Abstract
Current cell transplantation techniques are hindered by small graft size, requiring high cell doses to achieve therapeutic cardiac remuscularization. Enhancing the proliferation of transplanted human embryonic stem cell-derived cardiomyocytes (hESC-CMs) could address this, allowing an otherwise subtherapeutic cell dose to prevent disease progression after myocardial infarction. In this study, we designed a hydrogel that activates Notch signaling through 3D presentation of the Notch ligand Delta-1 to use as an injectate for transplanting hESC-CMs into the infarcted rat myocardium. After 4 weeks, hESC-CM proliferation increased 2-fold and resulted in a 3-fold increase in graft size with the Delta-1 hydrogel compared to controls. To stringently test the effect of Notch-mediated graft expansion on long-term heart function, a normally subtherapeutic dose of hESC-CMs was implanted into the infarcted myocardium and cardiac function was evaluated by echocardiography. Transplantation of the Delta-1 hydrogel + hESC-CMs augmented heart function and was significantly higher at 3 months compared to controls. Graft size and hESC-CM proliferation were also increased at 3 months post-implantation. Collectively, these results demonstrate the therapeutic approach of a Delta-1 functionalized hydrogel to reduce the cell dose required to achieve functional benefit after myocardial infarction by enhancing hESC-CM graft size and proliferation.
Keywords: cell transplantation, Notch signaling, cardiomyocyte, stem cells, cardiac regeneration, angiogenesis, vascularization
Graphical Abstract
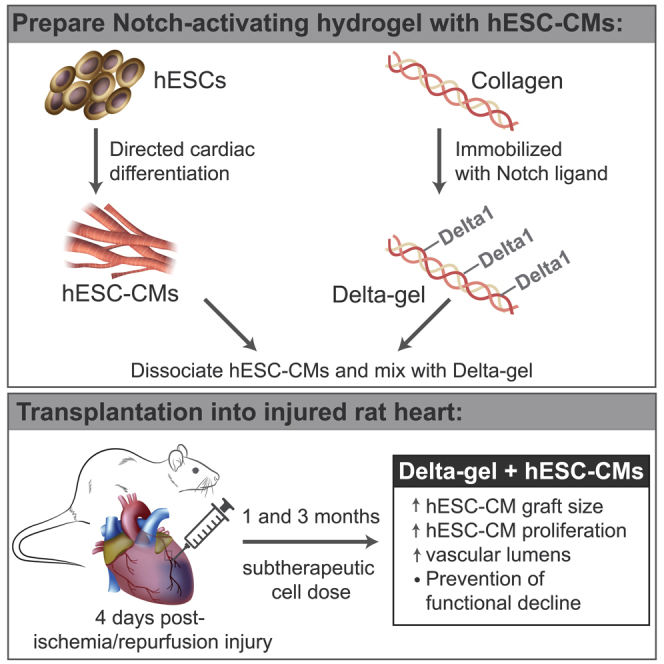
Transplantation of hESC-derived cardiomyocytes can remuscularize injured myocardium, but it requires a high therapeutic cell dose. Murry and colleagues developed a Notch-activating hydrogel used as a vehicle for transplantation of a subtherapeutic cell dose. When injected into injured rat myocardium, this hydrogel promoted hESC-cardiomyocyte proliferation, increased graft size, and prevented functional decline.
Introduction
Stem cell-derived cardiomyocyte cell therapy has been established as a promising strategy for cardiac repair following myocardial infarction. Numerous groups have demonstrated both long-term engraftment and increased cardiac function in acute models of myocardial infarction in rodents and large animals.1, 2, 3, 4, 5, 6, 7 However, established techniques for transplanting human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in vivo are hindered by small graft sizes, resulting from limited early cell retention and high rates of post-transplant cell death.8, 9, 10 Consequently, a high cell dose is required in order to achieve a therapeutic response after transplantation. Similar issues face other cell therapies, including those involving neural or islet cells.11,12 While tissue engineering strategies may address some of these limitations by implanting bulk tissues,13, 14, 15 other issues arise such as reduced electromechanical integration and the need for invasive implantation techniques.5 Thus, to facilitate the clinical translation and scalability of hESC-CM cell therapy, there is a need for methods to enhance graft size and to minimize the number of cardiomyocytes required for transplantation.
One strategy to address this is to enhance cardiomyocyte proliferation in vivo after transplantation. Notch signaling has been previously demonstrated to regulate cardiomyocyte proliferation,16, 17, 18, 19, 20, 21, 22 and full-length Notch ligands have been used to stimulate hESC-CM cell cycle activity in vitro.19 Direct cell-cell contact is typically required for Notch activation, as tension between the ligand and receptor likely exposes an extracellular cleavage site prior to releasing the Notch intracellular domain (NICD), which functions as a transcriptional cofactor. This presents a major hurdle in designing therapies utilizing Notch signaling, as ligands require immobilization and orientation through a signaling cell or surface to elicit a robust response.23,24 Previous studies have addressed this in vitro by activating Notch through ligand immobilization on plates or beads18,19,25,26 or by utilizing viral overexpression systems;17,18 however, these techniques are limited in their translational potential due to more complicated delivery techniques required.27,28
An alternative approach that is compatible with cell-based therapy is to immobilize Notch ligands within an injectable biomaterial. Many injectable materials have been investigated for myocardial transplantation, including naturally occurring extracellular matrix (ECM)-derived proteins as well as synthetic biomaterials;29,30 however, few studies have modified the materials to immobilize signaling proteins in order to manipulate cell fate.29,31 Notch activation has been achieved in this context through a self-assembling peptide functionalized with a peptide mimic of the Notch ligand Jagged-1; however, these studies were limited to c-kit+ rat progenitor cells,16 now known to have minimal cardiogenic potential.32,33 We hypothesized that Notch ligand immobilization onto a natural, 3D scaffold would allow for transient activation of the Notch pathway in stem cell-derived cardiomyocytes, which could be used to promote proliferation and enhance engraftment after transplantation into a cardiac injury model. Thus, we sought to design an approach that would be compatible with established hESC-CM cell therapy techniques, using an injectable biomaterial that gels in situ to allow for needle delivery of hESC-CMs and the Notch ligand into the myocardial wall.
In this study, we have developed a novel approach to reduce the required therapeutic dose of cells for myocardial repair by promoting proliferation of injected cardiomyocytes via immobilized Notch signaling in a conveniently injectable hydrogel scaffold. We designed a collagen-based hydrogel with the immobilized Notch ligand Delta-1, which is used to promote the proliferation of engrafted cardiomyocytes after transplantation through activating the Notch signaling pathway. This Delta-1-functionalized hydrogel was first validated in vitro, where activating Notch signaling resulted in an increase in hESC-CM proliferation. The hydrogel was then used as an injectate for hESC-CM transplantation into rat myocardial infarcts, resulting in a 2-fold increase in engrafted cardiomyocyte proliferation and a corresponding 3-fold increase in graft size compared to controls. Furthermore, implantation of the Delta-1-functionalized hydrogel with a subtherapeutic dose of hESC-CMs prevented functional decline in the heart at 3 months.
Results
Delta-1 Promotes Notch Signaling and hESC-CM Proliferation in 2D In Vitro
Notch signaling activation with the Notch ligand Delta-1 was first confirmed in 2D using a U2OS CSLluc/ren luciferase reporter cell line (Figure S1). The Delta-1 ligand was fused to an Fc domain, and this ligand was immobilized and presented in an oriented manner by binding it to anti-immunoglobulin G (IgG), which had been adsorbed to the 2D tissue culture polystyrene (TCPS) plates. In this context, peak luciferase expression occurred between 48 and 72 h with an increase of 3.5 ± 0.2-, 5.8 ± 0.2-, 6.1 ± 0.4-, and 4.6 ± 0.2-fold over uncoated TCPS and control surfaces that had been coated with IgG at 24, 48, 72, and 96 h, respectively (Figure S1A, p <0.005 at all time points), with expression returning to baseline by 7 days (Figure 1A). To promote attachment of hESC-CMs and permit longer-term culture on 2D surfaces, fibronectin was added, which further enhanced the response to Delta-1 (Figure S1B), likely due to more cell spreading and contact area on TCPS. Culturing high-purity hESC-CMs (>95% cTnT [cardiac troponin T]+) on Delta-1-coated surfaces (Delta) resulted in a significant increase in proliferation compared to hESC-CMs cultured on IgG-coated surfaces (control), measured by immunohistochemistry for double-labeled BrdU (5-bromo-2′-deoxyuridine)+/βMHC (β myosin heavy chain)+ cells. This proliferative response was dose-dependent, with absolute proliferation rates for Delta increasing by 15.1% ± 3.1%, 22.0% ± 6.7%, and 11.5% ± 2.9% on 5, 10, and 20 μg/mL surfaces, respectively, versus control (Figure S1C). This enhanced cell cycle activity also corresponded with a significant increase in cell number, suggesting that the cardiomyocytes completed mitosis rather than becoming tetraploid (Figure S1D). Addition of a γ-secretase inhibitor, which inhibits cleavage and release of the NICD, blocked the increase in cell number induced by Delta, consistent with a Notch-dependent effect. Interestingly, proliferation rates in control plates were also reduced by γ-secretase inhibition. Although we cannot rule out off-target effects of γ-secretase in this context, this suggests that Notch signaling may contribute to the basal proliferation rates of hESC-CMs in monolayers (Figure S1D).
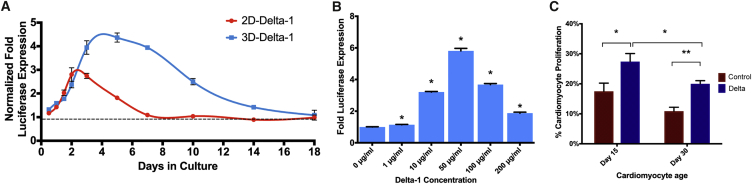
Figure 1.
Prolonged Notch Activation in 3D Gels Is Dose-Dependent and Promotes hESC-CM Proliferation
(A) Time course analysis of Notch-mediated luciferase expression in U2OS CSLluc/ren Notch reporter cell line indicates a prolonged Notch signal response in 3D culture conditions compared to the 2D Delta-1 platform. Luciferase signal is expressed normalized to IgG controls (2D or 3D) and plotted against days in culture. IgG controls were normalized to 1 (dashed line). (B) Dose-dependent Notch activation. Culturing the U2OS CSLluc/ren Notch reporter cell line in the 3D Delta-1 gel results in Delta-1 dose-dependent activation of Notch signaling, as indicated by fold luciferase expression compared to IgG control gels. (C) hESC-CMs in 3D-engineered cardiac tissues proliferate in response to Delta-1 (Delta). Cardiomyocyte proliferation was measured by histology as double-positive βMHC+/BrdU+ cells, which results in a significant increase in response to Delta with day 15 cells and day 30 cells over IgG controls (control). Note that although proliferation slows over time, significant augmentation by Delta is still possible at day 30. For (B), p-values were calculated using a one-way ANOVA with all samples compared to 0 μg/mL followed by Dunnett’s multiple comparison test. For (C), p-values were calculated using a multiple unpaired t-test without assuming a consistent SD. ∗p < 0.05, ∗∗p < 0.005. Error bars denote SEM. See also Figures S1 and S2.
Delta-1 Promotes Notch Signaling and hESC-CM Proliferation in 3D-Engineered Tissues In Vitro
Previous work with recombinant proteins showed that soluble Notch ligands do not induce signaling, whereas immobilizing the ligand to the tissue culture substrate induces robust signaling.24,34 We sought to achieve controlled Notch signaling in a 3D culture environment, so we designed a collagen-based scaffold with immobilized Delta-1 by cross-linking anti-IgG to collagen and subsequently loading the gel with Delta-1-Fc ligand or with IgG (control). We then validated Notch activation in vitro by forming engineered tissues using either the U2OS CSLluc/ren reporter cells or hESC-CMs. While direct, unoriented conjugation of Delta-1 did not significantly increase Notch signaling over controls in 3D collagen gels, we found that linking Delta-1 through an intermediate anti-IgG protein allowed for ligand orientation and resulted in a 3.7 ± 0.2-fold increase over control gels (p < 0.005), and a 3.1 ± 0.1-fold increase over unoriented Delta-1 (p < 0.005) (Figure S2A). This activation was further optimized by increasing ligand-collagen incubation time (Figure S2B), which led to a significant and dose-dependent increase in Notch signaling compared to the established 2D ligand coating platform (Figures 1A and 1B). Our finding of the requirement for Delta-1 orientation to elicit a response is consistent with previously published work demonstrating that Notch ligands must be immobilized onto a surface to effectively initiate Notch signaling.24,34 Based on our confirmation of this and these previously published studies demonstrating the ineffectiveness of soluble Notch ligands, we did not test the effect of soluble Delta-1 in our system. However in 3D Notch gels with oriented and immobilized Delta-1, Notch-driven luciferase expression peaked at day 5 with a 4.4 ± 0.2-fold increase over controls, and it remained 2.5 ± 0.1-fold higher than 3D control gels at day 10 (Figure 1A). Luciferase expression was still detectable after 2 weeks in 3D Delta-1 gels but declined back to baseline levels by day 18 (1.1 ± 0.2-fold). This represents a 3.9-fold increase in total Notch signal (area under the curve) when compared to 2D platforms.
When hESC-CMs were seeded into the Delta-1 gels to form engineered cardiac tissues, we found that hESC-CM proliferation was significantly increased compared to control (Figure 1C). Tissues formed with hESC-CMs at day 15 after the initiation of directed differentiation showed a proliferative rate of 17.5% ± 2.7% in control tissues compared to 27.4% ± 2.7% in Delta-1 tissues (p = 0.037). As expected, older hESC-CMs at day 30 after directed differentiation showed a lower basal rate of proliferation but also demonstrated a proliferative response to Delta-1 (20.0% ± 1.0% on Delta-1 compared to 10.9% ± 1.3% with control, p = 0.0002). These data demonstrate that the 3D Delta-1 gel induces Notch signaling and increases proliferation in human cardiomyocytes in engineered heart tissues.
Delta-1 Gel Increases Graft Size and Vascularization in Infarcted Hearts
Following in vitro validation of the collagen gel with immobilized Delta-1, we next investigated whether Notch signaling could enhance proliferation and improve cellular engraftment in vivo in a model of myocardial infarction. We modified established protocols in our laboratory for transplanting hESC-CMs into the infarcted rat heart with pro-survival cocktail1,5,6 to include gel functionalized with either IgG (control) or Delta-1 (Delta), as 50:50 v/v of the injectate along with implantation of 10 × 106 hESC-CMs. Unexpectedly, we found no significant difference in graft area (quantified from histology for βMHC) or infarct area (quantified from picrosirius red stain) identified between groups (Figures S3A and S3B). A previous study suggests that Notch signaling is inhibited by cyclosporine A,35 one of the components of our pro-survival cocktail. Indeed, in vitro addition of pro-survival cocktail components, including cyclosporine, resulted in a 2-fold reduction in the Notch signaling response in Delta gels compared to standard media treatment (p < 0.0001, Figure S3C). To validate this in vivo, we excluded the pro-survival cocktail and performed a pilot study (n = 2 per group) injecting a lower dose of cells (5 million cells per heart) in the same gel volume (either control + hESC-CMs or Delta + hESC-CMs) and found a 2-fold enhancement in graft size in the Delta gel (Figure S4). Based on these results, pro-survival cocktail was excluded from all subsequent studies.
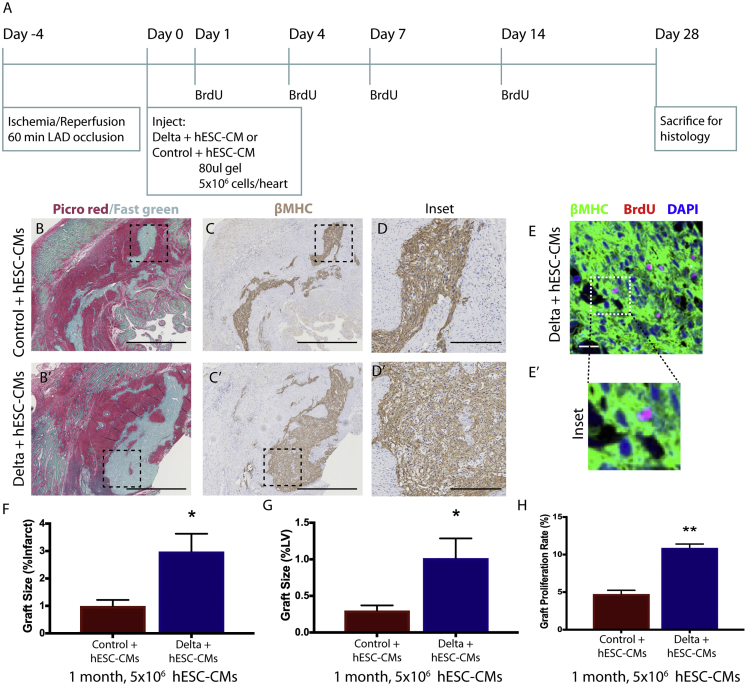
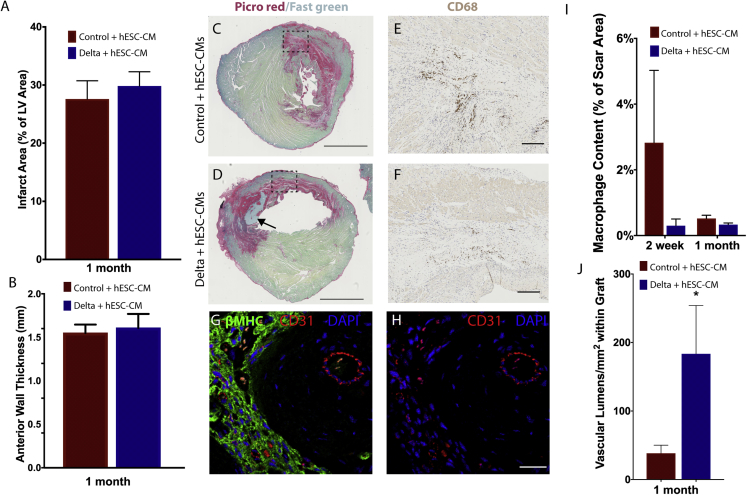
In the 2-week pilot study, graft size in the Delta-1 group was comparable to control grafts (with pro-survival cocktail) at 1 month, despite having only half the cell dose and half the time for in situ proliferation. This suggests that when coupled with pro-proliferative Notch signaling, fewer implanted cells may be required to achieve myocardial remuscularization. To test this with greater rigor, we systematically assessed graft size at 1 month after transplantation using a lower cell dose. Five million hESC-CMs were transplanted into the infarcted rat myocardium in either control or Delta gel (control + hESC-CMs and Delta + hESC-CMs, respectively) (Figure 2A; Figure S5). Four weeks after implantation, human myocardial grafts were identified within the infarct regions by histology for βMHC (Figure 2B–2D). Implantation of Delta + hESC-CMs resulted in a significant 3-fold increase in graft area compared to implantation of control + hESC-CMs, with grafts covering 3.0% ± 0.6% of the infarct region in Delta + hESC-CMs and only 1.0% ± 0.2% in control + hESC-CMs (p = 0.04, Figure 2F). This corresponded to human myocardial grafts that comprised 1.0% ± 0.3% of the left ventricle in Delta + hESC-CMs, a 3-fold increase over control + hESC-CMs (p = 0.016, Figure 2G). Consistent with the in vitro studies, Delta + hESC-CMs showed a higher level of cell cycle activity, identified by co-staining for BrdU and βMHC (animals were given a BrdU pulse at days 1, 4, 7, and 14 post-implantation). Delta + hESC-CMs were 10.9% ± 0.5% double-positive for BrdU+/βMHC+ compared to only 4.8% ± 0.5% in control + hESC-CMs, corresponding to a 2.3-fold increase in proliferation (p = 1.27−05, Figure 2H). Infarct area and anterior wall thickness were equivalent between groups (Figures 3A and 3B). Vascularization was assessed by histological staining for CD31 to label endothelial cells, followed by quantifying microvessel density within the graft region. Delta + hESC-CMs increased neovascularization by 4.4 ± 1.3-fold compared to control + hESC-CMs (p = 0.04, Figures 3G, 3H, and 3J). We also observed a decreasing trend of macrophage content normalized to infarct area at 2 and 4 weeks; however, due to high variability in the control group, this was not statistically different (Figures 3C–3F and 3I; Figures S4F and S4F′).
Figure 2.
Notch Signaling Enhances hESC-CM Engraftment and Proliferation at 1 Month Post-Implantation
(A) Experimental timeline. Four days after ischemia/reperfusion injury, 5 × 106 hESC-CMs were transplanted in either IgG- or Delta-1-modified gel (control + hESC-CMs and Delta + hESC-CMs, respectively), and tissues were harvested after 4 weeks. (B and B′) Collagenous scar area is identified by picrosirius red staining with a fast green counterstain to label healthy myocardium. Outline identifies region of interest. Scale bars, 1 mm. (C and C′) In serial sections, human myocardial grafts are identified by staining for β myosin heavy chain (βMHC, brown) with hematoxylin counterstain. Scale bars, 1 mm. (D and D′) Regions of interest outlined in previous panels are shown at higher magnification with staining for βMHC. Scale bars, 250 μm. (E) Proliferation of transplanted hESC-MCs is identified by histology. Tissue sections are stained with antibodies to detect βMHC (green), BrdU (pink), and nuclear counterstain of DAPI (blue). Scale bar, 200 μm. Region of interest outlined in (E) is shown at higher magnification in (E′). (F) Graft area normalized to left ventricular area is significantly augmented in Delta + hESC-CMs. (G) Graft area normalized to infarct area is significantly augmented in Delta + hESC-CMs. (H) Engrafted cardiomyocyte proliferation identified by βMHC+/BrdU+ cells is significantly enhanced in Delta + hESC-CMs. For (F)–(H), p-values were calculated using an unpaired two-tailed t-test. ∗p < 0.05, ∗∗p < 0.0001. Error bars represent SEM. See also Figures S3–S5.
Figure 3.
Notch Signaling Enhances Vascularization with Similar Infarct Size at 1 Month Post-Implantation
(A) Average infarct area is assessed by histology and normalized to left ventricular (LV) area at 4 weeks. (B) Anterior wall thickness by histology is shown for an area approximately 4 mm from the apex of the heart. Values are in mm. (C and D) Picrosirius red and fast green counterstain were used to identify fibrotic regions that are quantified in (A). Representative images are shown for both control + hESC-CMs (C) and Delta + hESC-CMs (D), where the black arrow identifies a region of human myocardial graft within the infarct. Scale bars, 2.5 mm. (E and F) Inflammatory response at 4 weeks by CD68 staining normalized to infarct area. Outlined regions of interest in (C) and (D) are shown at higher magnification in (E) and (F). Serial sections are stained with CD68 antibody to label monocytes and macrophages and visualized with diaminobenzidine (DAB) (brown). Scale bars, 200 μm. (G and H) Host-derived vessels are identified by staining with CD31 antibody (red) (G and H) with a double stain for βMHC (green) (G) to identify hESC-CM grafts within the infarct regions. Scale bar, 200 μm. (I) Level of inflammatory response is expressed as CD68+ area normalized to scar area by picrosirius red at 2 and 4 weeks. Values for 2 week time point were obtained through pilot study experiments shown in Figure S4. (J) Level of neovascularization is quantified as CD31+ lumens within βMHC+ graft regions. p-values were calculated using an unpaired two-tailed t-test. For (A), (B), (F), and (G), ∗p < 0.05. Error bars indicate SEM.
Transplantation with Delta-1 Gel Improves Cardiac Function in a Low Cell-Dose Model
Previous studies have established that transplantation of 10 million hESC-CMs was required to achieve therapeutic functional benefit in the infarcted athymic rat heart.6 Due to increased graft proliferation at 1 month, implantation of only 5 million hESC-CMs in the Delta-1-functionalized hydrogel resulted in increased graft size despite a lower cell dose. We hypothesized that this subtherapeutic cell dose could be lowered further and still result in therapeutic benefit post-infarct.
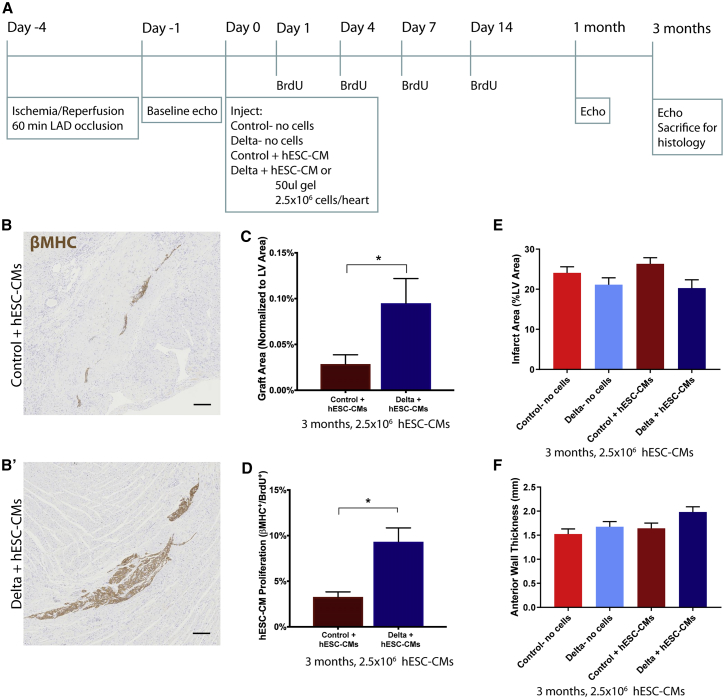
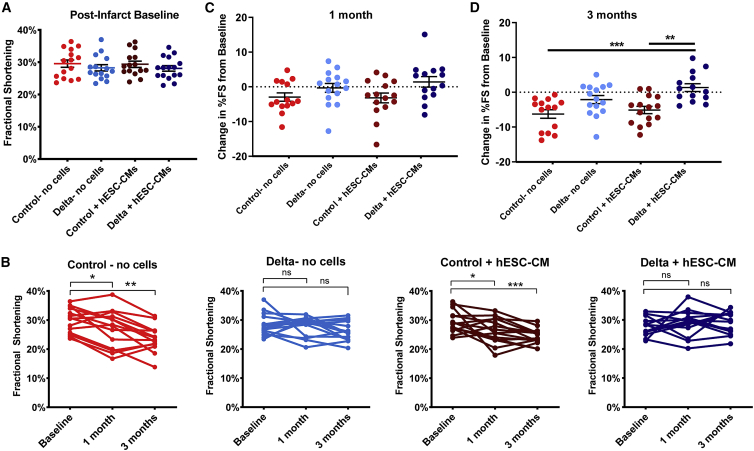
To provide a stringent test of the ability of the Delta-1-functionalized hydrogel to improve cardiac repair in the long-term with hESC-CMs, we delivered a subtherapeutic dose of cells (2.5 million cells per heart) to the infarcted myocardium and followed the animals by serial echocardiography for 3 months. Two additional gel-only control groups were added to evaluate the possible effects of the gel without the presence of cells, resulting in four groups total: control gel-no cells, Delta gel-no cells, control gel + hESC-CMs, and Delta gel + hESC-CMs (Figure 4A). As with the previous experiments, IgG protein was bound to the collagen instead of Delta-1 to evaluate the effects of an inactive protein for both control groups. Consistent with the data at 1 month post-transplant, implantation of Delta + hESC-CMs resulted in a significant increase in the βMHC+ graft area compared to implantation of control + hESC-CMs (Figures 4B and 4C). The graft size was smaller in both groups compared to our previous studies, likely due to the reduced cell dose. As anticipated based on our results at the 1 month endpoint, Delta + hESC-CMs exhibited a higher cell cycle activity, as identified by staining for double-positive BrdU+/βMHC+ cells after a series of four BrdU pulses during the first 2 weeks post-implantation (Figure 4D).
Figure 4.
Human Myocardial Graft Area is Significantly Increased 3 Months after Implantation with Notch Signaling
(A) Experimental timeline. Four days after ischemia/reperfusion injury, 2.5 × 106 hESC-CMs were transplanted within the IgG control gel or the Delta-1 gel (control + hESC-CMs and Delta + hESC-CMs, respectively), with two additional gel-only control groups (control-no cells and Delta-no cells). (B and B′) Representative images of hESC-CM grafts identified by staining for β myosin heavy chain (βMHC, brown) with hematoxylin counterstain for control + hESC-CMs (B) and Delta + hESC-CMs (B′). Scale bars, 200 μm. (C) βMHC+ graft area is normalized to the LV area. There is a substantial increase in graft area with the Delta + hESC-CMs. (D) Proliferating hESC-CMs are identified by double-labeled βMHC+/BrdU+ cells, and quantification is shown here. There is a significant increase in Delta + hESC-CM proliferation. (E) Infarct area, quantified by the picrosirius red area, is normalized to the LV area. There is a modest, non-significant reduction in infarct size with Delta + hESC-CM treatment. (F) Anterior wall thickness is shown in mm. There is a modest, non-significant increase in anterior wall thickness in the Delta + hESC-CMs. For (C)–(F), ∗p < 0.05. Error bars indicate SEM. p-values were calculated for (C) and (D) using an unpaired t-test and for (E) and (F) using a one-way ANOVA followed by Sidak’s multiple comparison test comparing control-no cells versus Delta-no cells, control-no cells versus control + hESC-CMs, Delta-no cells versus Delta + hESC-CMs, and control + hESC-CMs versus Delta + hESC-CMs.
Three months after implantation, there was no significant difference in histological infarct size (Figure 4E). Infarcts occupied 20.3% ± 2.1% of the left ventricular (LV) area in Delta + hESC-CMs hearts and 21.1% ± 1.7% in Delta-no cells hearts, compared to 26.3% ± 1.5% and 24.1 ± 1.5% in the control + hESC-CMs and the control-no cells groups (Figure 4E). There was no difference in anterior wall thickness in the Delta + hESC-CMs compared to all other groups (Figure 4F).
To determine whether the increased graft area observed in the Delta + hESC-CMs group impacted global heart function, echocardiography was performed on all animals at baseline post-injury, and at 1 and 3 months post-implantation. Measurements were conducted by two independent, blinded observers per animal and time point and showed high interobserver agreement (Figures S7A and S7B). All groups had comparable LV end-diastolic dimension and fractional shortening at the post-infarct baseline echocardiogram, suggesting comparable baseline infarct sizes prior to cell and gel implantation (Figure 5A; Figures S6C and S6D). LV end-diastolic and end-systolic dimensions increased across baseline at 1 and 3 months in all groups (Figures S6A and S6B), with no significant between-group differences, indicating no effect on negative remodeling (Figures S6C and S6D). However, in hearts receiving control gel + hESC-CMs, there was a significant reduction in fractional shortening from baseline to 1 month (from 29.4% ± 1.0% to 26.2% ± 1.1%), and further reduction to 24.4% ± 0.7% at 3 months (compared to baseline, p = 0.039 at 1 month, p = 0.0002 at 3 months, Figure 5B). This is similar to the progression of heart failure in the control gel-no cells group, (Figure 5B) and indicates that the dose of cells in this group is indeed subtherapeutic. In contrast, animals in the Delta gel + hESC-CMs group showed no progression of heart failure after transplantation (28.1% ± 0.9% fractional shortening at baseline, versus 28.7% ± 1.2% and 29.3 ± 1.0% at 1 and 3 months, respectively, Figure 5B). The change in fractional shortening from baseline trended toward improvement at 1 month (Figure 5C) and was significantly improved at 3 months when animals received Delta + hESC-CMs compared to control gel-no cells and control gel + hESC-CMs (Figure 5D). The Delta gel-no cells group also showed no significant decline in function over time with a mean fractional shortening of 28.3% ± 1.0%, 28.0% ± 0.9%, and 26.2% ± 0.8% at baseline, 1 month, and 3 months, respectively (Figure 5B). This suggests a benefit of Notch signaling on host cells in the infarcted heart, but unlike the Delta gel + hESC-CMs group, there was no statistically between-group improvement in function in the Delta gel-no cells group, indicating that hESC-CMs provide additional benefit. These data indicate that the Delta-1 gel augments the ability of an otherwise subtherapeutic dose of human cardiomyocytes to improve systolic function of the infarcted heart, in association with enhanced graft size and improved graft vascularization.
Figure 5.
Notch Signaling Maintains Heart Function at 3 Months with Implantation of a Subtherapeutic Dose of hESC-CMs
(A) Echocardiography was performed at baseline after ischemia/reperfusion injury with no differences between groups, indicating a similar extent of injury prior to implantation. (B) Individual fractional shortening data from baseline to 1 and 3 months are shown for all four implant groups. Note the decline in fractional shortening over time in control-no cells and control + hESC-CMs, whereas fractional shortening is maintained in Delta-no cells and Delta + hESC-CMs. Paired t-tests were performed comparing 1 and 3 months with baseline within each group. (C and D) Change in percent fractional shortening from baseline, at 1 month (C) and 3 months (D). Each data point represents the change in a heart’s function at the corresponding time point, with group means and standard error shown for each group. Control-no cells and control + hESC-CMs show a significantly greater decrease in fractional shortening at 3 months compared to the Delta + hESC-CMs as determined by a one-way ANOVA followed by Sidak’s multiple comparison test comparing control-no cells versus Delta-no cells, control-no cells versus control + hESC-CMs, control-no cells versus Delta + hESC-CMs, Delta-no cells versus Delta + hESC-CMs, and control + hESC-CMs versus Delta + hESC-CMs. For all panels, ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005. ns, not significant (p > 0.05). Error bars represent SEM. See also Figures S6–S7.
Discussion
Cell therapy methods for cardiac repair are hindered by small graft sizes resulting from limited retention and high cell death after implantation. This has traditionally required transplanting a high cell dose of hESC-CMs into the myocardium, which is a hurdle for translation of this therapeutic approach to the clinic. To address this, we developed a method to increase the proliferation of hESC-CMs after transplantation via Notch signaling activation, which resulted in increased graft size and maintained heart function with a normally subtherapeutic cell dose.
Notch ligand immobilization in 2D has been used to successfully mediate cell fate decisions in hESCs and hESC-CMs;19 however, we found that similar 2D surfaces begin to lose signal activity within 2–3 days in culture. In contrast, we demonstrate in Figure 1 that immobilizing a Notch ligand onto a 3D scaffold allowed for significant Notch signaling activation for up to 2.5 weeks of in vitro culture. These data indicate a robust increase in total Notch signal gained from the 3D system compared to 2D; however, the data also indicate that Notch signaling activation decreases back to baseline levels over time. This is important particularly for in vivo translation, because overly proliferative cardiomyocytes have been shown to contribute to decreased global heart function when the proliferative stimuli are overexpressed long-term in the heart.36,37 This highlights the importance of the transient proliferative cues achieved here with the Delta-1 gel, which we think allows for a balance between cardiomyocyte proliferation and the eventual maturation of de novo cardiomyocytes needed for functional improvement. Although we did not directly assess cardiomyocyte maturation in vivo in the context of this study, this has been characterized in previously published work in the adult and neonatal rat heart after human pluripotent stem cell-derived cardiomyocyte transplantation.38
Interestingly, by implanting hESC-CMs with the Delta-1 gel we could achieve human myocardial grafts that, 2 weeks after implantation, were comparable to the graft sizes that we have generated in previous studies that used twice the cell input number at 1 month.5,6 Specifically, we have shown previously that implantation of 10 × 106 hESC-CMs in pro-survival cocktail into the infarcted rat myocardium resulted in human cardiomyocyte grafts covering 1.1% of the LV area at 1 month,5 which is equivalent to the graft sizes reported here in the Delta + hESC-CMs group with half of the input cell number (5 × 106 hESC-CMs) and no PSCs (1.0% of LV area, Figure 2G), and nearly 3-fold the graft size in the control + hESC-CMs group. This suggests that any pro-survival effect lost by the removal of pro-survival cocktail in these experiments was compensated for and surpassed by the pro-proliferative effect of Notch signaling. It is possible that using a higher cell dose would have further augmented graft sizes, as well as enhanced the modest benefit in global heart function.
Based on these promising graft size results at 1 month we wanted to harness the pro-proliferative effect of the Delta-1 gel to reduce the required cell dose and asked whether a subtherapeutic dose of hESC-CMs could improve heart function in this context. For these experiments, we reduced the cell dose for the 3-month functional experiments, where only 2.5 × 106 hESC-CMs were implanted instead of 5 × 106 hESC-CMs implanted in the 1-month experiment. Importantly, the increase in hESC-CM proliferation and subsequent increase in graft size were identified at both 1 and 3 months post-transplantation of hESC-CMs in the Delta-1 gel, suggesting graft stability at later time points even with a reduced cell input. Indeed, Delta + hESC-CMs showed a maintenance of heart function during the 3-month time period following implantation, where control + hESC-CMs showed functional decline (Figure 5). Additionally, Delta + hESC-CMs showed statistically significant improvement in the change in fractional shortening from baseline when compared with control + hESC-CMs. Interestingly, a trend of improved fractional shortening from baseline at 1 and 3 months was identified in rats receiving Delta + hESC-CMs, suggesting that there may be combinatorial impact of the Notch activation and cell therapy in this context. We were, however, surprised to find no statistical difference in global heart remodeling between groups at 3 months, as shown by LV area dimensions (Figure S6). Instead, LV internal dimension (LVID) increased in all groups concurrently.
Based on results from previous studies by our group where animals received cell-free injections after an infarction,6 we anticipated that the control gel-no cells group would show a decline in heart function during 1 month and continue to decline during 3 months, which is consistent with the data shown here (Figure 5). Due to the previously reported evidence of Notch-mediated effects on cardiac repair in mice and zebrafish,20,39, 40, 41, 42, 43 we also hypothesized that the transient Notch signal in the Delta groups may stimulate an endogenous repair response in the rat host myocardium, resulting in some benefit gained by the Delta-1 signal without the presence of hESC-CMs in the Delta-no cells group. While Delta-no cells did not show a functional decline over time (Figure 5B), and there was a trend for improved change in fractional shortening from baseline compared to the control gel-no cells animals, this was not statistically different. Further evidence of an endogenous response from Delta is supported by the increase in vascularization and decrease in inflammation (Figures 3E–3J; Figure S4F and S4F′).
In conclusion, we developed a Notch-signaling hydrogel to promote the proliferation of hESC-CMs, which was validated in vitro using tissue engineering and translated in vivo as an injectate for hESC-CMs to increase graft size and maintain heart function. Implanting hESC-CMs in the Delta-1 gel resulted in a 2-fold increase in the proliferation of engrafted cardiomyocytes and corresponded to a 3-fold increase in graft size compared to cell transplantation with a control gel at 1 month, and, importantly, prevented functional decline with a subtherapeutic dose of cells at 3 months. Collectively, these results demonstrate the use of a Delta-1 functionalized hydrogel as a therapeutic approach to enhance hESC-CM graft size after implantation into injured heart through transiently stimulating proliferation, allowing an otherwise subtherapeutic dose of hESC-CMs to improve systolic function after an acute myocardial infarction.
Materials and Methods
2D Tissue Culture Platforms with Immobilized Notch Ligands
To achieve oriented immobilization of recombinant human Delta-1-Fc,24 tissue culture polystyrene culture plates were pre-coated with 20 mg/mL anti-IgG (human, Fc-specific, Sigma) for 1 h at 37°C. Wells were washed with PBS, blocked with 20% fetal bovine serum (FBS) in PBS for 1 h at room temperature, and subsequently incubated overnight with the Notch ligand Delta-1 (courtesy of Dr. Irwin Bernstein’s Laboratory, Fred Hutchinson Cancer Research Center [FHCRC]) or a control protein, human IgG (Sigma),44 to achieve oriented immobilization. A similar method has been previously described.23 2D cell culture experiments used Delta-1 ligand or IgG control at 10 μg/mL unless otherwise noted. Prior to cell culture, wells were washed three times with PBS to remove excess unbound ligand. In 2D experiments using hESC-CMs, 5 μg/mL fibronectin (Invitrogen) was added during overnight ligand incubation to promote cardiomyocyte attachment to the tissue culture surfaces.
Collagen Modification and Notch Ligand Immobilization
Carbodiimide chemistry was used to immobilize Delta-1-Fc onto solubilized collagen similar to previously described methods,45,46 where free carboxyl groups on the collagen are reacted to free amine groups to bind a secondary protein, that is, anti-IgG in all experiments, except for unoriented ligand immobilization experiments that used either IgG or Delta-1 bound directly to collagen. To activate the collagen, a stock concentration of 15 mg/mL rat tail collagen type 1 was dissolved in 0.1% acetic acid, diluted to 2.5 mg/mL using RPMI 1640 cell culture medium, and reacted with 1-ethyl-3-(dimethylaminopropyl)carbodiimide hydrochloride (EDC)/N-hydroxysulfosuccinimide (sulfo-NHS) on ice for 1 h, with a 10-fold molar excess of EDC (Thermo Fisher Scientific) to free carboxyl groups and 5 mM sulfo-NHS (Thermo Fisher Scientific). The solution was mixed periodically during the incubation period using a chilled 1-cc syringe. After 1 h, 2-mercaptoethanol (2-ME) was added at 20 mM to inactive the EDC, and the pH was adjusted to 7.0–7.2 using 1 M NaOH. Next, 200 μg/mL anti-human IgG (Fc domain-specific) was mixed with the collagen and incubated for 48 h at 4°C. Following the 48-h incubation, 100 μg/mL human recombinant Delta-1 or human IgG (Sigma) was added, and the solution was incubated for another 24 h at 4°C. Prior to the addition of cells, the solution was brought to a final concentration of 1.5 mg/mL collagen using cell culture medium (DMEM for U2OS luciferase characterization studies or RPMI 1640 for cardiomyocyte experiments), 1× HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 30% v/v unmodified rat tail collagen 1 (4 mg/mL stock, Thermo Fisher Scientific), and 1× Matrigel, and the pH was adjusted to 7.4 using 1 M NaOH. This entire process occurred with reagents, pipettes, and syringes maintained on ice.
Luciferase-Based Analysis of Notch Signaling
To validate the Delta-1-modified collagen, we used a luciferase-based assay to confirm bioactivity of the Notch signaling pathway.47 To achieve this, we used CSL-luciferase U2OS osteosarcoma cells (courtesy of Dr. Randall Moon’s Laboratory, University of Washington), which were engineered to express constitutively active Renilla and firefly luciferase under control of CSL (CBF1, Su(H), and Lag-1) expression (cells are referred to as U2OS CSLluc/ren) and the Dual-Luciferase reporter assay system (Promega) for both 2D and 3D Notch signaling activation experiments in vitro. U2OS CSLluc/ren cells were cultured in uncoated 10-cm TCPS (tissue culture polystyrene) plates and fed every 2–3 days with DMEM supplemented with 10% FBS, 2 mM l-glutamine, and 100 U/mL penicillin/100 μg/mL streptomycin, and passaged at 80% confluence using 0.25% trypsin-EDTA.
For 2D experiments, U2OS CSLluc/ren cells were replated in triplicate onto IgG- or Delta-1-coated wells in a 96-well plate at a density of 5,000 cells/well in standard culture medium. For 3D gel experiments, 25,000 U2OS CSLluc/ren cells were resuspended in 30 μL of IgG- or Delta-modified collagen and allowed to gel at 37°C in 96-well plates (in three to six replicates) for 1 h before adding an additional 200 μL of cell culture medium. Cell culture medium was replaced the following day to wash away excess unbound ligand, and it was changed daily thereafter. Bioactivity of implanted gels was assessed using gels formed in vitro with CSLluc/ren reporter cells and harvested at 72 h for luciferase analysis. For luciferase analysis, medium was removed and 100 or 200 μL of 1× passive lysis buffer (Promega) was added to 2D and 3D wells, respectively. Cells were lysed during a 10-min incubation followed by trituration and two to three repetitive freeze/thaw cycles at −80°C. 3D gels required an additional trituration step to fully disrupt the gels, which was accomplished using a handheld tissue homogenizer inserted into each sample tube and triturated for 15 s. Luminescence was recorded following the Dual-Luciferase reporter cells protocol (Promega). Briefly, 20 μL per sample was loaded in triplicate into optical 96-well plates, and background fluorescence was recorded over three individual reads on a plate luminometer. 20 μL of luciferase assay reagent II (LARII) was added to each well and firefly luciferase luminescence was recorded as before, followed by the addition of 20 μL Stop and Glo reagent to each well and luminescence recording. Notch-driven luciferase data are represented as normalized firefly to Renilla luminescence for each sample, expressed as the fold expression over the corresponding control (normalized to 1) for both 2D and 3D experiments.
hESC-Derived Cardiomyocyte Culture and Differentiation
Undifferentiated RUES2 hESCs were maintained as previously described5 on Matrigel (Corning Life Sciences) in mouse embryonic fibroblast (MEF)-conditioned media supplemented with 5 ng/mL basic fibroblast growth factor (bFGF) (Peprotech). Cardiomyocyte differentiation was performed as described previously using a combination of small molecules and growth factors.5 A high-density cell monolayer was pre-treated with 1 μM CHIR99021 for 6–24 h (Cayman Chemical) and induced with 100 ng/mL activin A (R&D Systems) and 1× Matrigel in RPMI 1640 with B27 supplement minus insulin (Life Technologies). Medium was changed after 18 h to RPMI 1640 with B27 supplement (minus insulin) supplemented with 1 μM CHIR99021 and 5 ng/mL BMP4 (R&D Systems). After 2 days, media were changed and supplemented with XAV939 (Tocris) for an additional 48 h. After day 7 of differentiation, RPMI 1640 with B27 supplement containing insulin (Life Technologies) was used to maintain cells. Beating was typically observed between days 7 and 10, and medium was changed every 2–3 days thereafter. Unless otherwise noted, cardiomyocytes were cryopreserved on day 21–24 of differentiation for long-term storage and thawed 2–3 days prior to implantation to allow for recovery.48 For cryopreservation, cells were heat shocked for 1 h at 42°C the day prior. After a 1-h pretreatment with 10 μM ROCK inhibitor Y-27632 (Tocris), cardiomyocytes were harvested by a brief incubation with EDTA and dispersed into single cells using 0.25% trypsin-EDTA (Life Technologies). Cells were washed and resuspended in CryoStor (Sigma), added to cryovials, and frozen to −80°C in a controlled rate freezer with a decrease in temperature of 1°C/min before transfer to liquid nitrogen for long-term storage. To thaw, cryovials were agitated briefly at 37°C, collected in RPMI 1640 supplemented with 200 U/mL DNase (VWR), and washed with basal medium. Cardiomyocytes were replated onto Matrigel-coated culture dishes in RPMI 1640 with B27 containing insulin and supplemented with 10 μM Y-27632 for the first 24 h. Flow cytometry for cardiac troponin T (1:100, Thermo Scientific) was used to characterize cardiomyocyte population purity after cardiac differentiation and again at the time of harvest for implantation.
In Vitro Cardiomyocyte Proliferation Experiments
HESC-CMs were replated into wells of a four-well Nunc chamber slide at 60,000 cells/well (Thermo Scientific) following immobilization of either IgG or Delta-1 and supplemented with 5 μg/mL fibronectin as described above. Culture media were changed the following day, including 10 μM BrdU (Sigma), and cells were fixed 24 h later with 4% paraformaldehyde. For experiments where cell number was the primary endpoint, 24-well plates were used instead of chamber slides, and they were coated with Delta-1 or IgG as described above. For these experiments, high-purity cardiomyocytes resulting from the differentiation protocol described above (97.1% ± 0.9% cTnT+ by flow cytometry) were replated at a density of 200,000 cells/well, medium was changed every other day, with or without the addition of the γ-secretase inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) (Sigma) at 5 μM, and cells were counted on a hemocytometer using trypan blue dye at day 7 post-replating. Engineered cardiac constructs were formed using methods previously described by our group.49, 50, 51 HESC-CMs were removed from monolayer culture using 0.25% trypsin-EDTA, washed twice with serum-free medium, resuspended in the modified collagen with IgG (control) or Delta-1, and added into sterile PDMS (polydimethylsiloxane) molds using a 1-cc syringe for 30-μL gels with 0.5 × 106 cells/gel in a PDMS mold. Collagen gels were allowed to polymerize by incubation at 37°C for 1 h, followed by the addition of RPMI 1640 + B27 containing insulin supplemented with 10 μM Y-27632 for the first 24 h. Medium was changed every other day, with 10 μM BrdU included 24 h prior to fixation with 4% paraformaldehyde.
Pro-Survival Cocktail Experiments
Initial implantation studies, which included pro-survival cocktail, were performed as previously described5,6 using a 1:1 mix of pro-survival cocktail with the modified collagen for implantation. As per the pro-survival cocktail protocol, animals received daily subcutaneous injections of 5 mg/kg cyclosporine A for 7 days, starting the day prior to implantation (for experiments shown in Figures S3A and S3B). For the set of experiments shown in Figure S3C, we investigated the effect of pro-survival cocktail on Notch signaling in vitro using the U2OS luciferase assay described above. U2OS cells were resuspended in DMEM containing the pro-survival cocktail, which includes 100 μM ZVAD (benzyloxycarbonyl-Val-Ala-Asp(O-methyl)-fluoromethyl ketone), 50 nM Bcl-xL BH4, 200 nM cyclosporine A, 100 ng/mL insulin-like growth factor (IGF)-1, and 50 μM pinacidil. In these studies and in the implantation studies including the pro-survival cocktail, the 50% v/v Matrigel described in the original reference5,6 was replaced with the modified collagen gel (immobilized with IgG or Delta-1). To mimic the daily cyclosporine injections given to rats during the first week following implantation, daily medium was supplemented with 200 nM cyclosporine A.
Because we found the pro-survival cocktail to have a negative impact on Notch signaling, it was omitted from all other implantation experiments and was only included in the experiments shown in Figure S3.
Surgical and Implantation Procedures
All animal procedures were performed in accordance with the US NIH Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Washington (UW) Institutional Animal Care and Use Committee. Male Sprague-Dawley athymic rats (8–12 weeks old, 250–300 g) (Envigo) were weighed and anesthetized with an intraperitoneal injection of 68.2 mg/kg ketamine (Zoetis) and 4.4 mg/kg xylazine (Akorn Animal Health), intubated, and mechanically ventilated. Core body temperature was maintained at 37°C by placing the rat on a warming pad and monitoring rectal temperature every 15 min. A local block of 1 mg/kg lidocaine (Hospira) and 1 mg/kg bupivacaine (Hospira) was injected subcutaneously at the incision site prior to surgery start. A thoracotomy was performed to expose the heart, and ischemia was induced by occluding the left descending coronary artery with a 7-0 suture for 60 min followed by reperfusion. The chest was closed aseptically and animal recovery was monitored. An analgesic dose of 1 mg/kg sustained-release buprenorphine (ZooPharm) was given subcutaneously before rehousing the animals the same evening. All animals received postoperative care twice daily for 2 days following surgery and were monitored daily until the second surgery.
Four days after ischemia/reperfusion (I/R), rats were again weighed and given 1 mg/kg sustained-release buprenorphine subcutaneously at least 1 h prior to the start of surgery. Animals were then anesthetized with 2.5%–5% isoflurane, mechanically ventilated, and a second thoracotomy was performed to expose the heart. For 1-month studies (Figures 2 and 3), 5 × 106 freshly harvested (not cryopreserved) RUES2 hESC-CMs were delivered in an 80-μL vol of either the IgG-collagen (control + hESC-CMs) or Delta-1-collagen gel (Delta + hESC-CMs) in two injections within the infarct region using a 26G needle. For the pilot subtherapeutic cell dose 2-week study (Figure S4), a normally subtherapeutic dose of 5 × 106 freshly harvested (not cryopreserved) RUES2 hESC-CMs was delivered in an 80-μL vol of Notch signaling or control gel (Delta + hESC-CMs and control + hESC-CMs, respectively). For 3-month studies (Figures 4 and 5; Figures S6 and S7), 2.5 × 106 (freshly harvested, not cryopreserved) RUES2 hESC-CMs were delivered in a 50-μL vol of Notch signaling or control gel (Delta + hESC-CMs and control + hESC-CMs, respectively) and additional control groups received either cell-free Delta-1 gel (Delta-no cells) or cell-free IgG control gel (control-no cells). After cell injection, the chest was closed aseptically and animal recovery was monitored. All animals received postoperative care twice daily for 2 days followed by monitoring three to four times per week until their endpoint. BrdU was administered via intraperitoneal injection at 10 mg/kg on days 1, 4, 7, and 14 after cell injection. Echocardiography, using either a GE Vivid 7 Dimension ultrasound or Visual Sonics Vevo 2100, was performed during the 3-month studies at 4 days after infarction (baseline) and at 1 and 3 months after cell injection. To perform echocardiography, animals were lightly anesthetized with 1%–2.5% isoflurane, and the LV end diastolic dimension (LVEDd), LV end systolic dimension (LVEDd), fractional shortening (%FS), and heart rate were recorded. Animals were euthanized at their endpoint using a lethal overdose of Beuthanasia (Merck Animal Health) administered via intraperitoneal injection. Hearts were collected, washed in cold PBS, perfused with PBS to flush out blood, and soaked in 150 mM KCl. During this time, a small sample of intestine was collected from each animal to serve as a BrdU-positive control.
Immunohistochemical Analysis
After euthanasia, hearts were fixed in 4% paraformaldehyde overnight, sectioned, processed, and stained with the appropriate primary and secondary antibodies. Each heart was sliced in 2-mm-thick sections, resulting in five sections per heart, which were embedded in paraffin and sectioned onto slides. For each immunohistochemical analysis, all visible tissue on each slide was analyzed unless noted below (refer to Figures S4D and S4D′ for an example). Infarcted myocardium was visualized using picrosirius red with a fast green counterstain, and the picrosirius red area was normalized to the LV area during analysis. Human cardiomyocyte grafts were identified by staining for βMHC (hybridoma supernatant, ATCC #CRL-2046) and visualized using either Alexa Fluor 488 goat anti-mouse (1:100, Molecular Probes) or an avidin-biotin goat anti-mouse antibody (1:100, Vector Laboratories) developed with diaminobenzidene (Vector Laboratories). To analyze BrdU incorporation, slides were first stained with the βMHC primary antibody as described above and then treated with 1.5 N HCl, followed by overnight incubation with a peroxidase-conjugated anti-BrdU primary antibody (1:40, Roche). Slides were then incubated with Alexa Fluor 488 goat anti-mouse (1:100, Molecular Probes) for βMHC and Alexa Fluor 594-tyramide (Thermo Fisher Scientific) to amplify BrdU. Cardiomyocyte proliferation was assessed by counting double-labeled BrdU+/βMHC+ cells, which was analyzed on images obtained from the infarct and border regions on a Nikon A1R confocal microscope. Vasculature was identified in the infarct and border regions using CD31/PECAM-1 primary antibody (1:100, Novus), and macrophages/monocytes were visualized across all tissue sections using CD68 primary antibody (1:100, Serotec).
Statistical Analysis
All histological measurements were performed using ImageJ, and statistical analyses were performed using Excel or GraphPad Prism. Statistical tests performed and significance values are indicated in the figure legends for each experiment. All values are reported as means, and error bars represent standard error of the mean (SEM).
Author Contributions
K.A.G. designed and performed experiments, analyzed data, and wrote the manuscript; K.A.M. performed experiments, analyzed data, and edited the manuscript; A.M.M. performed surgeries, echocardiography, and edited the manuscript; X.G. analyzed data and edited the manuscript; C.E.M. supervised all aspects of the study, designed experiments, edited the manuscript, and obtained funding for the research.
Conflicts of Interest
C.E.M. is a co-founder and an equity holder in Sana Biotechnology. The remaining authors declare no competing interests.
Acknowledgments
The authors thank Dr. Irwin Bernstein for providing the Delta-1 ligand and for helpful discussions regarding Notch signaling, Daniel Burnham and Alexander Moon for assistance with immunohistochemistry, Gabriel Rush for assistance with data collection, and Dr. Lil Pabon and Dr. Hans Reinecke for insightful scientific discussions. We gratefully acknowledge funding from the Foundation Leducq Transatlantic Network of Excellence and NIH grants R01HL146868, R01HL128362, U54DK107979, and P01HL094374 (to C.E.M.). K.A.G. was supported by NIH training grant T32EB001650 and an NSF Graduate Research Fellowship. We gratefully acknowledge the Tom and Sue Ellison Stem Cell Core of the Institute for Stem Cell and Regenerative Medicine for use of cell culture space and equipment. This research was supported by the Cell Analysis Facility Flow Cytometry and Imaging Core in the Department of Immunology at the University of Washington. We would like to acknowledge the Mike and Lynn Garvey Cell Imaging Lab at the Institute for Stem Cell and Regenerative Medicine (UW) and its director Dr. Dale Hailey for assistance with sample imaging and analysis. Histopathology work was provided by the Pathology Research Service laboratory at the University of Washington. We would like to thank Megan Larmore and Brian Johnson in the University of Washington Histology and Imaging Core for their assistance with whole-slide digital scanning.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.04.011.
Supplemental Information
References
- 1.Bargehr J., Ong L.P., Colzani M., Davaapil H., Hofsteen P., Bhandari S., Gambardella L., Le Novère N., Iyer D., Sampaziotis F. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat. Biotechnol. 2019;37:895–906. doi: 10.1038/s41587-019-0197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y., Ogasawara T., Okada K., Shiba N., Sakamoto K. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y.-W., Chen B., Yang X., Fugate J.A., Kalucki F.A., Futakuchi-Tsuchida A., Couture L., Vogel K.W., Astley C.A., Baldessari A. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018;36:597–605. doi: 10.1038/nbt.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbin K.A., Yang X., Murry C.E., Coulombe K.L.K. Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PLoS ONE. 2015;10:e0131446. doi: 10.1371/journal.pone.0131446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 7.Shiba Y., Filice D., Fernandes S., Minami E., Dupras S.K., Biber B.V., Trinh P., Hirota Y., Gold J.D., Viswanathan M., Laflamme M.A. Electrical integration of human embryonic stem cell-derived cardiomyocytes in a guinea pig chronic infarct model. J. Cardiovasc. Pharmacol. Ther. 2014;19:368–381. doi: 10.1177/1074248413520344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulot J.-S., Stillitano F., Salem J.E., Kovacic J.C., Fuster V., Hajjar R.J. Considerations for pre-clinical models and clinical trials of pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2014;5:1. doi: 10.1186/scrt390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller-Ehmsen J., Whittaker P., Kloner R.A., Dow J.S., Sakoda T., Long T.I., Laird P.W., Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell. Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M., Methot D., Poppa V., Fujio Y., Walsh K., Murry C.E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 11.Hughes A., Rojas-Canales D., Drogemuller C., Voelcker N.H., Grey S.T., Coates P.T. IGF2: an endocrine hormone to improve islet transplant survival. J. Endocrinol. 2014;221:R41–R48. doi: 10.1530/joe-13-0557. [DOI] [PubMed] [Google Scholar]
- 12.Marchionini D.M., Collier T.J., Camargo M., McGuire S., Pitzer M., Sortwell C.E. Interference with anoikis-induced cell death of dopamine neurons: implications for augmenting embryonic graft survival in a rat model of Parkinson’s disease. J. Comp. Neurol. 2003;464:172–179. doi: 10.1002/cne.10785. [DOI] [PubMed] [Google Scholar]
- 13.Fujita B., Zimmermann W.-H. Myocardial tissue engineering for regenerative applications. Curr. Cardiol. Rep. 2017;19:78. doi: 10.1007/s11886-017-0892-4. [DOI] [PubMed] [Google Scholar]
- 14.Haraguchi Y., Shimizu T., Yamato M., Okano T. Regenerative therapies using cell sheet-based tissue engineering for cardiac disease. Cardiol. Res. Pract. 2011;2011:845170. doi: 10.4061/2011/845170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vunjak-Novakovic G., Lui K.O., Tandon N., Chien K.R. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu. Rev. Biomed. Eng. 2011;13:245–267. doi: 10.1146/annurev-bioeng-071910-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boopathy A.V., Che P.L., Somasuntharam I., Fiore V.F., Cabigas E.B., Ban K., Brown M.E., Narui Y., Barker T.H., Yoon Y.S. The modulation of cardiac progenitor cell function by hydrogel-dependent Notch1 activation. Biomaterials. 2014;35:8103–8112. doi: 10.1016/j.biomaterials.2014.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campa V.M., Gutiérrez-Lanza R., Cerignoli F., Díaz-Trelles R., Nelson B., Tsuji T., Barcova M., Jiang W., Mercola M. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J. Cell Biol. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collesi C., Zentilin L., Sinagra G., Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. J. Cell Biol. 2008;183:117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung J.C., Paige S.L., Ratner B.D., Murry C.E., Giachelli C.M. Engineered biomaterials control differentiation and proliferation of human-embryonic-stem-cell-derived cardiomyocytes via timed Notch activation. Stem Cell Reports. 2014;2:271–281. doi: 10.1016/j.stemcr.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L., Borikova A.L., Ben-Yair R., Guner-Ataman B., MacRae C.A., Lee R.T., Burns C.G., Burns C.E. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA. 2014;111:1403–1408. doi: 10.1073/pnas.1311705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Amato G., Luxán G., de la Pompa J.L. Notch signalling in ventricular chamber development and cardiomyopathy. FEBS J. 2016;283:4223–4237. doi: 10.1111/febs.13773. [DOI] [PubMed] [Google Scholar]
- 22.Hashmi S., Ahmad H.R. Molecular switch model for cardiomyocyte proliferation. Cell Regen. (Lond.) 2019;8:12–20. doi: 10.1016/j.cr.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckstead B.L., Tung J.C., Liang K.J., Tavakkol Z., Usui M.L., Olerud J.E., Giachelli C.M. Methods to promote Notch signaling at the biomaterial interface and evaluation in a rafted organ culture model. J. Biomed. Mater. Res. A. 2009;91:436–446. doi: 10.1002/jbm.a.32214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varnum-Finney B., Wu L., Yu M., Brashem-Stein C., Staats S., Flowers D., Griffin J.D., Bernstein I.D. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 2000;113:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 25.Koyanagi M., Bushoven P., Iwasaki M., Urbich C., Zeiher A.M., Dimmeler S. Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ. Res. 2007;101:1139–1145. doi: 10.1161/CIRCRESAHA.107.151381. [DOI] [PubMed] [Google Scholar]
- 26.Vas V., Szilágyi L., Pálóczi K., Uher F. Soluble Jagged-1 is able to inhibit the function of its multivalent form to induce hematopoietic stem cell self-renewal in a surrogate in vitro assay. J. Leukoc. Biol. 2004;75:714–720. doi: 10.1189/jlb.1003462. [DOI] [PubMed] [Google Scholar]
- 27.Cox D.B., Platt R.J., Zhang F. Therapeutic genome editing: prospects and challenges. Nat. Med. 2015;21:121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin H., Kauffman K.J., Anderson D.G. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 2017;16:387–399. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 29.Purcell B.P., Elser J.A., Mu A., Margulies K.B., Burdick J.A. Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 2012;33:7849–7857. doi: 10.1016/j.biomaterials.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singelyn J.M., Christman K.L. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J. Cardiovasc. Transl. Res. 2010;3:478–486. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell B.P., Kim I.L., Chuo V., Guinen T., Dorsey S.M., Burdick J.A. Incorporation of sulfated hyaluronic acid macromers into degradable hydrogel scaffolds for sustained molecule delivery. Biomater. Sci. 2014;2:693–702. doi: 10.1039/C3BM60227C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sultana N., Zhang L., Yan J., Chen J., Cai W., Razzaque S., Jeong D., Sheng W., Bu L., Xu M. Resident c-kit+ cells in the heart are not cardiac stem cells. Nat. Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Berlo J.H., Kanisicak O., Maillet M., Vagnozzi R.J., Karch J., Lin S.C., Middleton R.C., Marbán E., Molkentin J.D. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckstead B.L., Santosa D.M., Giachelli C.M. Mimicking cell-cell interactions at the biomaterial-cell interface for control of stem cell differentiation. J. Biomed. Mater. Res. A. 2006;79:94–103. doi: 10.1002/jbm.a.30760. [DOI] [PubMed] [Google Scholar]
- 35.Pandey R., Botros M.A., Nacev B.A., Albig A.R. Cyclosporin a disrupts notch signaling and vascular lumen maintenance. PLoS ONE. 2015;10:e0119279. doi: 10.1371/journal.pone.0119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabisonia K., Prosdocimo G., Aquaro G.D., Carlucci L., Zentilin L., Secco I., Ali H., Braga L., Gorgodze N., Bernini F. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019;569:418–422. doi: 10.1038/s41586-019-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian Y., Liu Y., Wang T., Zhou N., Kong J., Chen L., Snitow M., Morley M., Li D., Petrenko N. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 2015;7:279ra38. doi: 10.1126/scitranslmed.3010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadota S., Pabon L., Reinecke H., Murry C.E. In vivo maturation of human induced pluripotent stem cell derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Reports. 2017;8:278–289. doi: 10.1016/j.stemcr.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao L., Ben-Yair R., Burns C.E., Burns C.G. Endocardial Notch signaling promotes cardiomyocyte proliferation in the regenerating zebrafish heart through Wnt pathway antagonism. Cell Rep. 2019;26:546–554.e5. doi: 10.1016/j.celrep.2018.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kratsios P., Catela C., Salimova E., Huth M., Berno V., Rosenthal N., Mourkioti F. Distinct roles for cell-autonomous Notch signaling in cardiomyocytes of the embryonic and adult heart. Circ. Res. 2010;106:559–572. doi: 10.1161/CIRCRESAHA.109.203034. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R., Han P., Yang H., Ouyang K., Lee D., Lin Y.F., Ocorr K., Kang G., Chen J., Stainier D.Y. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498:497–501. doi: 10.1038/nature12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gude N.A., Emmanuel G., Wu W., Cottage C.T., Fischer K., Quijada P., Muraski J.A., Alvarez R., Rubio M., Schaefer E., Sussman M.A. Activation of Notch-mediated protective signaling in the myocardium. Circ. Res. 2008;102:1025–1035. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Hiroi Y., Liao J.K. Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction. Trends Cardiovasc. Med. 2010;20:228–231. doi: 10.1016/j.tcm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delaney M.K., Liu J., Kim K., Shen B., Stojanovic-Terpo A., Zheng Y., Cho J., Du X. Agonist-induced platelet procoagulant activity requires shear and a Rac1-dependent signaling mechanism. Blood. 2014;124:1957–1967. doi: 10.1182/blood-2014-03-560821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osathanon T., Giachelli C.M., Somerman M.J. Immobilization of alkaline phosphatase on microporous nanofibrous fibrin scaffolds for bone tissue engineering. Biomaterials. 2009;30:4513–4521. doi: 10.1016/j.biomaterials.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y.H., Shoichet M.S., Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 47.DeForest C.A., Tirrell D.A. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater. 2015;14:523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- 48.Xu C., Police S., Hassanipour M., Li Y., Chen Y., Priest C., O’Sullivan C., Laflamme M.A., Zhu W.Z., Van Biber B. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen. Med. 2011;6:53–66. doi: 10.2217/rme.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhandari S. University of Washington, Seattle; 2015. Engineering a novel device to implement afterload on human stem cell-derived cardiac tissues. Master’s thesis. [Google Scholar]
- 50.Ruan J.L., Tulloch N.L., Saiget M., Paige S.L., Razumova M.V., Regnier M., Tung K.C., Keller G., Pabon L., Reinecke H., Murry C.E. Mechanical stress promotes maturation of human myocardium from pluripotent stem cell-derived progenitors. Stem Cells. 2015;33:2148–2157. doi: 10.1002/stem.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tulloch N.L., Muskheli V., Razumova M.V., Korte F.S., Regnier M., Hauch K.D., Pabon L., Reinecke H., Murry C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.