Abstract
Background
The central lymph node is the most common involvement for papillary thyroid carcinoma (PTC), which is correlated to recurrence and survival. But it is difficult to accurately evaluate lymph node prior to an operation. This retrospective study was designed to develop a risk model and risk stratification to preoperatively predict central lymph node metastasis (CLNM) in PTC and validate this model.
Methods
A series of 1,714 initial treatment PTC patients were enrolled. Among these patients, 1,001 patients were used to develop a predictive model and establish a stratification scoring system. This was validated through the remaining 713 patients.
Results
The multivariate analysis revealed that CLNM and lateral lymph node metastasis (LLNM) in ultrasound (US), tumor size, gender, capsule invasion in US, microcalcification and age were significant independent predictors for CLNM. The area under the curve (AUC) of the model was 0.778. Furthermore, the cutoff value to predict CLNM was 8 points, and the sensitivity and specificity were 77% and 65%, respectively. In the scoring system for CLNM, a score of ≤8, 8–18 and >18 were defined as low, intermediate and high risk, respectively. The risk of CLNM was approximately 30%, 60% and 80%, corresponding to the stratification. When validated, the model predicted the risk of CLNM with an AUC of 0.811, a sensitivity and specificity of 83% and 63%, respectively.
Conclusions
This study presented a predictive model to preoperatively assess the risk of CLNM in PTC. The predictive model performed well, but needed to be prospectively validated in external center.
Keywords: Central lymph node, multivariate analysis, papillary thyroid carcinoma (PTC), prediction, risk model
Introduction
Differentiated thyroid cancer (DTC) accounts for the vast majority (>90%) of all thyroid cancers (1), and papillary thyroid carcinoma (PTC) is the most common, which frequently exhibits nonaggressive behaviors with excellent prognosis, and has an overall 10-year survival of up to 96% (2) and an overall 15-year survival of >87% (3). Approximately 30–90% (4) of patients with PTC, however, will have clinical or occult cervical lymph node involvement, despite of the long-term survival. The central compartment lymph node (CLN) is the primary and most common region (5), which makes up 20–90% (6). It has been reported that the 10-year recurrence rates were approximately within 14–26%, and lymph node metastasis (LNM) was one of the independent risk factors for recurrence and survival (7). A retrospective study with large-scale patients who had PTC and were younger than 45 years old showed that overall survival (OS) was decreased for those with LNM, when compared to those without (hazard ratio:1.30, P=0.006) (8).
Yet one of the major clinical challenges is that there are no accurate and satisfying methods to directly evaluate preoperative CLN status (9-11).
Literatures associated with clinical and pathological factors or ultrasonographic features for predicting central lymph node metastasis (CLNM) had been previously reported (12-16). Moreover, a study developed a computer-aided diagnosis (CAD) system to identify and differentiate metastatic lymph nodes on ultrasound (US) (17). However, to our knowledge, few studies have established reliable prediction methods (18,19). One of previous studies predicted CLNM using simple indicators based on the CLN features seen in enhanced CT, but without considering the clinical characteristics of the patients (18). Although the other analyzed the clinical and ultrasonic characteristics, it included postoperative indicators such as extrathyroidal extension (ETE) (19). In addition, their research sample size was relatively small and neither of the models had been verified. Therefore, the present study aimed to develop a risk model and risk stratification to preoperatively predict CLNM in PTC based on clinical and sonographic features with a larger sample and to validate its effectiveness.
Methods
Participant population
This retrospective study protocol was approved by the Ethics Committee of our Institutional Review Board (IRB, No. B2018-064). Informed consent from enrolled patients was exempted by the IRB because of the retrospective nature of this study. The study was performed in accordance with the Declaration of Helsinki.
From January 2015 to May 2017, 2,098 consecutive patients who underwent near, sub-, or total thyroidectomy, and were pathologically confirmed with PTC at the Department of Head and Neck Surgery in our hospital, were included into the study. Patient inclusion criteria: (I) PTC confirmed by surgical pathology, (II) underwent central lymph node dissection (CLND), (III) age ≥18 years old. Exclusion criteria: (I) underwent a neck operation, (II) diagnosed with other types of thyroid tumor at the same time, (III) history of radiation therapy, (IV) unavailable to be completely evaluated by US. In our center, surgical procedures are performed in patients based on the recommendations of the National Comprehensive Cancer Network (NCCN) (20) and American Thyroid Association (ATA) (21). Prophylactic CLND is commonly performed in the central compartment (neck level VI), according to our institutional protocol, regardless of the clinical evidence of LNM. Lateral lymph node dissection (LLND) is performed when metastasis is highly suspicious at preoperative imaging examinations, including nodes at level II, III, IV and V. However, this is not routinely performed at level I unless there is clinical evidence to prove that this level involved.
Clinicopathological data source
Clinical data, such as age, gender and other basic information of patients, were collected from the electronic medical record through the registry. Pathologic information was gathered from postoperative pathology reports in the electronic medical record. All diagnoses were rendered and reported by pathologists who had 3–40 years of experience.
US examination
A comprehensive neck US examination was preoperatively performed for all patients in the supine position, with their neck extended, using a 5–18 MHz linear array transducer machine (iU22, Philips Medical Systems; Acuson Sequoia 512, Siemens Medical Solutions; LOGIQ S8 and E9, GE Medical Systems). These were performed by board-certified radiologists specializing in head and neck imaging, who have 3–20 years of experience. The radiologist who performed the US examination prospectively recorded the US features of the thyroid nodules and CLN status. If more than one nodule with suspicion of malignancy were found in thyroid gland, the maximum diameter of the most suspicious lesion was recorded and included in the data analysis. These images were completely stored.
Image interpretation
All images above were carefully evaluated by reviewers blinded to the pathology results, based on the Thyroid Imaging, Reporting and Data System criteria issued by the American College of Radiology in 2017 (2017 ACR TI-RADS) (22). Nodes with suspicious metastasis by US had the following characteristics, according to a previous study (23): microcalcifications, cystic aspect, peripheral vascularity, hyperechogenicity, round shape and loss of hilum.
Statistical analysis
Patients enrolled from January 2015 to May 2016 were assigned to the modeling group, while the remaining patients were assigned to the validation series. Statistical analysis was performed using SPSS version 19.0 software (IBM, Armonk, New York, USA). Continuous data were expressed as mean ± standard deviation (range), and categorical variables presented as a number and percentage. Chi-square test, t-test and Mann-Whitney U test were used to compare the characteristics between modeling and validation cohort. The univariate logistic regression was analyzed to identify risks associated with CLNM. Standardized by the z-score method, age and tumor size (expressed as z-age and z-size, respectively) were included in the multivariate regression. The predictive model was obtained from the forward stepwise multivariate logistic regression analysis. A score system was developed based on the standardized regression coefficient and odds ratio (OR). These scores were further divided into three groups, as follows: low, intermediate and high risk. In addition, the corresponding risk of CLNM was also evaluated. The overall differences were tested with Chi-square test. Bonferroni adjustment was used for post hoc multiple comparison test among groups. The receiver operating characteristic (ROC) curve with area under the curve (AUC) was used to evaluate the effectiveness and discrimination ability of the model and scoring system. An appropriate cut-off value was selected to calculate for the sensitivity and specificity of the model. Two-sided P values were calculated, and P<0.05 was considered statistically significant.
Results
Patient basic characteristics and US parameters
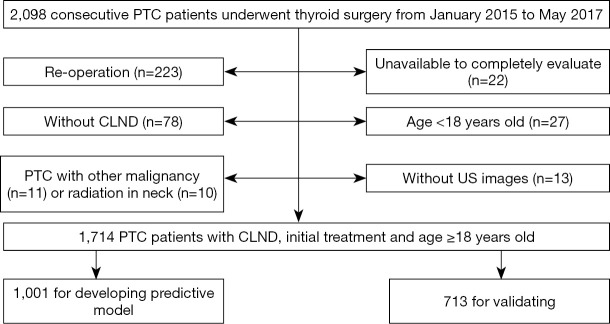
The study population is presented in a flow chart (Figure 1). Among the 2,098 patients, 384 patients were excluded: patients who had a re-operation (n=223); patients without CLND (n=78, large nodules diagnosed as benign by preoperative fine-needle aspiration biopsy, but occult PTC was confirmed by postoperative histopathology, or old patients who refused CLND); patients unavailable to be completely evaluated (n=35) due to large tumors or macrocalcifications, resulting in wide posterior acoustic shadowing; patients who were <18 years old (n=27); patients with other thyroid malignancies (n=11) or radiation history in the neck (n=10). In total, 1,714 PTC patients, who underwent CLND, received initial treatment, and were ≥18 years old, were included.
Figure 1.
Flow chart of the participant population. PTC, papillary thyroid carcinoma; CLND, central lymph node dissection; US, ultrasound.
Among the 1,001 patients enrolled in the modeling group, 285 (28.47%) were male and 716 (71.53%) were female, aged 18–79 years with a mean age of 40 years. Pathology revealed that 570 of 1,001 (56.94%) patients had CLNM, while 182 (28.17%) patients had lateral lymph node metastasis (LLNM). According to US results, 424 (42.36%) and 329 (32.87%) patients had positive nodes in central (uCLNM) and lateral (uLLNM) neck, respectively. Further, 713 cases with a mean age of 41 (range, 19–81) years were in the validation group, including 209 (29.31%) males and 504 (70.69%) females, and 406 (56.94%) of those were CLNM. The detailed data of the study cohort is summarized in Table 1.
Table 1. Demographics, tumor and cervical nodes characteristics of modeling and validation group.
| Characteristics | Modeling group (n=1,001) | Validation group (n=713) | P |
|---|---|---|---|
| Gender | 0.705 | ||
| Male | 285 (28.47) | 209 (29.31) | |
| Female | 716 (71.53) | 504 (70.69) | |
| Age (years) | |||
| Mean ± SD [range] | 40.65±11.67 [18–79] | 41.11±11.93 [19–81] | 0.429 |
| ≤24 | 53 (5.29) | 34 (4.77) | 0.947 |
| 25–34 | 296 (29.57) | 214 (30.01) | |
| 35–44 | 291 (29.07) | 195 (27.35) | |
| ≥45 | 361 (36.07) | 270 (37.88) | |
| Tumor size (mm) | |||
| Mean ± SD (range) | 15.63±10.08 (3.0–92.0) | 15.83±9.62 (3.0–67.0) | 0.685 |
| ≤10 | 366 (36.56) | 257 (36.04) | 0.609 |
| ≤20 | 443 (44.26) | 292 (40.95) | |
| ≤30 | 139 (13.89) | 105 (14.73) | |
| ≤40 | 30 (3.00) | 42 (5.89) | |
| >40 | 23 (2.30) | 17 (2.38) | |
| Cervical nodes | |||
| uCLNM& | 424 (42.36) | 356 (49.93) | 0.002 |
| uLLNM& | 329 (32.87) | 266 (37.31) | 0.064 |
| pCLNM§ | 570 (56.94) | 406 (56.94) | 1.000 |
| pLLNM§ | 282 (28.17) | 234 (32.82) | 0.042 |
&, CLNM/LLNM in ultrasound; §, CLNM/LLNM in pathology; CLNM, central lymph node metastasis; LLNM, lateral lymph node metastasis.
Risk factors of CLNM for PTC
Univariate and multivariate logistic regression
The results of the logistic regression analysis are presented in Table 2. It was found that uCLNM (OR =5.033), uLLNM (OR =3.905), uCapsule invasion (OR =2.205), punctate echogenic foci (PEF) (OR =2.441), multifocality (OR =2.263), male (OR =1.433), tumor size (OR =1.094), background (OR =0.559) and age (OR =0.964) were statistically significant variables associated with pCLNM (all P<0.05). Accompanied with nodular goiter and older age, CLNM trended to be less likely involved, while others had increased risk. Furthermore, the multivariate logistic regression results revealed that uCLNM (β=1.201) was the greatest contributor to the model, which was subsequently followed by uLLNM (β=0.758), z-size (β=0.465), gender (β=0.432), uCapsule invasion (β=0.411), PEF (β=0.404), and z-age (β=0.329). All these were independent predictors for CLNM, which were used to develop the following predictive model (Table 2).
Table 2. Uni- and multi-variate regression analysis of factors associated with CLNM.
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P | OR | OR (95% CI) | P | OR | OR (95% CI) | ||
| Gender | |||||||
| Male | 0.012 | 1.433 | 1.081, 1.900 | 0.016 | 1.495 | 1.077, 2.076 | |
| Age (years) | 0.000 | 0.964 | 0.953, 0.975 | 0.000 | 0.973 | 0.960, 0.986 | |
| Background | 0.001 | 0.095 | |||||
| GTβ | 0.000 | 0.559 | 0.409, 0.762 | 0.048 | 0.701 | 0.493, 0.996 | |
| HTγ | 0.052 | 0.896 | 0.629, 1.276 | 0.182 | 0.755 | 0.499, 1.414 | |
| Primary tumor | |||||||
| Size (mm) | 0.000 | 0.973 | 0.960, 0.986 | 0.000 | 1.056 | 1.032, 1.081 | |
| Location | |||||||
| Solitary lesion | 0.537 | – | – | – | – | – | |
| Middle third | 0.215 | 0.799 | 0.560, 1.139 | – | – | – | |
| Lower third | 0.205 | 0.772 | 0.517, 1.153 | – | – | – | |
| Isthmus | 0.986 | 0.992 | 0.429, 2.297 | – | – | – | |
| Multifocality | 0.000 | 2.263 | 1.666, 3.073 | 0.096 | 1.353 | 0.948, 1.932 | |
| Composition | |||||||
| Solid | 0.085 | 0.611 | 0.349, 1.071 | – | – | – | |
| Echogenicity | 0.010 | ||||||
| Hypoechoic | 0.637 | 1.195 | 0.570, 2.503 | – | – | – | |
| Very hypoechoic | 0.554 | 0.801 | 0.385, 1.668 | – | – | – | |
| Shape | |||||||
| Taller-than-wide | 0.617 | 0.933 | 0.711, 1.224 | – | – | – | |
| Margin | 0.073 | ||||||
| ETEδ | 0.035 | 1.748 | 1.040, 2.939 | 0.811 | 0.929 | 0.507, 1.701 | |
| Echogenic foci | 0.000 | 0.070 | |||||
| Macrocalcifications | 0.199 | 1.780 | 0.738, 4.293 | 0.672 | 1.241 | 0.456, 3.382 | |
| Peripheral (rim) | 0.891 | 1.113 | 0.243, 5.089 | 0.880 | 0.870 | 0.143, 5.287 | |
| PEFλ | 0.000 | 2.441 | 1.801, 3.309 | 0.010 | 1.576 | 1.116, 2.226 | |
| Distance (mm) | 0.000 | 0.728 | 0.626, 0.846 | 0.920 | 1.009 | 0.841, 1.212 | |
| uCapsule◎ | 0.000 | 2.205 | 1.709, 2.846 | 0.018 | 1.484 | 1.070, 2.057 | |
| TI-RADS$ Categories | 0.141 | 1.044 | 0.986, 1.107 | – | – | – | |
| uCLNM& | 0.000 | 5.033 | 5.033, 3.791 | 0.000 | 3.498 | 2.561, 4.778 | |
| uLLNM& | 0.000 | 3.905 | 3.905, 2.894 | 0.000 | 1.965 | 1.399, 2.761 | |
β, nodular goiter; γ, Hashimoto’s thyroiditis; δ, extrathyroidal extension; λ, punctate echogenic foci; ◎, capsule invasion in ultrasound; $, Thyroid Imaging, Reporting and Data System; &, CLNM/LLNM in ultrasound. CLNM, central lymph node metastasis; LLNM, lateral lymph node metastasis.
Predictive model and stratification
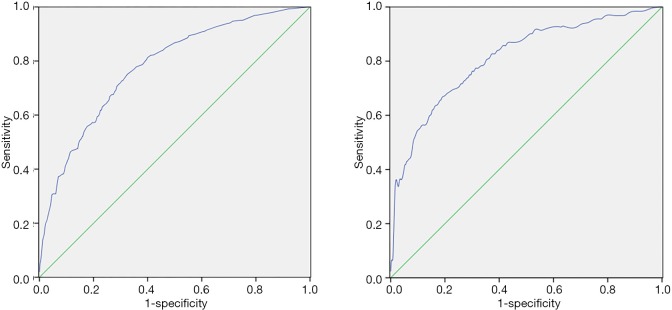
The β-value of z-age was the smallest. The β-value of other variables was divided by the sum of all regression coefficients (expressed in weight). The points of the predictors were assigned by the results, in which weight was multiplied by the corresponding OR. The reference baseline was assigned as 0 point (Table 3). The AUC for the modeling serie was 0.778 (95% CI: 0.751–0.803), suggesting good discrimination (Figure 2A). When a score of >8 was used as the cut-off point, the sensitivity and specificity were 77.0% and 65.0%, respectively. Furthermore, the rate of CLNM per two points and accumulative rate were calculated, as demonstrated in Table 4. According to a cut-off 8 and 18 points, the system was classified into three groups: low, intermediate and high risk of CLNM groups. The risk for these groups was approximately 30%, 60% and 80%, respectively. Higher scoring categories had a higher rate of metastasis. The overall positive rates of each group were compared using Chi-square test (χ2=203.20, P<0.000). After further adjustment for significant levels (α=0.05/β=0.017) in the multiple-group comparison, it was found that there was statistical significance between any two groups (P<0.000).
Table 3. Scoring system for CLNM prediction.
| Predictors | P | β | Weight※ | OR | Points# assigned |
|---|---|---|---|---|---|
| uCLNM& | 0.000 | 0.300 | |||
| Yes | 1.201 | 3.355 | 12.4 | ||
| No | 0 | ||||
| uLLNM& | 0.000 | 0.189 | 5.0 | ||
| Yes | 0.758 | 2.185 | |||
| No | 0 | ||||
| z-Size¥ | 0.000 | 0.465 | 0.116 | ||
| >4 | 6.061 | 8.5 | |||
| ≤4 | 2.535 | 3.5 | |||
| ≤3 | 2.423 | 3.4 | |||
| ≤2 | 1.794 | 2.5 | |||
| ≤1 | 0 | ||||
| Gender | 0.009 | 2.0 | |||
| Male | 0.432 | 0.108 | 1.532 | ||
| Female | 0 | ||||
| uCapsule | 0.006 | ||||
| Yes | 0.411 | 0.103 | 1.499 | 1.8 | |
| No | 0 | ||||
| PEF | 0.016 | ||||
| Yes | 0.404 | 0.101 | 1.477 | 1.8 | |
| No | 0 | ||||
| z-Age¥ | 0.000 | 0.329 | 0.082 | ||
| ≤24 | 2.020 | 2.0 | |||
| 25–34 | 2.197 | 2.2 | |||
| 35–44 | 1.389 | 1.4 | |||
| ≥45 | 0 |
&, CLNM/LLNM in ultrasound; ¥, standardized by z-score; β, standardization regression coefficient; ※, weight was each β of the predictor divided by the sum of all β; #, points were weight multiplied by OR. CLNM, central lymph node metastasis; LLNM, lateral lymph node metastasis; OR, odds ratio.
Figure 2.
The ROC. (A) Modeling group; (B) Validation group. The AUC of the model was 0.778 (95% CI: 0.751–0.803) with a sensitivity of 77.0% and a specificity of 65.0% using a cut-off value of 8 points. The predictive model yielded an AUC of 0.811 (95% CI: 0.781–0.839) with a cut-off value of 8 points, and the model had sensitivity of 82.5% and specificity of 63.2% in validation group. ROC, receiver operating characteristic; AUC, area under the curve.
Table 4. Scoring system and stratification associated with CLNM in modeling.
| Score | pCLNM§ (n) | Total (n) | pCLNM (%) | Accumulation rate (%) | Stratification | Risk of CLNM (%)@ |
|---|---|---|---|---|---|---|
| ≤2 | 15 | 90 | 16.67 | 31.87 | Low | 30 |
| ≤4 | 26 | 100 | 26.00 | |||
| ≤6 | 45 | 126 | 35.71 | |||
| ≤8 | 45 | 95 | 47.37 | |||
| ≤10 | 46 | 76 | 60.53 | 63.47 | Intermediate | 60 |
| ≤12 | 28 | 46 | 60.87 | |||
| ≤14 | 32 | 45 | 71.11 | |||
| ≤16 | 26 | 45 | 57.78 | |||
| ≤18 | 40 | 59 | 67.80 | |||
| ≤20 | 54 | 74 | 72.97 | 83.70 | High | 80 |
| ≤22 | 42 | 55 | 76.36 | |||
| ≤24 | 56 | 65 | 86.15 | |||
| ≤26 | 63 | 70 | 90.00 | |||
| ≤28 | 32 | 35 | 91.43 | |||
| ≤30 | 10 | 10 | 100.00 | |||
| >30 | 10 | 10 | 100.00 |
§, CLNM confirmed by pathology in modeling; @, estimated by accumulation rate. CLNM, central lymph node metastasis.
Model validation
A series of 713 patients met the inclusion and exclusion criteria applied to the validation, the predictive model yielded an AUC of 0.811 (95% CI: 0.781–0.839; Figure 2B). With a cut-off value of 8, which is categorized as intermediate or high for predicted probability, the present model had a sensitivity and specificity of 82.5% and 63.2%, respectively.
Discussion
This study has established a risk model and risk stratification to preoperatively predict CLNM in PTC using a larger data. All the indicators were collected prior to the operation. Seven variables considered in the model, in order, were uCLNM, uLLNM, size, gender, uCapsule invasion, PEF and age. The model demonstrated higher predictive value, either in modeling group or in validation cohort, which showed good consistency and discrimination ability. In our study, the incidence of CLNM in the modeling group was 56.94% (570/1,001), and the sensitivity and specificity of US for detecting CLNM was 58.07% (331/570) and 78.19% (337/431), respectively. According to our model, higher scores represented greater weight. The AUC of the model was 0.778 (95% CI: 0.751–0.803), and the sensitivity and specificity was 77% and 65%, respectively, using the cut-off value of 8 points. The sensitivity approximately increased by 19% based on the present data. When applied to the validation group, remarkable efficiency could be observed, with an increase of 83% for sensitivity. In addition, risk stratification also had a pretty good discrimination, as confirmed by the Bonferroni test. Thus, the present preliminary study preoperatively improved the risk assessment for CLNM.
Despite of the predilection for lymphatic spread in PTC, the challenge is that there are presently no favorable and accurate methods for pre- and intraoperative lymph node assessment. The diagnostic accuracy of US for CLNM in PTC has a wide range of 38–84% for sensitivity and 72–93% for specificity, and analogously, a CT range of 50–57% for sensitivity and 85–91% for specificity (9,10,24). Despite all this, risk factors correlated to CLNM in PTC have already been studied from different aspects (12-16). However, the predictive factors vary, and are not well-defined. Furthermore, merely few of these have further explored the relative contribution to CLNM (18,19). Therefore, the present retrospective research developed a risk model and risk stratification and both performed well.
A similar prediction established by Xiang et al. (19), which included 392 patients with cervical lymph node-negative PTC, indicated that CLNM had a sensitivity and specificity of 86.2% and 70.4%, respectively, with an index point of ≥2. However, some of the indices enrolled in their model were collected from postoperative pathological examinations and they did not stratify the scores. In addition, the predictive score system was not validated. Hence, it is not a practical and convenient model for use in clinical practice. Thus,we only included preoperative indices compared to their study. According to the coefficients, an easy-to-apply scoring system was developed, which would allow both radiologists and surgeons to conveniently calculate and express the probability of patient risk in CLNM, and this was validated by the subsequent 713 patients. In order to determine the risk correlation to direct clinical application, the scores were classified into different groups based on the risks of CLNM.
In the present model, uCLNM and uLLNM were the first two predictors. US, which has been recommend by ATA, ACR and NCCN guidelines (20-22), has become the preferred method to evaluate thyroid nodules and cervical nodes. Based on the experience of our investigators, US has a sensitivity of 58.07% and 85.46%, and a specificity of 78.19% and 87.86% for assessing CLNM and LLNM, respectively, which were consistent with previous studies (9,10,24). However, this was not sensitive enough to detect CLNM, particularly for patients with a thick neck, or lower paratracheal and retropharyngeal nodes, which may be obscured by the sternum or tracheal air shadow. Thus, other parameters are required to predict the risk of CLNM. The relationship between CLNM and LLNM would be expected, because tumor cells spread in a general stepwise dissemination through the lymphatic system in PTC, which spread from the thyroid gland to the central and lateral compartments on each side of the thyroid tumor. Then, opposite lateral and mediastinal LNM would follow suit (24-26). This suggests that LLNM may be accompanied by CLNM in almost all cases, with the exception of those that involve skip metastasis. Furthermore, uLLNM was correlated to a higher rate of CLNM, when compared with negative lateral neck lymph nodes (P<0.000, OR =3.905). This result was similar to that in previous studies, which was within 82.9–96.6% (24-26). It is conceivable that most PTCs with lateral LNMs have been strongly associated with ipsilateral CLNM. The newest ATA guideline also suggests that prophylactic central-compartment neck dissection should be considered in patients that clinically involved lateral neck nodes (cN1b), although there was weak recommendation and low-quality evidence (21). Therefore, selective CLND may be helpful when these independent features are noted on the preoperative examination of young PTC patients with suspicious large thyroid nodules, specially patients with clinically positive LLN.
Tumor size, which is defined as the size of the maximum diameter independent of tumor number in multifocal tumors, is included in many staging systems, such as the AGES, MACIS and AJCC/TNM classification (27). In the AJCC/TNM classification, primary tumor T stage is determined by tumor size and ETE (28). In the present series, a larger size was more frequently positive for regional lymph nodes, which is consistent with a previous retrospectively study that enrolled 3,219 patients, and revealed that tumor size was the strongest predictor of microscopic node metastasis and lymph node recurrence in a series of clinically node-negative PTC patients (27). The 2015 ATA consensus statement recommends therapeutic CLND for any patient with clinically positive nodes, and prophylactic CLND for patients with T3 and T4 primary tumors, without evidence of nodal metastases, or with known LLNM, or if information could be obtained which would guide the further steps in the therapy (21). Furthermore, clinicians should be aware that the prophylactic dissection of PTC with a large size should be considered to minimize lymph node metastases or recurrence. The new edition of the AJCC/UICC TNM staging system removed microscopic ETE from the staging, designating patients as having either disease limited to the thyroid or gross ETE. This may be contributed to two recent studies. In a study conducted by Woo et al., it was concluded that the presence of minimal ETE had no significant influence on recurrence-free survival (RFS) in solitary PTC (29). The other conducted by Radowsky et al. revealed that the outcome was worse in patients with gross ETE than in patients with microscopic local invasion (30). The present univariate analysis revealed that uCapsule was associated with CLNM, and reached a statistical significance. Therefore, performance of US in detecting capsule status was further analyzed. The uCapsule assessment had a sensitivity and specificity of 71.05% (486/684) and 89.27% (283/317), respectively, in the present modeling group. Patients with encapsulated carcinoma did not usually have distant metastasis, but exhibited an indolent biologic behavior (31). Thus, preoperative assessment of the capsule would be of great value for further clinical management.
Histologically, calcification was classified as either psammoma bodies, stromal calcification, or bone formation (32), and psammoma bodies was significantly associated with malignancy with a high specificity of 87.8% in thyroid nodules (33). PEF appears as hyperechoic spots of approximately 1 mm in diameter in US, although this may not exactly correspond to psammoma bodies during the cytological or histological examination, and the mechanism of these formations remains controversial. Bai et al. found that the presence of psammoma bodies was significantly correlated with gross LNM (32). The present model was in line with this study, which had an OR of 1.477 and assigned 1.8 points for PEF. However, it was acknowledged that the PEF was not confirmed by pathology, which requires further in-depth studies.
Compared with adults, PTC in the pediatric population exhibits differences in pathophysiology, clinical presentation, and long-term outcomes. Merely patients who were ≥18 years old were included. Furthermore, these patients were divided into groups every 10 years in the present model. Similar to other studies, younger age raised the risk of CLNM. The eighth TNM/AJCC edition (28) used a cut-off of 55 years to evaluate the clinical stage, although the age cut-off of either 45 or 55 years remains controversial (34,35) for prognosis prediction. Although it is a problem, the investigators consider that the cut-off age should be used to assess its impact in CLNM. First, the end point is extremely different, which is metastasis vs. survival. The former investigates the risk of CLNM in patients with PTC, while other studies investigated the mortality risk. Furthermore, there are no sufficient and convincing evidence to support any cut-off age, at present. Finally, the majority of studies have indicated that younger patients tend to have metastasis, which contradicts with the TNM staging. Notwithstanding, age remains as an important indicator, which should be analyzed properly and combined with other factors, in order to thoroughly evaluate patients, and make optimal care decisions.
At the same time, the investigators acknowledge several limitations. First, the present study was retrospective in nature, and may suffer from bias. Second, this approach could have prevented the study from identifying important US findings that could provide a clue to diagnose in real time, which might have influenced the evaluation of the investigators. Third, the nodes of US were matched with that of the pathologic examination based on region, and not node to node. There was a possibility that the nodes identified at US may not match those identified in surgical specimens. Finally, the present model was verified through a retrospective cohort series in our center. Therefore, these needs to be further and prospectively validated in external center patients before application to clinical practice.
Conclusions
The present study contributed a feasible and effective prediction, either to radiologists or surgeons, which may guide clinical decision-making and optimize the therapy regimen, thereby promoting the avoidance of excessive or insufficient treatment. But it needed to be validated further.
Acknowledgments
We thank Jibin Li for his assistance with statistical analysis.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study protocol was approved by the Ethics Committee of our Institutional Review Board (IRB) (No. B2018-064). Informed consent from enrolled patients was exempted by the IRB because of the retrospective nature of this study. The study was performed in accordance with the Declaration of Helsinki.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs.2020.03.02). Min Xu was studying for her master’s degree in the Department of Ultrasound and Electrocardiogram, Sun Yat-Sen University Cancer Center when the study was carried out. But now she works in the Department of Ultrasound, The First Affiliated Hospital, College of Medicine, Zhejiang University. The other authors have no conflicts of interest to declare.
References
- 1.Sherman SI. Thyroid carcinoma. Lancet 2003;361:501-11. 10.1016/S0140-6736(03)12488-9 [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Uhry Z, Guizard AV, et al. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol 2015;39:511-8. 10.1016/j.canep.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 3.Sciuto R, Romano L, Rea S, et al. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol 2009;20:1728-35. 10.1093/annonc/mdp050 [DOI] [PubMed] [Google Scholar]
- 4.Caron NR, Clark OH. Papillary thyroid cancer: surgical management of lymph node metastases. Curr Treat Options Oncol 2005;6:311-22. 10.1007/s11864-005-0035-9 [DOI] [PubMed] [Google Scholar]
- 5.Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am 1996;5:43-63. 10.1016/S1055-3207(18)30404-6 [DOI] [PubMed] [Google Scholar]
- 6.Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery 2003;134:946-54; discussion 954-5. 10.1016/S0039-6060(03)00424-0 [DOI] [PubMed] [Google Scholar]
- 7.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 2002;26:879-85. 10.1007/s00268-002-6612-1 [DOI] [PubMed] [Google Scholar]
- 8.Adam MA, Pura J, Goffredo P, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol 2015;33:2370-5. 10.1200/JCO.2014.59.8391 [DOI] [PubMed] [Google Scholar]
- 9.Wu LM, Gu HY, Qu XH, et al. The accuracy of ultrasonography in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma: a meta-analysis. Eur J Radiol 2012;81:1798-805. 10.1016/j.ejrad.2011.04.028 [DOI] [PubMed] [Google Scholar]
- 10.Suh CH, Baek JH, Choi YJ, et al. Performance of CT in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid cancer: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2017;38:154-61. 10.3174/ajnr.A4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DH, Kim YK, Yu HW, et al. Computed tomography for detecting cervical lymph node metastasis in patients who have papillary thyroid microcarcinoma with tumor characteristics appropriate for active surveillance. Thyroid 2019;29:1653-9. 10.1089/thy.2019.0100 [DOI] [PubMed] [Google Scholar]
- 12.Qu H, Sun GR, Liu Y, et al. Clinical risk factors for central lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2015;83:124-32. 10.1111/cen.12583 [DOI] [PubMed] [Google Scholar]
- 13.Suman P, Wang CH, Abadin SS, et al. Risk factors for central lymph node metastasis in papillary thyroid carcinoma: a National Cancer Data Base (NCDB) study. Surgery 2016;159:31-9. 10.1016/j.surg.2015.08.032 [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui S, White MG, Antic T, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid 2016;26:807-15. 10.1089/thy.2015.0429 [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Xiao C, Chen J, et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer 2019;19:622. 10.1186/s12885-019-5835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei X, Wang M, Wang X, et al. Prediction of cervical lymph node metastases in papillary thyroid microcarcinoma by sonographic features of the primary site. Cancer Biol Med 2019;16:587-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Baek JH, Kim JH, et al. Deep learning-based computer-aided diagnosis system for localization and diagnosis of metastatic lymph nodes on ultrasound: a pilot study. Thyroid 2018;28:1332-8. 10.1089/thy.2018.0082 [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Su X, Chen W, et al. A mathematical model using computed tomography for the diagnosis of metastatic central compartment lymph nodes in papillary thyroid carcinoma. Eur Radiol 2014;24:2827-34. 10.1007/s00330-014-3335-2 [DOI] [PubMed] [Google Scholar]
- 19.Xiang Y, Lin K, Dong S, et al. Prediction of central lymph node metastasis in 392 patients with cervical lymph node-negative papillary thyroid carcinoma in Eastern China. Oncol Lett 2015;10:2559-64. 10.3892/ol.2015.3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: thyroid carcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- 21.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017;14:587-595. 10.1016/j.jacr.2017.01.046 [DOI] [PubMed] [Google Scholar]
- 23.Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J 2013;2:147-59. 10.1159/000354537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim E, Park JS, Son KR, et al. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 2008;18:411-8. 10.1089/thy.2007.0269 [DOI] [PubMed] [Google Scholar]
- 25.Machens A, Hinze R, Thomusch O, et al. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg 2002;26:22-8. 10.1007/s00268-001-0176-3 [DOI] [PubMed] [Google Scholar]
- 26.Lee YS, Lim YS, Lee JC, et al. Clinical implication of the number of central lymph node metastasis in papillary thyroid carcinoma: preliminary report. World J Surg 2010;34:2558-63. 10.1007/s00268-010-0749-0 [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Fukushima M, Higashiyama T, et al. Tumor size is the strongest predictor of microscopic lymph node metastasis and lymph node recurrence of N0 papillary thyroid carcinoma. Endocr J 2013;60:113-7. 10.1507/endocrj.EJ12-0311 [DOI] [PubMed] [Google Scholar]
- 28.Amin MB, Edge SB. editors. AJCC cancer staging manual. 8th ed. New York: Springer, 2017. [Google Scholar]
- 29.Woo CG, Sung CO, Choi YM, et al. Clinicopathological significance of minimal extrathyroid extension in solitary papillary thyroid carcinomas. Ann Surg Oncol 2015;22 Suppl 3:S728-33. 10.1245/s10434-015-4659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radowsky JS, Howard RS, Burch HB, et al. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014;24:241-4. 10.1089/thy.2012.0567 [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Hirokawa M, Uruno T, et al. Biological behavior and prognosis of encapsulated papillary carcinoma of the thyroid: experience of a Japanese hospital for thyroid care. World J Surg 2008;32:1789-94. 10.1007/s00268-008-9482-3 [DOI] [PubMed] [Google Scholar]
- 32.Bai Y, Zhou G, Nakamura M, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol 2009;22:887-94. 10.1038/modpathol.2009.38 [DOI] [PubMed] [Google Scholar]
- 33.Remonti LR, Kramer CK, Leitão CB, et al. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 2015;25:538-50. 10.1089/thy.2014.0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganly I, Nixon IJ, Wang LY, et al. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid 2015;25:1106-14. 10.1089/thy.2015.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shteinshnaider M, Muallem Kalmovich L, Koren S, et al. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: a comparative study. Thyroid 2018;28:201-9. 10.1089/thy.2017.0265 [DOI] [PubMed] [Google Scholar]