosis. P. fermentans YSH secretions (IC50) showed cellular cytotoxicity in human tongue squamous carcinoma (HSC4, RRID:CVCL_1289) cells (85% apoptosis) similar to the cytotoxicity of cisplatin whereas only 21% apoptosis was observed in human epithelial normal (KDR, RRID:CVCL_9V14) cells. The prophylactic efficacy of reference yeast, which regarded as a reference, was not comparable to P. fermentans YSH illustrating strain-dependent properties of bioactivities on oral disease control and prevention. According to our result, the main cytotoxicity is related to apoptosis mechanisms induced by apoptosis genes inducing BAX and CASP. However, follow-up researches should be performed to recognize the compounds to be utilized as effective anticancer therapeutics.
Keywords: Anticancer, Apoptosis, Cytotoxicity, Yeast
Introduction
Oral squamous cell carcinoma is involved in about 94% of all oral malignancies. Numerous reports have been estimated that 9500 deaths of mouth and oropharynx cancer occurred every year (Udeabor et al. 2012). Oral cancer in Asian countries (especially in East Asia) as significantly different from those in western countries which are in the first place for men (Herman et al. 2015). Microbial composition in people with squamous cell carcinoma (SCC) has been evaluated by numerous researchers, which have indicated that microbial flora differed among healthy individuals and patients (Bolz et al. 2014). Besides, multiple parameters such as age, smoking cigarettes and age can affect microbial composition as well as SCC progression (Marttila et al. 2015). Though it has yet not been substantiated the oral flora carcinogenesis, cytokines have the potential to alter metabolic pathways and malignant transformation (Lee et al. 2015). Hence, patients with poor oral health were more undergoing oral tumor risk (Satheeshkumar and Mohan 2014). However, regardless of the challenges facing pharmacological cancer therapy, there is a limited therapeutic option in advanced oral cancer (Phuphanich et al. 2005). The phenomenon of induction of apoptosis by certain microorganisms may offer a complementary or alternative strategy for solid tumor treatment (Ghoneum et al. 2005). Subsequently, particular attention increased for determining mediators that induce apoptosis and suppress the metastasis of oral squamous cell carcinoma with minimum side effects. The most of the probiotic microorganisms in human microbiota belong to the genera of lactic acid producing bacteria, but also, several strains of yeast that present in fermented dairy products are considered as probiotics (Pinpimai et al. 2015). Among the multiple yeasts, Saccharomyces boulardii, Saccharomyces cerevisiae also several yeast strains belonging to Pichia, Candida, and Kluyveromyces are familiar probiotics because of their potential health benefits (Burns and Rowland 2000). Saccharomyces cerevisiae has been previously reported that can induce apoptosis in breast and tongue cancer (Ghoneum et al. 2008). Apoptosis is associated with the activating of several factors in apoptosis signaling pathways (Ren et al. 2015). The immunopotentiating activity of polysaccharides extracted from various sources is well documented. Among the polysaccharides of microbial origin, pichilan obtained from Pichia fermentans has been previously reported its immunostimulating activity (Desboeufs et al. 1988). Expression of apoptosis-related genes has been known as substantial factors that affect apoptosis (Hassan et al. 2014), as well are directly or indirectly involved in initiating apoptotic signals through the mitochondrial pathway (Jee-Youn et al. 2006). To ascertain the mechanisms underlying apoptosis induction, we examined the pivotal genes implicated in apoptosis pathways including anti-apoptotic genes (ERBB2, ERBB3), BCL2 family of anti-and pro-apoptotic-related genes (BCL-2, BCL-XL, and BAX), caspase-8 and -9 as two starter genes in and TNF alpha and intrinsic apoptosis pathway, respectively. Hence, the aim of the current study was to examine the anticancer activity of P. fermentans against oral squamous cell carcinoma (OSCC) and highlighted the related apoptotic molecular pathway.
Materials and methods
Materials
Yeast Malt Broth (YMB), yeast malt agar (YMA), Methanol, l-glutamine, Penicillin, Streptomycin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Formaldehyde, Phosphate-buffered saline (PBS), 4′,6-diamidino-2-phenylindole (DAPI), Trypsin-EDTA, Methylene blue, Sodium citrate solution and Fetal bovine serum (FBS) were purchased from Sigma Co. (St. Louis, MO, USA). Roswell Park Memorial Institute (RPMI) 1640 was obtained from Gibco Co. (Uxbridge, UK), AnnexinV-FITC/PI apoptosis kit (eBioscience, San Diego, ca.), RNX-plus solution (Sina Clone, Iran), Prime Script RT Reagent kit, SYBR Green PCR master mix (Takara Bio Inc., Tokyo, Japan), Mueller-Hinton agar plates (Oxiod Limited., Basingstoke, England) were used in present study. The HSC4 and KDR cells were purchased from National Cell Bank, (Pasteur Institute, Iran).
Yeast isolation
The traditional yogurt sample (10 g) from eastern Azerbaijan, a north-west area in Iran, was first suspended in 10 mL buffered sodium citrate solution (2% w/v), and gradually homogenized with stomacher (Seward Laboratory Systems Inc., Bohemia, NY, http://www.seward.co.uk) for about 5 min. Thereafter, the homogenized sample (about 1 mL) was dissolved in 14 mL liquid YMB media and incubated overnight at 37 °C. Finally, the yeast colony was isolated on YMA by using a streak plated procedure similar to the previous condition.
Molecular identification
The isolated yeast cells were placed in YMB media (25 mL) under the aerobic condition for 14 h at 37 ºC. For molecular identification, the method was completely used that has been previously described by Saber et al. (2017b).
Cell-free supernatant preparation
The reference yeast (P. fermentans PTCC 5296) and isolated yeast were cultured into culture tube 50 mL in YMB media under the aerobic condition at 37 ºC. To prepare cell-free supernatant (CFS), the method was completely used that has been previously described by Saber et al. (2017a). The filtrate (0.2 µm membrane filter; Millipore, Eschborn, Germany) sterile supernatant was applied for the treatment of HSC4 (OSCC cell line) and KDR (normal epithelial cell line).
Cell lines and cell culture media
The HSC4 (https://web.expasy.org/cellosaurus/CVCL_1289) and KDR (https://web.expasy.org/cellosaurus/CVCL_9V14) cell lines were cultured in RPMI 1640 supplemented with 10% (v/v) inactivated FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin (Bairoch 2018). Each cell lines with cell seeding density 4 × 104 (cell/cm2) were seeded in 25 cm2 T-flask in a humidified atmosphere containing 5% CO2 at 37 ºC.
MTT assay
The HSC4 and KDR cell lines (1.2 × 104 cells per well; 96-well microplate) were cultured for 24 h and incubated at 37 °C, 5% CO2, and then exposed to treatment by sterile yeast’s cell-free supernatant. The IC50 concentration of yeast supernatant was estimated in KDR cells by using MTT assay that has been explained by Haghshenas et al. (2015). The IC50 of cisplatin (2.5 µM) for the HSC4 cell line was used as a positive control group.
DAPI staining
The determination of nuclear morphological changes was performed by using the DAPI staining method (Nami et al. 2014). The HSC4 and KDR cell lines (total of 3.6 × 105 cells per well; 6-well culture plate) were cultured for 24 h and incubated standard culture conditions. Cells (3.6 × 105 cells/well) were incubated in 6-well culture plate that was covered by a sterile coverslip. After achieving sufficient confluence (50%), an untreated control group, treated with yeast supernatant (60 µL) and cisplatin (2.5 µM) as positive control were incubated for 24 h in standard culture condition. Next, both cell lines fixed with paraformaldehyde (4%), permeabilized with Triton-X100 (0.1%) for another 5 min and then stained with 50 µL diluted DAPI dye (1:2000) for nearly 3 min at ambient temperature. Slides were washed by PBS (pH 7.2) and alteration of nuclear morphology evaluated by fluorescent microscopy (Olympus BX64, Olympus, Japan) with U-MWU2 fluorescence filter (excitation filter BP 330e385, dichromatic mirror DM 400, emission filter LP 420).
Flow cytometry
The assessment of cellular apoptosis was performed using the FITC Annexin V apoptosis detection kit according to the standard protocol. Both cell lines were washed twice by PBS and once by binding buffer (1×) and the supernatant was removed after centrifugation in each washing stage. The cells were then resuspended in 100 µL of 1× binding buffer and all the contents were transferred into a 5 ml tube. Afterward, FITC conjugated Annexin V (5 µL) was added to 100 µL of the cell suspension and incubated for about 15 min in the dark condition at room temperature. After washing with 1× binding buffer, cells were completely resuspended in binding buffer (200 µL), and then propidium iodide solution (5 µL) was added to the cells and investigated by flow cytometry. Our results were analyzed by Cell Quest Pro software (BD Biosciences, San Jose, ca., USA). Analyses were carried out on 150,000 cells at a rate of 900 cells/s.
Real-time PCR amplification
All treated/untreated cells were thoroughly washed 3 times in PBS (pH 7.2). Total RNA of cells was extracted by the RNX-plus solution kit according to the manufacturer’s guidance. Quality and quantity assessments were assessed using agarose gel electrophoresis and UV spectrophotometry, respectively. The extracted RNA (1 µg/µL) was applied for complementary DNA (cDNA) synthesis using a Primescript RT reagent kit based on the manufacturer’s instructions. Details of the specific primers presented in Table 1. All experiments were conducted in a volume of 20 µL and investigated by ROTOR-GENETM 6000 Real-time analyzer (Corbett, Cambridge, UK). Each well included: 2.5 µL of 2× power SYBR green PCR master mix (Takara Bio Inc., Tokyo, Japan), 1 µL of cDNA template, 1 µL of specific primer and 10.5 µL of nuclease-free water. PCR cycling conditions were as follow 1 cycle of 94 °C for 10 min, 40 cycles of 95 °C for 15 s, 56–62 °C for 30 s, and 72 °C for 25 s. Data analysis was performed by Pfaffl’s model and the threshold cycle (CT) value was normalized to the GAPDH expression rate as an internal control (Pfaffl 2001).
Table 1.
Primers sequences for RT-PCR amplification
| Gene name and symbol | Sequence (5′–3′) | Amplicon size (bp) | TM | |
|---|---|---|---|---|
| F | R | |||
| GAPDH |
F:5′-AAGCTCATTTCCTGGTATGACAACG-3′ R:5′-TCTTCCTCTTGTGCTCTTGCTGG-3′ |
126 | 60.3 | 61.1 |
| ERBB 2 |
F: 5′-TGTGACTGCCTGTCCCTACAA-3′ R: 5′-CCAGACCATAGCACACTCGG-3′ |
152 | 62.8 | 62 |
| ERBB 3 |
F: 5′-GACCCAGGTCTACGATGGGAA-3′ R: 5′-GTGAGCTGAGTCAAGCGGAG-3′ |
100 | 62.7 | 62.5 |
| CASPAS 8 |
F: 5′-GACAGAGCTTCTTCGAGACAC-3′ R: 5′-GCTCGGGCATACAGGCAAAT-3′ |
116 | 60.1 | 63 |
| CASPAS 9 |
F: 5′-CTCAGACCAGAGATTCGCAAAC-3′ R: 5′-GCATTTCCCCTCAAACTCTCAA-3′ |
116 | 60.9 | 60.5 |
| BAX |
F: 5′-CCCGAGAGGTCTTTTTCCGAG-3′ R: 5′- CCAGCCCATGATGGTTCTGAT-3′ |
155 | 62.1 | 61.9 |
| BCL-2 |
F: 5′- GGTGGGGTCATGTGTGTGG-3′ R: 5′-CGGTTCAGGTACTCAGTCATCC-3′ |
99 | 62.6 | 61.8 |
| BCL-XL |
F: 5′- GAGCTGGTGGTTGACTTTCTC-3′ R: 5′-TCCATCTCCGATTCAGTCCCT-3′ |
119 | 60.6 | 61.9 |
Statistical analysis
The statistical analysis was analyzed by SPSS software version 16.0 (SPSS Inc, Chicago, IL, USA). The Kolmogorov–Smirnov test (KS test) was conducted for testing the normal distribution of data. Tukey’s post hoc test and ANOVA were performed for multiple mean comparisons of groups and statistical data analysis, respectively. p-value ≤ 0.05 was considered statistically significant and data were calculated as the mean ± SD for three repetitions of each experiment.
Results
Molecular identification
According to ITS1-5.8 s-ITS2 sequence-based identification through blast methods and considering actual threshold values of taxonomical cutoff (Khodadadi et al. 2017), the isolated yeast identified to P. fermentans YSH strain with high homology (99% to 100%).
Toxicity assay
The cellular cytotoxicity efficacy of P. fermentans YSH metabolites and specific reference yeast was performed by MTT assay against epithelial normal cell lines (KDR) and OSCC cell lines (HSC4). Also, time and dose dependence of cytotoxic effect induced by P. fermentans YSH secretions against HSC4 cancer cell line were assessed at 6, 12, and 24 h. The survival rates for HSC4 cells reduced slowly followed by an increasing incubation period and specified doses of secretions. Our results indicated that HSC4 cells growth was considerably inhibited after 24 h treatment, while the data for 6 and 12 h incubation were not significant (p ≤ 0.05) and didn’t provide.
The cytotoxic activities of metabolites against HSC4 cells differed markedly from reference strain-treated or un-treated groups, but the best results were observed by applying 60 µL/mL metabolites for 24 h incubation (Fig. 1). In addition, KDR normal cells treated with P. fermentans YSH secretions showed no toxic effects and more than 91% of the cells grown well.
Fig. 1.
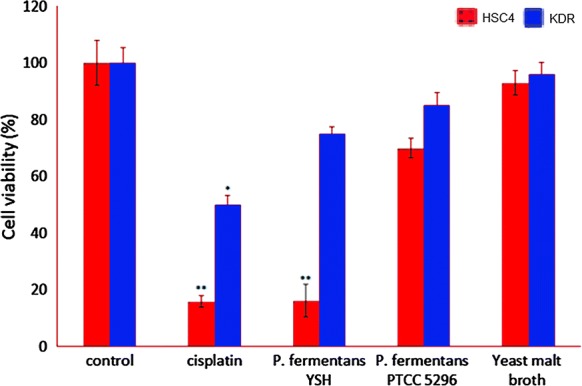
Effect of P. fermentans YSH secretion metabolites on the viability of HSC4 cancer cells and KDR normal cell by 60 µL for 24 h incubation. Data are expressed as mean viability ± SD (error bars). Asterisks denote statistically significant differences (**p ≤ 0.01, *p ≤ 0.05). Untreated cells were used as negative control and cisplatin was used as a positive control. Yeast malt broth as yeast culture medium was used to determine its effect on cell viability. All experiments were performed independently in triplicate
Morphological change observations
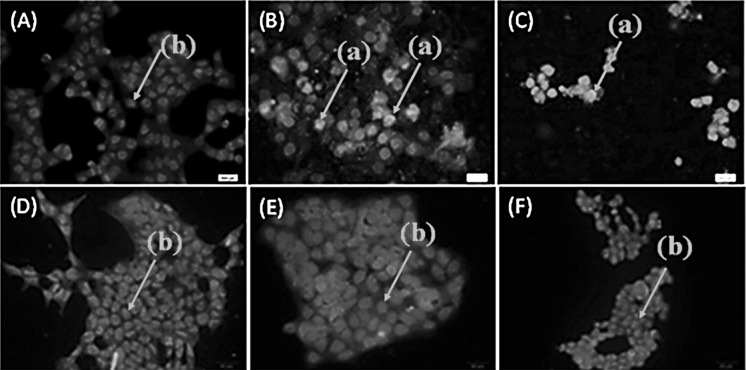
The result of DAPI staining indicated significant alteration including nuclear condensation, which detected in the treated HSC4 cells but not in healthy (KDR) cells (Fig. 2).
Fig. 2.
DAPI staining of treated/untreated HSC4 and KDR cells. A Represents untreated HSC4, B treated with cell-free supernatant (60 µL/mL) HSC4, and C treated with cisplatin (IC50) HSC4. D Represent untreated KDR, E treated with cell-free supernatant (60 µL/mL) KDR, and F treated with cisplatin (IC50) KDR cells. The incubation time was 24 h. The arrows either labeled with an (a) or (b) point to condensed and normal cell nuclei respectively. The condensed nuclei represent apoptotic/necrotic cells
Flow cytometry assay
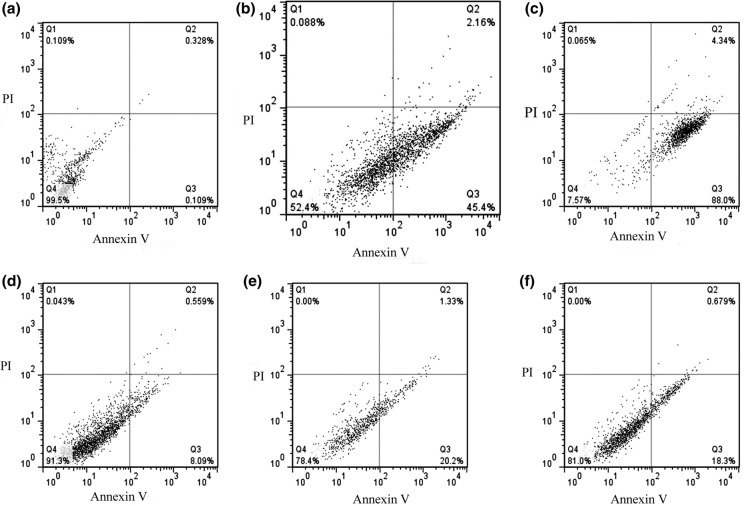
According to flow cytometry data, P. fermentans YSH (60 µL/mL) significantly caused an increase in the proportion of apoptotic cells in HSC4 cancer cells in comparison to normal cells (p ≤ 0.05) (Fig. 3). As shown in Fig. 3, in treated HSC4 and KDR cells, the rate of early apoptosis were 45.4% and 20.3% and late apoptosis 2.16% and 1.33%, respectively (p ≤ 0.05) (Fig. 3b, e). Our result indicated that P. fermentans YSH induced apoptosis in HSC4 cancer cells similar to cisplatin as approved chemotherapy drugs.
Fig. 3.
Flow cytometric analysis of treated/untreated HSC4 and KDR cells. a–c Represent untreated, treated with cell-free supernatant (60 µL/mL), and treated with cisplatin (IC50) at HSC4 cells and d–f represent untreated, treated with cell-free supernatant (60 µL/mL), and treated with cisplatin (IC50) at KDR cells respectively for 24 h incubation. Dots with Annexin V−/PI+ (Q1), Annexin V+/PI+ (Q2), Annexin V+/PI− (Q3), and Annexin V−/PI− (Q4) and feature represent necrotically, late apoptotic, early apoptotic, and viable intact cells respectively. All experiments were performed in triplicate
The levels of genes expression
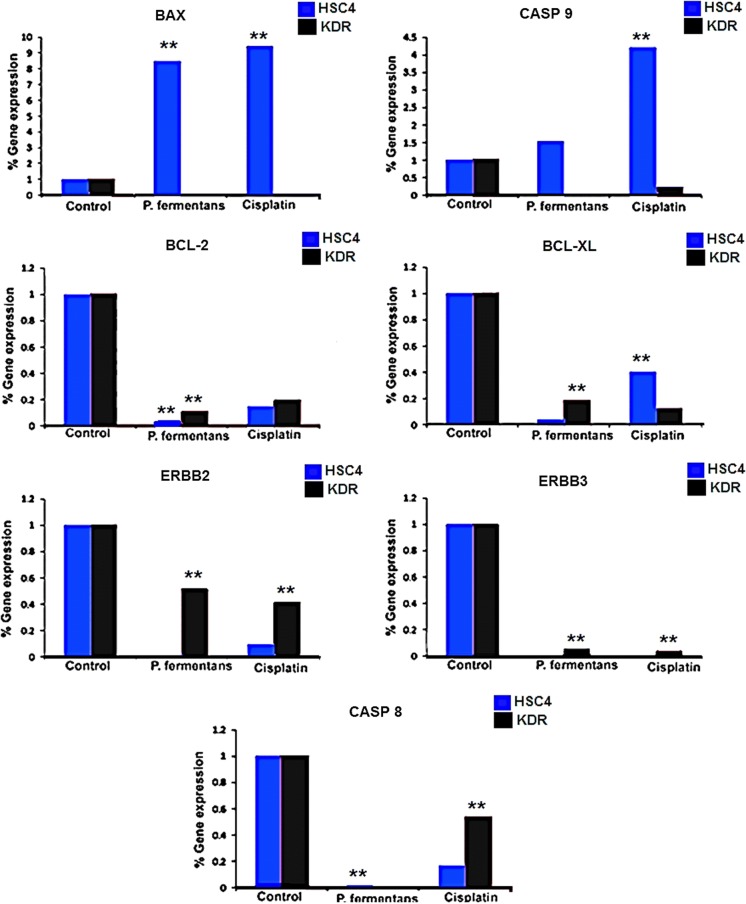
The expression of anti-apoptotic genes ERBB2, ERBB3, BCL-2, BCL-XL and starter gene in the extrinsic apoptotic pathway (caspase-8) substantially reduced by P. fermentans YSH in treated cells versus control cell lines (Fig. 4). The reduction of declared genes by P. fermentans YSH was similar to cisplatin while the level of CASP 9 (starter gene in mitochondrial apoptosis pathway) and BAX (crucial gene in extrinsic IL-3 mediated apoptosis pathway) genes were considerably different among the P. fermentans YSH and cisplatin-treated cells (Fig. 4). P. fermentans YSH increased the BAX gene expression in HSC4 treated cells whereas cisplatin up-regulated the expression of CASP 9 indicating different inducing pathways of apoptosis.
Fig. 4.
Apoptosis-related gene expression ratio in treated (60 µL P. fermentans YSH secretion metabolites) and untreated control HSC4 and KDR cells for 24 h. Asterisks denote statistically significant differences (**p ≤ 0.01). All experiments were performed independently in triplicate. (Color figure online)
Discussion
The anticancer activity of probiotic strains via apoptosis induction in tumor cells can be a capable strategy in treating many disorders. Therefore, much attention has been paid to finding new yeast strains with probiotic properties. By considering yeasts advantages as compared to probiotic bacteria, such as their higher resistance than bacteria, their capability to protect against pathogenic bacteria and toxic compounds, having a diverse enzymatic profile and more effective in the human immune system (Moslehi-Jenabian et al. 2010), as well Saccharomyces cerevisiae clinical cancer prevention effects as a probiotic in double-blind studies (Consoli et al. 2015), the most investigations have been focused on common species like S. cerevisiae, and other important probiotic yeasts that may be more effective and should not be overlooked. Our results reveal that P. fermentans YSH metabolite can be considered as a potential candidate for treating oral squamous carcinoma through performing additional advanced experiments in the future. Although a wide range of studies has been carried out regarding the effects of probiotic yeasts on different cancers, its exact mechanism and active compound(s) are not clear precisely. Besides, probiotics have been continually used in many cases including scientific research, pharmaceutical, and dietary supplements. Recently, the efficacy of probiotics in the oncology, immunology, and biology areas have significantly improved (Chong 2014). The probiotic effects have been recommended in the molecular and cellular as well as the clinical field for cancer treatment that often includes chemotherapy (Ciorba et al. 2015). In the current study, cytotoxicity effects of metabolites secreted by yeast on the OSCC cells (HSC4), were investigated using the MTT assay. The CFS at concentration 60 µL was as specified as the IC50 of P. fermentans YSH against HSC4 tumor cells. To determine the potential of yeasts’ CFS in induced DNA damage, all treated/untreated groups both HSC4/KDR cell lines were stained by DAPI and investigated using fluorescence microscopy to assess P. fermentans YSH secretions-efficacy against cell viability.
According to our data, the apoptosis process might be regarded as a key cytotoxic mechanism in yeast metabolites against cancer cells. Hence, P. fermentans YSH strain metabolites can be employed as a promising alternative for nutraceutical or pharmaceutical, due to it doesn’t have any side effect on normal cells. Collectively, secreted metabolites more specifically induced the apoptotic signaling pathways in cancerous cells compared with normal cell lines (Fig. 2). Flow cytometry dot-plots analysis was achieved by both treated and untreated control groups illustrated that the production of yeast secretory metabolites by P. fermentans YSH caused to increase in apoptosis in cancer cells (47.56%) but developing apoptosis in normal cells was only 21.53%. Overall, these findings recommend that the induction of apoptosis by the secretory metabolites of yeast in OSCC more than healthy cells. Moreover, necrosis incidence (0.0%) resulted from P. fermentans YSH demonstrates the lower necrotic cytotoxicity in HSC4 and KDR cells. Cisplatin is used as standard chemotherapy treatment for oral cancer patients is defined as a positive control group. The flow cytometry results in treated cells with cisplatin exhibited that cellular apoptosis is induced by 88% of HSC4 cells and 18.9% of KDR cells. Among the platinum family of anticancer compounds, cisplatin has shown very effective against human embryonal carcinoma (Tripathi et al. 2011), but unfortunately, its use is significantly limited by severe dose-limiting side effects such as nephrotoxicity, autotoxicity, and peripheral neurotoxicity (Karasawa and Steyger 2015). The findings of this current study showed the P. fermentans YSH secretions could effectively suppress the human oral squamous carcinoma (HSC4) growth through inhibiting cell proliferation with a dose-dependent feature. Effects of P. fermentans YSH on important apoptotic pathway genes expression were indicated by using real time-PCR analysis. Applying P. fermentans YSH lead to the significant downregulation in ERBB 2, ERBB 3, BCL-2 and BCL-XL, CASP 8 in all treated groups compared with control groups (Fig. 4). Additionally, the reduction of referred genes expression through P. fermentans YSH in all treated cells was similar to cells that were treated with cisplatin. In contrast, the level of CASP 9 and BAX, respectively, as two effective factors in intrinsic and extrinsic apoptosis signaling pathways were markedly different in P. fermentans YSH compared with cisplatin-treated cells (Fig. 4). The over-expression of BAX and CASP9 genes in HSC4 treated cells with P. fermentans YSH secretions reduced proliferation and induced cellular apoptosis (Fig. 4). The spontaneous release of cytochrome c (Cyt c) from mitochondria and binding to Apaf-1, induced apoptosome formation and activated caspase-9, as the initiator of the intrinsic apoptosis pathway (Ashkenazi 2002). Anti-apoptotic BCL2 family (BCL2, BCL-XL) regulates the mitochondria-initiated caspase activation pathway by preventing the release of cytochrome c in the presence of apoptotic stimuli (Ashkenazi et al. 1999). The human research has revealed that consumption of fermented dairy products such as milk and probiotic yogurt can be effective in colorectal cancer prevention (Yang et al. 2014). Mechanisms of mentioned microorganisms include stimulating inflammatory cytokines production in the host, enzyme activity alteration, prevention of carcinogen uptake and followed by a decrease in mutagenicity and also production of anticancer and cytotoxic agents (Habil et al. 2014). The lovastatin (LV) in red yeast reduced proliferation and induced cell apoptosis in both colorectal cancer cells HT-29 and HCT-116 (Mee et al. 2008). Based on our findings, P. fermentans YSH may produce metabolites that inhibit the SCC cell growth, which confirmed through the results of MTT assay, flow cytometry, DAPI staining, and real-time PCR. However, those produced low toxicity on the viability of KDR normal cell lines. Overall, the results of the present study demonstrated the safety of declared metabolites for the consumption of individual as pharmaceutical/nutraceutical compounds as a preventive therapy.
Conclusions
Supernatants from cultures of P. fermentans YSH from traditional yogurt, as well supernatants from a reference yeast, were applied to cultures of human oral squamous cells (OSCC) and of normal epithelial cells (KDR), either alone or with the anticancer agent cisplatin, to investigate their potential therapeutic properties. The cytotoxicity results show P. fermentans YSH significantly inhibition on the cell growth of HSC4 cancer cells similar to the anticancer efficacy of cisplatin on the HSC4 cancer cell. In addition, P. fermentans YSH secretions exhibited non-cytotoxicity to KDR as an epithelial normal cell.
Acknowledgements
The facilities support of the Tabriz University of Medical Sciences is gratefully acknowledged.
Authors’ contributions
HBS performed the experiment; ZA participated in the protocol design and provided help; AS and HBS prepared the manuscript; AYKH participated in the discussion, protocol design, edit and revised the manuscript. All authors read and approved the final manuscript for submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Please contact the author for data requests.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
This study has “Not applicable” any individual person’s data.
Ethics approval and consent to participate
This study has “Not applicable” any individual person’s data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investig. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairoch A. The cellosaurus, a cell-line knowledge resource. J Biomol Technol. 2018;29:25–38. doi: 10.7171/jbt.18-2902-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J, Dosa E, Schubert J, Eckert AW. Bacterial colonization of microbial biofilms in oral squamous cell carcinoma. Clin Oral Investig. 2014;18:409–414. doi: 10.1007/s00784-013-1007-2. [DOI] [PubMed] [Google Scholar]
- Burns A, Rowland I. Anti-carcinogenicity of probiotics and prebiotics. Curr Issues Intest Microbiol. 2000;1:13–24. [PubMed] [Google Scholar]
- Chong ES. A potential role of probiotics in colorectal cancer prevention: review of possible mechanisms of action. World J Microbiol Biotechnol. 2014;30:351–374. doi: 10.1007/s11274-013-1499-6. [DOI] [PubMed] [Google Scholar]
- Ciorba MA, Hallemeier CL, Stenson WF, Parikh PJ. Probiotics to prevent gastrointestinal toxicity from cancer therapy: an interpretive review and call to action. Curr Opin Support Palliat Care. 2015;9:157–162. doi: 10.1097/SPC.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli ML, da Silva RS, Nicoli JR, Bruna-Romero O, da Silva RG, de Vasconcelos Generoso S, Correia MI. Randomized clinical trial: impact of oral administration of Saccharomyces boulardii on gene expression of intestinal cytokines in patients undergoing colon resection. J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115584387. [DOI] [PubMed] [Google Scholar]
- Desboeufs J, Ralambosoa C, Astoin J, Joram C, Salhi SL, Bastide JM. Structural characterization of pichilan, a beta-d-glucan immunostimulant from Pichia fermentans. Chem Pharm Bull. 1988;36:2766–2771. doi: 10.1248/cpb.36.2766. [DOI] [PubMed] [Google Scholar]
- Ghoneum MHJ, Brown J, Gollapudi S. Human squamous cell carcinoma of the tongue and colon undergoes apoptosis upon phagocytosis of Saccharomyces cerevisiae, the baker’s yeast, in vitro. Anticancer Res. 2005;25:981–989. [PubMed] [Google Scholar]
- Ghoneum M, El-Din NKB, Noaman E, Tolentino L. In vivo tumor inhibitory effects of Saccharomyces cerevisiae on Ehrlich carcinoma-bearing mice. Cancer Immunol Immunother. 2008;57:581–592. doi: 10.1007/s00262-007-0398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habil N, Abate W, Beal J, Foey AD. Heat-killed probiotic bacteria differentially regulate colonic epithelial cell production of human beta-defensin-2: dependence on inflammatory cytokines. Benef Microbes. 2014;5:483–495. doi: 10.3920/BM2013.0061. [DOI] [PubMed] [Google Scholar]
- Haghshenas B, Nami Y, Abdullah N, Radiah D, Rosli R, Khosroushahi AY. Anticancer impacts of potentially probiotic acetic acid bacteria isolated from traditional dairy microbiota. LWT-Food Sci Technol. 2015;60:690–697. doi: 10.1016/j.lwt.2014.09.058. [DOI] [Google Scholar]
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Herman MP, Dagan R, Amdur RJ, Morris CG, Werning JW, Vaysberg M, Mendenhall WM. Postoperative radiotherapy for patients at high risk of recurrence of oral cavity squamous cell carcinoma. Laryngoscope. 2015;125:630–635. doi: 10.1002/lary.24938. [DOI] [PubMed] [Google Scholar]
- Jee-Youn K, Su-Mi K, Jeong-Hun K, Ji-Hye Y, Jin-Hae P, Jae-Hoon P. Interaction of pro-apoptotic protein HGTD-P with heat shock protein 90 is required for induction of mitochondrial apoptotic cascades. FEBS Lett. 2006;580:3270–3275. doi: 10.1016/j.febslet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015;237:219–227. doi: 10.1016/j.toxlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi H, Karimi L, Jalalizand N, Adin H, Mirhendi H. Utilization of size polymorphism in ITS1 and ITS2 regions for identification of pathogenic yeast species. J Med Microbiol. 2017;66:126–133. doi: 10.1099/jmm.0.000426. [DOI] [PubMed] [Google Scholar]
- Lee CH, Chang JS, Syu SH, Wong TS, Chan JY, Tang YC, Yang ZP, Yang WC, Chen CT, Lu SC, Tang PH, Yang TC, Chu PY, Hsiao JR, Liu KJ. IL-1beta promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230:875–884. doi: 10.1002/jcp.24816. [DOI] [PubMed] [Google Scholar]
- Marttila E, Uittamo J, Rusanen P, Lindqvist C, Salaspuro M, Rautemaa R. Site-specific acetaldehyde production and microbial colonization in relation to oral squamous cell carcinoma and oral lichenoid disease. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:697–699. doi: 10.1016/j.oooo.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Mee YH, Navindra PS, Yanjun Z, David H. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J Nutr Biochem. 2008;19:448–458. doi: 10.1016/j.jnutbio.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslehi-Jenabian S, Lindegaard L, Jespersen L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients. 2010;2:449–473. doi: 10.3390/nu2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami Y, Abdullah N, Haghshenas B, Radiah D, Rosli R, Khosroushahi AY. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe. 2014;28:29–36. doi: 10.1016/j.anaerobe.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuphanich S, Baker SD, Grossman SA, Carson KA, Gilbert MR, Fisher JD, Carducci MA. Oral sodium phenylbutyrate in patients with recurrent malignant gliomas: a dose escalation and pharmacologic study. Neuro Oncol. 2005;7:177–182. doi: 10.1215/S1152851704000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinpimai K, Rodkhum C, Chansue N, Katagiri T, Maita M, Pirarat N. The study on the candidate probiotic properties of encapsulated yeast, Saccharomyces cerevisiae JCM 7255, in Nile Tilapia (Oreochromis niloticus) Res Vet Sci. 2015;102:103–111. doi: 10.1016/j.rvsc.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhu H, Chi C, Yang F, Xu X. MiRNA-139 regulates oral cancer Tca8113 cells apoptosis through Akt signaling pathway. Int J Clin Exp Pathol. 2015;8:4588–4594. [PMC free article] [PubMed] [Google Scholar]
- Saber A, Alipour B, Faghfoori Z, Khosroushahi AY. Secretion metabolites of dairy Kluyveromyces marxianus AS41 isolated as probiotic, induces apoptosis in different human cancer cell lines and exhibit anti-pathogenic effects. J Funct Foods. 2017;34:408–421. doi: 10.1016/j.jff.2017.05.007. [DOI] [Google Scholar]
- Saber A, Alipour B, Faghfoori Z, Mousavi Jam A, Yari Khosroushahi A. Secretion metabolites of probiotic yeast, Pichia kudriavzevii AS-12, induces apoptosis pathways in human colorectal cancer cell lines. Nutr Res. 2017;41:36–46. doi: 10.1016/j.nutres.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Satheeshkumar PS, Mohan MP. Malignant potential of oral submucous fibrosis due to intraoral extraction wounds and poor oral hygiene. Oral Oncol. 2014;50:e5–e6. doi: 10.1016/j.oraloncology.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Tripathi R, Samadder T, Gupta S, Surolia A, Shaha C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol Cancer Ther. 2011;10:255–268. doi: 10.1158/1535-7163.MCT-10-0606. [DOI] [PubMed] [Google Scholar]
- Udeabor SE, Rana M, Wegener G, Gellrich NC, Eckardt AM. Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol. 2012;4:28. doi: 10.1186/1758-3284-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, McCullough ML, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, Flanders WD, Campbell PT. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2014;32:2335–2343. doi: 10.1200/JCO.2014.55.3024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the author for data requests.