Introduction
A 82-year-old man presented with asthenia, dyspnea and fever for 2 days and was admitted from the emergency department to our intensive care unit with acute hypoxemic respiratory failure. His past medical history included a myeloperoxidase-antineutrophil cytoplasmic autoantibody-associated vasculitis with renal impairment, diagnosed 4 years earlier and treated with steroids and methotrexate for 2 years, resulting in a persistent chronic kidney disease. Following admission and endotracheal intubation for mechanical ventilation, a thoracic CT-scan (see Supplementary material) disclosed bilateral interstitial lung infiltrates, with multiple patterns including micronodules, ground-glass and tree-in-bud opacities. Bronchoalveolar lavage (BAL) was performed in the right middle lobe (75 mL/150 mL). Macroscopic examination of BAL fluid revealed a pink color; microscopic examination (Fig. 1) revealed a total cell count (220 cell/µL) in the normal range, but a high lymphocyte differential count (24%, CD3 82% and CD19 2%) with a CD4/CD8 T-cell ratio of 1.7. A complete microbiological workup was performed, which allowed to diagnose COVID-19 pneumonia, without concomitant bacterial infection. No recurrence of vasculitis was identified. Despite symptomatic treatment, the patient died after 13 days in the ICU. Here, we point out the high lymphocyte count in BAL fluid during COVID-19 pneumonia. Data about alveolar cellularity in this novel viral infection are urgently needed, which may provide clues for testing immunomodulatory treatments [1] in the most severely affected patients.
Fig. 1.
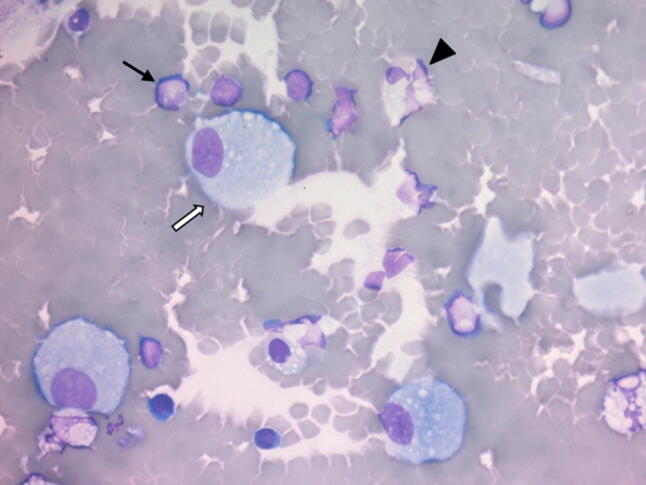
May-Grunwald Giemsa stained cells from bronchoalveolar lavage fluid after cytocentrifugation (40X). Black arrow: lymphocyte; white arrow: macrophage; arrowhead: neutrophil
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
GV had full access to all the data and takes responsibility for the integrity of the data. GV drafted the manuscript. AF performed the pathological examination of BAL, participated in the images acquisition and interpretation, and revised the manuscript. VL participated in the images interpretation and helped to revise the manuscript. MF revised the manuscript. All authors read and approved the final version to be submitted.
Funding
No funding.
Compliance with ethical standards
Conflict of interest
The authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Statement of human and animal rights
Authors declare that Human rights are followed in this study.
Informed consent
Authors declare that the informed oral consent for publication has been obtained from the patient’s next of kin then from the patient himself.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reference
- 1.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


