Abstract
Background
To determine the role of HOXD10 in pancreatic cancer.
Methods
A stable HOXD10-expressing PANC-1 cell line was established. Proliferation rates were detected by 5-Ethynyl-2'-deoxyuridine (Edu) staining while invasion was evaluated by Transwell assay. The expression levels of different proteins were analyzed by Western blotting. A subcutaneous xenograft of pancreatic cancer was established in nude mice, and the tumor weight and body weight were monitored. The in-situ expression of relevant markers in the tumor tissues was detected by immunohistochemistry.
Results
HOXD10 overexpression significantly decreased the proliferation rates of PANC-1 cells, and down-regulated Ki67 and Survivin (P<0.05). In addition, the invasive capacity (P<0.05) and the levels of vascular endothelial growth factor (VEGF) and MMP-14 were also significantly decreased (P<0.05) in the cells overexpressing HOXD10. Consistent with this, high levels of HOXD10 were associated with an increase in E-cadherin (P<0.05) and a decrease in N-cadherin (P<0.05) expression. Furthermore, the HOXD10-overexpressing xenografts were significantly smaller (P<0.05) and had fewer Ki67, VEGF, and N-cadherin-positive cells (P<0.05).
Conclusions
HOXD10 acts as a tumor suppressor in pancreatic cancer, and inhibits the proliferation, invasion, and epithelial-mesenchymal transition of the tumor cells.
Keywords: Pancreatic cancer, HOXD10, malignant biological behavior, epithelial-mesenchymal transition
Introduction
Pancreatic cancer is a malignancy of the digestive system, with steadily increasing global incidence and a dismal 5-year survival rate (1). The outcomes of immunotherapy, surgery and radiation therapy have so far been unsatisfactory due to the considerable side effects (2). Therefore, it is necessary to identify novel therapeutic targets in order to improve patient prognosis. The homeobox (Hox) family members function as oncogenes or tumor suppressors across different cancer types (3). Among these, HOXD10 is an inhibitor of tumor metastasis (4), and is significantly down-regulated in breast cancer, glioma, gastric cancer and liver cancer (5-8). However, its expression levels and pathological relevance in pancreatic cancer have thus far not been reported. The aim of this study was to determine the influence of HOXD10 on the proliferation, invasion, and tumor forming capacity of pancreatic cancer cells.
Methods
Experimental reagents
Dulbecco’s modified Eagle’s medium (DMEM; 12100-046), fetal bovine serum (FBS; 10082-147), trypsin (25200-056), and penicillin-streptomycin (15140-122) were purchased from Gibco, USA. The bicinchoninic acid assay (BCA) kit (P0012S) was purchased from Shanghai Beyotime Biotechnology Institute, and Transwell inserts (3374) were acquired from Corning, USA.
Cell culture and transfection
Human pancreatic cancer tumor cell line PANC-1 was obtained from the Cell Bank of the Typical Culture Preservation Committee of the Chinese Academy of Sciences. The cells were cultured in a high-glucose DMEM supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C under 5% CO2. The medium was changed every 2–3 days, and the cells were harvested with 0.25% trypsin at the logarithmic growth phase for various assays. The cells were seeded into a 6-well plate at the density of 5×105/well, and cultured for 24 h until they reached 80% confluency. They were then, transfected with the HOXD10 construct or the empty pcDNA plasmid using LipofecamineTM2000 as per the manufacturer’s instructions (9).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from PANC-1 cells using Trizol, and the A260/A280 was measured with a NanoDrop spectrophotometer. Reverse transcription and polymerase chain reaction (PCR) were conducted according to the kit instructions. The reaction conditions included pre-denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30s, extension at 72 °C for 30 s, and final extension at 72 °C for 10 min. The amplified products were analyzed by 2% agarose gel electrophoresis.
5-Ethynyl-2'-deoxyuridine (Edu) staining
The PANC-1 cells were seeded into a 96-well plate at the density of 1×105/well, and cultured overnight. The medium was replaced with 100 µL fresh medium supplemented with 50 µ 5-Edu per well, and the cells were incubated for 2 h. After washing twice with PBS, the cells were fixed and stained, and observed under a fluorescence microscope.
Transwell assay
The Transwell inserts were coated with Matrigelin serum-free medium at 37 °C for 5 h. The suitably treated PANC-1 cells were seeded into the upper chambers in 100 µL serum-free medium per well, and the lower chambers were filled with 600 µL medium supplemented with 20% FBS. After a 24 h culture, the Transwell inserts were removed and washed twice with PBS, fixed with 5% glutaraldehyde, stained with 0.1% crystal violet for 30 min, and observed under a fluorescence microscope.
Establishment of xenograft model
Nude mice were anesthetized by intraperitoneal injection of 1% sodium pentobarbital, and inoculated subcutaneously with 1×106 control or HOXD10-overexpressing PANC-1 cells in 100 µL medium (5 per group). The tumor nodules were measured every day, and the mice were weighed every 5 days. Thirty days after inoculation, the mice were sacrificed, and the tumors were harvested and weighed.
Immunohistochemistry
Tumor tissues were embedded in paraffin and cut into thin sections. After routine de-waxing, the sections were heated in citrate buffer solution at 95 °C for 10 min, cooled to room temperature, and incubated with 3% hydrogen peroxide for 10 min. The sections were then washed 3 times with PBS, blocked with 5% BSA at 37 °C for 30 min, and incubated with primary antibodies against HOXD10 (ab172865), Ki67 (ab15580), Survivin (ab76424), vascular endothelial growth factor(VEGF) (ab2350), MMP-14 (ab3644), E-cadherin (ab15148), N-cadherin (ab18203), and actin (ab8227) (all from Abcam, USA) at 37 °C for 1 h. After washing 3 times with phosphate-buffered saline (PBS), the sections were incubated with biotin-labeled secondary antibody at 37 °C for 30 min, washed again as described, and incubated with the SABC reagent at 37 °C for 30 min. Following the final washing step, color was developed using 3, 3'-Diaminobenzidine (DAB), and the sections were counter-stained with hematoxylin, dehydrated, cleared, and sealed with neutral gum. The percentage of immuno-positive cells was analyzed by Image J.
Statistical analysis
All data were analyzed by the SPSS17.0 software and expressed as mean ± SD. The groups were compared by analysis of variance, and P<0.05 was considered statistically significant.
Results
HOXD10 overexpression inhibited the growth and invasion of PANC-1 in vitro
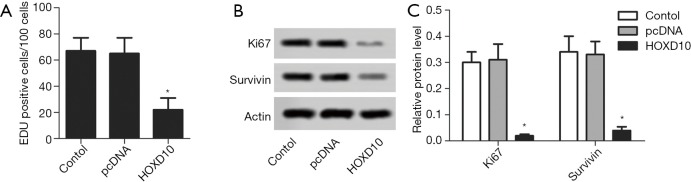
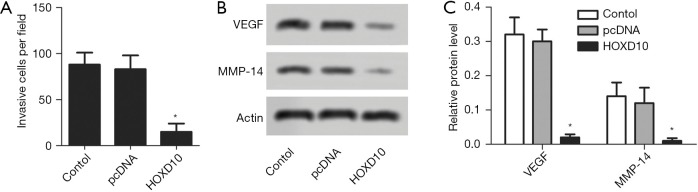
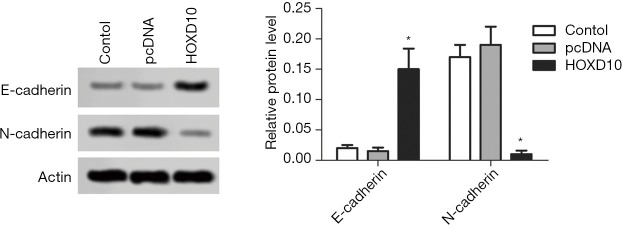
The biological role of HOXD10 in pancreatic cancer cells was determined by ectopically expressing the gene in PANC-1 cells (Figure 1). As shown in Figure 2A, the percentage of EDU-positive proliferating cells was significantly lower in the HOXD10 group compared to the control group (P<0.05), which correlated to a marked decline in the levels of Ki67 and Survivin (P<0.05; Figure 2A,B). Furthermore, HOXD10 overexpression significantly decreased the proportion of invasive cells (P<0.05; Figure 3A), in addition to down-regulation of VEGF and MMP-14 (P<0.05; Figure 3B,C). Consistent with this, E-cadherin levels were significantly increased in the HOXD10-overexpressing cells (P<0.05, Figure 4), while that of N-cadherin was significantly decreased (P<0.05, Figure 4), indicating epithelial-to-mesenchymal transition (EMT) inhibition.
Figure 1.
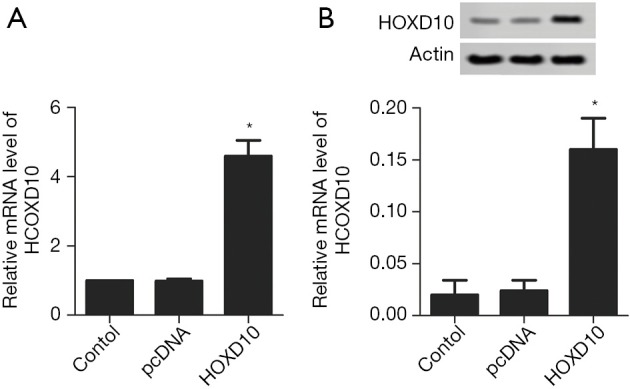
Validation of pcDNA-HOXD10 transfection in PANC-1 cells. HOXD10 mRNA (A) and protein (B) levels in the control and HOXD10-overexpressing cells were tested. *, P<0.05 with t-test, data are shown as mean ± SEM of at least 3 replicates.
Figure 2.
Cell proliferation in HOXD10 transectd PANC-1 cells. (A) Percentage of proliferating PANC-1 cells in the indicated groups. Ki67 and Survivin levels in the control and HOXD10 cells were tested with western blotting (B) and semi-quantified (C). *, P<0.05 with t-test, data are shown as mean ± SEM of at least 3 replicates.
Figure 3.
Cell invasion in HOXD10 transectd PANC-1 cells. (A) The proportion of invading cells in the control and HOXD10 groups tested with transwell assay. (B,C) VEGF and MMP-14 expression levels in the indicated groups. *, P<0.05 with t-test, data are shown as mean ± SEM of at least 3 replicates. VEGF, vascular endothelial growth factor.
Figure 4.
E-cadherin and N-cadherin levels in the control and HOXD10-overexpressing PANC-1 cells. *, P<0.05 with t-test, data are shown as mean ± SEM of at least 3 replicates.
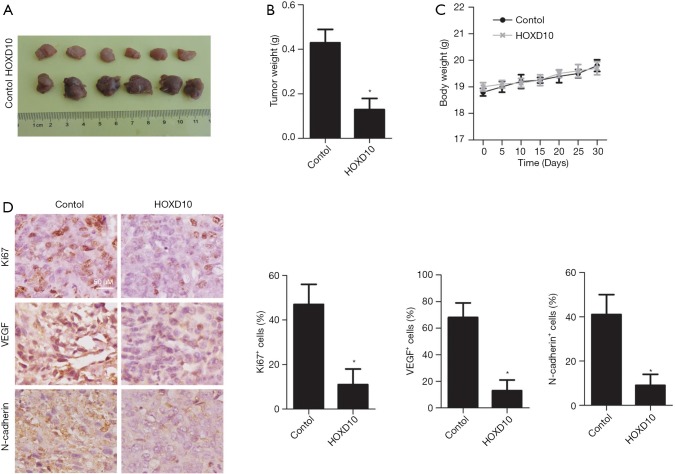
HOXD10 overexpression inhibited the growth of PANC-1 xenografts
Compared to the control group, the HOXD10-overexpressing tumors were significantly smaller (P<0.05; Figure 5A,B), whereas the body weight of the tumor-bearing mice were similar in both groups (Figure 5C). Consistent with this, the number of Ki67, VEGF, and N-cadherin positive cells were significantly greater in the HOXD10 versus control tumor tissues (P<0.05; Figure 5D).
Figure 5.
Effects of HOXD10 overexpression in PANC-1 xenograft mice. (A,B) Tumor weight and volume in the control and HOXD10 groups. (C) Body weight of the mice in different groups. (D) Ki67, VEGF, and N-cadherin expression levels in the control and HOXD10 xenografts. *, P<0.05 with t test, data are shown as mean ± SEM of at least 3 replicates. VEGF, vascular endothelial growth factor.
Discussion
The Hox gene superfamily encodes transcription factors regulating differentiation and morphogenesis-related genes (10) and is frequently dysregulated during tumorigenesis and metastasis (11). HOXD10 is an established tumor suppressor, although its role in pancreatic cancer has not been reported previously. We found that HOXD10 expression significantly decreased the proliferation rates and invasiveness of pancreatic cells in vitro and in vivo, indicating that it acts as a tumor suppressor.
Uncontrolled cell proliferation is a hallmark of cancer, and is accompanied by a surge in the expression of Ki67, which is in fact a prognostic indicator for pancreatic cancer (12). Survivin, an anti-apoptotic protein that enhances tumor cell proliferation and infiltration, is also highly expressed in pancreatic cancer tissues (13). We found that both survival-related factors were up-regulated in the PANC-1 cells, and decreased significantly upon ectopic expression of HOXD10. Therefore, unsurprisingly, the PANC-1 cells overexpressing HOXD10 also formed smaller tumors in vivo. This is consistent with the finding of Yang et al. (14) in whichHOXD10 overexpression significantly reduced the colony forming ability of bile duct cancer cells. In addition, high levels of HOXD10 significantly inhibited the proliferation of the human hepatobiliary carcinoma RBE and HCCC-9810 cell lines (15).
Metastasis is a critical factor determining cancer-related mortality (16), and depends on multiple factors. For instance, high levels of VEGF induce angiogenesis and improve tumor vascular permeability, thereby facilitating tumor cell entry into circulation and eventual metastasis (17). VEGF is frequently overexpressed in pancreatic cancer tissues compared to the normal adjacent tissues (18). MMP-14, a member of the matrix metalloproteinase family, is also aberrantly expressed in cancer and promotes tumor cell metastasis (19). In pancreatic cancer, MMP-14 further activates MMP-2 and MMP-9, which correlate to greater invasion and metastasis (20). Xi et al. (21) found that HOXD10overexpression in colorectal cancer cells significantly decreased VEGF levels and retarded tumor growth. Consistent with this, Jin et al. (22) found that overexpression of HOXD10 significantly inhibited the invasiveness of nasopharyngeal carcinoma cells. We found that HOXD10 significantly down-regulated VEGF and MMP-14 in PANC-1 cells, and decreased the number of VEGF-positive cells in the xenografts, indicating that HOXD10 can inhibit the invasion of pancreatic cancer cells.
EMT, or the transformation of epithelial cells into mesenchymal cells, is a key driver of metastasis since it endows the tumor cells with greater motility and invasiveness. Therefore, increased EMT of tumor cells is indicative of poor prognosis (23). At the molecular level, EMT is characterized by the down-regulation of E-cadherin and a concomitant increase in N-cadherin levels (24). HOXD10 significantly up-regulated E-cadherin and down-regulated N-cadherin in the PANC-1 cells, indicating that it likely inhibits the EMT and metastasis of pancreatic cancer cells.
In summary, HOXD10 overexpression inhibited the proliferation, invasion, EMT, and in vivo expansion of PANC-1 cells, indicating its potential as a therapeutic target in pancreatic cancer.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.03.28). The authors have no conflicts of interest to declare.
References
- 1.Ciofoaia V, Haddad NG, Smith JP. How many pancreatic cysts are out there and how to best manage them? Transl Cancer Res 2018;7:S500-5. 10.21037/tcr.2018.03.30 [DOI] [Google Scholar]
- 2.Negoi I, Beuran M, Hostiuc S, et al. Glycosylation alterations in acute pancreatitis and pancreatic cancer: CA19-9 expression is involved in pathogenesis and maybe targeted by therapy. Ann Transl Med 2019;7:S306. 10.21037/atm.2019.10.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer 2010;10:361-71. 10.1038/nrc2826 [DOI] [PubMed] [Google Scholar]
- 4.Xiao H, Li H, Yu G, et al. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol Rep 2014;31:1832-8. 10.3892/or.2014.3048 [DOI] [PubMed] [Google Scholar]
- 5.Makiyama K, Hamada J, Takada M, et al. Aberrant expression of HOX genes in human invasive breast carcinoma. Oncol Rep 2005;13:673-9. [PubMed] [Google Scholar]
- 6.Wang L, Chen S, Xue M, et al. Homeobox D10 gene, a candidate tumor suppressor, is downregulated through promoter hypermethylation and associated with gastric carcinogenesis. Mol Med 2012;18:389-400. 10.2119/molmed.2011.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu X, Chen D, Cui Y, et al. Targeting microRNA-23a to inhibit glioma cell invasion via HOXD10. Sci Rep 2013;3:3423. 10.1038/srep03423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Ding C, Chen C, et al. miR-224 promotion of cell migration and invasion by targeting Homeobox D 10 gene in human hepatocellular carcinoma. J Gastroenterol Hepatol 2014;29:835-42. 10.1111/jgh.12429 [DOI] [PubMed] [Google Scholar]
- 9.Hu Q, Li J, Meng H, et al. Effects of RegIV on proliferation, invasion, and migration of pancreatic cancer cells PANC-1. Shandong Medical Journal 2017;57:1-4. [Google Scholar]
- 10.Botas J. Control of morphogenesis and differentiation by HOM/Hox genes. Curr Opin Cell Biol 1993;5:1015-22. 10.1016/0955-0674(93)90086-6 [DOI] [PubMed] [Google Scholar]
- 11.Yachi K, Tsuda M, Kohsaka S, et al. miR-23a promotes invasion of glioblastoma via HOXD10-regulated glial-mesenchymal transition. Signal Transduct Target Ther 2018;3:33. 10.1038/s41392-018-0033-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin R, Smyrk TC, Reed NR, et al. Combining clinicopathological predictors and molecular biomarkers in the oncogenic K-RAS/Ki67/HIF-1alpha pathway to predict survival in resectable pancreatic cancer. Br J Cancer 2015;112:514-22. 10.1038/bjc.2014.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Wang Z, Zhu H. Progress of survivin in pancreatic carcinoma. Journal of Medical Postgraduates 2009;22:306-10. [Google Scholar]
- 14.Yang H, Mi J, Yu D, et al. Role of HOXD10 in radiosensitivity of cholangiocarcinoma cells. Tumor 2016;36:986-93. [Google Scholar]
- 15.Yang H, Zhou J, Mi J, et al. HOXD10 acts as a tumor-suppressive factor via inhibition of the RHOC/AKT/MAPK pathway in human cholangiocellular carcinoma. Oncol Rep 2015;34:1681-91. 10.3892/or.2015.4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han H, Wang D, Yang M, et al. High expression of RACK1 is associated with poor prognosis in patients with pancreatic ductal adenocarcinoma. Oncol Lett 2018;15:2073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu DM, Li DC, Zhang ZX, et al. Effect of endothelial PAS domain protein 1 and hypoxia inducible factor 1alpha on vascular endothelial growth factor expression in human pancreatic carcinoma. Chin Med J (Engl) 2008;121:2258-64. 10.1097/00029330-200811020-00010 [DOI] [PubMed] [Google Scholar]
- 18.Li CL, Cui YF, Du XF, et al. Clinical significance of matrix metalloproteinases-9 and vascular endothelial growth factor expression in the invasion and metastasis of pancreatic carcinoma. World Chinese Journal of Digestology 2008;16:1012-6. 10.11569/wcjd.v16.i9.1012 [DOI] [Google Scholar]
- 19.Soubani O, Ali AS, Logna F, et al. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis 2012;33:1563-71. 10.1093/carcin/bgs189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu B, Zhang K, Li S, et al. HIC1 attenuates invasion and metastasis by inhibiting the IL-6/STAT3 signalling pathway in human pancreatic cancer. Cancer Lett 2016;376:387-98. 10.1016/j.canlet.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 21.Xi CL, Gong ZL, Zhou QC, et al. Mechanism of HOXD10 gene inhibiting on growth of colorectal cancer cells. J Chinese Journal of Immunology 2018;34:1804-8. [Google Scholar]
- 22.Jin QZ, Meng YY, Huang DD, et al. Adjustment effect of homeobox gene D10 on malignant biological behaviors of human nasopharyngeal carcinoma. J Chinese Journal of Health Laboratory Technology 2016;29:1297-300. [Google Scholar]
- 23.Ma RR, Gong HB, Gong J, et al. Relationship between epithelial to mesenchymal transition and prognosis in pancreatic cancer. World Chinese Journal of Digestology 2016;24:686-91. 10.11569/wcjd.v24.i5.686 [DOI] [Google Scholar]
- 24.Gaianigo N, Melisi D, Carbone C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers (Basel) 2017;9:122. 10.3390/cancers9090122 [DOI] [PMC free article] [PubMed] [Google Scholar]