Isoforms of protein kinase B (also known as AKT) play important roles in mediating insulin and growth factor signals. Previous studies have suggested that the AKT2 isoform is critical for insulin-regulated glucose metabolism, while the role of the AKT1 isoform remains less clear. This study focuses on the effects of AKT1 on the adaptive response of pancreatic β cells. Using a mouse model with inducible β-cell-specific deletion of the Akt1 gene (βA1KO mice), we showed that AKT1 is involved in high-fat-diet (HFD)-induced growth and survival of β cells but is unnecessary for them to maintain a population in the absence of metabolic stress.
KEYWORDS: β-cell proliferation, β-cell apoptosis, protein kinase B (AKT1), high-fat diet (HFD), endoplasmic reticulum (ER) stress
ABSTRACT
Isoforms of protein kinase B (also known as AKT) play important roles in mediating insulin and growth factor signals. Previous studies have suggested that the AKT2 isoform is critical for insulin-regulated glucose metabolism, while the role of the AKT1 isoform remains less clear. This study focuses on the effects of AKT1 on the adaptive response of pancreatic β cells. Using a mouse model with inducible β-cell-specific deletion of the Akt1 gene (βA1KO mice), we showed that AKT1 is involved in high-fat-diet (HFD)-induced growth and survival of β cells but is unnecessary for them to maintain a population in the absence of metabolic stress. When unchallenged, βA1KO mice presented the same metabolic profile and β-cell phenotype as the control mice with an intact Akt1 gene. When metabolic stress was induced by HFD, β cells in control mice with intact Akt1 proliferated as a compensatory mechanism for metabolic overload. Similar effects were not observed in βA1KO mice. We further demonstrated that AKT1 protein deficiency caused endoplasmic reticulum (ER) stress and potentiated β cells to undergo apoptosis. Our results revealed that AKT1 protein loss led to the induction of eukaryotic initiation factor 2 α subunit (eIF2α) signaling and ER stress markers under normal-chow-fed conditions, indicating chronic low-level ER stress. Together, these data established a role for AKT1 as a growth and survival factor for adaptive β-cell response and suggest that ER stress induction is responsible for this effect of AKT1.
INTRODUCTION
Pancreatic β-cell mass and function are critical for maintaining metabolic homeostasis. To accommodate increased insulin demand, β cells adapt by expanding their mass to maintain euglycemia (1, 2). The adaptive response of β cells to a high-fat diet (HFD) has been studied extensively (3–6). Taken together, these studies demonstrate that HFD consumption induces the growth of β cells to produce more insulin in an effort to lower blood glucose. However, this adaptive response is limited. When stimulation is persistent, the adaptive proliferation response eventually fails and β cells undergo apoptosis. While the molecular switch for turning the adaptive response to apoptosis remains unknown, extensive stress to β cells has been observed during progression, including oxidative, inflammatory, amyloidal, and endoplasmic reticulum (ER) stress (1, 7, 8). Among these, ER stress, which is induced by HFD feeding, has been linked to β-cell apoptosis in diabetic models (9, 10). Recently, the balance between XBP1 and CHOP, two proteins involved in ER stress regulation, has been demonstrated to influence the switch between adaptive proliferation and apoptosis (11).
Protein kinase B, or AKT, is a serine/threonine kinase that plays important roles in multiple cellular processes, including cell growth, survival, and metabolism. Mutations of the genes AKT1 and AKT2, which transcribe the two major isoforms of AKT, have been associated with insulin resistance and diabetes (12–15). In particular, AKT2 was found to be required for maintaining metabolic homeostasis, as Akt2−/− mice develop insulin resistance (77). Likewise, AKT2 is found to indirectly affect adaptive islet growth through regulating peripheral glucose metabolism. However, a direct function of AKT isoforms in the adaptive response of β cells beyond regulating glucose metabolism is ambiguous (16–21).
A tentative role of AKT1 in the regulation of growth and survival of pancreatic β cells has been indicated (16, 19). The ectopic overexpression of constitutively active AKT1 in β cells leads to a dramatic increase in islet mass (17, 23, 24). In addition, the deletion of Pten (phosphatase and tensin homologue deleted on chromosome 10) in β cells, which leads to constitutive activation of AKT, resulted in increased β-cell proliferation, enhanced islet mass, and hypoglycemia in mice (25, 26). However, in mice expressing a kinase-dead form of AKT1 in β cells or deficient for AKT1, normal β-cell mass and morphology are observed (22, 27). These studies suggest that AKT1 activation, while capable of inducing β-cell hyperproliferation, is not necessary for the physiological function and maintenance of β cells. The role of AKT1 in the adaptive response of β cells, however, is unexplored.
In this study, we developed a β-cell Akt1 deletion mouse model (βA1KO; Akt1loxP/loxP, Mip-CreERT+) to specifically address the role of AKT1 in β cells. By inducing adult-onset deletion of Akt1 specifically in β cells, our data demonstrated that AKT1 is not required for the maintenance of β-cell mass in the unchallenged physiological euglycemia state. However, it is indispensable for the adaptive growth response of β cells in response to HFD feeding to meet HFD-induced metabolic challenges. We further demonstrated that AKT1 loss results in chronic, low-level ER stress and makes β cells more susceptible to ER stress-induced cell death.
RESULTS
AKT1 is unnecessary for physiological maintenance of β cells but required for adaptive β-cell response to metabolic stress.
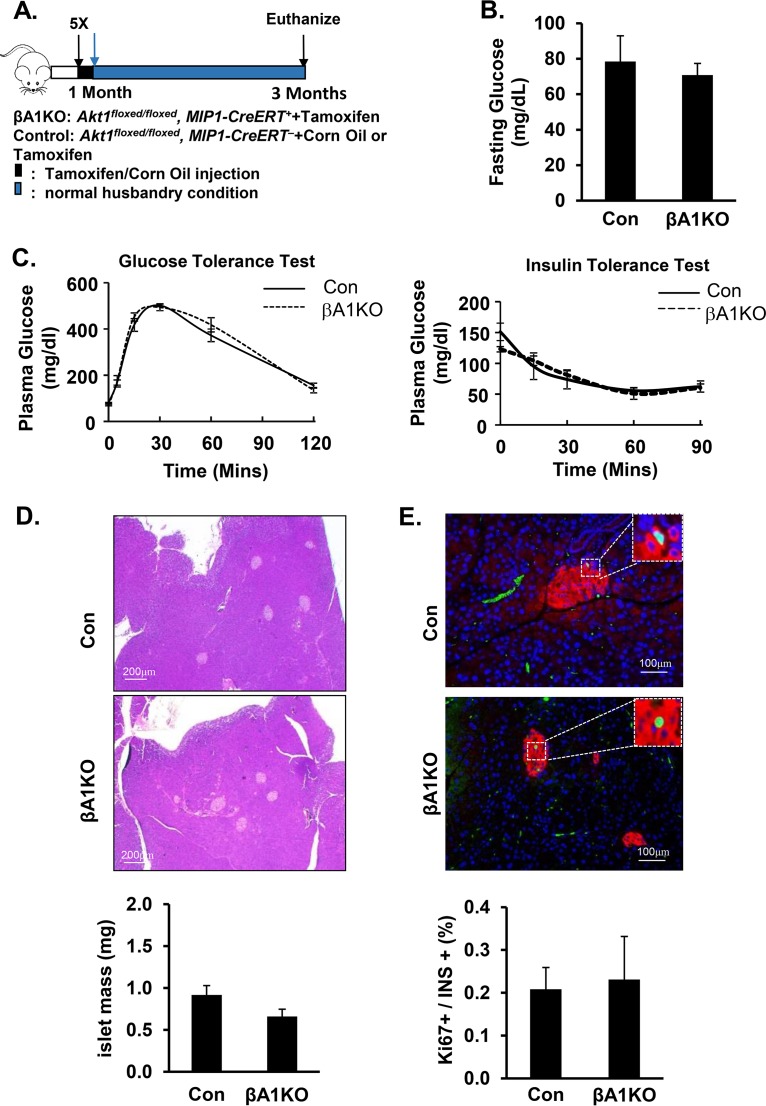
To specifically address the function of the AKT1 protein on pancreatic β cells, we created a β-cell-specific Akt1 deletion mouse model, βA1KO mice (Akt1loxP/loxP, Mip-CreERT+ mice treated with tamoxifen). Akt1 deletion was induced in these mice by administering 5 injections of tamoxifen starting at 4 weeks of age (Fig. 1A). Using Akt1loxP/loxP, Mip-CreERT+, Rosa26YFP (βA1KO-YFP) mice, where β cells with Akt1 deletion are labeled with yellow fluorescent protein (YFP), our data indicated that this protocol can induce Akt1 deletion in at least 95% of β cells (see Fig. S2A in the supplemental material). Immunoblot analysis of isolated islets confirmed that tamoxifen treatment induced the loss of AKT1 protein and reduced phosphorylation of its substrate, PRAS40 (Fig. S2B and C). There is also no compensatory increase of AKT2 for the loss of AKT1 in islets (Fig. S2C). AKT3 is not detectable in islets (27, 28). At 3 months of age, there was no statistically significant difference in fasting plasma glucose levels between the βA1KO and control mice (Fig. 1B). Further analysis indicated that β-cell-specific loss of AKT1 did not affect the overall glucose metabolism. No statistically significant differences were observed in either ipGTT or ipITT response between the βA1KO and control mice (Fig. 1C). We then examined the morphology of the pancreatic tissues. Analysis of islet area and β-cell proliferation indicated that losing AKT1 protein did not impact the proliferative ability of β cells or the mass of the islets (Fig. 1D and E). Similar results were also observed in mice carrying a germ line deletion of Akt1 (data not shown), consistent with previous loss-of-function studies of AKT1 (22, 27).
FIG 1.
AKT1 deficiency does not impact the physiological maintenance of pancreatic β cells. (A) Illustration of the protocol for inducing Akt1 deletion with tamoxifen in pancreatic β cells. (B) Plasma glucose determined in control versus βA1KO mice after 16 h of fasting (n = 14 for control; n = 5 for βA1KO). (C, left) Glucose tolerance test (ipGTT) performed with intraperitoneal injection of 2 mg/kg (body weight) glucose after 16 h of fasting. (C, right) Insulin tolerance test (ipITT) performed with 0.5U/g (body weight) insulin intraperitoneal injection after 5 h of fasting (n = 9 for control, n = 4 for βA1KO). (D) The top two panels are representative H&E staining images of control (Con) and βA1KO mouse pancreas. The bottom panel shows islet mass of control (Con) and βA1KO mice (n = 4 to 5/group). (E) The top two panels are representative images of Ki67/insulin/4′,6-diamidino-2-phenylindole (DAPI)-stained pancreas sections from the indicated mouse groups (red, insulin; green, Ki67; blue, DAPI). The bottom panel shows the quantification of the percentage of Ki67-positive β cells versus total insulin-positive β cells in the indicated groups. Three sections (240 μm apart) were analyzed for each mouse (n = 5).
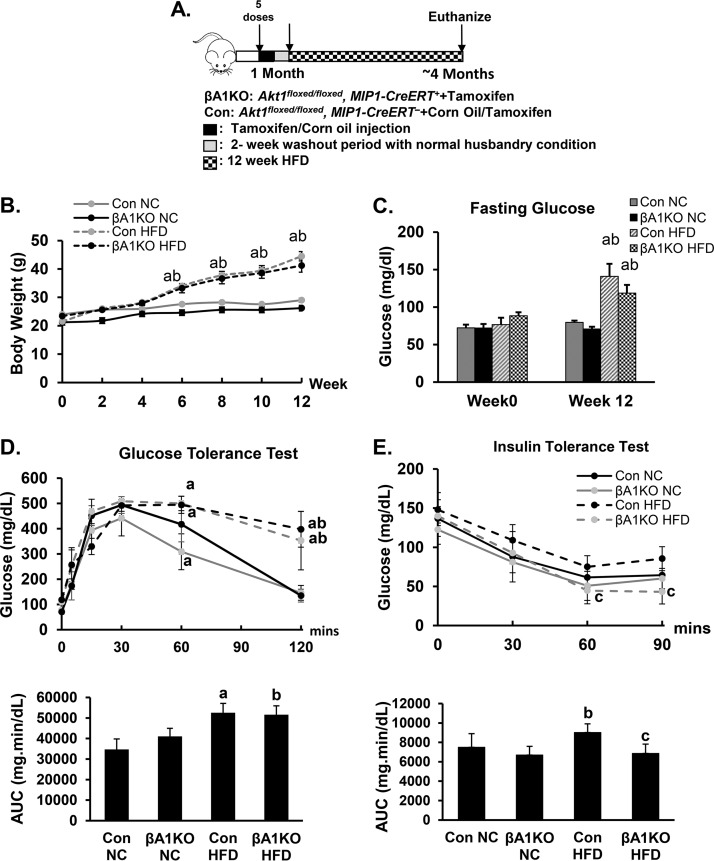
Pancreatic β cells are known to be extremely slow at replenishing themselves under normal physiological conditions (29). In adulthood, adaptive proliferation or apoptosis of β cells occurs in response to a wide range of pathophysiological conditions, including insulin resistance. The inability of β cells to adapt and maintain euglycemia is closely associated with prediabetic and diabetic conditions in humans (30–32). To address the role of AKT1 in this adaptive response, we exposed the control and βA1KO mice to either normal chow (NC) or HFD feeding (Fig. 2A and B). After a 12-week feeding of HFD, both βA1KO and control mice displayed hyperglycemia, as expected (Fig. 2C). No difference was observed in body weight and fasting plasma glucose level between βA1KO and control mice when fed the same diet (Fig. 2B and C). There also were no major differences in glucose levels during ipGTT between the control and βA1KO mice when fed the same diet (Fig. 2D). The calculated areas under the curve (AUC) were also similar for control and βA1KO mice when exposed to the same diet (data not shown).
FIG 2.
Metabolic status of control and βA1KO mice during HFD feeding. (A) Illustration demonstrating the protocol of the HFD feeding experiment. (B) Body weight of mice in different groups (n = 5 to 14 animals per group). (C) Fasting glucose level of control (Con) and βA1KO mice before and after HFD feeding (n = 14 and 9 for control NC and control HFD, respectively; n = 5 for both βA1KO NC and βA1KO HFD). (D, top) Glucose tolerance test (ipGTT) (n = 14 and 9 for control NC and control HFD, respectively; n = 5 for both βA1KO NC and βA1KO HFD). (Bottom) Area under the curve (AUC) analysis for the ipGTT curve. (E, top) Insulin tolerance test (ipITT) (n = 9 and 10 for control NC and control HFD, respectively; n = 4 and 5 for βA1KO NC and βA1KO HFD, respectively). (Bottom) AUC analysis for ipITT curve. For statistical analysis, letters indicate the following: a, control HFD is significantly different from control NC, P ≤ 0.05; b, βA1KO HFD is significantly different from βA1KO NC, P ≤ 0.05; c, βA1KO HFD is significantly different from Con HFD.
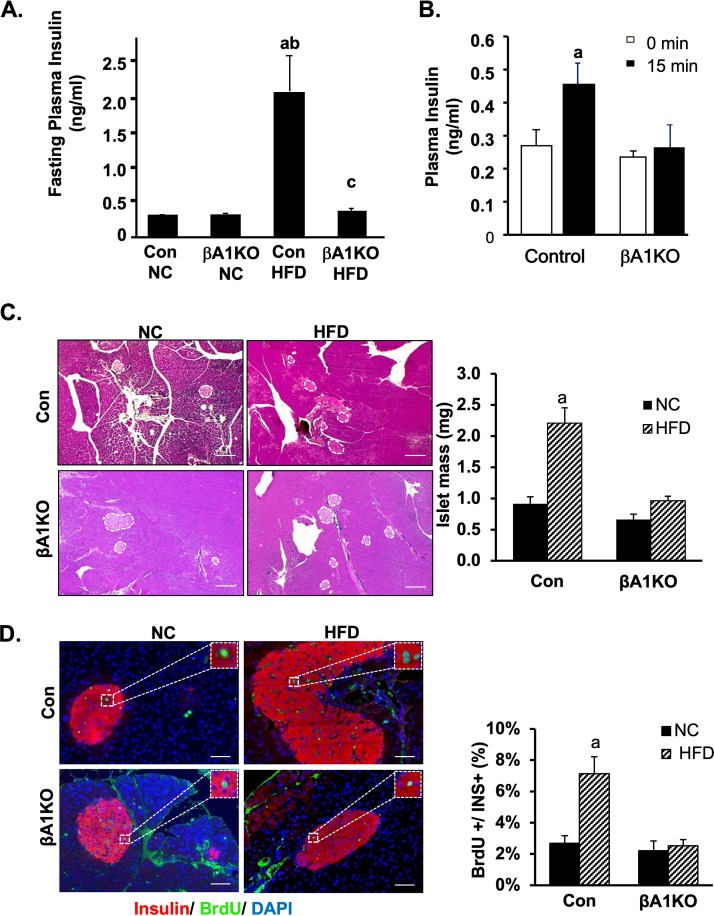
In control mice, feeding of HFD induced moderate but insignificant resistance to exogenous insulin treatment (Fig. 2E). Plasma glucose levels at different time points during ipITT and AUC examination are higher in control animals fed HFD than in those fed chow diet. The βA1KO mice on HFD, however, did not develop similar resistance to insulin when they were fed HFD. The AUC of ipITT for βA1KO mice on HFD is more similar to that of the control and βA1KO mice fed normal chow. To address how this may occur, we first analyzed the fasting plasma insulin levels. Our data show that unlike the control mice, the βA1KO mice on HFD do not experience hyperinsulinemia (Fig. 3A). The fasting plasma insulin levels in βA1KO mice on HFD is less than 50% of that of the control mice on HFD. Similarly, glucose injection did not induce elevated plasma insulin in the βA1KO mice, as it did in the controls (Fig. 3B), supporting a dysfunctional adaptive response of the βA1KO β cells.
FIG 3.
AKT1 is necessary for HFD to induce proliferation in β cells. (A) Measured fasting insulin (n = 7 for Con NC; n = 5 for βA1KO NC; n = 6 for Con HFD; n = 5 for βA1KO HFD). (B) Glucose-stimulated insulin release 15 min after injection of glucose to control and βA1KO mice (n = 9 and 8 for control and βA1KO at 0 min, respectively; n = 9 and 3 for control and βA1KO at 15 min, respectively). (C, left) Representative H&E staining pictures of pancreas from control mice (Con) and βA1KO mice on either NC or HFD. Islet area is circled with dashed lines in the picture (scale bars, 200 μm). (C, right) Islet mass of control and βA1KO mice fed with either NC or HFD (n = 5 for Con NC; n = 4 for βA1KO NC; n = 3 for both Con HFD and βA1KO HFD). Three sections 240 μm apart were analyzed for each mouse. All islets were counted for each section for statistical analysis. (D, left) Representative picture of BrdU/insulin/DAPI staining of pancreas from the indicated mouse group (red, insulin; green, BrdU; blue, DAPI; scale bars, 100 μm). (D, right) Quantification of the percentage of BrdU-positive β cells versus total insulin-positive β cells in the indicated groups (n = 5 for Con NC; n = 4 for βA1KO NC; n = 3 for both Con HFD and βA1KO HFD). Three sections 240 μm apart were analyzed for each mouse. All islet cells were counted from each section for statistical analysis. For statistical analysis, letters indicate the following: a, control HFD is significantly different from control NC, P ≤ 0.05; b, βA1KO HFD is significantly different from Con HFD, P ≤ 0.05; c, for each type of mouse, the insulin level at 15 min after glucose injection is significantly different from the fasting insulin level.
β cells adapt to the increased insulin demand by increasing the secretion of insulin as well as inducing the mass of β cells for long-term adaptation (3, 33). To explore the mechanisms for this lack of insulin response to HFD and glucose stimulation, here, we investigated the adaptive growth of β cells, as AKT1 is a growth/survival kinase. While AKT1 did not affect the steady-state islet mass, the ability of HFD to induce hypertrophy of islets is severely impaired in the βA1KO mice (Fig. 3C). In response to HFD, the total islet area increased significantly by 2- to 3-fold in control mice. In βA1KO mice, an insignificant increase of less than 1.2-fold was observed. As HFD is expected to induce the proliferation of β cells, we determined the proliferation rate in β cells using bromodeoxyuridine (BrdU) labeling. The BrdU-positive β cells increased by approximately 3-fold (7.13% ± 1.08% versus 2.53% ± 0.39%) due to HFD feeding in control mice, whereas this rate did not differ between HFD- and NC-fed βA1KO mice (2.69% ± 0.63% versus 2.21% ± 0.33%) (Fig. 3D). Collectively, these data indicate that AKT1 participates in the growth of β cells as they respond to metabolic stress induced by HFD. Specifically, the lack of AKT1 prevented β-cell proliferation in response to HFD feeding.
AKT1 loss induces ER stress and subjects β cells to stress-induced apoptosis.
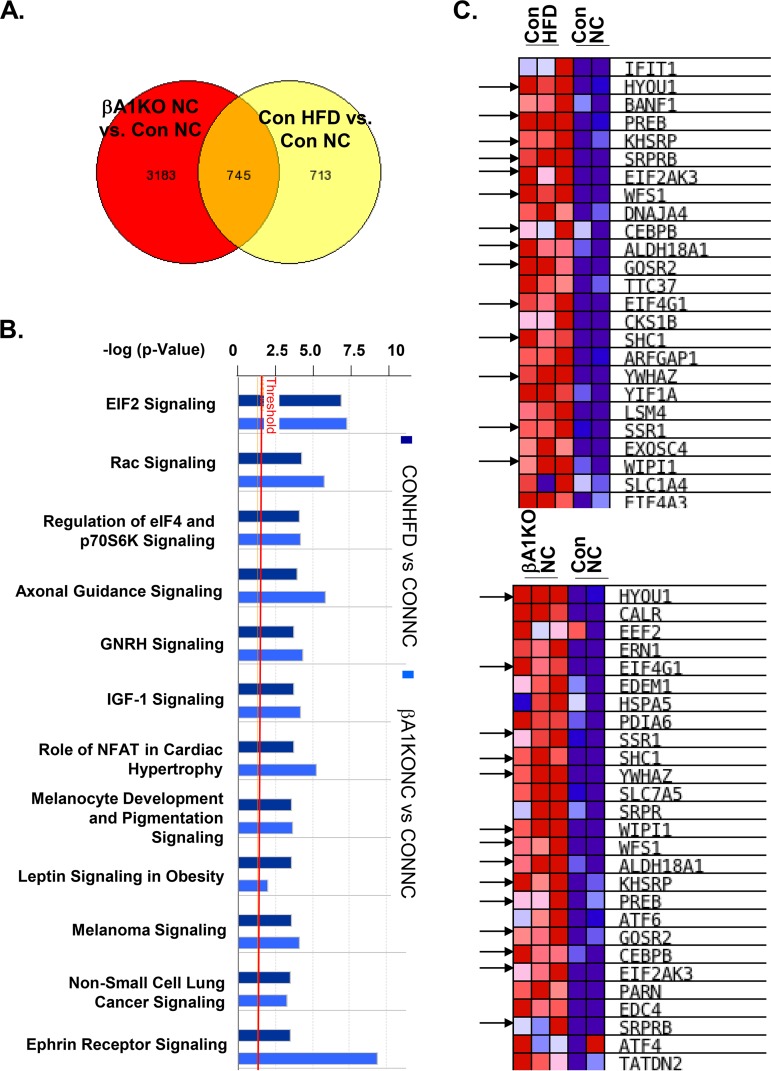
To address the molecular signals by which AKT1 may affect β-cell adaptive responses, we isolated RNA from islets of the βA1KO and control mice fed either NC or HFD and performed RNA-sequencing (RNA-seq) analysis. Our analysis identified 745 genes that were mutually modified by both Akt1 deletion and HFD feeding, suggesting that the cellular responses regulated by these genes were changed similarly by HFD feeding and Akt1 deletion in β cells (Fig. 4A). Ingenuity Pathway Analysis (IPA) of these 745 genes identified the eukaryotic initiation factor 2 (eIF2) signaling pathway as the most significantly altered signaling induced by both HFD feeding and by Akt1 deletion (Fig. 4B and Fig. S3A). eIF2 is essential for the integrated stress response, and upregulation of eIF2 signaling is an indication of cellular stress. Transcriptome changes have been used as indications for chronic ER stress in neurons previously (34, 35). Thus, we further compared the transcriptome of genes involved in ER stress signals. Our analysis revealed that a substantial number of the ER stress genes altered by HFD feeding in control mice (Fig. 4C, top) overlap those altered by Akt1 deletion in normal chow mice (Fig. 4C, bottom).
FIG 4.
AKT1 deficiency in β cells induces eIF2 and ER stress signals. (A) Venn diagram demonstrating genes mutually changed by HFD versus NC and by Akt1 deletion versus controls. Genes that increased or decreased more than 2-fold between the indicated groups were included in the analysis. RNA-seq data obtained with islets (n = 3) isolated from different groups of mice were analyzed. Con NC, control mice fed normal chow; Con HFD, HFD-fed control mice; βA1KO NC, mice with Akt1 deletion in β cells fed on NC; βA1KO HFD, mice with Akt1 deficiency in β cells fed on HFD. (B) Ingenuity Pathway Analysis (IPA) identified eIF2 signaling to be the most upregulated signal after HFD feeding in control mice or due to AKT1 deficiency in β cells. (C) Gene set enrichment analysis (GSEA) shows ER stress marker genes in the indicated groups of mice. Arrows indicate genes that were upregulated in both control mice and AKT1 deficiency groups.
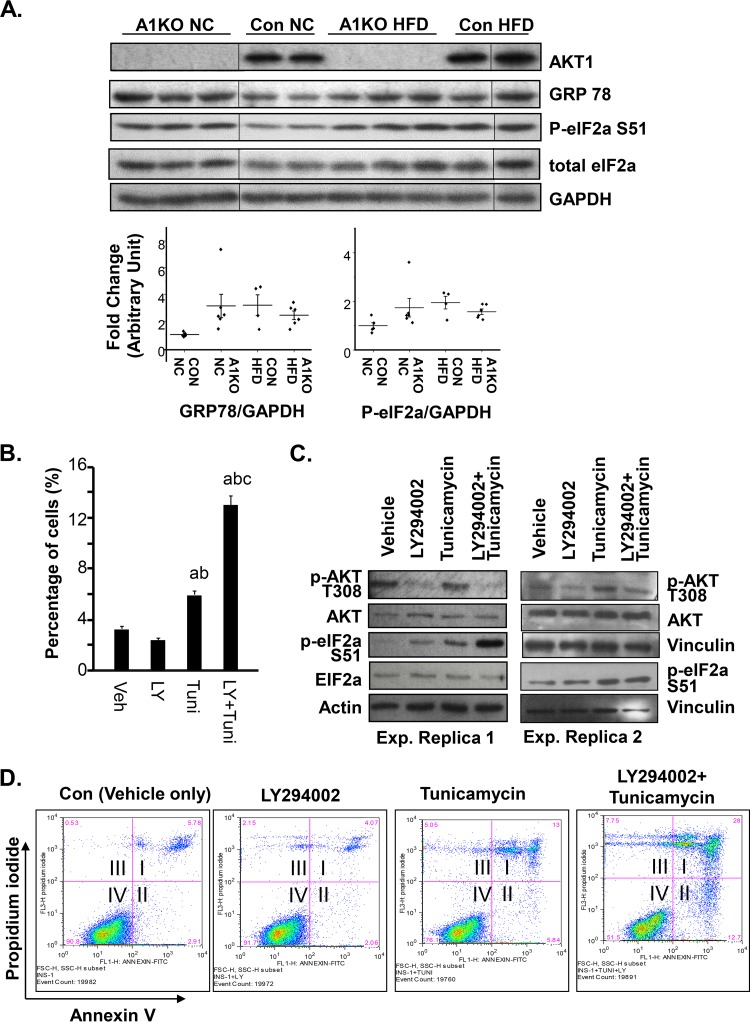
This transcriptome analysis suggests that AKT1 loss in β cells induces a stress state similar to that of HFD feeding. In HFD-fed mice, eIF2α is phosphorylated on s51 to mediate the response to HFD-induced stress to β cells (Fig. S3B). To confirm that AKT1 loss indeed affected ER stress signaling in β cells, we evaluated the protein levels of several ER stress markers, including p-PERK (phosphorylated endoplasmic reticulum-resident protein kinase, T980), GRP78 (78-kDa glucose-regulated protein), and phosphorylated eIF2α in control and Akt1−/− islets isolated from mice fed either NC or HFD for 4 months. Consistent with an effect of HFD on ER stress signaling (36, 37), our analysis showed that HFD induced moderate upregulation of GRP78 and phosphorylation of eIF2α (Ser51) in the control islets (Fig. 5A). Loss of Akt1 led to moderately elevated protein levels of GRP78 and p-eIF2α under normal chow conditions. The levels of GRP78 and p-eIF2α in islets of these NC-fed AKT1-deficient mice are comparable to that of control islets of HFD-fed mice. No further effect of HFD is observed in the AKT1-deficient islets. We were not able to detect PERK and phosphorylated PERK in the islets (data not shown). These analyses confirm the observation based on our RNA-seq analysis and suggest that AKT1 deficiency induced basal ER stress signals in β cells when they are not stressed with HFD.
FIG 5.
AKT1 deficiency causes mild ER stress and subjects β cells to cell death. (A) Western blot analysis of islets isolated from mice in the indicated groups. A1KO NC, Akt1−/− mice fed normal chow; Con NC, Akt1+/+ mice fed normal chow; Con HFD, Akt1+/+ mice fed high-fat diet; A1KO HFD, Akt1−/− mice fed a high-fat diet. AKT1, GRP78, p-eIF2α (Ser51), and total eIF2α were detected with GAPDH as a loading control. Image is representative of 3 blots. The dot plot under the blots indicates densitometry quantification of each biological sample (n = 5 for Con NC; n = 6 for A1KO NC; n = 4 for Con HFD; n = 6 for A1KO HFD). The relative densitometry quantification for each band is compared to the average relative densitometry quantification of Con NC on the same blot. Each dot represents one sample. The line indicates the average for all samples in the same group. (B) Quantification of annexin V+ INS-1 cells after treatment with the indicated chemicals for 48 h (n = 3; Con, control; +LY, LY294002 treatment; +Tuni, tunicamycin treatment). Annexin V+ cells are in quadrant II. (C) Two representative Western blots show the p-AKT (Thr308) and p-eIF2α (Ser51) levels of INS-1 cells exposed to LY294002, tunicamycin, or a combination of LY294002 and tunicamycin. (D) Representative flow cytometry plots of INS-1 cells treated with LY294002, tunicamycin, or LY29002 and tunicamycin. The y axis indicates propidium iodide staining. The x axis indicates annexin V staining. Quadrant I, annexin-positive and propidium iodide-positive cells; quadrant II, annexin-positive and propidium iodide-negative cells; quadrant III, annexin-negative and propidium iodide-positive cells; quadrant IV, annexin-negative and propidium iodide-negative cells. For statistical analysis, letters indicate the following: a, significantly different from Con (vehicle) group, P < 0.05; b, significantly different from +LY group, P < 0.05; c, significantly different from the +Tuni group, P < 0.05.
Elevated fatty acid and obesity have been reported to directly induce ER stress in β cells (36, 37). This ER stress is partially responsible for the eventual failure of β cells to compensate for hyperglycemia developed in individuals with insulin resistance (38). Our observation that the eIF2 signaling pathway is induced along with ER stress genes in response to HFD feeding is consistent with this understanding of β-cell response to HFD feeding. As a similar induction of eIF2, GRP78, and the ER stress signaling pathway is observed in the βA1KO islets of mice fed with normal chow, we hypothesize that the lack of AKT1 protein triggers low levels of ER stress that prepares the β cells to either more readily respond to stress or fail faster due to this chronic stress. To test this hypothesis, we either left INS-1 cells untreated or pretreated them with LY294002 to block AKT signaling, followed by tunicamycin treatment to induce ER stress (39). While LY294002 treatment alone had very little effect on the percentage of apoptotic cells positive for annexin V, tunicamycin induced apoptosis in these cells. Combination treatment of tunicamycin and LY294002 synergistically increased the percentage of apoptotic cells (Fig. 5B and D). Our analysis further showed that inhibiting AKT activity (with LY294002) leads to increased phosphorylation of eIF2α (Fig. 5C), confirming the results observed in the islets (Fig. 5A). Combination treatment of LY294002 and tunicamycin led to a synergistic increase of eIF2α phosphorylation, suggesting that AKT inhibition promoted ER stress signals to accelerate apoptosis.
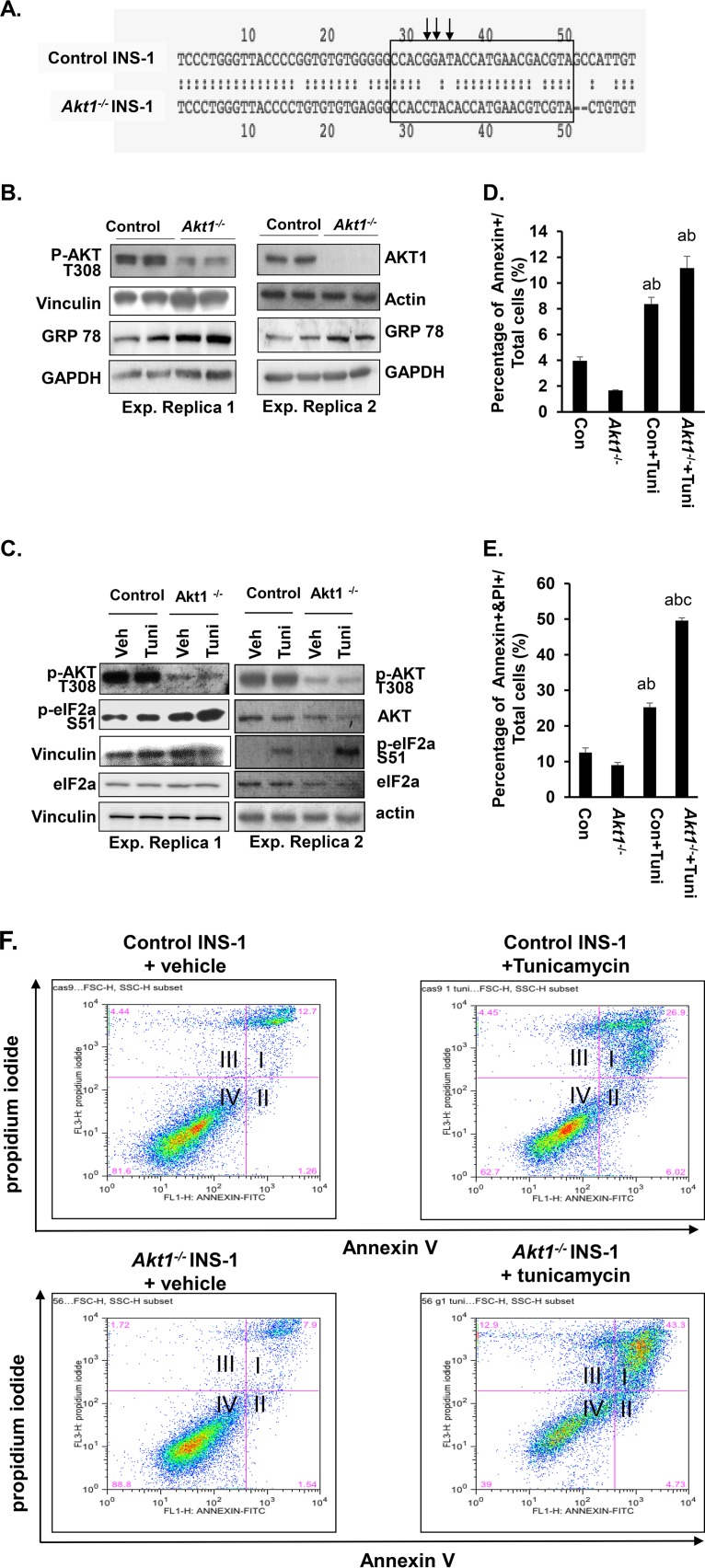
These data are further corroborated by studies where Akt1 is deleted in INS-1 cells using CRISPR/Cas9 technology (Fig. 6A and Fig. S4A). Similar to what was observed with LY294002 treatment (Fig. 5C), AKT1 loss caused an upregulation of GRP78 in INS-1 cells (Fig. 6B), although a modest change in basal eIF2α phosphorylation was observed (Fig. 6C). Tunicamycin, which induced more robust phosphorylation of eIF2α when combined with LY294002, also induced similar synergistic effects with Akt1 deletion (Fig. 6C). These data confirm the role of AKT1 in adaptation to the ER stress signal. Likewise, cells lacking AKT1 were more susceptible to tunicamycin-induced cell death (Fig. 6D to F), consistent with a synergistic effect of AKT1 inhibition and ER stress on cell survival. Of note, AKT1 loss induced a moderate increase in apoptotic cells. A synergistic effect is observed with AKT1 loss and LY294002 treatment (Fig. S4B), suggesting that the regulation of cell survival by phosphatidylinositol 3-kinase signal in β cells is not dependent solely on AKT1 as the survival kinase. Together, these in vivo and in vitro data suggest that β cells lacking AKT1 are undergoing low levels of ER stress. This moderately increased ER stress prepares the cells for ER stress-induced apoptosis.
FIG 6.
AKT1 deficiency in INS-1 cells induced GRP78 expression and activation of eIF2α signaling pathway and sensitized cells to tunicamycin. (A) Partial sequence of Akt1 exon 1. The box is the genetic sequence targeted by the guide RNA (TACGTCGTTCATGGTATCCGTGG), and the arrows point to mutated base pairs. (B) Two representative Western blots show the loss of AKT1 protein and upregulation of GRP78 in Akt1−/− INS-1 cells. (C) Two representative Western blots show the p-AKT (Thr308) and p-eIF2α (Ser51) level of INS-1 cells and Akt1−/− cells with or without tunicamycin treatment (Tuni, tunicamycin). (D) Quantification of the annexin V+ cells in the indicated groups (n = 3). Annexin V+ cells are in quadrant II. (E) Quantification of the percentage of annexin V+ and PI+ cells in the indicated groups (n = 3). Annexin V+ and PI+ cells are in quadrants I and III. (F) Representative plots for flow cytometry experiments of control INS-1 and Akt1−/− cells exposed to tunicamycin. Quadrant I, annexin-positive and propidium iodide-positive cells; quadrant II, annexin-positive and propidium iodide-negative cells; quadrant III, annexin-negative and propidium iodide-positive cells; quadrant IV, annexin-negative and propidium iodide-negative cells. For statistical analysis, letters indicate the following: a, significantly different from Con INS-1, P < 0.05; b, significantly different from Akt1−/− INS-1 group, P < 0.05; c, significantly different from Con INS-1 +Tuni group, P < 0.05.
AKT1 loss sets up β cells for apoptosis in response to metabolic stress in mice.
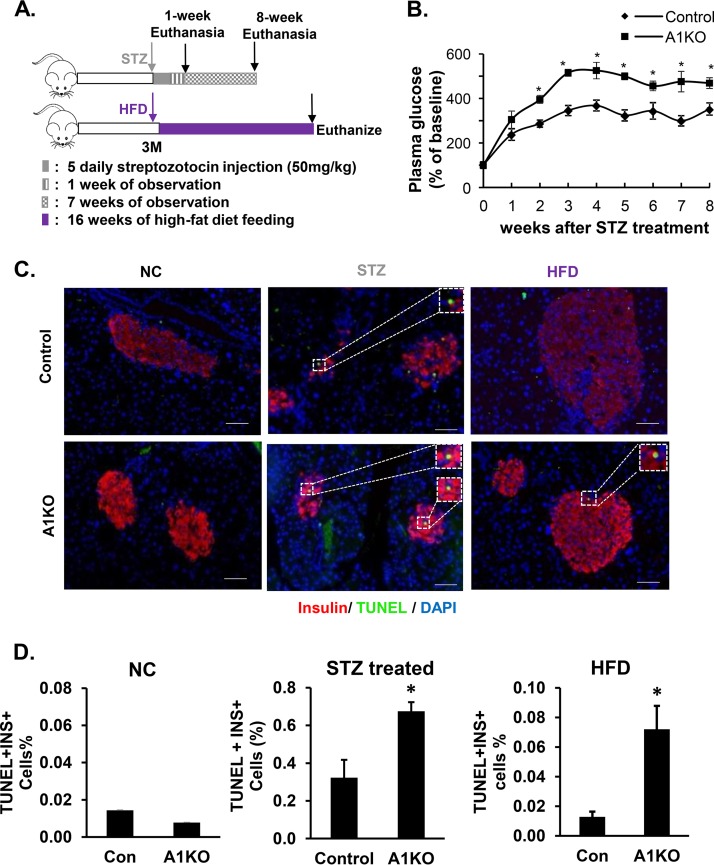
To further confirm this observation in vivo, we tested if AKT1-deficient β cells presented higher cell apoptosis rates upon metabolic stress. We used two approaches to induce stress/cell death of β cells: administration of streptozotozin (STZ), a β-cell toxin, and HFD feeding (Fig. 7A). In the STZ-treated mice, plasma glucose started to rise beginning at 1 week after the initiation of STZ treatment and persisted through the 8-week study period (Fig. 7B). We collected pancreatic tissues 1 week after the last dose of STZ treatment in a subgroup of animals to assess the apoptosis rate using terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis (Fig. 7C and D). Our analysis shows that the percentage of apoptotic β cells in the AKT1-deficient islets is significantly higher than that of the controls (Fig. 7D, middle).
FIG 7.
β cells deficient in AKT1 were subjected to cell death upon high-fat diet (HFD) and streptozotocin (STZ)-induced stress. (A) Experiment design. (B) Plasma glucose level increased after STZ treatment. More severe hyperglycemia is observed in A1KO versus Con mice upon STZ treatment. *, P ≤ 0.05 compared to the controls at the same time point. (C) Representative pancreatic pictures of TUNEL staining in Con and A1KO mice after HFD or STZ treatment (red, insulin; green, TUNEL; blue, DAPI). Scale bars, 100 μm. (D, left) Quantification of the percentage of TUNEL-positive β cells in Con and A1KO mice fed with NC. (D, middle) Quantification of the percentage of TUNEL-positive β cells in control and Akt1-deficient mice treated with STZ. (D, right) Quantification of the percentage of TUNEL-positive β cells in Con and A1KO mice fed with HFD. *, P ≤ 0.05 compared to the controls.
For the HFD experiment, although the apoptosis rate was low in both control and AKT1-deficient β cells (Fig. 7D, left), HFD indeed triggered an approximately 7-fold increase of the apoptotic β cells in the AKT1-deficient β cells, whereas no detectable increase is observed due to HFD feeding in the control β cells (Fig. 7C and D, right). Taken together, these in vivo observations validate our in vitro observations that mild baseline ER stress induced by AKT1 loss made the β cells more susceptible to cell death when exposed to metabolic stress.
DISCUSSION
Adaptive response is a major mechanism by which β cells respond to metabolic changes to cope with the changing demand for insulin release. Under conditions such as obesity and insulin resistance, fatty acids and glucose serve as major signals that control β-cell growth and survival (1). Here, using a novel β-cell-specific Akt1 knockout mouse model, we addressed the role of the AKT1 isoform in the adaptive cell growth response of β cells to metabolically induced stress (i.e., HFD). Several findings are reported in this study. First, we showed that the AKT1 protein is not required for the physiological maintenance of pancreatic β cells when metabolic stress is not present. Second, we identified that AKT1 is required for HFD-induced β-cell proliferation. Third, we discovered that deficiency in the AKT1 function induces mild ER stress signals in β cells and that this effect prepares β cells for metabolic stress-induced apoptosis.
Adaptive β-cell proliferation is observed concurrently with hyperglycemia in both NOD mice and Zucker rats (40, 41). Treatment with HFD and STZ, which leads to hyperglycemia, was also shown to induce proliferation of β cells (42, 43). Recent glucose infusion and in vitro studies exposing isolated mouse islets to glucose showed that pancreatic β cells proliferate in response to glucose by maintaining an elevated cyclin D2 level (44, 45). Previous studies have indicated an important role of G1/S checkpoint regulators in β-cell growth (46–50). Other studies recognized the contribution of a number of signaling pathways, including signals downstream of platelet-derived growth factor, hepatocyte growth factor, insulin growth factor 1/insulin, and glucagon-like peptide 1, in the regulation of β-cell proliferation (51–54).
As AKT is a major kinase mediating these signaling pathways, the decline of its function has the potential to cause the inability of β cells to maintain and increase their mass, resulting in the deficiency of islet function. Mutations and variants of genes encoding AKT have been correlated with diabetic symptoms, including fasting insulin levels in humans, indicating a role for AKT in maintaining islet function (14, 15, 18, 55). However, genetic studies targeting AKT have not led to a clear understanding of how AKT isoforms play a role in β cells (16, 18–22). While AKT2 was found to indirectly influence adaptive islet growth by regulating peripheral glucose metabolism, previous loss- and gain-of-function studies are inconsistent on the role of AKT1 in β cells as a cell growth and survival kinase (19, 20, 22). In the current study, we showed that AKT1 is indeed necessary to sustain the HFD-induced growth of β cells. This is despite the observation that it is not required in the maintenance of β cells when the demand for increased insulin output is not present, i.e., physiological maintenance of β cells in euglycemic mice maintained on normal chow. In a previous study, glucose infusion in cultured islets induced AKT phosphorylation at 15 min (56). The upregulation of cyclin D2 following AKT activation was found to be important for β-cell proliferation in response to such glucose infusion. It was later determined that IRS2 and mTOR mediate the upregulation of cyclin D2 and proliferation of β cells (57). The significance of AKT upregulation by glucose was not explored. The data we present here suggest that the early activation of AKT by glucose in the previous study (56) is functionally relevant to the proliferative response induced by glucose observed for the β cells.
Metabolic stress has been identified as the major mechanism that stimulates β-cell proliferation and later induces their death during the development of type 2 diabetes (1, 9, 10, 58). We have shown previously that the adaptive response to oxidative stress in hepatocytes is regulated by the PTEN/AKT signaling pathway (39). In this study, we identified that the loss of AKT1 kinase leads to chronic ER stress in β cells, similar to HFD feeding. In mice with intact AKT1, long-term HFD induces ER stress and is accompanied by increased β-cell proliferation (59). In the adult-onset β-cell-specific AKT1-deficient mice, we discovered that chronic, low-level ER stress is accompanied by the inability of β cells to proliferate and enhance β-cell apoptosis upon long-term HFD. We also identified enhanced eIF2α signaling as being associated with β-cell apoptosis. This finding is consistent with previous findings that reduced eIF2α phosphorylation is associated with AKT hyperphosphorylation, which together contribute to β-cell proliferation (60). Our conclusions are further supported by a genomic study where several unfolded protein response (UPR) markers were discovered to be downregulated in the replicating pancreatic β cells in mice (61).
The timing and the level of ER stress signals may affect how β cells respond to ER stress induction. It was reported that the induction of ER stress by treatment with subthreshold doses of tunicamycin and thapsigargin promotes β-cell proliferation, while high doses lead to the induction of cleaved caspase and downregulation of PCNA (59). In vitro short-term treatment with chemical chaperones to reduce ER stress blocked HFD-induced β-cell proliferation, suggesting that at least acute ER stress induction is necessary to induce proliferation of β cells (59). On the other hand, acute relief of ER stress by reducing insulin production was also shown to promote β-cell proliferation (60). The observation that both induction and relief of ER stress are linked to β-cell proliferation may be partially explained by the involvement of different ER stress signaling pathways and the specific factors that induce ER stress. Three distinct stress sensing signaling pathways are elicited to resolve ER stress: the IRE1α/XBP1, ATF6, and PERK/eIF2α pathways (10, 58, 62). When the Ire1a gene was deleted in β cells, HFD was no longer able to stimulate β-cell proliferation, indicating that IRE1α protein serves as a sensor for HFD-induced ER stress and facilitates β-cell proliferation (63). Under a glucose-stimulated ER stress condition, dispersed rodent and human islets were shown to rely on ATF6 for proliferation (59). ER stress induced by neonatal PERK deletion led to the failure of β-cell proliferation fairly quickly after birth, suggesting the inhibition of PERK function directly suppresses β-cell proliferation and promotes death (64, 65). eIF2α, the primary ER signal that we found to be upregulated by AKT1 loss, is phosphorylated by PERK and is the major mediator of PERK-regulated ER stress responses. Thus, AKT1-regulated eIF2α may utilize the ER stress signal to control adaptive β-cell proliferative responses.
Understanding the physiological processes that can trigger the proliferation of β cells holds significant promise for future therapeutic development to increase β-cell mass. Our work reported here shows that AKT1 is required for HFD to induce the adaptive proliferation and survival of β cells with a process mediated by ER stress. This work, which uncovered an important molecular signal that controls the adaptive regeneration response of β cells, is significant for understanding how β cells fail during the development of diabetes.
MATERIALS AND METHODS
Animals.
Akt1loxP/loxP, Mip-CreERT+ mice were generated by crossing the Akt1loxP/loxP mice with Mip-CreERT+ mice (C57BL/6 background) (66, 67). These mice were also crossed with Rosa26YFP mice to test the efficiency of the deletion. Initial analysis indicated no gross differences between the CreERT+ versus CreERT− mice (see Fig. S1A in the supplemental material). Whether the mice were fasting or fed, plasma glucose also did not differ significantly between the two strains of mice (68.5 ± 9.6 versus 61 ± 3.8 mg/dl). Subcutaneous (s.c.) injections of tamoxifen (Sigma-Aldrich, St. Louis, MO), every other day for a total of 5 injections, were used to induce β-cell-specific deletion of Akt1 (βA1KO) (68, 69). The efficiency of this injection protocol is assessed with yellow fluorescent protein (YFP) expression, AKT1 protein levels, and phosphorylation of its substrate, pPRAS40, in βA1KO mouse islets (Fig. S2). Tamoxifen-injected Akt1loxP/loxP, Mip-CreERT− mice were also used in the initial analysis as controls and shown to have no effects on islet mass or glucose homeostasis, as evaluated by glucose tolerance testing and body weight (Fig. S1). More importantly, tamoxifen injection did not affect the ability of a high-fat diet (8 weeks) to induce islet mass (Fig. S1B). Thus, corn oil- and tamoxifen-injected Akt1loxP/loxP, Mip-CreERT− mice were used as controls. Akt1−/− (A1KO) mice with mixed background were also used in this study with background-matched Akt1+/+ mice as controls. All mice were housed in a temperature-, humidity-, and light-controlled room (12-h light/dark cycle) and were allowed free access to food and water. All experiments were conducted according to the Institutional Animal Care and Use Committee of the University of Southern California research guidelines.
Cell line and cell culture.
The INS-1 cell line was a generous gift from the Jacobson laboratory (Vanderbilt University). The INS-1 cells were cultured with RPMI 1640 medium (Corning Cellgro) supplemented with 10% fetal bovine serum (FBS; purchased from Atlanta Biologics, USA) and 1% PENSTREP (penicillin-streptomycin) antibiotics (supplied by the University of Southern Californian Cell Culture Core Facility). The Akt1−/− INS-1 cell line was generated using CRISPR technology. Plasmids expressing Cas9 protein and the LentiCRISPRv2 plasmid were provided by Ping Wang at the University of Southern California. The LentiCRISPRv2 plasmid then was engineered to include the guidance RNA to target exon 1 of the Akt1 gene (sequence, TACGTCGTTCATGGTATCCGTGG). Both plasmids were delivered to INS-1 cells using the lentiviral transfection system. Successfully transduced cells were selected with 1.5 μg/ml puromycin for 14 days. Approximately 1 out of every 80 single-cell clones chosen for amplification did not express AKT1 protein, and these cells were renamed “Akt1−/− INS-1 cells.” The control cells for Akt1−/− INS-1 cells were naive INS-1 cells that were transfected with plasmid expressing Cas9 protein only.
In vitro treatment.
For each experiment, 2.5 × 105 cells were seeded in 6-well plates for 48 h. Before treatment, cells were synchronized by maintenance in FBS-free RPMI 1640 medium overnight. The following day, treatments of LY294002 (5 μM; Sigma), tunicamycin (2.5 μM; Sigma), or both chemicals were applied to the cells depending on the experimental designs. Protein samples were collected after 6 h of LY294002 and/or tunicamycin treatment. Serum was added back to the medium at the start of treatment.
STZ injection and HFD feeding.
Streptozotocin (STZ; Sigma) injections were conducted daily for 5 days at a dosage of 50 mg/kg of body weight as previously described (25, 68, 69). The high-fat diet (HFD) was PicoLab 5053. This diet contains 60.3% of the calories from fat content, while 18.4% and 21.3% of the calories are from protein and carbohydrates, respectively (70).
Plasma glucose assay and insulin analysis.
Glucose levels were determined by measuring the tail vein blood samples with the Abbott Freestyle glucose monitoring system (25, 68). Fasting glucose was measured after overnight (approximately 16 h) fasting. For the intraperitoneal glucose tolerance test (ipGTT), a 30% glucose solution (2 mg/kg body weight) was injected intraperitoneally after overnight (approximately 16 h) fasting. For the intraperitoneal insulin tolerance test (ipITT), insulin in sterile phosphate-buffered saline (PBS) (0.1 U/ml) was injected intraperitoneally at a dose of 0.5 U/g body weight after approximately 5 h of fasting. Blood samples for plasma insulin analysis were collected into a sterile Eppendorf tube with anticoagulant via vena cava at terminal necropsy or via orbital vein following glucose injection. The collected samples then were centrifuged at 2,000 × g for 15 min at room temperature. The insulin level in the plasma samples was measured by following procedures of the mouse insulin enzyme-linked immunosorbent assay kit (Alpco).
Islet mass determination.
For each mouse, pancreatic tissue was dissected and processed as described previously (70, 71). Tissue sections were stained for hematoxylin and eosin (H&E) for morphological analysis. Three sections per mouse, with 240-μm vertical distance between sections, were analyzed to obtain the ratio of islet area to pancreas area for individual animals. This number then was multiplied by the individual pancreas weight to obtain islet mass value for each mouse. The average islet mass for each group of animals was calculated and graphed with standard deviations. Islet and pancreas areas were measured using Axiovision 4.5 software (Zeiss, Thornwood, NY) or ImageJ software as previously described (25, 68).
Determination of cell proliferation and cell death.
BrdU (1 mg/ml in drinking water) was given to mice for 5 days before the end of the study to label the proliferating cells (72). Paraffin-embedded tissue sections were stained with insulin to visualize β cells. Cell proliferation then was evaluated with immunohistochemical analysis using an anti-BrdU antibody or anti-Ki67 antibody (68, 69). Multiple sections (at least 3) were used for quantitative analysis. A minimum of 3,000 insulin-positive cells was counted for each mouse. The mitotic index was determined using the percentage of BrdU/insulin (or Ki67/insulin) double-positive cells in all insulin-positive cells. The following antibodies were used: insulin (1:100; Abcam, Carlsbad, CA), BrdU (1:100; BD Pharmingen, San Jose, CA), and Ki67 (1:100; number12202; CST) monoclonal antibodies.
β-Cell apoptosis was evaluated using the TUNEL assay kit on paraffin-embedded tissue samples per the manufacturer’s instructions (Roche, Indianapolis, IN) (68, 69). Multiple tissue sections were used to ensure that at least 1,500 insulin-positive cells were counted in the STZ experiment. In the HFD experiment, at least 4,000 insulin-positive cells were counted due to the low abundance of TUNEL-positive cells. The cell apoptosis ratio was determined using the percentage of TUNEL/insulin double-positive cells among all insulin-positive cells (26).
Mouse islet isolation, protein extraction, and RNA extraction.
Immediately after each mouse was euthanized, the pancreas was perfused with collagenase P solution (0.8 mg/ml in Hanks’ balanced salt solution [HBSS] buffer with 1 mM HEPES and 1 mM MgCl2) and then digested at 37°C for 17 min. The HBSS buffer and HEPES were purchased from Corning Cellgro. MgCl2 (1 mM) was prepared by dissolving MgCl2·6H2O in water, followed by filtration (0.22 μm). The digested tissue samples were further purified using Ficoll gradients with densities of 1.108, 1.096, 1.069, and 1.037 (Cellgro) (26). Islets between layers 1.096 and 1.069 were collected and handpicked for either protein extraction or RNA extraction (25, 68). Protein extraction was performed by adding cell lysis buffer containing 1 mM sodium pyrophosphate, 10 mM β-glycerol phosphate, 10 mM sodium fluoride, 0.5 mM sodium orthovanadate, 1 μM microcystin, and protease inhibitor cocktail set II (Calbiochem) (73, 74). RNAs were extracted by following the instructions of the commercial RNeasy minikit (Qiagen).
RNA sequencing.
Total RNA from islets isolated from the control and βA1KO mice fed normal chow or HFD was used for RNA-seq analysis (n = 3). cDNA library preparation and sequencing were performed by the University of Southern California Molecular Genomics Core. cDNA libraries were sequenced on an Illumina HiSeq2500 with a single 75-bp read, with 25 million reads per sample. FASTQ files were generated by CASAVA (v1.8.2) and further analyzed by Patrek Flow software. Reads were mapped to the mouse transcriptome (RefGene v1.1.17) using TOPHAT2, allowing two mismatches and a maximum of 20 multiple hits. The gene expression values (quartiles were used for analysis. Normalized data with differentially expressed genes are further analyzed using Ingenuity Pathway Analysis (IPA) and gene set enrichment analysis (GSEA) software.
Immunoblotting.
Cell or islet tissue lysates with equal amounts of protein (30 μg) were subjected to SDS-PAGE, followed by transference to polyvinylidene fluoride membranes for immunoblotting (75, 76). The membranes were probed with antibodies against p-PERK (Thr980) (number 3179; Cell Signaling Technologies), total PERK (number 3192; Cell Signaling Technologies), p-AKT (Thr308) (number 9275; Cell Signaling Technologies), AKT1 (number 2938; Cell Signaling Technologies), total AKT (number 9272; Cell Signaling Technologies), p-eIF2α (Ser51) (number 9721; Cell Signaling Technologies), total eIF2α (number 9722; Cell Signaling Technologies), GRP78 (sc-13968; Santa Cuz), pFOXO1(Thr24)/pFOXO3a(Thr32) (number 9464; Cell Signaling Technologies), FOXO1 (number 9454; Cell Signaling), p-PRAS40 (Thr246) (number 2640; Cell Signaling Technologies), PRAS40 (number 2610; Cell Signaling Technologies), actin and vinculin (Sigma), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling Technologies). All antibodies were diluted 1:1,000 with 1% bovine serum albumin (in phosphate-buffered saline with Tween 20).
Flow cytometry analysis for PI and annexin V.
INS-1 cells (2.5 × 105 cells/well) were seeded in six-well plates in RPMI medium. After 48 h, cells were treated with dimethyl sulfoxide or with LY294002 (5 μM), tunicamycin (2.5 μM), or both. Media containing detached and floating cells were collected. The rest of the cells were trypsinized, gently washed with sterile PBS, and resuspended in 1× annexin binding buffer (ABB). Annexin protein (400 ng per 1 × 106 cells) then was added to cells and incubated for 15 min at room temperature away from light. Propidium iodide (PI; 1 mg/ml) then was added to each sample (2.5 μg/ml per sample) and incubated for approximately 2 min. Flow cytometry was performed on 20,000 cells to quantify the cells labeled with annexin V only, PI only, or both. Flow cytometry analysis then was performed (39). 1× ABB (500 ml) was prepared by mixing 5 ml 1 M HEPES, 4.38 g of NaCl, 101.6 mg MgCl2·6H2O, and 132.3 mg CaCl2·2H2O in 500 ml deionized water and filtering the solution through a 0.45-μm filter.
Statistics.
Student’s t tests were performed on data analyzing only two groups to compare the differences between control and experimental mice. For multigroup comparisons, statistical analysis was calculated by a one-way analysis of variance in GraphPad Prism 6 with Bonferroni correction as a post hoc test for comparing the differences between groups on quantified data. Data are presented as means ± standard errors of the means.
Supplementary Material
ACKNOWLEDGMENTS
We have no competing interests to declare.
Part of this work was supported by R01 DK084241-01. B. L. Stiles also acknowledges support from NCI R01CA154986-01. Z. Peng acknowledges a fellowship from the California Institute of Regenerative Medicine.
Footnotes
Supplemental material is available online only.
REFERENCE
- 1.Prentki M, Nolan CJ. 2006. Review series islet B cell failure in type 2 diabetes. J Clin Investig 116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rieck S, Kaestner KH. 2010. Expansion of β-cell mass in response to pregnancy. Trends Endocrinol Metab 21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golson ML, Misfeldt AA, Kopsombut UG, Petersen CP, Gannon M. 2010. High fat diet regulation of β-cell proliferation and β-cell mass. Open Endocrinol J 4:10.2174/1874216501004010066. doi: 10.2174/1874216501004010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE. 2005. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia 48:1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- 5.Ahrén J, Ahrén B, Wierup N. 2010. Increased β-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets 2:353–356. doi: 10.4161/islets.2.6.13619. [DOI] [PubMed] [Google Scholar]
- 6.Ng S-F, Lin RCY, Laybutt DR, Barres R, Owens JA, Morris MJ. 2010. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 7.Maclean N, Ogilvie RF. 1955. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes 4:367–376. doi: 10.2337/diab.4.5.367. [DOI] [PubMed] [Google Scholar]
- 8.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. 2014. β-Cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J Clin Endocrinol Metab 99:1983–1992. doi: 10.1210/jc.2014-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laybutt DR, Preston AM, Åkerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. 2007. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca SG, Gromada J, Urano F. 2011. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab 22:266–274. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JY, Luzuriaga J, Maxwell EL, West PK, Bensellam M, Laybutt DR. 2015. The balance between adaptive and apoptotic unfolded protein responses regulates β-cell death under ER stress conditions through XBP1, CHOP and JNK. Mol Cell Endocrinol 413:189–201. doi: 10.1016/j.mce.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 12.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O'Rahilly S, Barroso I. 2004. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan K, Kimber WA, Luan J, Soos MA, Semple RK, Wareham NJ, O'Rahilly S, Barroso I. 2007. Analysis of genetic variation in Akt2/PKB-beta in severe insulin resistance, lipodystrophy, type 2 diabetes, and related metabolic phenotypes. Diabetes 56:714–719. doi: 10.2337/db06-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaney JM, Gordish-Dressman H, Harmon BT, Bradbury MK, Devaney SA, Harris TB, Thompson PD, Clarkson PM, Price TB, Angelopoulos TJ, Gordon PM, Moyna NM, Pesca LS, VIsich PS, Zoeller RF, Seip RL, Seo J, Kim BH, Tosi LL, Garcia M, Li R, Zmuda JM, Delmonico MJ, Lindsay RS, Howard BV, Kraus WE, Hoffman EP. 2011. AKT1 polymorphisms are associated with risk for metabolic syndrome. Hum Genet 129:129–139. doi: 10.1007/s00439-010-0910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmon BT, Devaney SA, Gordish-Dressman H, Reeves EK, Zhao P, Devaney JM, Hoffman EP. 2010. Functional characterization of a haplotype in the AKT1 gene associated with glucose homeostasis and metabolic syndrome. Hum Genet 128:635–645. doi: 10.1007/s00439-010-0891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. 2001. Akt1/PKB is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 17.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. 2001. Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Investig 108:1631–1638. doi: 10.1172/JCI200113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzone D, Fröjdö S, Disse E, Debard C, Laville M, Pirola L, Vidal H. 2008. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia 51:512–521. doi: 10.1007/s00125-007-0913-8. [DOI] [PubMed] [Google Scholar]
- 19.Wan M, Easton RM, Gleason CE, Monks BR, Ueki K, Kahn CR, Birnbaum MJ. 2012. Loss of Akt1 in mice increases energy expenditure and protects against diet-induced obesity. Mol Cell Biol 32:96–106. doi: 10.1128/MCB.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treins C, Alliouachene S, Hassouna R, Xie Y, Birnbaum MJ, Pende M. 2012. The combined deletion of S6K1 and Akt2 deteriorates glycemic control in a high-fat diet. Mol Cell Biol 32:4001–4011. doi: 10.1128/MCB.00514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. 2009. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab 10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. 2004. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Investig 114:928–936. doi: 10.1172/JCI200420016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. 2001. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat Med 7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 24.Blandino-Rosano M, Alejandro EU, Sathyamurthy A, Scheys JO, Gregg B, Chen AY, Rachdi L, Weiss A, Barker DJ, Gould AP, Elghazi L, Bernal-Mizrachi E. 2012. Enhanced beta cell proliferation in mice overexpressing a constitutively active form of Akt and one allele of p21Cip. Diabetologia 55:1380–1389. doi: 10.1007/s00125-012-2465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng N, Yang KT, Bayan JA, He L, Aggarwal R, Stiles JW, Hou X, Medina V, Abad D, Palian BM, Al-Abdullah I, Kandeel F, Johnson DL, Stiles BL. 2013. PTEN controls β-cell regeneration in aged mice by regulating cell cycle inhibitor p16ink4a. Aging Cell 12:1000–1011. doi: 10.1111/acel.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiles BL, Kuralwalla-Martinez C, Guo W, Gregorian C, Wang Y, Tian J, Mark A, Magnuson MA, Wu H. 2006. Selective deletion of Pten in pancreatic β cells leads to increased islet mass and resistance to STZ-induced diabetes. Mol Cell Biol 26:2772–2781. doi: 10.1128/MCB.26.7.2772-2781.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzzi F, Xu L, Zuellig RA, Boller SB, Spinas GA, Hynx D, Chang Z, Yang Z, Hemmings BA, Tschopp O, Niessen M. 2010. Differential effects of protein kinase B/Akt isoforms on glucose homeostasis and islet mass. Mol Cell Biol 30:601–612. doi: 10.1128/MCB.00719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandel ES, Hay N. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res 253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 29.Butler PC, Meier JJ, Butler AE, Bhushan A. 2007. The replication of β cells in normal physiology, in disease and for therapy. Nat Rev Endocrinol 3:758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 30.Maedler K, Schumann DM, Schulthess F, Oberholzer J, Bosco D, Berney T, Donath MY. 2006. Aging correlates with decreased β-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for fas and pancreatic duodenal homeobox-1. Diabetes 55:2455–2462. doi: 10.2337/db05-1586. [DOI] [PubMed] [Google Scholar]
- 31.Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E. 2015. Natural history of β-cell adaptation and failure in type 2 diabetes. Mol Aspects Med 42:19–41. doi: 10.1016/j.mam.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. 2003. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 33.Gupta D, Jetton TL, LaRock K, Monga N, Satish B, Lausier J, Peshavaria M, Leahy JL. 2017. Temporal characterization of β cell-adaptive and -maladaptive mechanisms during chronic high-fat feeding in C57BL/6NTac mice. J Biol Chem 292:12449–12459. doi: 10.1074/jbc.M117.781047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesnokova E, Bal N, Kolosov P. 2017. Kinases of eIF2a switch translation of mRNA subset during neuronal plasticity. Int J Mol Sci 18:2213. doi: 10.3390/ijms18102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alanko J, Mai A, Jacquemet G, Schauer K, Kaukonen R, Saari M, Goud B, Ivaska J. 2015. Integrin endosomal signalling suppresses anoikis. Nat Cell Biol 17:1412–1421. doi: 10.1038/ncb3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. 2004. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology 145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 37.Omikorede O, Qi C, Gorman T, Chapman P, Yu A, Smith DM, Herbert TP. 2013. ER stress in rodent islets of Langerhans is concomitant with obesity and β-cell compensation but not with β-cell dysfunction and diabetes. Nutr Diabetes 3:e93. doi: 10.1038/nutd.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cnop M, Foufelle F, Velloso LA. 2012. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med 18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Zeng N, Li Y, He L, Xu X, Galicia V, Deng C, Stiles BL. 2011. Adaptive basal phosphorylation of eIF2 is responsible for resistance to cellular stress-induced cell death in Pten-null hepatocytes. Mol Cancer Res 9:1708–1717. doi: 10.1158/1541-7786.MCR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE. 2001. Cell mass dynamics in Zucker diabetic fatty rats: rosiglitazone prevents the rise in net cell death. Diabetes 50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 41.Wang RN, Bouwens L, Klöppel G. 1994. Beta-cell proliferation in normal and streptozotocin-treated newborn rats: site, dynamics and capacity. Diabetologia 37:1088–1096. doi: 10.1007/BF00418372. [DOI] [PubMed] [Google Scholar]
- 42.Mosser RE, Maulis MF, Moullé VS, Dunn JC, Carboneau BA, Arasi K, Pappan K, Poitout V, Gannon M. 2015. High-fat diet-induced-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am J Physiol Endocrinol Metab 308:E573–E582. doi: 10.1152/ajpendo.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Wang Z, Huang Y, Wang J. 2011. Effects of chronic administration of alogliptin on the development of diabetes and β-cell function in high fat diet/streptozotocin diabetic mice. Diabetes Obes Metab 13:337–347. doi: 10.1111/j.1463-1326.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 44.Salpeter SJ, Klochendler A, Weinberg-Corem N, Porat S, Granot Z, Shapiro AMJ, Magnuson MA, Eden A, Grimsby J, Glaser B, Dor Y. 2011. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic β-cells through glycolysis and calcium channels. Endocrinology 152:2589–2598. doi: 10.1210/en.2010-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocaña A. 2007. A new model to induce-cell replication. Diabetes 56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cozar-Castellano I, Takane KK, Bottino R, Balamurugan AN, Stewart AF. 2004. Induction of cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes 53:149–159. doi: 10.2337/diabetes.53.1.149. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, Kim SK. 2009. Polycomb protein Ezh2 regulates pancreatic β-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. 2006. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 49.Harb G, Vasavada RC, Cobrinik D, Stewart AF. 2009. The retinoblastoma protein and its homolog p130 regulate the G1/S transition in pancreatic beta-cells. Diabetes 58:1852–1862. doi: 10.2337/db08-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vasavada RC, Cozar-Castellano I, Sipula D, Stewart AF. 2007. Tissue-specific deletion of the retinoblastoma protein in the pancreatic β-cell has limited effects on β-cell replication, mass, and function. Diabetes 56:57–64. doi: 10.2337/db06-0517. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. 2000. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem 275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK. 2011. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 478:349–355. doi: 10.1038/nature10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Haeften TW, Twickler TB. 2004. Insulin-like growth factors and pancreas beta cells. Eur J Clin Investig 34:249–255. doi: 10.1111/j.1365-2362.2004.01337.x. [DOI] [PubMed] [Google Scholar]
- 54.Vilsbøll T. 2009. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab 11:11–18. doi: 10.1111/j.1463-1326.2009.01073.x. [DOI] [PubMed] [Google Scholar]
- 55.Minic M, Rocha N, Harris J, Groeneveld MP, Leiter S, Wareham N, Sleigh A, De Lonlay P, Hussain K, O'Rahilly S, Semple RK. 2017. Constitutive activation of AKT2 in humans leads to hypoglycemia without fatty liver or metabolic dyslipidemia. J Clin Endocrinol Metab 102:2914–2921. doi: 10.1210/jc.2017-00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jetton TL, Everill B, Lausier J, Roskens V, Habibovic A, LaRock K, Gokin A, Peshavaria M, Leahy JL. 2008. Enhanced beta-cell mass without increased proliferation following chronic mild glucose infusion. Am J Physiol Endocrinol Metab 294:E679–E687. doi: 10.1152/ajpendo.00569.2007. [DOI] [PubMed] [Google Scholar]
- 57.Stamateris RE, Sharma RB, Kong Y, Ebrahimpour P, Panday D, Ranganath P, Zou B, Levitt H, Parambil NA, O’Donnell CP, García-Ocaña A, Alonso LC. 2016. Glucose induces mouse b-cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes 65:981–995. doi: 10.2337/db15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walter P, Ron D. 2011. The unfolded protein response. Science 334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 59.Sharma RB, Arvan P, Alonso LC. 2015. Insulin demand regulates β cell number via the unfolded protein response. J Clin Investig 125:3831–3846. doi: 10.1172/JCI79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szabat M, Page MM, Panzhinskiy E, Skovsø S, Mojibian M, Fernandez-Tajes J, Bruin JE, Bround MJ, Lee JT, Xu EE, Taghizadeh F, O’Dwyer S, van de Bunt M, Moon KM, Sinha S, Han J, Fan Y, Lynn FC, Trucco M, Borchers CH, Foster LJ, Nislow C, Kieffer TJ, Johnson JD. 2016. Reduced insulin production relieves endoplasmic reticulum stress and induces B cell proliferation cell metabolism article reduced insulin production relieves endoplasmic reticulum stress and induces B cell proliferation. Cell Metab 23:179–193. doi: 10.1016/j.cmet.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Klochendler A, Caspi I, Corem N, Moran M, Friedlich O, Elgavish S, Nevo Y, Helman A, Glaser B, Eden A, Itzkovitz S, Dor Y. 2016. The genetic program of pancreatic β-cell replication in vivo. Diabetes 65:2081–2093. doi: 10.2337/db16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hetz C. 2012. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 63.Xu T, Yang L, Yan C, Wang X, Huang P, Zhao F, Zhao L, Zhang M, Jia W, Wang X, Liu Y. 2014. The IRE1α-XBP1 pathway regulates metabolic stress-induced compensatory proliferation of pancreatic β-cells. Cell Res 24:1137–1140. doi: 10.1038/cr.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. 2002. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22:3864–3874. doi: 10.1128/mcb.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang W, Feng D, Li Y, Iida K, Mcgrath B, Cavener DR. 2006. PERK EIF2AK3 control of pancreatic B cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Shearin AL, Monks BR, Seale P, Birnbaum MJ. 2016. Lack of AKT in adipocytes causes severe lipodystrophy. Mol Metab 13:472–479. doi: 10.1016/j.molmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, Bernal-Mizrachi E, Elghazi L, Roe MW, Labosky PA, Myers MG, Gannon M, Powers AC, Dempsey PJ. 2010. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic cre transgene expression in the brain. Diabetes 59:3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang KT, Bayan JA, Zeng N, Aggarwal R, He L, Peng Z, Kassa A, Kim M, Luo Z, Shi Z, Medina V, Boddupally K, Stiles BL. 2014. Adult-onset deletion of Pten increases islet mass and beta cell proliferation in mice. Diabetologia 57:352–361. doi: 10.1007/s00125-013-3085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayan JA, Peng Z, Zeng N, He L, Chen J, Stiles BL. 2015. Crosstalk between activated myofibroblasts and β cells in injured mouse pancreas. Pancreas 44:1111–1120. doi: 10.1097/MPA.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Debebe A, Medina V, Chen C-Y, Mahajan IM, Jia C, Fu D, He L, Zeng N, Stiles BW, Chen C-L, Wang M, Aggarwal K-R, Peng Z, Huang J, Chen J, Li M, Dong T, Atkins S, Borok Z, Yuan W, Machida K, Ju C, Kahn M, Johnson D, Stiles BL. 2017. Wnt/β-catenin activation and macrophage induction during liver cancer development following steatosis. Oncogene 36:6020–6029. doi: 10.1038/onc.2017.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He L, Gubbins J, Peng Z, Medina V, Fei F, Asahina K, Wang J, Kahn M, Rountree CB, Stiles BL. 2016. Activation of hepatic stellate cell in Pten null liver injury model. Fibrogenesis Tissue Repair 9:8. doi: 10.1186/s13069-016-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galicia VA, He L, Dang H, Kanel G, Vendryes C, French BA, Zeng N, Bayan J, Ding W, Wang KS, French S, Birnbaum MJ, Rountree CB, Stiles BL. 2010. Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology 139:2170–2182. doi: 10.1053/j.gastro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding W, Dang H, You H, Steinway S, Takahashi Y, Wang H-G, Liao J, Stiles B, Albert R, Rountree CB. 2012. miR-200b restoration and DNA methyltransferase inhibitor block lung metastasis of mesenchymal-phenotype hepatocellular carcinoma. Oncogenesis 1:e15. doi: 10.1038/oncsis.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sundaresan G, Paulmurugan R, Berger F, Stiles B, Nagayama Y, Wu H, Gambhir S. 2004. MicroPET imaging of Cre–loxP-mediated conditional activation of a herpes simplex virus type 1 thymidine kinase reporter gene. Gene Ther 11:609–618. doi: 10.1038/sj.gt.3302194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li C, Li Y, He L, Agarwal AR, Zeng N, Cadenas E, Stiles BL. 2013. PI3K/AKT signaling regulates bioenergetics in immortalized hepatocytes. Free Radic Biol Med 60:29–40. doi: 10.1016/j.freeradbiomed.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jia C, Medina V, Liu C, He L, Qian D, Tu T, Okamoto CT, Stiles BL. 2017. Crosstalk of LKB1-regulated and PTEN-regulated signals in liver morphogenesis and tumor development in mice. Hepatol Commun 1:153–167. doi: 10.1002/hep4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB III, Kaestner KH, Bartolomer MS, Shulman GI, Birnbaum MJ. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.