Abstract
Balsamia, a hypogeous, sequestrate genus in the Helvellaceae, has been characterized variously as having three to eight species in North America, and these have been considered either different from or conspecific with European species. No available modern systematic treatment of Balsamia exists to allow for accurate identification at the species level. We sequenced DNA from recent western North American Balsamia collections, assessed relationships by sequence similarity, and identified molecular taxonomic units. From these data, we determined which matched descriptions and types of named species. ITS sequences supported 12 Balsamia species in western North America, five originally described by Harkness and Fischer and seven new species that we describe here. No sequences from Balsamia collections in western North America were nested among those of European species. We found no clear evidence for separation of Balsamia into multiple genera.
Keywords: hypogeous fungi, Pezizomycotina, Pseudobalsamia, sequestrate fungi, truffles
INTRODUCTION
Balsamia is a genus of hypogeous, ectomycorrhizal, sequestrate fungi (truffles) in the Helvellaceae (O’Donnell et al. 1997, Læssøe & Hansen 2007, Lumbsch & Huhndorf 2007). No comprehensive molecular phylogeny of Helvellaceae exists. The few phylogenetic studies of Pezizales that include the Helvellaceae agree that Balsamia is sister to Helvella sensu stricto (e.g. O’Donnell et al. 1997, Læssøe & Hansen 2007, Hansen & Pfister 2006).
The historical literature on Balsamia raises important questions regarding the integrity of the genus and the number of species: One genus or two? How many Balsamia species occur in western North America? Do European Balsamia species occur in North America? Vittadini (1831) described the genus Balsamia initially from Europe based on B. vulgaris, to which Fischer (1897) and Harkness (1899) added species from North America. Fischer (1907) later described the genus Pseudobalsamia based on P. setchellii from western North America. Gilkey (1916) accepted Pseudobalsamia and concluded that Balsamia was restricted to Europe whereas Pseudobalsamia was restricted to North America. However, Trappe (1975) synonymized Pseudobalsamia with Balsamia.
Harkness (1899) recognized seven Balsamia species from California. Four he described as new: B. alba, B. filamentosa, B. magnata, and B. nigrans (as nigrens); and three he considered as species described from Europe: B. platyspora, B. polysperma, and B. vulgaris. In addition, W.A. Setchell sent a Californian specimen to E. Fischer (1907) who described it as Pseudobalsamia setchelli to increase the total to eight species in California.
Gilkey (1916, 1954) synonymized Balsamia alba, B. filamentosa, and Pseudobalsamia setchelli with P. magnata, and reduced B. nigrans to P. magnata var. nigrans (as nigrens). Later, however, Gilkey re-elevated her var. nigrans to species level as P. nigrans (Gilkey 1954). Gilkey (1916) reclassified the three collections identified by Harkness as B. platyspora, B. polysperma, and B. vulgaris to P. magnata. Later Gilkey (1939) identified B. platyspora in a Canadian collection. Thus at various times, Gilkey recognized one, two, or three Balsamia species in North America. Based on Gilkey’s revisions (1916, 1939, 1954) and Trappe’s (1975) synonomyzing of genera, all collections of Balsamia and Pseudobalsamia from California and the Pacific Northwest would be identified as one of three species: B. magnata, B. nigrans, or B. platyspora.
Taxonomic uncertainty has limited our ability to recognize fungal species and confirm the identity of rare fungal species (Molina 2008). No modern systematic treatment of Balsamia has been published, leading to earlier generic and species names being retained ad hoc and applied to collections from California and the Pacific Northwest. For example, Grubisha et al. (2005) reported collections of B. magnata, B. nigrens (sic), and B. setchellii from the Channel Islands (California). Frank et al. (2006) submitted an ITS sequence to GenBank from an oak-associated specimen from Oregon under the name B. alba. Palfner & Agerer (1998) recognized an ectomycorrhizal morphotype formed by B. alba on roots of Pinus jeffreyi. Bonito et al. (2013) used DNA sequences of Balsamia cf. setchellii (as Balsamia sp. in Smith et al. 2007) as the outgroup collection for a multi-locus phylogeny of Tuberaceae. Allen et al. (1999) identified a Balsamia associated with Adenostoma fasciculatum (Rosaceae) in southern California as B. vulgaris. In California and the Pacific Northwest, B. alba, B. nigrans, and B. platyspora have been recognized as rare, special status, or sensitive species (Castellano et al. 1999, ORBIC 2016).
Molecular data are critical to verify Balsamia species distinctions and to appropriately apply names that should be used for these collections. The nuclear ribosomal internal transcribed spacer (ITS) region has been recognized as a standard of comparison among fungal species (Schoch et al. 2012, Kõljalg et al. 2013, Ryberg 2015).
Our goals were to (1) determine whether ITS sequences support three species for western North America as determined by Gilkey (1916, 1939, 1954) or more than three species as described by Harkness (1899) and Fischer (1907); (2) correlate molecular data with morphological characters that can be used to identify and differentiate Balsamia species from western North America; and (3) determine whether Pseudobalsamia is distinct from Balsamia and should be retained as a separate genus. We sequenced ITS ribosomal DNA from recent collections, assessed relationships by sequence similarity, and then correlated sequence groups with type descriptions. Because we did not obtain ITS sequences from type specimens, we selected epitypes and emended species descriptions based on fresh collections. For collections that did not match one of the named species, we described new species.
MATERIALS AND METHODS
DNA extraction, PCR amplification, sequencing and phylogenetic analysis
DNA was obtained from fresh or dry specimens. Samples were extracted in 2 % cetyltrimethyl ammonium bromide (CTAB) with chloroform and amplified in PCR with fungal primers ITS1F and ITS4 (White et al. 1990, Gardes & Bruns 1993). An initial 3 min at 93 °C was followed by 30 cycles of 30 s at 95 °C, 2 min at 54 °C and 3 min at 72 °C, with a final cycle for 10 min at 72 °C, modified from Frank et al. (2010). PCR products were purified with QIAquick PCR Purification kits (QIAGEN, Valencia, CA), prepared with BigDye Terminator Ready Reaction Mix 3.1 and sequenced in an ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA) at the Biotechnology Center at Southern Oregon University. We obtained molecular data by sequencing the ITS region, including ITS1, the 5.8S ribosomal DNA gene, and ITS2, with forward primers ITS1F and ITS1, and reverse primer ITS4 (Frank et al. 2010).
Sequences were edited with Chromas v. 1.45 (McCarthy 1998); contigs were assembled with Sequencher v. 4.7 (Gene Codes Corp. Ann Arbor, MI). Sequences were compared to fungal sequences in GenBank (www.ncbi.nlm.nih.gov) with BLAST (Altschul et al. 1990, Thompson et al. 1997). Multiple sequence alignments of the ITS region, from our sequences and from GenBank, were generated with MAFFT v. 7 (Katoh et al. 2002, Katoh and Standley 2013) and the sequences reviewed in Clustal X and edited in Mesquite (Maddison & Maddison 2011). DNA sequences were deposited in GenBank (Accession numbers KF983477–KF983491, KM115877–KM115881, KU170027–KU170042, and KY706376–KY706390).
Phylogenetic trees, using parsimony and maximum likelihood with 1000 bootstrap replicates and 1000 jack-knife replicates, were generated using PAUP v. 4.10b10 (Swofford 2002). Consensus trees with 50 % majority-rule were generated using a tree-bisection-reconnection branch-swapping algorithm. All characters were given equal weight; gaps were treated as missing. Maximum likelihood trees with 100 bootstrap replicates were also generated using PhyML through the phylogeny.fr portal using the substitution model HKY85 (Dereeper et al. 2008). Consensus trees were examined to confirm branch positions.
Morphology
We examined specimens from western North America obtained from the following sources (http://sweetgum.nybg.org): Oregon State University (OSC), Southern Oregon University (SOC), University of California, Berkeley (UC), National Fungus Collections (BPI) and the Florida Museum of Natural History (FLAS), as well as permanent microscopic sections of Harkness collections from OSC (duplicates at BPI). We also obtained fresh specimens collected from southern Oregon and California. We compared these with descriptions in Tulasne & Tulasne (1851), Harkness (1899), Fischer (1907, 1908), Gilkey (1916, 1939, 1954), Ławrynowicz (1988), Pegler & Spooner (1993), and Montecchi & Sarasini (2000).
Emended descriptions were based on epitypes selected by D. Southworth. The International Code of Nomenclature for algae, fungi and plants (Melbourne Code) allows for designation of an epitype when the holotype or isotype cannot be “critically identified” because of a lack of diagnostic features or when no molecular sequences could be obtained from the type to confirm the current application of the name (McNeill et al. 2012). No material was available for sequencing from type collections, which were in too poor condition for a comprehensive morphological assessment. We assigned Harkness species names to MOTUs that segregated by ITS sequences based on original descriptions, on tissue and spores prepared from Harkness collections, and on habitat and location of the holotype.
For the Harkness type specimens and other collections that Harkness identified, we examined resin-embedded sections, stained with toluidine blue O or safranin-fast green, housed at OSC with duplicates at BPI. From fresh and dried specimens, hand sections were mounted in water and ascospores measured (20 randomly selected spores per collection) at 1000 × with a Leica DMLB microscope and SPOT RT software (Diagnostic Instruments Inc., Sterling Heights, MI). Digital images were captured with a SPOT RT camera. Because means and medians did not differ (paired t-test, P = 0.853, Minitab v. 15), spore dimensions were reported as mean and range (in parentheses) for length, width, and the ratio of length to width (Q). Terminology for description of ascomata tissues follows Ulloa & Hanlin (2012); dimensions of peridial warts are reported as height × width. Holotypes, epitypes, and additional specimens were deposited in the OSC herbarium.
RESULTS
Phylogenetic analyses
The ITS data matrix consisted of 15 taxa with 63 sequences, 58 from vouchered specimens of Balsamia, four from ectomycorrhizas, and one from the outgroup Barssia (Table I). The edited alignment consisted of 1 109 characters: 484 parsimony informative, 442 constant, 183 non-informative.
Table 1.
Collections of Balsamia species and Bassia oregonensis with ITS GenBank Accession numbers from which sequences were used in phylogenetic analyses.
| Taxon | Fungariuma | Voucherb | Collection locale | ITS GenBank | Type designation |
|---|---|---|---|---|---|
| Balsamia alba | OSC157708 | T37489 | USA: CA: Placer Co. | KY706381 | |
| OSC130666 | T6253 | USA: OR: Jackson Co. | KF983482 | ||
| OSC157720 | NS1740 | USA: CA: Modoc Co. | KY706376 | ||
| OSC80952 | T19926g | USA: CA: Fresno Co. | AY558741 | ||
| UC1999421 | T17852g | USA: CA: Fresno Co. | AY558742 | ||
| OSC157711 | T37496 | USA: CA: Placer Co. | KY706384 | ||
| OSC157712 | T37499 | USA: CA: Placer Co. | KY706385 | ||
| OSC157715 | T37509 | USA: CA: Placer Co. | KY706387 | ||
| OSC157709 | T37490 | USA: CA: Placer Co. | KY706382 | epitype | |
| OSC157719 | T37520 | USA: CA: Shasta Co. | KY706390 | ||
| Balsamia cascadensis | OSC151392 | S1522 | USA: OR: Jackson Co. | KF983483 | holotype |
| OSC151391 | S1521 | USA: OR: Jackson Co. | JN022501 | paratype | |
| Balsamia filamentosa | OSC157710 | T37491 | USA: CA: Placer Co. | KY706383 | |
| OSC157713 | T37502 | USA: CA: Placer Co. | KY706386 | ||
| OSC157716 | T37511 | USA: CA: Placer Co. | KY706388 | epitype | |
| OSC130728 | T19467 | USA: CA: Contra Costa Co. | KM115880 | ||
| OSC131295 | T18019 | USA: CA: Ventura Co. | KF983484 | ||
| Balsamia latispora | OSC148026 | AM-CA11-021 | USA: OR: Riverside Co. | MF098665 | paratype |
| OSC157702 | T37338 | USA: CA: Siskiyou Co. | KY706378 | paratype | |
| OSC157704 | T37345 | USA: CA: Siskiyou Co. | KY706380 | paratype | |
| OSC66585 | T35434 | USA: OR: Jefferson Co. | KF983487 | holotype | |
| OSC157701 | T37336 | USA: CA: Siskiyou Co. | KY706377 | paratype | |
| OSC49763 | T11671 | USA: CA: El Dorado Co. | KU170033 | paratype | |
| OSC48551 | T9530 | USA: CA: Sierra Co. | KU170037 | paratype | |
| OSC130672 | T8082 | USA: CA: Sierra Co. | KU170036 | paratype | |
| OSC131289 | T7574 | USA: CA: Siskiyou Co. | KF983486 | paratype | |
| OSC130676 | T11394 | USA: CA: San Bernardino Co. | KU170032 | paratype | |
| OSC151399 | S1648 | USA: OR: Jackson Co. | KF983485 | paratype | |
| OSC157703 | T37340 | USA: CA: Siskiyou Co. | KY706379 | paratype | |
| OSC62003 | T25894 | USA: OR: Jackson Co. | KU170034 | paratype | |
| OSC80116 | T26983 | USA: OR: Jackson Co. | KU170035 | paratype | |
| Balsamia lazyana | OSC130670 | T19348 | USA: OR: Josephine Co. | KM115879 | holotype |
| Balsamia limuwensis | OSC130981 | LG1115 | USA: CA: Santa Barbara Co. | KU170027 | holotype |
| OSC49229 | T11111 | USA: CA: Marin Co. | KU170029 | paratype | |
| Balsamia magnata | OSC157718 | T37519 | USA: CA: Shasta Co. | KY706389 | |
| OSC151398 | S1843 | USA: OR: Jackson Co. | KF983477 | ||
| OSC151397 | S1386 | USA: OR: Jackson Co. | KF983478 | epitype | |
| JLF3324 | USA: CA: Mendocino Co. | KU170038 | |||
| OSC151393 | F1943 | USA: OR: Marion Co. | KF983479 | ||
| OSC130663 | T2050 | USA: OR: Columbia Co. | KF983480 | ||
| OSC151395 | S1860 | USA: OR: Jackson Co. | KF983481 | ||
| Balsamia nigrans | OSC151401 | S1061 | USA: OR: Josephine Co. | FJ789590 | |
| OSC130695 | T8431 | USA: CA: Mariposa Co. | KP859264 | ||
| SOC871 | S871 | USA: CA: Tehama Co. | GU184098 | ||
| OSC130700 | T19921 | USA: CA: Fresno Co. | EU669383 | epitype | |
| Balsamia pallida | OSC130727 | T17945 | USA: CA: Riverside Co. | KU170040 | holotype |
| Balsamia platyspora | O-F245397 | Norway: Ostfold | KP149493 | ||
| Balsamia quercicola | OSC151402 | S558 | USA: OR: Jackson Co. | DQ453695 | paratype |
| FLAS_F58857 | MES84 | USA: CA: Yolo Co. | KF983490 | paratype | |
| FLAS_F58860 | SRC868 | USA: CA: Yuba Co. | KF983491 | holotype | |
| Balsamia setchellii | FLAS_F58859 | SRC395 | USA: CA: Yuba Co. | DQ974730 | epitype |
| OSC80893 | SRC396 | USA: CA: Yuba Co. | KU170028 | ||
| OSC140476 | L5470 | USA: OR: Josephine Co. | KP859277 | ||
| OSC49779 | T11706 | USA: AZ: Gila Co. | KU170030 | ||
| Balsamia trappei | OSC149793 | D3000 | Canada: Alberta | KU170041 | paratype |
| OSC131300 | T20842 | USA: WA: Pend Oreille Co. | KU170042 | holotype | |
| Balsamia vulgaris | AH44223h | M2404 | Italy: Reggio Emilia | KM243646 | |
| AH44224h | M2403 | Italy: Reggio Emilia | KM243647 | ||
| OSC149589 | S2036 | Cyprus: Kelefos Bridge | KM115881 | ||
| Balsamia ectomycorrhiza | 7992.6.Rf | UK: England | EU668245 | ||
| UE_ITA248c | Italy: Perugia | JX474845 | |||
| AZA731e | France | JX989935 | |||
| AR1166d | Canada: NW Terr. | JX630958 | |||
| Barssia oregonensis | OSC151400 | F2161 | USA: OR: Marion Co. | KF983489 |
aIndex Herbariorum http://sweetgum.nybg.org/ih/
bAM, A Mujic; D, RM Danielson; F, JL Frank; G, HM Gilkey; H, HW Harkness; L, S Loring; LG, L Grubisha; M, A Montecchi; MES, ME Smith; NS, N. Siegel; S, D Southworth; SRC, Sierra Research Center; T, JM Trappe.
gIzzo et al. (2005). Note: The accession numbers of these two collections are reversed in GenBank.
hCrous et al. (2014).
ITS sequences supported 12 ITS molecular operational taxonomic units (MOTUs) in North America (Fig. 1). Bootstrap analysis separated these into two major branches, one with eight MOTUs from North America and one with European species and two MOTUs from North America (Fig. 1). We assigned five MOTUs to described species: B. alba, B. filamentosa, B. magnata, B. nigrans, and B. setchellii (Harkness 1899, Fisher 1907); and five to new species: B. cascadensis, B. latispora, B. lazyana, B. limuwensis, and B. trappei. In addition we described B. pallida and B. quercicola with ambiguous positions. The position of B. quercicola was driven mainly by a large deletion. We did not attempt to verify identification of European species.
Fig. 1.
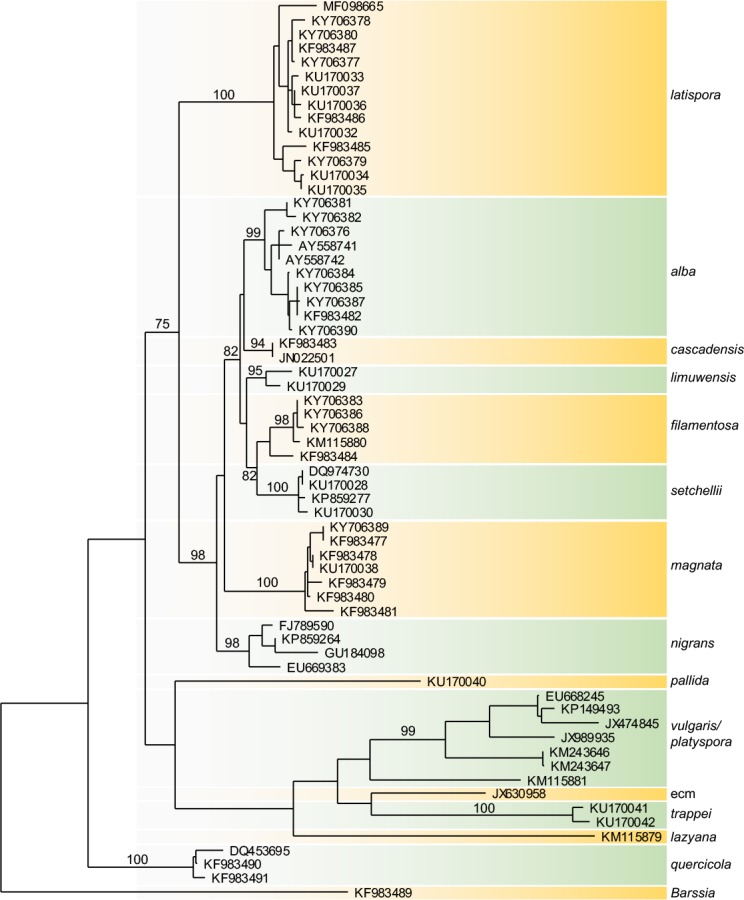
Maximum likelihood tree inferred from ITS rDNA showing relationships among North American species of Balsamia. Bootstrap values (>50 %) from the parsimony analysis (1000 replicates) using PAUP* are shown above branches.
Morphological variation
Morphological distinctions among species were sometimes subtle as a consequence of inherent variation, environmental effects, and uncertain developmental stage. Relative maturity clearly differed among and within collections, though developmental stages were difficult to assess. As specimens dried, the gleba color of immature specimens sometimes changed from white to golden brown and shrank, while the gleba color of more mature specimens remained white and did not shrink. The shrinking of glebal tissue in immature specimens resulted in enlarged glebal chamber openings and narrowed the spacing of peridial warts.
Because ascospores in Balsamia species are not actively discharged from asci, the relative maturity of asci and ascospores is difficult to determine precisely. However, hand-sectioned specimens differed in the ease with which spores dispersed from asci (as viewed under the compound microscope). We interpreted specimens with readily dispersed spores as more mature than those in which most spores remained inside the asci. Ascospores in less mature specimens had thinner spore walls and were shorter than in fully mature specimens. The number of guttules per spore varied among collections within a species, possibly due to developmental stage.
Dimensions of ascomata, warts, asci, and ascospores varied considerably. For example, width of peridial warts on individual specimens ranged from 200–700 μm. Although spore dimensions tended to be similar within a given ascus, spore size varied by up to 30 % within a single ascoma for some species. Furthermore, different regions in an individual ascoma often differed in spore dimensions. This may have been due to differences in maturity or to normal size variation. Ascoma size was a poor indicator of developmental stage. Although all ascomata must have grown from a small initial structure to the final size, some small (<1 cm) ascomata were fully mature while some large ones (1–2 cm) were immature.
TAXONOMY
Balsamia Vittad., Monogr. Tuberac. (Milano): 30. 1831, emend. D. Southw. & Trappe
Type species: Balsamia vulgaris Vitt.
Original type description (Vittadini 1831, translation D. Southworth): Characters. Fruiting body pliant, sessile, rootless [hyphal cords], always closed, chambered-fleshy [gleba]. Sporangia [asci] oblong, membranous, stalked, 8-spored, embedded in the walls of the chambers and arranged in rows. Spores cylindrical, smooth, hyaline.
Observations: Fruiting body hypogeous, lacking root and branch, exterior warty, interior chambered, with the peridium inserted from the surface and variously folded on itself, chambered-fleshy. Flesh [gleba] always white, with veins markedly pallid when immature, at last nearly liquifying; the inside of the chambers lined with short hairs. Spores white, hyaline, containing 1–2 large vesicles (perhaps little spores?) and as many smaller ones. Natural History. The fungi fleshy-soft, not very persistent. Habit altogether of the genus Tuber, with which they are still commonly confused but distinct by the softness of the fruiting body, the form and nature of the spores, etc. They grow together with true truffles and frequently with those produced in autumn and winter. Edible, but less valued than truffles and often even noxious.
Emended description: Ascomata sequestrate, subglobose, often compressed, irregularly lobed and invaginated to form an apical depression, 5–30 × 5–30 mm; peridial warts pale orange, reddish brown, brown, or black; warts pointed to rounded or nearly flat, 100–350 × 150–700 μm; gleba white, drying to golden when immature and remaining white when mature; chambers open or closed - a condition in which the chamber walls are pressed together to create narrow channels; some of the channels open to the exterior (venae externae) in the area of the apical depression. Peridium surface with sparse, pointed cystidium-like cells, ±8 μm wide at base, protruding up to 25 μm, with walls 1 μm thick; outer peridial layer 2–4 cells thick; cells isodiametric, 20–25 μm wide, to radially elongate, 11–20 μm × 22–33 μm; with walls 2–4 μm thick, red-brown; inner layer of hyaline, isodiametric cells 10–15 μm wide, with walls 1 μm thick; with scattered brown cells up to 27 μm wide, with walls 2 μm thick. Gleba of loosely interwoven hyphae, 4–5 μm wide. Chambers when immature lined with a palisade of paraphyses, at maturity the hyphal lining of interwoven paraphyses. Asci hyaline, citriform when immature, maturing to ovoid, 65–100 × 30–35 μm, with walls 1–1.5 μm, stalk 5 × 25 μm visible in immature specimens, 8-spored. Ascospores hyaline, smooth (but see Commentary), ellipsoid to cylindric, 14.5–40 × 8–20 μm, Q = 1.3–3.0 with 1–3 guttules.
Habit, habitat, and distribution: Hypogeous under diverse host trees including conifers (Abies, Pinus, Pseudotsuga), hardwoods (Alnus, Arbutus, Corylus, Quercus, Populus), or shrubs (Adenostoma, Arctostaphylos) widespread in the PNW and western USA and Canada.
Commentary: In some specimens of B. setchellii, B. filamentosa, and B. nigrans collected 1898 to 1904, spores exhibited a pattern of transverse, slightly oblique ridges (0.9 μm wide) visible at 100× (oil immersion) with Nomarski optics. We interpret these as artifacts of shrinkage due to a combination of uncertain factors, e.g., developmental stage, specimen condition, and fixation and drying procedures. They may reflect an underlying structure, e.g., of cell wall microfibril orientation and/or the subtending cytoskeleton. We attempted to induce this pattern in recent specimens by fixation in formalin and/or ethanol followed by air-drying. Less than 1 % of spores and only those with broken cell walls exhibited transverse ridges; no ridges were observed in fully developed spores with filled guttules.
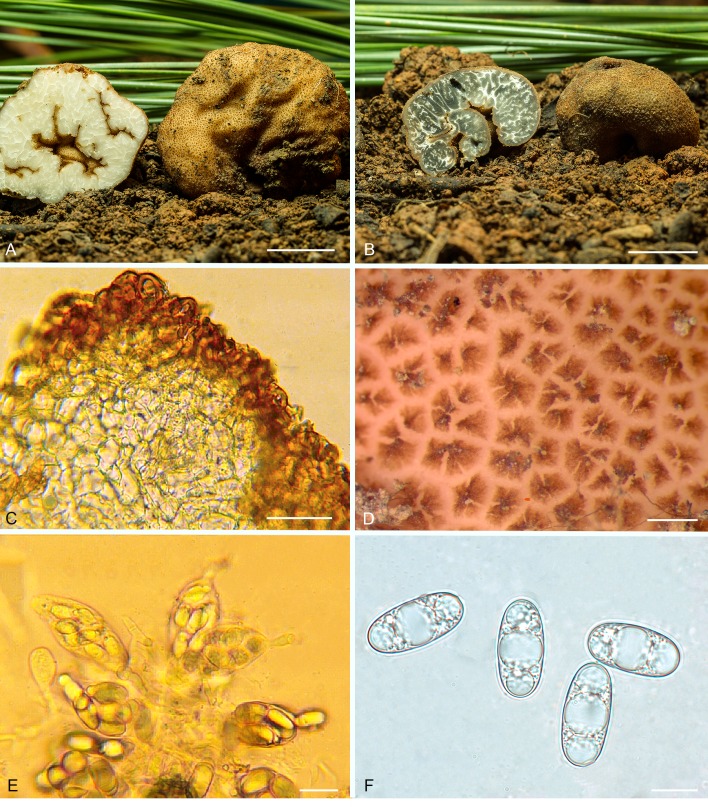
Balsamia alba Harkn., Proc. Calif. Acad. Sci. Bot. 1: 264. 1899, emend. D. Southw. & Castellano, Fig. 2.
Fig. 2.
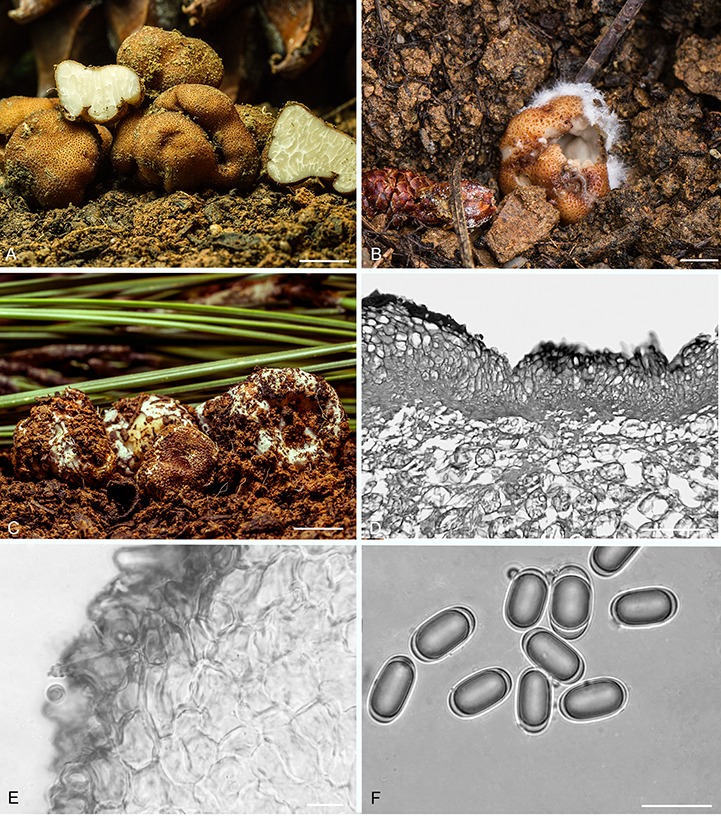
Balsamia alba. A. Fresh ascomata showing peridial surface, gleba, and peridium in section (OSC 157712). B. Ascoma in situ with white alien hyphae (OSC 157714). C. Ascoma with white alien mycelium covering the peridium (OSC 157708). D. Peridium showing outer wart layer, inner peridial layer and glebal structure. E. Peridium with outer warty layer of thick-walled cells. F. Ascospores. C–F (OSC 130666). Bars A = 10 mm, B–C = 5 mm, D = 100 μm, E–F = 25 μm.
Diagnosis: Peridium of red-brown warts often covered with white hyphae to which soil particles adhere thus rendering it “dirty white” as described by Harkness. The warts of B. alba are low, rounded to pointed, less red than in B. magnata. In addition, the spores of B. alba have a slightly narrower aspect (mean Q = 2.1) and the inner peridial tissue is significantly thicker than in B. magnata.
Isotype: USA, California, Placer Co., Wire Bridge, south of Auburn at end of Rattlesnake Lane, at Rattlesnake Bar, Folsom Lake State Recreation Area, under Quercus, Feb. c. 1898, H.W. Harkness 129, Gilkey 1033 (OSC81203).
Epitype: USA, California, Placer Co., Auburn State Recreation Area, Foothill Rd., 0.8 km from Upper Lake Clementine Day Use area to the right after entering at trailhead entering meadow, 38°55.0′N 120°58.4′W, 540 m, under Ceanothus, Pinus ponderosa, P. sabiniana, 11 Dec. 2016, M. Castellano (designated here OSC157709, MBT381870), GenBank accession number ITS: KY706382.
Original type description (Harkness 1899, p. 264): “Large, color dirty white, subglobose, fissured, deeply verrucose; gleba firm; asci ellipsoidal, 8-spored; spores cylindrical, guttulate, 12 × 18 μ. Type: Harkness 129. Under oaks, Wire Bridge, Placer County, Calif., February. This fungus is remarkable for the density of the gleba and the small number of its asci”.
Emended description: Ascomata sequestrate, subglobose to reniform with invaginations, 7–20 × 6–12 mm, warts dark reddish brown, pale tan between warts. Peridial surface often covered with white hyphae between or over warts with adhering soil particles; thus the surface may appear “dirty white;” peridial warts low, flat to rounded or obtusely pointed, 100–220 × 260–430 μm. Under a compact white hyphal layer, warts flattened to create a brown layer, 50–60 μm thick; gleba pale yellow when fresh, chambers mostly appressed. Peridium wart surface partially covered with white mycelium comprised of loosely woven, branched, hyaline hyphae either 5–6.5 μm wide, lacking clamp connections, but bulging on one side of septum, or 2.5–3 μm wide, with clamp connections and encrusted walls. Under the white hyphae are warts comprised of 4–6 layers of reddish brown cells that are ± isodiametric (20 × 17 μm) to radially elongate, 22–30 × 11–14 μm, with walls 6–7 μm thick, intergrading to radially elongated yellow to hyaline cells of similar size, with walls 4 μm thick, intergrading to cells with walls 1 μm thick. On warts lacking white surface hyphae are scattered pointed cystidium-like cells up to 35 μm long. Gleba of loosely interwoven hyphae (textura intricata), 4–7 μm wide; chambers lined with a palisade of paraphyses 70–120 × 4–5 μm. Asci hyaline, elongate to ellipsoid, 23–25 × 30–70 μm, 8-spored. Ascospores hyaline, smooth, ellipsoid, 24.9 × 12.4 (21–28 × 11–13.5) μm, Q = 2.1 (1.7–2.3), with 1–3 guttules.
Habit, habitat, and distribution: Hypogeous in variously mixed forests under Abies concolor, Picea engelmannii, Pinus jeffreyi, P. lambertiana, P. ponderosa, P. sabiniana, Pseudotsuga menziesii, Quercus garryana, Q. kelloggii, and Quercus species; Sierra Nevada and Klamath-Siskiyou mountains and foothills.
Additional materials examined: USA, California, Fresno Co., Sierra National Forest, McKinley Grove Rd. junction with FS Rd. 10S13, 37°0.8′N 119°4.8′W, 2050 m, under Abies concolor, Pinus jeffreyi, 17 Aug. 1995, T. Bruns SNF222 (OSC130690, UC1999421), GenBank accession number ITS: AY558742; Ross Creek Drainage, Turtle Creek, 36°57′N 119°6′W, 1463 m, under Abies concolor, Pinus lambertiana, P. ponderosa, 25 June 1997, L. Criley (OSC80952), GenBank accession number ITS: AY558741; Kern Co., Sequoia National Forest, Kern Gulch Campground, 35°40.3′N 118°28.3′W, 665 m, under Arctostaphylos, Pinus ponderosa, Quercus, 24 Apr. 1996, M. Castellano (OSC131296); Modoc Co., Modoc National Forest, Warner Mtns., 0.8 km north of Hwy 299, road to Stough Reservoir, under Abies concolor, 11 Jun. 2016, N. Siegel 1740 (OSC157720), GenBank accession number ITS: KY706376; Placer Co., Auburn State Recreation Area, Foothill Blvd., 0.8 km from Upper Lake Clementine Day Use Area at trailhead entering meadow, 38°57.0′N 120°58.4′W, 540 m, under Ceanothus, Pinus ponderosa, P. sabiniana, Pseudotsuga menziesii, Quercus, 11 Dec. 2016, M. Castellano (OSC157708), GenBank accession number ITS: KY706381; Old Foothill Blvd. at Mammoth Bar OHV Area, downhill, 38°55.3′N 121°0.9′W, 350 m, under Ceanothus, Pinus ponderosa, P. sabiniana, Quercus, 11 Dec. 2016, M. Castellano (OSC157711), GenBank accession number ITS: KY706384 (OSC157712), GenBank accession number ITS: KY706385 (OSC157714); (OSC157715), GenBank accession number ITS: KY706387; Shasta Co., I-5 Exit 707 in median strip, headed north, at state transportation property turnoff, 40°56.2′N 122°25.1′W, 395 m, in disturbed soil and gravel and under Pinus ponderosa, Pseudotsuga menzeisii, 12 Dec. 2016, M. Castellano (OSC157719), GenBank accession number ITS: KY706390; Oregon, Jackson Co., Jacksonville Cemetery, 425 m, under Pinus ponderosa, Quercus garryana, Q. kelloggii, 1 Apr. 1981, M. Castellano (OSC130666), GenBank accession number ITS: KF983482.
Commentary: Recent collections from Placer Co., CA, near the original Harkness site included specimens with and without the “dirty white” peridial coating. This supports the interpretation that the white mycelium, which sometimes has clamp connections, consists of secondary fungi parasitic on some, but not all specimens of B. alba. We were unable to obtain clean sequences of the white mycelium. No spores of other fungi, e.g., Sepedonium or Hypomyces, have been observed on the “dirty white” specimens. Specimens of other Balsamia species, for example, B. cascadensis and B. nigrans, are sometimes covered with white hyphae.
The isotype of B. alba (OSC81203) is in poor condition and stored in an unknown liquid. Ascospores from sections prepared at BPI from the type collection measured 19–25 × 11–13 μm, longer than those reported in the original description and closer to spore sizes in the epitype. Peridial surface warts observed on the type specimen measured 100 × 200 μm, dimensions consistent with the epitype.
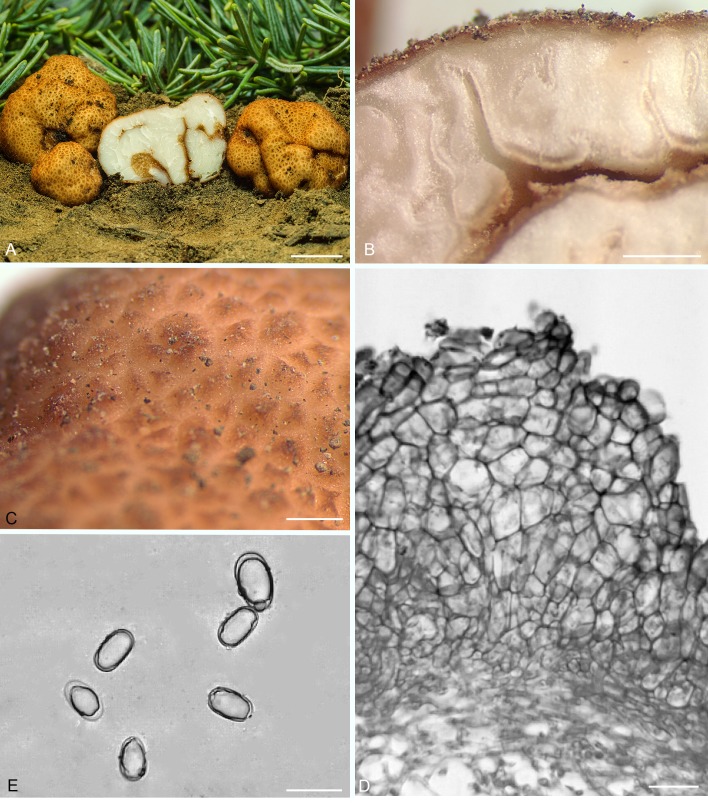
Balsamia cascadensis D. Southw., J.L. Frank & Castellano, sp. nov. MycoBank MB822496. Fig. 3.
Fig. 3.
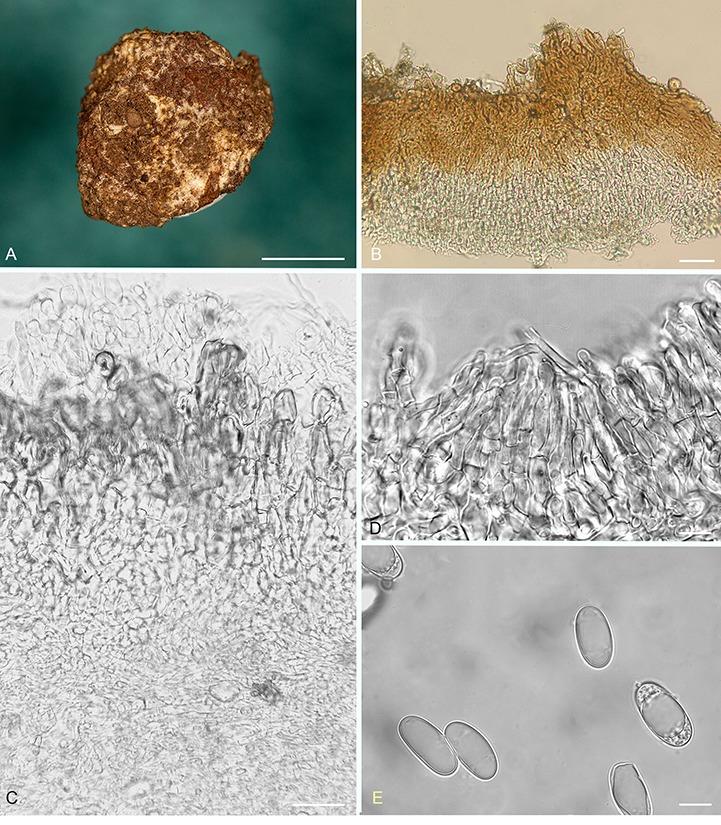
Balsamia cascadensis. A. Dried ascoma showing peridial surface partially covered by white alien mycelium (holotype; OSC 151392). B. Peridium showing outer wart layer and inner peridial layer (OSC 151390). C. Peridium showing outer wart layer covered by alien hyphae, and inner peridial layer (OSC 151392). D. Paraphyses lining glebal cavity (OSC 151392). E. Ascospores (OSC 151390). Bars A = 5 mm, B = 25 μm, C–E = 10 μm.
Etymyology: Named for the Cascade Range in southern Oregon.
Diagnosis: Peridium of red-brown warts sometimes covered with white hyphae to which soil particles adhere. Balsamia cascadensis is distinguished by narrow spores (mean width, 11.6 μm; Q = 2.1) from all other Balsamia species except B. lazyana, which has even narrower spores (mean width = 10.1 μm).
Holotype: USA, Oregon, Jackson Co., Bureau of Land Management, Cascade-Siskiyou National Monument, Chinquapin Mtn., T39S R3E section 35, 42°8.3′N 122°26.0′W, 1860 m, under Abies concolor, 17 Jun. 2010, C. Reha (OSC151392), GenBank accession number ITS: KF983483.
Ascomata sequestrate, subglobose, 7–12 × 5–12 mm; warts brown 110–160 × 200–360 μm, rounded to pointed; with white hyphae between or over warts with adhering soil particles, thus the surface appears “dirty white;” gleba white, chamber walls pressed together. Peridium wart surface partially covered with white mycelium comprised of loosely woven, branched, hyaline hyphae 5–6.5 μm wide, lacking clamp connections, but bulging on either side of septum; or hyaline hyphae, 2.5–3 μm wide, with clamp connections and encrusted walls; or brown hyphae, 5 μm wide, smooth, with clamp connections. Warts of 4–6 layers of reddish brown cells, radially elongate, 18 × 22 μm, with walls 4 μm thick, intergrading to radially elongated yellow to hyaline cells, 20–22 × 10–12 μm, with walls 1 μm thick. Gleba hyphae branched, loosely interwoven, 4–7 μm wide; chambers lined with a palisade of paraphyses 110–120 × 2–8 μm. Asci hyaline, obovoid, 29 × 50 μm, 8-spored. Ascospores hyaline, smooth, ellipsoid to nearly cylindric, 23.7 × 11.6 (21–27 × 11–13.5) μm, Q = 2.1 (1.6–2.3); 1–3 guttules.
Habit, habitat, and distribution: Hypogeous under Abies concolor, Pinus jeffreyi, or Quercus garryana, southern Oregon mountains.
Additional materials examined: USA, Oregon, Jackson Co., Bureau of Land Management, Cascade-Siskiyou National Monument, Chinquapin Mtn., T39S R3E section 35, 42°8.3′N 122°26.0′W, 1860 m, under Abies concolor, 17 Jun. 2010, C. Hill (OSC151391), GenBank accession number ITS: JN022501; Josephine Co., Bureau of Land Management, Grants Pass Resource Area, French Flat, T40S R8W, section 15, NAD83 N447555 E4660130, alt. 450 m, under Pinus ponderosa, Quercus garryana, 29 May 2011, D. Southworth 1832 (OSC151390).
Commentary: Balsamia cascadensis is morphologically similar to B. alba with a white hyphal coating that is “dirty white”. However, ITS sequences support its separation as a new species. The diverse nature of the white hyphae on the peridium supports the interpretation that the white mycelium is a parasitic fungus or group of fungi. Two of the hyphal types resembled those seen on B. alba at the type locality. In addition, there was a third hyphal type (brown with clamp connections). Attempts to sequence alien mycelia were not successful.
Balsamia filamentosa Harkn., Proc. Calif. Acad. Sci. Bot. 1: 264. 1899, emend. D. Southw., J.L. Frank & Castellano, Fig. 4.
Fig. 4.
Balsamia filamentosa. A. Ascoma showing peridial surface, gleba, and peridium in section (epitype; OSC 157716). B. Parasitized ascoma showing peridial surface, gleba and peridium (OSC 157717). C. Peridium with outer wart layer with red-brown outer cells and hyaline inner cells (OSC 131291). D. Peridial surface showing wart arrangement (OSC 157713). E. Immature asci (OSC 131291). F. Ascospores (OSC 157713). Bars A–B = 10 mm, C = 50 μm, D = 300 μm, E = 30 μm, F = 10 μm.
Diagnosis: Balsamia filamentosa is similar to B. magnata, but with warts slightly wider, more rounded, and browner, and with slightly larger spores (25.6 × 12.8 μm; Q = 2.0) than B. magnata (24.5 × 12.1 μm; Q = 2.0). Balsamia filamentosa differs from B. limuwensis which has shorter and narrower spores (24.1 x 12.1 μm; Q = 2.0) and from B. setchellii which has slightly shorter spores with a wider aspect (24.6 x 13.1 μm; Q = 1.9).
Isotype: USA, California, Placer Co., Auburn, under Heteromeles arbutifolia, Feb. 1898, H.W. Harkness 236 (OSC157736).
Epitype: USA, California, Placer Co., Auburn State Recreation Area, Old Foothill Blvd. at Mammoth Bar OHV Area, downhill from main road near fence line, 38°55.3′N 121°0.9′W, 280 m, under Ceanothus, Pinus ponderosa, P. sabiniana, 11 Dec. 2016, M. Castellano (designated here OSC157716, MBT381871), GenBank accession number ITS: KY706388.
Original type description (Harkness 1899, p. 265): “Large, oblong or irregularly globose, color ferruginous brown; densely verrucose; gleba filamentous; veins irregular; parenchyma pellucid; asci ellipsoid, markedly stipitate, 42 × 24 μ; spores cylindrical, having two to three oil globules, 18 × 12 μ. The oil globules disappear from view when placed in a medium denser than that of water”.
Emended description: Ascomata sequestrate, subglobose, 10 × 10–30 mm, invaginated at apex; peridial warts reddish brown to brown; warts rounded, sometimes ribbed, 80–100 × 200–520 μm; gleba white, chamber walls appressed; a few appressed channels open through the peridium (venae externae) at the invaginations. Peridium outer pigmented layer 2–4 cells thick, of brown, isodiametric cells, 20–25 μm wide, or elongate cells, 33 × 15 μm, with walls 2–4 (–6.5) μm thick; occasional surface cells protrude as pointed cystidium-like cells, up to 25 μm from peridial surface, with walls 1 μm thick; outer pigmented layer intergrades to an inner hyaline layer of isodiametric cells, 10–15 μm wide or elongate cells 25–33 × 11–17 μm, with walls 1 μm thick, with scattered brown cells, up to 27 μm wide, with walls 2 μm thick. Gleba of branched, interwoven hyphae, 4–5 μm wide; when immature, chambers lined with paraphyses, up to 66 μm long; at maturity, opposing chamber walls pressed against each other. Asci hyaline, ellipsoid to citriform, 55–65 × 30–35 μm, 8-spored, with walls 1 μm thick. Ascospores hyaline, smooth, ellipsoid, 25.6 × 12.8 (24–28 × 11.5–14.5) μm, Q = 2.0 (1.6–2.4), containing 1–3 guttules.
Habit, habitat, and distribution: Hypogeous in mixed conifer-hardwood stands with Arbutus menziesii, Pinus ponderosa, P. sabiniana, Pseudotsuga macrocarpa, Ps. menziesii, Quercus douglasii, Q. kelloggi, and Q. wislizeni in foothills of the Sierra Nevada and Klamath Mountains in California and in the Pinal Mountains of Arizona.
Additional materials examined: USA: Arizona, Gila Co., Pinal Mountains, Signal Peak, 2135 m, under Arctostaphylos, Pseudotsuga macrocarpa, P. menziesii, 8 Mar. 1991, J.M. Trappe 11706 (OSC49779), GenBank accession number ITS: KU170030; California, Los Angeles Co., Angeles National Forest, Upper Shake campground, under Pseudotsuga macrocarpa, Quercus, 14 Mar. 1993, K. Schaffroth (OSC131291), GenBank accession number ITS: KM115877; Placer Co., Auburn State Recreation Area, Old Foothill Blvd. at Mammoth Bar OHV Area, downhill, 38°55.3′N 121°0.9′W, 350 m, under Ceanothus, Pinus ponderosa, P. sabiniana, 11 Dec. 2016, M. Castellano (OSC157713), GenBank accession number ITS: KY706386, something is missing here (OSC157717); along Foothill Blvd. 0.8 km from Upper Lake Clementine Day Use area, at trailhead entering meadow, 540 m, under Ceanothus, Pinus sabiniana, Pseudotsuga menziesii, Quercus, 11 Dec. 2016, M. Castellano (OSC157710), GenBank accession number ITS: KY706383; San Diego Co., Fry Creek campground near Palomar observatory, 1585 m, under Pinus, Quercus, 3 Mar. 1990, G. Menser (OSC130677); Trinity Co., Shasta-Trinity National Forest, Hwy 299 west of Willow Creek, Burnt Ranch roadside west of Weaverville, 10 Dec. 2014, S. Loring, L. Sims,(OSC149794); Yuba Co., Sierra Research Station, near Englebright Reservoir, Koch Natural Area, 685 m, under Pinus sabiniana, Quercus douglasii, Q. wislizeni, 5 Feb. 2002, M.E. Smith SRC396 (OSC80893), GenBank accession number ITS: KU170028; Oregon, Douglas Co., Azalea exit I-5, under Pseudotsuga menziesii, 26 Nov. 1996, M. Amaranthus (OSC131299); Josephine Co., 605 m, under Arbutus menziesii, Pseudotsuga menziesii, Quercus kelloggii, 13 Dec. 2009, S. Loring 5470 (OSC140476), GenBank accession number ITS: KP859277.
Commentary: Balsamia filamentosa, B. limuwensis, and B. setchellii form a species complex distinguished by ITS sequences. Morphological separation of these species requires measurements of at least 20 spores to get average spore dimensions with which to compare species. In original descriptions (Harkness 1899, Fischer 1907), spore lengths given for B. filamentosa (18 × 12 μm) were shorter than those given for P. setchellii (21–28 × 10–12 μm). However, spore dimensions given for B. filamentosa were similar to those for B. magnata, which were also shorter than those of modern collections (Harkness 1899). Thus the Harkness spore measurements appear imprecise. Morphological characters combined with ITS barcodes (Fig. 1) supported the distinction between B. filamentosa and B. magnata made by Harkness (1899) and rejected the synonymy of B. filamentosa with P. magnata as proposed by Gilkey (1916).
Balsamia latispora D. Southw., J.L. Frank & Castellano, sp. nov. MycoBank MB807885. Fig. 5.
Fig. 5.
Balsamia latispora. A. Ascomata showing peridial surface, gleba, and peridium in section (OSC 157702). B. Ascoma in section showing peridium and gleba (OSC 157702). C. Peridial surface showing wart arrangement (OSC 157702). D. Peridial outer warty layer (OSC 131289). E. Ascospores (holotype; OSC 66585). Bars A = 10 mm, B = 1 mm, C = 250 μm, D = 30 μm, E = 20 μm.
Etymology: Lati (Latin) = wide + spora, referring to the relatively wide spores.
Diagnosis: Balsamia latispora is distinguished by low warts and broad spores, 13.7 (11.5–15.5) μm wide, and the lowest length-to-width ratio (Q = 1.7) of any Balsamia species in western North America.
Holotype: USA, Oregon, Jefferson Co., Deschutes National Forest, Jack Creek headwaters, 44°29′ N 121°42′ E 940 m, under Abies grandis, 21 May 1998, B. Fondrick, T. O’Dell (OSC66585), GenBank accession numbers ITS: EU837226, KF983487.
Ascomata sequestrate, subglobose, irregularly lobed and invaginated 7–10 × 10–25 mm; peridial warts red to reddish-brown, flat to rounded, 100–250 tall × 750 μm, some tripartite; pale reddish between warts; gleba white to pale yellow; chambers mostly laterally compressed, partly open, occasional chambers open to exterior in deep invaginations. Peridium wart surface uneven with rounded cells or pointed cystidium-like cells, 15 ×18 μm; wart outer layer 2–3 cells thick, of brown cells, 15–20 μm wide, with walls 2–3 μm thick, grading into hyaline cells, 15–30 μm wide, with walls 1 μm thick; space between warts with 1–2 pigmented cell layers over 1–2 hyaline inflated cell layers, subtended by a layer 30–40 μm thick of isodiametric cells, 4–6 μm in wide, grading into the trama. Gleba of interwoven hyphae (textura intricata), 4–6 μm wide; chambers lined with a weft of irregularly branched, septate hyphae, 3–5 μm wide. Asci hyaline, subglobose to citriform, 45 × 35 μm, 8-spored, stalked. Ascospores hyaline, smooth, ellipsoid, 22.0 × 13.1 (19–25.5 × 11–15.5) μm, Q = 1.7 (1.5–2.0), containing one guttule.
Habit, habitat, and distribution: Hypogeous under Abies grandis, A. x shastensis, A. procera, Pinus jeffreyi, P. ponderosa, Pseudotsuga macrocarpa, Ps. menziesii, Quercus garryana, and Q. kelloggii, widespread from the Sierra Nevada range in southern California through the Klamath Mountains in California and Oregon, and the central Cascade Range of Oregon and in Canada.
Additional materials examined: USA, California: El Dorado Co., Ice House Resort, 1645 m, under Pseudotsuga menziesii, 15 May 1990, C. Thayer (OSC49763), GenBank accession number ITS: KU170033; Placer Co., Auburn Dec. c. 1898, H.W. Harkness 214b (OSC157742); under Ceanothus, in sandy soil, Dec. 1898, H.W. Harkness 220b (OSC130980); Placer Co., Auburn, among shrubs under vegetable mould, Dec. 1898, H.W. Harkness 222 (OSC157743); west of Yuba Gap I-80 exit 160, 39°18.6′N 120°37.8′W, 1920 m, under Abies concolor, Pinus jeffreyi, P. ponderosa, Quercus, 27 June 1996, M. Castellano (OSC131298); Riverside Co., Hwy 74 at McGaugh Rd., under Pseudotsuga macrocarpa, Quercus agrifolia, Q. dumosa, Q. wizlizeni, 24 May 2011, A. Mujic CA11-021 (OSC148026), GenBank accession number ITS: MF098665; San Bernardino Co., San Bernardino Mountains, Camp Seeley, under Pinus ponderosa, Pseudotsuga macrocarpa, Quercus chrysolepis, 29 May 1983, D. Hayward (OSC130676), GenBank accession number ITS: KU170032; Shasta Co., Whiskeytown National Recreation Area, T32N R7W section 25, under Pinus attenuata, Quercus kelloggii, 11 Mar. 2008, J.L. Frank, Southworth 1161 (OSC157733); (OSC157732); (OSC157730); (OSC157731); 14 Apr. 2008, J.L. Frank, (OSC157734); Sierra Co., Plumas National Forest, San Francisco State University field campus, Hwy 49, under Abies concolor, A. magnifica, 15 Jun. 1984, Y. Wang (OSC130672), GenBank accession number ITS: KU170036; Wild Plum campground, before bridge near entrance downhill towards main road, 39°34.1′N 120°36.2′W, 1355 m, under Abies concolor, Pseudotsuga menziesii, Quercus kelloggii, 13 Jun. 1987, M. Amaranthus (OSC48551), GenBank accession number ITS: KU170037; Tahoe National Forest, Yuba Pass campground, 2045 m, under Abies concolor, A. magnifica, Pinus contorta, 9 Jun. 1987, M. Castellano (OSC48552); Siskiyou Co., Klamath National Forest, Carter Meadows summit 1890 m, under Abies concolor, A. magnifica var. shastensis, 1 Oct. 1983 J.M. Trappe 7574 (OSC131289), GenBank accession number ITS: KF983486; Shasta-Trinity National Forest at Harris Springs Fire Station, 41°27.4′N 121°47.1′W, 1510 m, under Abies concolor, P. ponderosa, 18 May 2016, M. Castellano (OSC157705); along Forest Service road 15, 1 km from upper entrance to Harris Springs campground, north side of road, 41°28.1′N 121°46.9′W, 1565 m, under Abies concolor, Pinus lambertiana, P. ponderosa, 18 May 2016, M. Castellano (OSC157701), GenBank accession number ITS: KY706377; (OSC157702), GenBank accession number ITS: KY706378; (OSC157703), GenBank accession number ITS: KY706379; (OSC157706); (OSC157707); (OSC157738); (OSC157704), GenBank accession number ITS: KY706380; Oregon, Deschutes Co., Cold Springs campground, under Pinus ponderosa, 7 May 1970, J.M. Trappe 2165 (OSC130671); 15 June 1975, R. Fogel 1165 (OSC157741); Douglas Co., Umpqua National Forest, Demonstration site, Watson Falls block, T27S R3E section 2, 43°14.8′N 122°26.1′W, 945 m, under Abies concolor, Pseudotsuga menziesii, 14 May 1996, D. Luoma 8831 (OSC123230); D. Luoma 8852 (OSC123232); Dog Prairie block, T27S R4E section 26, 43°11.7′N 122°18.9′W, 1585 m, under Abies procera, Pseudotsuga menziesii, 28 May 1996, D. Luoma 8894 (OSC123235); Jackson Co., west of Brown Mountain, 42°21.3′N 122°22.7′W, 1705 m, under Abies concolor, Pinus ponderosa, Pseudotsuga menziesii, June 1997, D. Laury (OSC80116), GenBank accession number ITS: KU170035; (OSC62003), GenBank accession number ITS: KU170034; China Gulch, under Pseudotsuga menziesii, Quercus garryana, 26 Jan. 2011, C. Reha (OSC151399), GenBank accession number ITS: KF983485; Jefferson Co., Riverside Campground, 16 km west of Sisters, 44°26.7′N 121°38.1′W, under Pinus ponderosa, Pseudotsuga menziesii, 28 May 2009, M. Castellano (OSC149004); Wasco Co., 5 km west of Pine Grove, under Pinus ponderosa, Quercus garryana, 2 May 1969, J.M. Trappe 1828 (OSC27826).
Commentary: Sequences of the ITS separated the western North American species B. latispora from the European species B. platyspora, which also has broad spores. Balsamia platyspora was described as “globose, about the size of a horse-bean, rufous, with the interstices of the minute warts of a light yellow tint, from the exposure of the internal substance. Cells minute, sporidia at first oblong-elliptic with a large globose nucleus and a number of minute granules” (Berkeley 1844). Dimensions of B. platyspora spores have been reported as 20–24 μm, Q = 1.6–1.7 (Montecchi & Sarasini 2000) and 21–26 × 13–15 μm, Q = 1.5–1.7 (Pegler et al. 1993). Two California collections identified by Harkness as B. platyspora (H222) and B. polysperma (H220b) have similar ascospores [20–24 × 9–11(–13) μm and 21 × 13 μm, respectively], minute warts, and chambers and canals partly filled with paraphyses suggesting that these collections are B. latispora.
Balsamia latispora may be a species complex. However, the difference in base pairs among branches is not consistently > 2 %. Thus there is not sufficient support within B. latispora to merit splitting.
Balsamia lazyana Trappe, D. Southw. & Amaranthus, sp. nov. MycoBank MB807880. Fig. 6A–D.
Fig. 6.
Balsamia lazyana (A–D; holotype; OSC 130670) and B. limuwensis (E–F; holotype; OSC 13098). A. Dried ascoma showing peridial surface, gleba, and peridium in section. B. Peridial surface showing wart arrangement. C. Peridial outer wart layer. D. Ascospores. E. Peridial surface with warts. F. Ascospores. Bars A = 5 mm, B = 400 μm, C = 30 μm, D = 10 μm, E = 200 μm, F = 10 μm.
Etymology: lazyana refers to the type locality, the Lazy A Ranch in the Applegate Valley near Grants Pass, Oregon.
Diagnosis: The combination of minute warts, open glebal chambers, and narrow spores (Q = 2.3) distinguishes B. lazyana from other Balsamia species.
Holotype: USA, Oregon, Josephine Co., Ruch, 12108 North Applegate Rd., Missouri Flat, Lazy A Ranch 42°19′N 123°15′W, under Pinus ponderosa and Pseudotsuga menziesii, 30 Nov. 1996, M. Amaranthus (OSC130670), GenBank accession numbers ITS: KF983488, KM115879.
Ascomata sequestrate, irregularly lobed to indented and reniform, 6–11 × 4–5 mm; peridial warts orange-red to reddish-brown, reddish between warts, flat or rounded to acute, 250–400 μm wide × 300 μm tall, often compound with 3–4 sections; surface of immature specimens mostly smooth with widely spaced warts, 100 x 100 μm; gleba white to pale tan; chambers open, 0.5–1 mm wide narrowing at maturity; chamber openings to exterior rare. Peridium wart surface uneven with rounded cells, 25 × 30 μm (Fig); wart outer layer of reddish-brown cells, 2–3 cells thick, wide, with walls 2–4(–9) μm thick, spheroidal to elongate, grading into yellow to hyaline, isodiametric cells, 25–30 μm, with walls 1 μm thick; inner layer of interwoven, hyaline hyphae, 10 μm wide, with walls 1 μm thick, grading into gleba matrix. Gleba of densely interwoven hyphae (textura intricata), 3–4 μm wide; chambers lined with a palisade of uniseriate paraphyses, 3–4 × 50 μm, with rounded or clavate tips; paraphyses tightly aligned in immature specimens becoming disorganized at maturity. Asci hyaline, elongate to citriform, 60 × 40 μm, stalked up to 20 μm long, 8-spored. Ascospores hyaline, smooth, ellipsoid, 23.5 × 10.1 (19.5–27 × 8–11.5) μm, Q = 2.3 (2.0–2.7), 1(–3) guttules.
Habit, habitat, and distribution: Hypogeous under variously mixed conifer-hardwoods: Arbutus menziesii, Arctostaphylos, Pinus ponderosa, Pseudotsuga menziesii, Quercus garryana, and Q. kelloggii.
Additional materials examined: USA, California, Sonoma Co., Lake Ralphine, under Arctostaphylos, 20 Mar. 1977, R. Heblack (OSC131292); Oregon, Josephine Co., north of Selma, under Quercus kelloggii, 26 Oct. 1971, J.M. Trappe 2969 (OSC130665); Ruch, 12108 North Applegate Rd., Missouri Flat, Lazy A ranch, 415 m, under Arbutus menziesii, Pinus ponderosa, Pseudotsuga menziesii, Feb. 1988, D. Parrish (OSC48654); under Arbutus menziesii, Pseudotsuga menziesii, 24 Mar. 1991, D. Arthur (OSC130668); under Pinus ponderosa, Pseudotsuga menziesii, Quercus garryana, 25 Jan. 1990, M. Amaranthus (OSC130667); 20 Mar. 1996, M. Amaranthus (OSC130669), GenBank accession number ITS: KM115878.
Commentary: Balsamia lazyana has the narrowest spores (mean width 10.1 μm; Q = 2.3) of any North American Balsamia species. Balsamia cascadensis is closest in spore width to B. lazyana but has spores that are significantly wider (mean width 11.6 μm).
Balsamia limuwensis D. Southw., J.L. Frank & Castellano, sp. nov. MycoBank MB815758. Fig. 6E–F.
Etymology: Limuw, the Native American Chumash name for Santa Cruz Island.
Diagnosis: Balsamia limuwensis (spore mean = 24.1 x 12.1 μm) is similar to B. filamentosa (spore mean = 25.6 x 12.8 μm) and B. setchellii (spore mean = 24.6 x 13.1 μm) but differs from them in slightly shorter and narrower spores.
Holotype: USA, California, Santa Barbara Co., Santa Cruz Island, 34°01′N, 119°46′W, 150 m, associated with Pinus muricata and Quercus, Mar. 2001, L. Grubisha 1115 (OSC130981), GenBank accession number ITS: KU170027.
Ascomata sequestrate, subglobose, 10 × 10–20 mm, invaginated at apex; peridial warts reddish-brown to brown, yellow-brown to brown between warts; warts rounded to obtusely pointed, 75–150 × 230–380 μm; gleba white, chambers closed, chamber walls appressed. Peridium outer pigmented layer 2–4 cells thick, of brown, isodiametric cells, 20 μm wide, with walls 2–4 μm thick, some cells radially aligned; sparse, pointed cystidium-like cells on surface, ±8 μm wide at base, protruding up to 25 μm from surface, with walls 1 μm thick; inner layer of hyaline, isodiametric cells, 10–15 μm wide, with walls 1 μm thick; with scattered brown cells, up to 27 μm wide, with walls 2 μm thick. Gleba composed of loosely interwoven hyphae surrounding asci and extending into chambers as paraphyses, 3–5 μm wide, some branched. Asci hyaline, citriform to ovoid, 27–32 × 48–58 μm, 8-spored. Ascospores hyaline, smooth, ellipsoid, 24.1 × 12.1 (20–31 × 10–14.5) μm, Q = 2.0 (1.7–2.4); 1–3 guttules.
Habit, habitat, and distribution: Hypogeous under Pinus muricata, Pinus spp., or Quercus agrifolia and other low elevation coastal Quercus spp., at low elevations in coastal California.
Additional materials examined: USA, California, Marin Co., Inverness, 150 m, under Quercus, 13 Feb. 1989, C. Thayer (OSC49229), GenBank accession number ITS: KU170029; Santa Barbara Co., Santa Cruz Island, Pelican Bay, under Pinus, Quercus, 18 Jan. 2002, J.M. Trappe 28212 (OSC130713).
Commentary: ITS sequences support the separation of this species from B. filamentosa and B. setchellii.
Balsamia magnata Harkn., Proc. Calif. Acad. Sci. Bot. 1: 264. 1899, emend. D. Southw. & Castellano, Fig. 7.
Fig. 7.
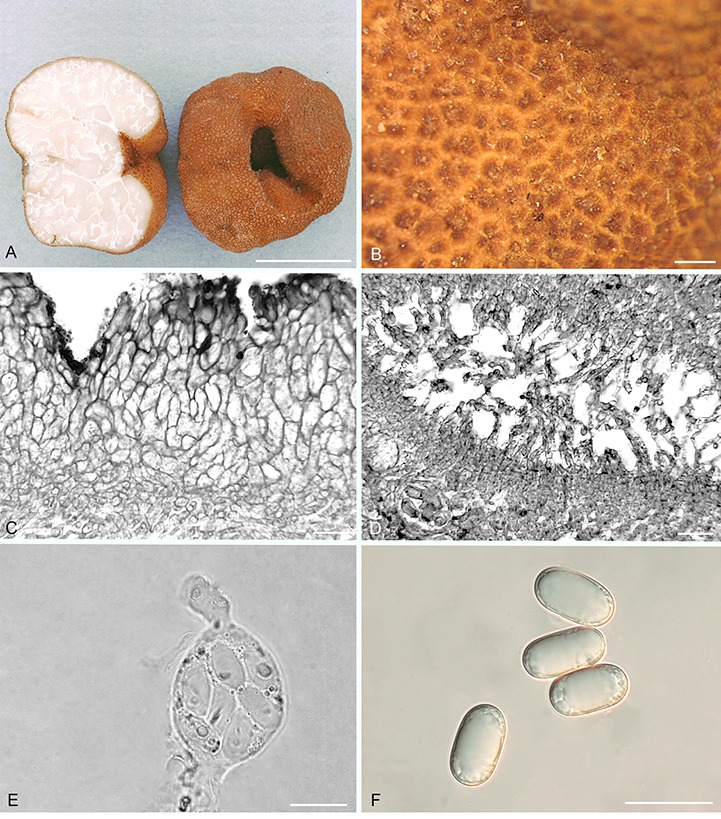
Balsamia magnata. A. Ascomata showing peridial surface, gleba, and peridium in section (OSC 56611). B. Peridial surface showing wart arrangement (OSC 130724). C. Peridium showing outer wart layer and inner peridial layer (OSC 130724). D. Glebal opening with scattered hyphae (OSC 131286). E. Immature ascus with stipitate base and capitate apex (OSC 130724). F. Ascospores (OSC 151395). Bars A = 10 mm, B = 500 μm, C = 15 μm, D = 25 μm, E = 20 μm, F = 25 μm.
Synonym: Pseudobalsamia magnata (Harkn.) Gilkey, Univ. Calif. Publ. Bot. 6: 292. 1916.
Diagnosis: Red-brown, relatively large pointed warts, often appearing compound (tripartite) and longitudinally ribbed. Ascospores 20–28.5 × 10–14.5 μm; Q = 1.7–2.4.
Isotype: USA, California, Placer Co., Auburn, in forests, May ca. 1898, Harkness 185 (OSC 81168).
Epitype: USA, Oregon, Jackson Co., Bureau of Land Management, Wellington Butte 2 km below saddle, 42°17.65′N, 123°5.20′W, 840 m, under Quercus kelloggii, 7 May 2010, C. Reha, D. Southworth 1386 (designated here OSC151397, MBT381872), GenBank accession number ITS: KF983478.
Original type description (Harkness 1899, p. 264): Subglobose, densely verrucose, 2.5 cm in diam., color orange red; cavity gyrose; gleba white, firm; asci subovate, 8-spored, 50 × 38 μ; spores cylindrical, containing from one to three oil globules, 18 × 8 μ″.
Emended description: Ascomata sequestrate, subglobose to irregularly lobed, often deeply invaginated, 8–20 × 6–10 mm; peridial warts reddish-brown, pale reddish-brown between warts; acutely to obtusely pointed, 150–300 × 250–500 μm, often compound of 3–5 segments and longitudinally ribbed; gleba white when fresh, immature gleba becomes brown when dry; chamber walls appressed, some opening to the exterior in deep folds. Peridium warty pigmented outer 2 to 4 layers of reddish-brown cells, 10 × 15 μm, with walls 4–8 μm thick, sometimes radially elongate; grading into a hyaline layer four cells thick, cells 10 × 15 μm, with walls 4 μm thick, then to an inner layer of interwoven hyphae, up to 10 μm wide, with walls 1 μm thick. Gleba of pseudoparenchyma (textura angularis) with cells 12–20 μm wide, grading to tightly to loosely interwoven hyphae (textura intricata), 4–6 μm wide; chambers lined with branched paraphyses, 3 μm wide × 60–100 μm long. Asci hyaline, obovate, 60–70 × 30–40 μm, with stalk 5 μm wide × 25 μm long with a swollen base, 8-spored. Ascospores hyaline, smooth, ellipsoid 24.5 × 12.1 (20–28.5 × 10–14.5) μm, Q = 2.0 (1.7–2.4), 1(–3) guttules.
Habit, habitat, and distribution: Hypogeous commonly under Pseudotsuga menziesii, but also in conifer-hardwood forests: Abies concolor, Alnus, Arbutus menziesii, Pinus coulteri, P. contorta var. contorta, P. jeffreyi, P. muricata, P. ponderosa, P. sabiniana, and Quercus agrifolia, Q. chrysolepis, or Q. kelloggii, widespread in mountains of the PNW, California, Montana and Arizona.
Additional materials examined: USA, Arizona, Coconino Co., Kaibab Plateau, Jacob Lake, under Pinus ponderosa, 6 June 1981, J. States (OSC40562); California, Butte Co., near Inskip, 1370 m, under Abies concolor, Pseudotsuga menziesii, Quercus kelloggii, 16 June 1980, R. Molina (OSC131286); El Dorado Co., University of California, Blodgett forest, in mixed forest, 6 May 1983, J.M. Trappe 7297 (OSC131288); Fresno Co., Sierra National Forest, Ross Creek Drainage, Turtle Creek, 36°54′N 119°6′W, 1465 m, under Abies concolor, Pinus lambertiana, P. ponderosa, 25 June 1997, B. Feno 13 (OSC130693); Los Angeles Co., Angeles National Forest, Angeles Forest Hwy milepost 14.21, Baughman Spring, 34°21.4′N 118°6.6′W, 1210 m, under Pinus coulteri, Populus, 24 Apr. 1996, M. Castellano (OSC130692); near Upper Shake campground, under Pseudotsuga macrocarpa, Quercus, 18 Apr. 1993, M. Castellano (OSC130687); Marin Co., Mill Valley, N.L. Gardner, Gilkey 1190 (OSC130977, UC46); 28 Dec. 1902, W. Setchell (OSC130982, UC33); Mariposa Co., Yosemite National Park, 26 June 2006, M. Meyer, M.E. Smith 691 (FLAS-F-58858); Mendocino Co. Mendocino National Forest, Croney Ridge 1340 m, under Abies concolor, Pinus ponderosa, Pseudotsuga menziesii, Quercus kelloggii, 8 May 2014, N. Siegel, J.L. Frank 3324, GenBank accession number ITS: KU170038; Napa Co., Pope Valley, Cleary Reserve, under Pinus, Quercus, 19 Dec. 1967, D. Largent 3490 (OSC157737); Placer Co., Auburn, H.W. Harkness 221b (OSC157739); under shrubby thickets, Dec. 1898, H.W. Harkness 231; Wire Bridge, under Ceanothus, May c. 1898, H.W. Harkness, 185 (OSC81168 – isotype); Plumas Co., Lassen National Forest, Jennie Springs Study site, 40°24.2′N 121°5.3′W, 1850 m, under Abies concolor, 9 June 1994, J. Waters (OSC58431); 14 June 1994, J. Waters (OSC58456); Riverside Co., San Bernardino National Forest, Hwy 243, milepost 15.50, under Arctostaphylos, Pinus attenuata, P. coulteri, Quercus agrifolia, 24 Apr. 1995, M. Castellano (OSC61547); San Francisco Co., sand dunes, under Ceanothus, 23 Mar. 1905, N.L. Gardner, H.W. Harkness 280 (OSC130979); Santa Clara Co., Alma, Tevis Ranch, under Pinus, 14 Jan. 1921, H.E. Parks (OSC146628); Shasta Co., I-5 Exit 707 in median strip, headed north, at state transportation property turnoff, 40°56.2′N 122°25.1′W, 395 m, in disturbed soil and gravel and under Pinus ponderosa, Pseudotsuga menzeisii, 12 Dec. 2016, M. Castellano (OSC157718), GenBank accession number ITS: KY706389; Stanislaus Co., Minnear Camp, Del Puerto Creek rd., milepost 11.8, under Pinus sabiniana, Quercus wislizeni; 28 Mar. 1985, D. Luoma (OSC46919); (OSC131290); Tuolomne Co., Stanislaus National Forest, Evergreen Rd., 1370 m, under Pinus ponderosa, 28 Apr. 1991, C. Thayer (OSC130691); Montana, Gallatin Co., Bozeman, Wiegand Garden, 30 June 1958, F.B. Cotner (OSC146631); Oregon, Benton Co., Oregon State University McDonald Forest, Soap Creek, 215 m, under Pseudotsuga menziesii, 8 Jan. 1983, D. Grandorff, R. Housen (OSC41111); Oak Creek gate, 180 m, under Pseudotsuga menziesii, 18 Apr. 1976, B. Ames (OSC130724); under Pseudotsuga menziesii, 14 Nov. 1982, P. Rawlinson (OSC131287); Philomath, Evergreen Rd., under Pseudotsuga menziesii, 4 Jan. 1992, F. Morris (OSC56611); Columbia Co., Scappoose, 105 m, under Alnus, Pseudotsuga menziesii, 8 Nov. 1969, H & R Oswald (OSC130663), GenBank accession number ITS: KF983480; west of Scappoose, 105 m, 29 Oct. 1971, D. & C. Goetz, H. & R. Oswald (OSC131235); near Scappoose, 105 m, 20 Oct. 1972, H. & R. Oswald (OSC131237); Douglas Co., Bureau of Land Management, Beatty Creek Research Natural Area, near reference point 51050, under Arbutus menziesii, Pinus jeffreyi, 26 Oct. 1997, J.M. Trappe 21422 (OSC61685); under Pseudotsuga menziesii, 24 Nov. 1997, G. Palfner (OSC60785); (OSC60841); upper Beatty Creek, under Arbutus menziesii, Pinus jeffreyi, 26 Oct. 1997, J.M. Trappe 21442 (OSC61680); North Bank Habitat Management Area, under Abies grandis, Pseudotsuga menziesii, Tsuga heterophylla, 2 Dec. 1997, J.M. Trappe 22328 (OSC61681); Jackson Co., Butte Falls R32S T2E sec20, under Quercus garryana, 9 Apr. 2015, S. Loring 13911 (OSC149590); Cascade-Siskiyou National Monument, Mariposa Reserve, under Pinus ponderosa, Pseudotsuga menziesii, Quercus garryana, 8 June 11, J.L. Frank (OSC151398), GenBank accession number ITS: KF983477; Cove Rd., under Pseudotsuga menziesii, Quercus garryana, 10 June 2011, D. Southworth 1860 (OSC151395), GenBank accession number ITS: KF983481; Heppsie Mountain, under Abies concolor, 1 June 2010, J.D. Scelza (OSC151394); Phoenix, 42°15.6′N 122°53.3′W, 625 m, under Arbutus menziesii, Quercus, D. Laury (OSC81162); Josephine Co., Deer Creek Center, under Pinus ponderosa, Quercus garryana, 7 Jan. 2007, S. Gladish (OSC151396); Ruch, 12108 North Applegate Rd., Missouri Flat, Lazy A ranch, 415 m, under Arbutus menziesii, Pseudotsuga menziesii, 4 Jan. 1987, D. Parrish (OSC48655); 2 Dec. 1995, D. Arthur (OSC49789); 24 Mar. 1991, J.M. Trappe 17560 (OSC55429); Lane Co., Willamette National Forest, H.J. Andrews Experimental Forest, stand L104, 44°13.5′N 122°12′W, 625 m, under Pseudotsuga menziesii, Tsuga heterophylla, 21 May 1992, D. Luoma 1181 (OSC56959); Winberry Park, under Cornus, Pseudotsuga menziesii, 23 Dec. 1973, H. & B. Pruitt (OSC40204); under Pseudotsuga menziesii, 24 Dec. 1975, H. Pruitt (OSC131285); Fall Creek Dam, under Pseudotsuga menziesii, mixed forest, 2 Dec. 1971, H. & B. Pruitt (OSC131236); Linn Co., Brownsville, Kirk drive, 120 m, under Pinus, Pseudotsuga menziesii, 3 Dec. 1988, N. Wedam (OSC49120); Marion Co., Willamette National Forest, Detroit, under Pseudotsuga menziesii, Quercus garryana, 4 May 2011, J.L. Frank 1943 (OSC15139), GenBank accession number ITS: KF983479; Polk Co., Silver Falls State Park, 5 Nov. 1926, S. Zeller 6994 (OSC146629); Wasco Co., Sunflower Flat, southeast of Bear Springs Ranger Station, 2970 m, under Pinus ponderosa, Quercus garryana, 3 May 1969, J.M. Trappe 1827 (OSC27827); Yamhill Co., Yamhill River, Flying M Ranch, under Pseudotsuga menziesii, 2 Mar. 1985, H. Pavelek (OSC130674).
Commentary: Ascospores from the isotype (Harkness 185) measured 19–29 × 10–14.5 μm, Q = 1.4–2.0 and thus were larger than originally reported (18 × 8 μm) in an unknown mounting medium. Gilkey (1916, 1939, 1954) reported ascospore dimensions of 20–24 × 12–14 μm for the type collection. Warts from the type specimen measured 300–400 μm wide × 250–500 μm tall.
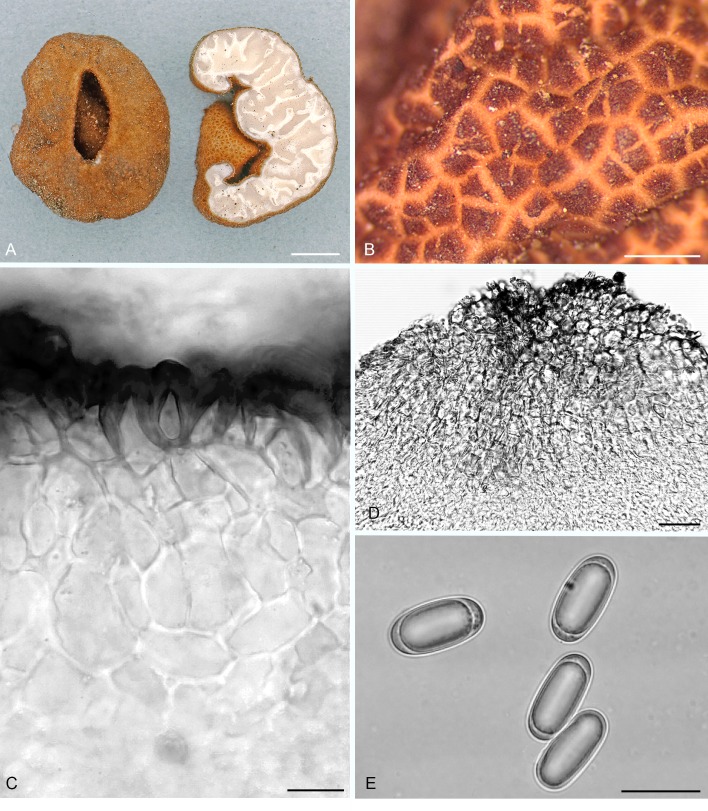
Balsamia nigrans Harkn., Proc. Calif. Acad. Sci. Bot. 1: 264. 1899, emend. D. Southw. & Castellano, Fig. 8.
Fig. 8.
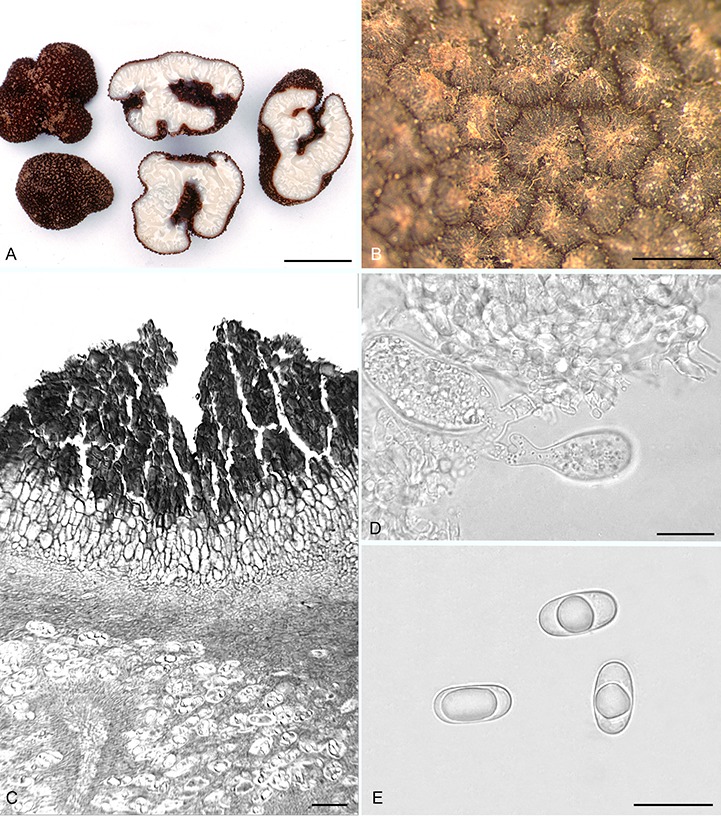
Balsamia nigrans. A. Ascomata showing peridial surface, gleba, and peridium in section (OSC 157740). B. Peridial surface warts (epitype; OSC 130700). C. Peridium showing outer wart layer, inner peridial layer and gleba (holotype; OSC 38607). D. Immature asci (OSC 157740). E. Ascospores (OSC 130983). Bars A = 20 mm, B = 600 μm, C = 25 μm, D = 20 μm, E = 25 μm.
Synonyms: Pseudobalsamia magnata var. nigrens (Harkn.) Gilkey, Univ. Calif. Publ. Bot. 6: 294. 1916.
Pseudobalsamia nigrens (Harkn.) Gilkey, North American Flora 2: 18. 1954.
Diagnosis: Black to dark reddish brown peridium composed of warts that are 250–500 × 300–600 μm, pointed, often longitudinally ribbed. Spore means 25.4 × 13.2 μm, Q = 1.9.
Isotype: USA, California, Placer Co., Auburn, beneath Ceanothus, May, ca. 1898, Harkness 180 (OSC 38607).
Epitype: USA, California, Fresno Co., Sierra National Forest, Ross Creek drainage, Turtle Creek, 36°57′N, 119°6′W, 1465 m, under Abies concolor, Pinus lambertiana, P. ponderosa, 25 Jun. 1997, L. Criley (designated here OSC130700, MBT381873), GenBank accession number ITS: EU669383.
Original type description (Harkness 1899, p. 264): “Medium, semiglobose, irregular, black, verrucose, warts with polygonal base; openings stellar, sometimes extending through the mass; cavities large and somewhat regular; gleba white, firm, crossed by white wavy lines. Asci semiglobose or ellipsoid, briefly stipitate, 8-spored, 48 × 32 μ; ascospores oblong-elliptic, guttulate, 26 × 12 μ”.
Emended description: Ascomata sequestrate, subglobose or irregularly lobed with deep or convoluted invaginations, 10–40 × 9–20 mm; peridial warts black or dark reddish-brown, some with reddish-brown hyphal tufts at the apex, brown to black between warts; warts pointed, 300–600 μm wide × 250–500 μm tall, often with longitudinal ribs. Gleba white when fresh becoming yellow when dry; chambers with white lining, open to appressed, some appressed chambers open to the exterior. Peridium wart surface uneven with rounded cells and scattered, pointed cystidium-like cells, 23–35 μm long, 10 μm at base; outer pigmented layer 4–8 cells thick, of brown cells, 20 × 30 μm, with walls 6 μm thick, grading to yellow to hyaline isodiametric cells, 30 μm wide, with walls 1–4 μm thick, pigmented and hyaline cells radially aligned, especially under the wart apex; warts subtended by a textura angularis with cells 7–9 μm wide. Gleba of loosely interwoven hyphae (textura intricata), 4–6 μm wide; chambers lined with paraphyses, 60–65 μm long × 4 μm wide, arranged in a palisade when immature, becoming disordered when mature. Asci hyaline, globose to citriform, 50 × 30 μm, with walls 1.5 μm thick, stalked, 8-spored. Ascospores hyaline, smooth, ellipsoid, 25.4 × 13.2 (22–29 × 10.5–15.5) μm, Q = 1.9 (1.5–2.5); one guttule.
Habit, habitat, and distribution: Hypogeous under Abies concolor, Pinus attenuata, P. jeffreyi, P. lambertiana, P. ponderosa, P. sabiniana, Pseudotsuga macrocarpa, Ps. menziesii, Quercus chrysolepis, Q. garryana, Q. kelloggii, and Q. wislizeni; widespread in Sierra Nevada and Cascade Mountains and foothills.
Additional specimens examined: USA, California, Fresno Co., Sierra National Forest, Ross Creek Drainage, Turtle Creek, 36°54′N 119°6′W, 1465 m, under Abies concolor, Pinus lambertiana, P. ponderosa, 25 Jun. 1997, K. Pendleton 15 (OSC130693); Teakettle Research Natural Area, 2040 m, under Abies magnifica, 24 Jun. 1996, M. Castellano (OSC131297); Lake Co., Boggs Mountain State Forest, intersection of roads 400 and 410, T11N R8W, sec. 12, 1005 m, under Pinus ponderosa, Pseudotsuga menziesii, Quercus kelloggii, 1 Mar. 1990, M. Castellano, D. Luoma (OSC157740); Los Angeles Co., Angeles National Forest, Upper Shake campground, under Pseudotsuga macrocarpa, Quercus, 14 Mar. 1993, K. Schaffroth (OSC130698); 24 Mar. 1993 (OSC131294); Mariposa Co., Greely Hill Rd. Hwy 120, under Lithocarpus densiflora, Pinus sabiniana, Quercus chrysolepis, Q. wislizeni, 27 Mar. 1985, M. Castellano (OSC130695), GenBank accession number ITS: KP859264; Placer Co., Auburn, under Ceanothus, Quercus May 1898, H.W. Harkness 180 (OSC38607 – isotype); H.W. Harkness 186; Auburn State Recreation Area, Foothill Blvd., 0.8 km from Upper Lake Clementine Day Use area at trailhead entering meadow, 38°57.0′N 120°58.4′W, 540 m, under Ceanothus, Pinus ponderosa, P. sabiniana, 14 Jan. 2017, N. Siegel (OSC157728); N. Siegel (OSC157727); Old Foothill Blvd. past Mammoth Bar OHV Area south side, trail on small hill, 38°55.6′N 121°0.4′W, 420 m, under Ceanothus, Pinus sabiniana, Quercus, 14 Jan. 2017, N. Siegel (OSC157725); N. Siegel (OSC157726); Tehama Co. Whiskeytown National Recreation Area, under Pinus attenuata, Quercus kelloggii, 1 May 2006, J.L. Frank (OSC151400), GenBank accession number ITS: GU184098; Tuolomne Co., Hwy J20, east of Groveland, south of old Hwy 120, under Pinus lambertiana, P. ponderosa, 27 Mar. 1985, M. Castellano (OSC130673); Oregon, Benton Co, near Philomath, milepost 4.2 along Beaver Creek rd., south of Decker Rd., 400 m, under Pseudotsuga menziesii, 6 Jun. 2009, S. Donovan (OSC158069); west of Philomath along Beaver Creek rd., 6.6 km from Decker rd., 380 m, under Pseudotsuga menziesii, 3 June 2006, S. Donovan (OSC112221); Woods Ck Rd, 460 m, under Pseudotsuga menziesii, 9 Jun. 1972, R. Fogel (OSC130983); Jackson Co., Applegate T37S R4W sec 33 sw1/4, 6 Dec. 1998, R. Young (OSC61957); Josephine Co., Missouri Flat, Ramsgate Ranch, 380 m, under Pinus ponderosa, 24 Mar. 1991, M. Amaranthus (OSC130697); Bureau of Land Management, Waldo Hill, 610 m, under Pinus jeffreyi, 19 Oct. 1968, J.M. Trappe 1737 (OSC130699); Rogue River-Siskiyou National Forest, Eight Dollar Mountain Rd., Josephine camp, across green bridge, T38S R8W, section 19, NAD83 443146 4677394, 400 m, under Pinus ponderosa, Quercus garryana, 11 May 2007, S. Gladish (OSC151401), GenBank accession number ITS: FJ789590; Polk Co., Mill Creek, 170 m, 19 Jun. 1989, W. Bushnell (OSC158068); Yamhill Co., Yamhill River, Flying M Ranch, under Pseudotsuga menziesii, 2 Mar. 1985, H. Pavelek (OSC130696).
Commentary: Ascospores from the isotype (Harkness 180) measured 23–29 × 13–17 μm, Q = 1.5–2.2, slightly wider than in the original description. Balsamia nigrans is an orthographic correction of the original spelling, B. nigrens, to its proper Latin spelling nigrans and does not affect authorship of the name.
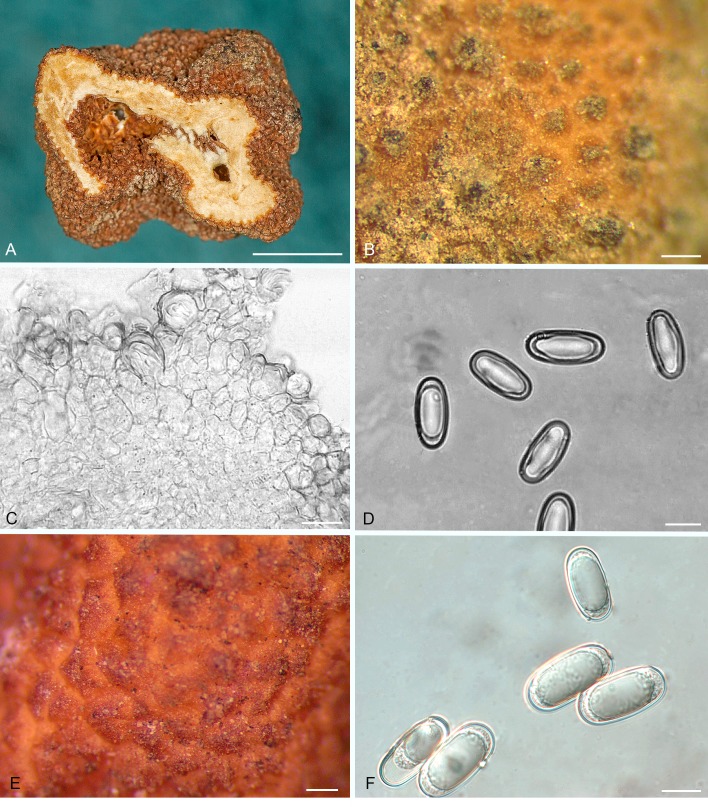
Balsamia pallida D. Southw., J.L. Frank & Castellano, sp. nov. MycoBank MB815825. Fig. 9A–C.
Fig. 9.
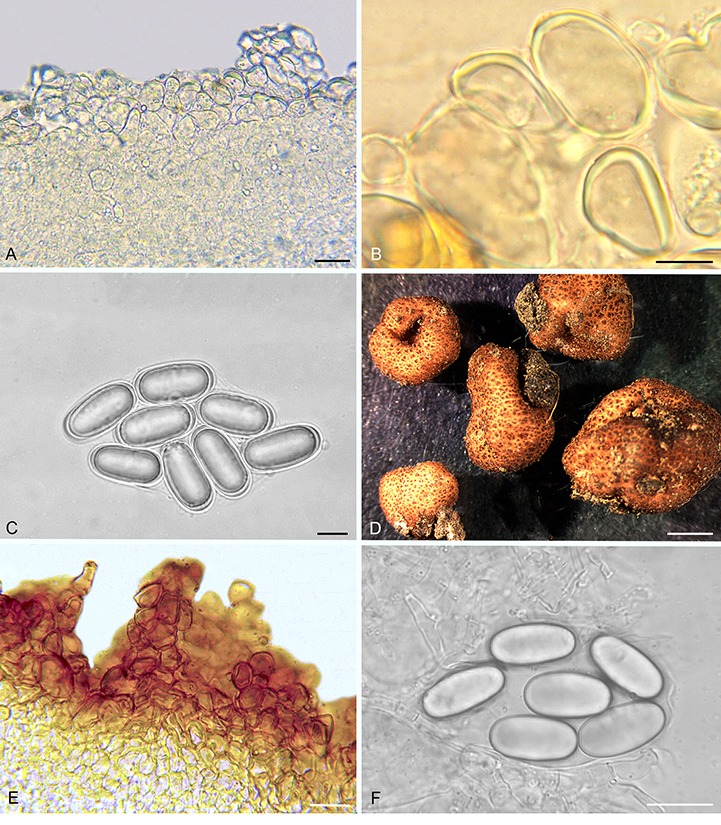
Balsamia pallida (A–C; holotype; OSC 130727) and B. quercicola (D–F). A. Peridium showing wart layer and inner layer in section. B. Peridial warts with thick-walled cells. C. Ascospores. D. Ascomata showing surface warts (OSC 151402). E. Wart structure showing thick-walled cells (FLAS 58860). F. Ascospores (FLAS 58860). Bars A = 25 μm, B = 10 μm, C = 10 μm, D = 3 mm, E–F = 20 μm.
Etymology: pallid refers to the pale orange peridium.
Diagnosis: The low warts (30–50 μm tall) and pale orange peridium differentiate this species from all other Balsamia species, yet DNA clearly places it in the genus. Open glebal chambers resemble those of B. lazyana, but the paraphyses lining the chambers are shorter. The habitat in chaparral under Adenostoma fasciculatum is distinctive.
Holotype: USA, California, Riverside Co., Skinner Lake, 33º35′N 117 º03′W, 450 m, under Adenostoma fasciculatum, 18 Jun. 1996, M.F. Allen (OSC130727), GenBank accession number ITS: KU170040.
Ascomata sequestrate, subglobose, flattened, irregularly lobed and invaginated, 10–15 × 5–8 mm; peridial warts and space between warts pale orange with occasional pale tan streaks, warts pointed, 100 μm wide × 30–50 μm tall; gleba yellow-brown to pale yellow-tan, with open chambers to 0.5 mm wide and 2 mm long; where appressed, chambers open to the exterior. Peridium wart surface with protruding cells; outer pigmented layer 1–2 cells thick, yellow, isodiametric to elongate, 19 × 29 μm wide, with walls 2–3.5 μm thick, grading to hyaline isodiametric to elongate cells, 16 × 26 μm, with walls 1 μm thick. Gleba of interwoven hyphae, ±4 μm wide; chambers lined with paraphyses, 3 × 100 μm. Asci hyaline, ampulliform when immature, becoming ovoid to citriform at maturity, 60–80 × 30 μm, stipitate 6 μm wide × 11–15 μm long, with walls 1–2 μm thick, 8-spored. Ascospores hyaline, smooth, ellipsoid, 26.9 × 12 (25–29 × 11–13) μm, Q = 2.2 (2.0–2.5); with one guttule.
Habit, habitat, and distribution: Hypogeous under Adenostoma fasciculatum (Rosaceae) in southern California chapparral.
Commentary: This species is known only from the type collection. Other hypogeous sequestrate fungi were collected with B. pallida in chaparral (Allen et al. 1999). Adenostoma fasciculatum belongs to Rosaceae, a group of plants which is not typically considered to be ectomycorrhizal, but Allen et al. (1999) found evidence of some ectomycorrhizal roots with unusual morphologies. Thus, B. pallida is putatively an ectomycorrhizal associate of A. fasciculatum.
Balsamia quercicola D. Southw., M.E. Smith & J.L. Frank, sp. nov. MycoBank MB807879. Fig. 9D–F.
Etymology: Quercus and -icola (Latin) = inhabitant of oak woodlands.
Diagnosis: Balsamia quercicola can be distinguished by the brown color of the peridium and the compact gleba. Mature ascospores of B. quercicola (mean spore length = 28.7 μm) are the longest of any Balsamia species in western North American species.
Holotype: USA, California, Yuba Co., University of California Sierra Foothill Research and Extension Center, Koch Natural Area, 39 º15′N 121 º18′′W, 370 m, under Pinus sabiniana, Quercus douglasii, Q. wislizeni, 15 Jan. 2005, M.E. Smith SRC 868 (FLAS-F-58860). GenBank accession number ITS: KF983491.
Ascomata sequestrate, subglobose to irregularly lobed, often deeply invaginated, 4–11 × 3–8 mm; peridial warts reddish-brown, pale reddish-brown between warts; warts rounded to pointed, 100–200 × 200–700 μm; gleba white when fresh, pale tan when dry; chamber walls appressed. Peridial wart surface with sparse cystidium-like cells protruding about ±8 μm, wart outer pigmented layer 3–4 cells thick, reddish-brown, isodiametric to radially elongate cells, 20–25 μm wide, with walls 2–4 μm thick, grading to hyaline isodiametric cells, 15–20 μm wide, with walls 1 μm thick, then grading to smaller cells, 8 μm wide. Gleba of loosely interwoven hyphae, ±4 μm wide. Asci hyaline, subglobose to citriform, 60 × 30 μm, 8-spored. Ascospores hyaline, smooth, cylindrical, 28.7 × 12.9 (25.5–33 × 10.5–16) μm, Q = 2.2 (2.0–2.5); with three guttules.
Habit, habitat, and distribution: Hypogeous under Pinus ponderosa, P. sabiniana, Quercus douglasii, Q. garryana, or Q. wislizeni; in the Sierra Nevada foothills and in interior valleys of California and Oregon.
Additional materials examined: USA, California, Los Angeles Co., Angeles National Forest, Upper Shake campground, under Pseudotsuga macrocarpa, Quercus, 14 Mar. 1993, K. Schaffroth (OSC130688); (OSC130689); Placer Co., Auburn State Recreation Area, Old Foothill Blvd. at Mammoth Bar OHV Area, downhill next to road, 38°55.3′N 121°0.9′W, 350 m, under Ceanothus, Pinus ponderosa, P. sabiniana, 14 Jan. 2017, N. Siegel (OSC157723); pullout past Mammoth Bar OHV Area, uphill south side, east side of huge rock, 38°55.3′N 121°0.8′W, 375 m, under Pinus sabiniana, Quercus, 14 Jan. 2017, N. Siegel (OSC157724); 0.8 km from Upper Lake Clementine Day Use area at trailhead entering meadow, 38°57.0′N 120°58.4′W, 540 m, under Ceanothus, Pinus ponderosa, P. sabiniana, 14 Jan. 2017, N. Siegel (OSC157729); Yolo Co., Davis, under Pinus ponderosa, Quercus, 6 Nov. 2004, M.E. Smith 84 (FLAS-F-58857), GenBank accession number ITS: KF983490; Oregon, Jackson Co., Whetstone Savanna Preserve, under Quercus garryana, 22 Nov. 2003, M.E. Smith (OSC151402), GenBank accession number ITS: DQ453695.
Commentary: Balsamia quercicola is differentiated by the large deletion in the ITS1 region yielding an ITS of ~600 bp, in contrast to ~700 bp in most other Balsamia species. In addition, mature ascospores (mean length = 28.7 μm) of B. quercicola, the longest of any western North American species, are comparable in length to those of B. vulgaris from Europe reported as 28 μm (Tulasne & Tulasne 1851); 26–32 μm (Pegler et al. 1993); and 25–30 μm (Montecchi & Sarasini 2000).
Balsamia setchellii (E. Fisch.) Trappe, D. Southw. & Castellano, comb. nov. MycoBank MB825537. Fig. 10.
Fig. 10.
Balsamia setchellii. A. Ascoma showing peridial surface, gleba, and peridium in section (OSC 79995). B. Peridial surface showing wart pattern in surface view (OSC 131295). C. Wart structure showing thick-walled cells. D. Cross-section of peridial surface showing wart structure (isotype; OSC 130978). E. Ascospores (OSC 131295). Bars A = 5 mm, B = 750 μm, C = 20 μm, D = 30 μm, F = 20 μm.
Basionym: Pseudobalsamia setchelli E. Fisch., Ber. Deutsch. Bot. Ges. 25: 374. 1907.
Diagnosis: Balsamia setchellii (spore means 24.6 x 13.1 μm, Q = 1.9) is distinguished from B. filamentosa (means 25.6 x 12.8 μm; Q = 2.0) by its slightly shorter and wider spores. Balsamia setchellii is distinguished from B. limuwensis (mean spore width = 12.1 μm) by its broader spores. ITS sequences clearly separate B. setchellii, B. filamentosa, and B. limuwensis.
Isotype: USA, California, Alameda Co., Berkeley, under Pinus radiata, 19 Nov. 1904, N.L. Gardner 212 (OSC130978).
Epitype: USA, California, Yuba Co., University of California Sierra Foothill Research and Extension Center, Koch Natural Area 39°14′N, 121°19′W, 370 m, under Pinus sabiniana, Quercus douglasii, and Q. wislizeni, 15 Dec. 2004, M.E. Smith SRC395 (designated here FLAS-F-58859, MBT381874), GenBank accession number ITS: DQ974730.
Original type description (Fischer 1907 p. 374; transl. D. Southworth and J. M. Trappe): “Pseudobalsamia setchelli gen. et sp. nov. The fruiting bodies are penetrated by venae externae [external veins] which either radiate from a pitted depression on the outer surface to the interior or show an irregular course and open to several points on the outer surface and which are lined by a more or less distinct hyphal palisade. The fruiting body surface is covered with a warty pseudoparenchymatous rind (peridium). The asci generally appear irregularly distributed between the venae externae that are evenly situated in the fruit body tissue (gleba). They are mostly ellipsoid to citriform or broadly fusiform, and contain in an irregular arrangement eight ellipsoid ascospores with slightly thickened, colorless, smooth membranes (cell walls). In Ps. setchelli the asci are 50–70 μ long and 25–35 μ in cross section. The ascospores are 21–28 × 10–12 μm”.
Emended description: Ascomata sequestrate, subglobose, invaginated at apex, 10 × 10–20 mm; peridial warts reddish-brown to brown, pale reddish between warts; warts rounded, 200–350 × 300–750 μm, ribbed from tip to base; gleba white when fresh, drying to yellow-brown; most chamber walls appressed, some open; a few appressed channels open through the peridium (venae externae) at the invaginations. Peridium outer pigmented layer 2–4 cells thick, of brown, isodiametric cells, 20 μm wide, or elongate cells, 12 x 24 um, with walls 4-6 μm thick, some cells radially aligned; sparse, pointed cystidium-like cells on surface, ±8 μm wide at base, protruding up to 25 μm from surface, with walls 1 μm thick; inner layer of hyaline, isodiametric cells, 13–17 μm wide, with walls 1 μm thick; with scattered brown cells, up to 27 μm wide, with walls 2 μm thick. Gleba chambers lined with white hyphae; trama surrounding asci of loosely interwoven hyphae, 4–5 μm wide, extending into chambers as paraphyses, up to 80 um long with rounded or capitate ends. Asci hyaline, stalked, citriform to ovoid, 65–100 × 30–35 μm; with walls 1–1.5 μm; 8-spored. Ascospores hyaline, smooth, ellipsoid, 24.6 × 13.1 (21–28 × 11–15) μm, Q = 1.9 (1.6–2.4); 1–3 guttules.
Habit, habitat, and distribution: Hypogeous under Arbutus menziesii, Arctostaphylos spp., Pinus coulteri, P. contorta var. contorta, P. muricata, P. radiata, P. sabiniana, Pseudotsuga menziesii, Quercus agrifolia, Q. chrysolepis, Q, douglasii, Q. kelloggii, and Q. wislizeni, in the Sierra Nevada foothills, Klamath Mountains, and California Coast Range.
Additional specimens examined: USA, California, Contra Costa Co., Mount Diablo State Park, 305 m, under Pinus coulteri, P. contorta var. contorta, P. sabiniana, Quercus agrifolia, 2 Feb. 1996, C. Thayer (OSC130728), GenBank accession number ITS: KM115880; Kern Co, Hwy 218 at gate 5 km from summit, under Pinus ponderosa, Quercus kelloggii, 19 Apr. 1993, M. Castellano (OSC79995), GenBank accession number ITS: KU170031; Lake Co., 3 km west of Middletown, 17 Jan. 1936, H.L. Mason (OSC130712); McLaughlin Reserve, under Pinus sabiniana, Quercus durata, 18 Jan. 2004, M.E. Smith 62 (FLAS-F-58856); Ventura Co., Los Padres National Forest, milepost 40 on Hwy 33, 34°37.5′N 119°22.3′W, 1920 m, under Arctostaphylos, Pinus muricata, Quercus chrysolepis, 23 Apr. 1996, M. Castellano (OSC131295), GenBank accession number ITS: KF983484.
Commentary: Fischer (1908) distinguished P. setchelli from B. filamentosa despite a note from W.A. Setchell, “I have examined the types of Harkness’s Balsamia filamentosa, B. alba and B. nigrens. Externally and in asci, shape of spores, etc., these species are all very close and resemble our No. 212 (p. 155)”. ITS barcodes support Fischer’s claim that these are distinct taxa.
Balsamia trappei D. Southw., J.L. Frank & Castellano, sp. nov. MycoBank MB815757. Fig. 11.
Fig. 11.
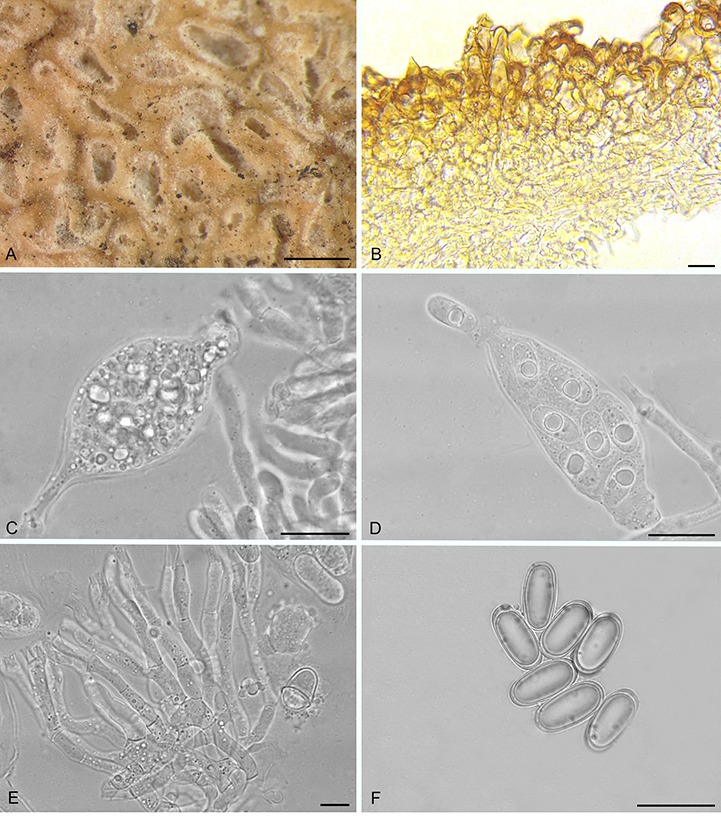
Balsamia trappei (holotype; OSC 131300). A. Glebal chambers in section. B. Peridial surface showing wart structure. C. Immature asci with stipitate base and capitate apex. D. Ascus showing release of ascospore. E. Paraphyses. F. Ascospores. Bars A = 1 mm, B = 25 μm, C–D = 20 μm, E = 10 μm, F = 25 μm.
Etymology: Named in honor of James Martin Trappe who collected the type specimen as well as many other Balsamia specimens in the Pacific Northwest.
Diagnosis: Balsamia trappei is distinguished by glebal chambers that remain open at maturity. Ascospores of B. trappei (mean = 24.8 x 12.3 μm, Q = 2.0) are similar to those of B. alba (mean = 24.9 x 12.4 μm, Q = 2.1) and B. magnata (mean = 24.5 x 12.1 μm, Q = 2.0), but B. alba has larger warts (260-430 μm wide x 100-220 μm tall) that are rounded to obtuse, never pointed, and dark reddish brown in color with pale tan between warts. Balsamia magnata has wider and taller warts (250-550 μm wide x 150-300 μm tall) that are pointed and longitudinally ribbed, and reddish brown in color with pale reddish brown between warts. Warts in B. trappei are the narrowest and shortest (175-350 μm wide x 100-120 μm tall) of the three; warts are also rounded to pointed, and brown in color with reddish brown between warts.
Holotype: USA, Washington, Pend Oreille Co., Pend Oreille State Park, 48º05′N 117º20′W, under Abies grandis, 13 July 1997, J.M. Trappe 20842 (OSC131300), GenBank accession number ITS: KU170042.
Ascomata sequestrate, subglobose to irregularly lobed, 15–20 mm; peridial warts brown, reddish brown between warts; warts pointed to rounded, 175–350 μm wide × 100–120 μm tall; gleba white to tan, chambers sinuous, open to 1 mm. Peridial wart surface with occasional protruding cells, rounded or with pointed tips to ±8 μm long; outer pigmented layer 4–6 cells thick, of reddish-brown cells, 25 x 45 μm, with walls 2–6 μm thick, grading into less pigmented cells, with walls 1–2 μm thick and then into hyaline cells approximately 18 x 24 μm. Gleba of loosely interwoven hyphae, 4 μm wide; chambers lined with paraphyses, 2–7 μm wide, with clavate to acuminate end cells. Asci hyaline, saccate-capitate with stipitate base, with walls 1 μm thick, 35 × 75 μm wide, 8-spored. Ascospores hyaline, smooth, ellipsoid, 24.8 × 12.3 (24–26 × 11.5–13.5) μm, Q = 2.0 (1.7–2.2), with one guttule.
Habit, habitat, and distribution: Hypogeous under Abies grandis and Pseudotsuga menziesii or with hardwoods, e.g., Alnus, Populus and Salix; in the intermountain region of Idaho, Oregon, Washington, and Alberta, Canada; and in Alaska.
Additional specimens examined: Canada, Alberta, Calgary, under Populus balsamifera, 7 Sept. 1980, J. Zak, R. Danielson 3000 (OSC149793), GenBank accession number ITS: KU170041. USA, Alaska, Palmer, Old Palmer Hwy, near Eklutna power plant, under Alnus, Salix, 3 Sept. 1974, V. Wells 6216 (OSC149792); Idaho, Idaho Co., near Florence, under Abies, 13 Aug. 1964, A.H. Smith 69265 (OSC149791); Oregon, Union Co., Starkey Experimental Forest and Range, Battle Creek, 1430 m, under Abies grandis, Pseudotsuga menziesii, 15 Oct. 1976, J.M. Trappe 4769 (OSC131284).
Commentary: Of the North American species, B. trappei most closely resembles the European B. vulgaris in morphology and ITS barcode.
DISCUSSION
Using ITS rDNA we were able to resolve the differences in the number of Balsamia species proposed by Harkness (1899) and by Gilkey (1916, 1939, 1954). Phylogenetic analysis supported recognition of multiple Balsamia species for western North America as described by Harkness (1899), rather than one to three species as proposed by Gilkey (1916, 1939, 1954). We described seven new species (B. cascadensis, B. latispora, B. lazyana, B. pallida, B. limuwensis, B. quercicola, and B. trappei) from North America. Thus, based on ITS barcodes, morphological characters, distribution, and habitat factors, 12 Balsamia species occur in western North America (Table 2).
Table 2.
Morphological characters that delineate species of Balsamia based on specimens for which we have ITS sequences.
| Species | Wart color, width x depth-to-cleft (μm), shape | Peridium pigmented cell size, wall thickness; surface features | Spore size (um); Q (ratio L/W) | Gleba chambers open or appressed |
|---|---|---|---|---|
| alba | white hyphae over brown to red-brown warts, 170–350 x 100–200, flat to rounded or pointed | 1–4 layers, 15 x 20–25 μm, 3–5 μm, surface hyphae hyaline, 6–7 μm wide or with scattered pointed cystidium-like cells to 35 μm | 24.9 (21–28) x 12.4 (11–13.5) Qav 2.1 (1.7–2.3) | partly open |
| cascadensis | white hyphae over brown warts, 200–360 x 110–160, rounded to pointed | 4–6 layers, 18 x 22, 4 μm; surface hyphae hyaline, 6–7 μm wide | 23.7 (21–27) x 11.6 (11–13.5) Q 2.1 (1.6–2.3) | appressed |
| filamentosa | red-brown, 200–520 um x 80–200, rounded to obtusely pointed | 2–4 layers, isodiametric 20–25 or elongate 15 × 33, 2–4 μm; sparse cystidium-like cells to 25 μm | 25.6 (24–28) x 12.8 (11.5–14.5) Q 2.0 (1.6–2.4) | appressed |
| latispora | red to red-brown, 240–300 x 100–250, flat to rounded | 2–3 layers, 15–20 μm , 2–3 μm; pointed or rounded cystidium-like cells | 22.0 (19–25.5) x 13.1 (11–15.5) Q 1.7 (1.5–2) | mostly appressed |
| lazyana | orange-red to red-brown, 250–400 x (50–)100–300, flat to rounded, often compound 3–4 lobed | 2–3 layers, 22–28 μm, 2–4 μm; pointed cystidium-like cells to 25 μm | 23.5 (19.5–27) x 10.1 (8–11.5) Q 2.3 (2–2.7) | open to 1 mm |
| limuwensis | red-brown to brown, 230–380 x 75–150, rounded to obtusely pointed | 2–4 layers, isodiametric 20 μm, 2–4 μm, pointed cystidium-like cells | 24.1 (20–31) x 12.1 (10–14.5) Q 2.0 (1.7–2.4) | appressed |
| magnata | red-brown, 250–500 x 150–300, pointed, often compound of 3 small warts | 2–4 layers, 10–15 μm, 4–8 μm | 24.5 (20–28.5) x 12.1 (10–14.5) Q 2.0 (17–2.4) | slightly open to appressed |
| nigrans | black to dark red-brown, 300–600 x 250–500, pointed, often ribbed | 4–8 layers, 20 x 30 μm, 6 μm; cystidium-like cells sparse 8 μm wide up to 90 μm long | 25.4 (22–29) x 13.2 (10.5–15.5) Q 1.9 (1.5–2.5) | appressed |
| pallida | pale orange to pale tan, 30–50 x 100, pointed | 3–4 layers, isodiametric to elongate 16–26 μm, 2–3.5 μm | 26.9 (25–29) x 12.0 (11–13) Q 2.2 (2–2.5) | open to 2 mm |
| quercicola | red-brown, 200–700 x 100–200, rounded to pointed | 3–4 layers isodiametric to elongate 20–25 μm, 2–4 μm; cystidium-like cells 8 μm long | 28.7 (25.5–33) x 12.9 (10.5–16) Q 2.2 (2.0–2.5) | appressed |
| setchellii | red-brown to brown, 300–750 x 200–350, rounded to obtusely pointed | 2–4 layers elongate 12 x 24 μm or isodiametric 20 μm, 4 μm, sparse cystidium-like cells to 25 μm | 24.6 (21–28) x 13.1 (11–15) Q 1.9 (1.6–2.4) | appressed |
| trappei | brown to red-brown, 170–350 x 100–120, pointed to rounded | 4–5 layers, 25 x 45 μm, 2–6 μm | 24.8 (24–26) x 12.3 (11.5–13.5) Q 2.0 (1.7–2.2) | open to 1 mm, sinuous |
Identification of Balsamia species was challenging due to variability in morphological characters, particularly among specimens of different developmental stages. The best indicator of ascoma maturity appeared to be the immediate release of ascospores from cut specimens. In mature specimens, ascospores dispersed quickly; the gleba remained white and did not shrink as the specimen dried leaving peridial warts fully expanded. In contrast, in immature specimens the ascospores remained in the asci. After drying, immature gleba tissue turned golden brown and shrank, thus opening cavities and moving peridial warts closer together.
We revisited the question of whether Balsamia and Pseudobalsamia are separate genera, and observed no consistent pattern based on ITS sequences to show separation between the two genera. Fischer’s original description of Pseudobalsamia (translated above) did not clearly distinguish between Balsamia and Pseudobalsamia though he later described Balsamia as having open chambers and Pseudobalsamia as having chambers more or less filled with hyphae (Fischer 1907, 1908, 1938). Gilkey (1916) applied Pseudobalsamia to Harkness’s Balsamia collections from California, but reported that there was no evidence for separation into two “distinct phylogenetic lines”. We did not observe morphological distinctions between the Pseudobalsamia isotype and the Balsamia species that we examined. Here we take the conservative position and recognize a single genus, Balsamia. This is appropriate because this has been the status quo accepted by most taxonomists since Pseudobalsamia was synonymized with Balsamia by Trappe (1975). However, it should be noted that the basal nodes in our phylogeny received moderate to weak bootstrap support so sequencing of additional molecular markers in the future should be useful to test this hypothesis.
The relative paucity of Balsamia collections and the rarity of certain species have limited our systematic analyses. The ITS barcodes proved useful for distinguishing Balsamia species. ITS data suggest that the patterns of endemism between North America and Europe in Balsamia parallel patterns found in Tuber and related Tuberaceae whereby species are rarely or never shared across continents (Bonito et al. 2013). However, further sampling of additional specimens from Asia and Europe is needed to clarify evolutionary patterns within Balsamia and among the other genera of Helvellaceae.
ACKNOWLEDGEMENTS
We thank Dr. A. Rossman, National Fungus Collections, Beltsville, MD, who graciously made available all of the deteriorating Harness wet collection to be sectioned with splits of each collection deposited at BPI and OSC. We thank Noah Siegel for access to Balsamia specimens and photographs; Anne Molia for a specimen of B. vulgaris from Crete; and Chelsea Reha, Claudia Hill, and Kerry Hofsess for field collecting in southern Oregon. Research was funded in part by the U.S. Department of Interior, Bureau of Land Management Oregon/Washington: Inter-agency Special Status Sensitive Species Program (ISSSSP), Agreement No. L10AC20020. Participation by M.E. Smith was made possible via support from the Institute of Food and Agriculture Sciences at University of Florida and funding from the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number FLA-PLP-005289. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the U.S. Government. Mention of trade names or commercial products does not constitute their endorsement by the U.S. Government.
REFERENCES
- Allen MF, Egerton-Warburton LM, Allen EB, et al. (1999). Mycorrhizae in Adenostoma fasciculatum Hook. & Arn.: a combination of unusual ecto- and endo-forms. Mycorrhiza 8: 225–228. [Google Scholar]
- Altshul SF, Gish W, Miller W, et al. (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Benucci GMN, Raggi L, Albertini E, et al. (2014). Assessment of ectomycorrhizal biodiversity in Tuber macrosporum productive sites. Mycorrhiza 24: 281–292. [DOI] [PubMed] [Google Scholar]
- Berkeley MJ. (1844). Notices of British fungi. Annals and Magazine of Natural History 13: 340–359. [Google Scholar]
- Bidartondo MI, Read DJ. (2008). Fungal specificity bottlenecks during orchid germination and development Molecular Ecology 17: 3707–3716. [DOI] [PubMed] [Google Scholar]
- Bonito G, Smith ME, Nowak M, et al. (2013). Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified Southern Hemisphere sister lineage. PLoS ONE 8(1): e52765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano MA, Smith JE, O’Dell T, et al. (1999). Handbook to strategy 1 fungal taxa from the Northwest Forest Plan. Gen. Tech. Rep. PNW-GTR-476. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. [Google Scholar]
- Dereeper A, Guignon V, Blanc G, et al. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research 36 (Web Server issue): W465-9. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. (1897). Ascomyceten: Tuberaceen und Hemiasceen. In: Die Pilze Deutschlands, Österreichs und der Schweiz, Vol 1, part 5 (Rabenhorst L, ed): 1–108. Leipzig, Germany: E. Kummer. [Google Scholar]
- Fischer E. (1907). Über einige kalifornische Hypogaeen. Berichte der Deutschen Botanischen Gesellschaft 25: 373–376. [Google Scholar]
- Fischer E. (1908). Zur Morphologie der Hypogaeen. Botanische Zeitung 66: 141–168. [Google Scholar]
- Fischer E. (1938). Tuberineae. In Engler A., Harms H. Natürlichen Pflanzenfamilien 5b (8): 1–42. [Google Scholar]
- Frank JL, Barry S, Southworth D. (2006). Mammal mycophagy and dispersal of mycorrhizal inoculum in Oregon white oak woodlands. Northwest Science 80: 264–273. [Google Scholar]
- Frank JL, Coffan RA, Southworth D. (2010). Aquatic gilled mushrooms: Psathyrella fruiting in the Rogue River in southern Oregon. Mycologia 102: 93–107. [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizas and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Gilkey HM. (1916). A revision of the Tuberales of California. University of California Publications in Botany 6: 275–356. [Google Scholar]
- Gilkey HM. (1939). Tuberales of North America. Oregon State Monographs. Studies in Botany 1: 1–63. [Google Scholar]
- Gilkey HM. (1954). Tuberales. North American Flora 2: 1–36. [Google Scholar]
- Grubisha LC, Trappe JM, Bruns TD. (2005). Preliminary record of ectomycorrhizal fungi on two California Channel Islands. In: Proceedings of the Sixth California Islands Symposium, Ventura, CA, Dec. 1-3, 2003 (Garcelon DK, Schwemm CA, eds): 171–183. National Park Service Technical Publication CHIS-05-01, Institute for Wildlife Studies, Arcata, CA. [Google Scholar]
- Hansen K, Pfister DH. (2006). Systematics of the Pezizomycetes - the operculate discomycetes. Mycologia 98: 1029–1040. [DOI] [PubMed] [Google Scholar]
- Harkness HW. (1899). Californian hypogæous fungi. Proceedings of the California Academy of Science, Series 3, Botany 1: 241–286. Online at http://www.biodiversitylibrary.org/item/48989#page/387/mode/1up [Google Scholar]
- Harmaja H. (2003). Notes on Clitocybe s. lato (Agaricales). Annales Botanici Fennici 40: 216. [Google Scholar]
- Izzo AD, Meyer M, North M, et al. (2005). Hypogeous ectomycorrhizal fungal species on roots and in small mammal diet in a mixed-conifer forest. Forest Science 51: 243–254. [Google Scholar]
- Katoh K, Misawa K, Kuma K, et al. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. (2013). MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kõljalg US, Nilsson RH, Abarenkov K, et al (2013). Towards a unified paradigm for sequence-based identification of fungi. Molecular Ecology 22: 5271–5277. [DOI] [PubMed] [Google Scholar]
- Læssøe T, Hansen K. (2007). Truffle trouble: what happened to the Tuberales? Mycological Research 111: 1075–1099. [DOI] [PubMed] [Google Scholar]
- Ławrynowicz M. (1988). Grzypy (Mycota): workowce (Ascomycetes), jeleniakowe (Elaphomycetales), truflowe (Tuberales) Vol. 18 Państwowe Wydawnictwo Nankowe; Warsaw, Poland. [Google Scholar]
- Lumbsch HT, Huhndorf SM. (2007). Outline of Ascomycota - 2007. Myconet 13: 1–58. Online at archive.fieldmuseum.org/myconet/outline.asp. [Google Scholar]
- Maddison WP, Maddison DR. (2011). Mesquite: a modular system for evolutionary analysis. Version 2.75. Online at http://mesquiteproject.org. [Google Scholar]
- McCarthy C. (1998). Chromas 1.45. Southport, Queensland, Australia: School of Health Science, Griffith University. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al (2012). International Code of Nomenclature for algae, fungi and plants (Melbourne Code), adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Koeltz Scientific Books, Königstein, Germany: Online at www.iapt-taxon.org/nomen. [Google Scholar]
- Molina R. (2008). Protecting rare, little known, old-growth forest-associated fungi in the Pacific Northwest USA: a case study in fungal conservation. Mycological Research 112: 613–38. [DOI] [PubMed] [Google Scholar]
- Montecchi A, Sarasini M. (2000). Fungi Ipogei D′Europa. Trento, Italy: Associazione Micologica Bresadola. [Google Scholar]
- Nilsson RH, Tedersoo L, Abarenkov K, et al. (2012). Five simple guidelines for establishing basic authenticity and reliability of newly generated fungal ITS sequences. MycoKeys 4: 37–63. [Google Scholar]
- O’Donnell K, Cigelnik E, Weber NS, et al. (1997). Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia 89: 48–85. [Google Scholar]
- [ORBIC] Oregon Biodiversity Information Center (2016). Rare, Threatened and Endangered Species of Oregon. Institute for Natural Resources, Portland State University, Portland, Oregon: 133 p. Online at http://inr.oregonstate.edu/orbic/rare-species/rare-species-oregon-publications. [Google Scholar]
- Palfner G, Agerer R. (1998). Balsamia alba Harkness + Pinus jeffreyi Grev. & Balf. Descriptions of Ectomycorrhizae 3: 1–6. [Google Scholar]
- Pegler DN, Spooner BM, Young TWK. (1993). British truffles. Kew, UK: Royal Botanic Gardens. [Google Scholar]
- Roy M, Rochet J, Manzi S, et al. (2013). What determines Alnus-associated ectomycorrhizal community diversity and specificity? A comparison of host and habitat effects at a regional scale. New Phytologist 198: 1228–1238. [DOI] [PubMed] [Google Scholar]
- Ryberg M. (2015). Molecular operational taxonomic units as approximations of species in the light of evolutionary models and empirical data from Fungi. Molecular Ecology 24: 5770–5777. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf A, et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Science USA 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Douhan GW, Rizzo DM. (2007). Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytologist 174: 847–863. [DOI] [PubMed] [Google Scholar]
- Swofford DL. (2002). PAUP* 4.1: Phylogenetic analysis using parsimony (*and other methods), version 4. Sunderland, MA: Sinauer. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, et al. (1997). The Clustal X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timling I, Dahlberg A, Walker DA, et al (2012). Distribution and drivers of ectomycorrhizal fungal communities across the North American Arctic. Ecosphere 3: 11–24. [Google Scholar]
- Trappe JM. (1975). Generic synonyms in the Tuberales. Mycotaxon 2: 109–122. [Google Scholar]
- Trappe JM. (1979). The orders, families, and genera of hypogeous Ascomycotina (truffles and their relatives). Mycotaxon 9: 297–340. [Google Scholar]
- Tulasne LR, Tulasne C. (1851). Fungi Hypogæi. Histoire et Monographie des Champignon Hypogés (Reimpression 1970): 123–125. Apud Friedrich Klincksieck, Paris, France. [Google Scholar]
- Ulloa M, Hamlin RT. (2012). Illustrated Dictionary of Mycology. 2nd edn St. Paul, MN: APS Press. [Google Scholar]
- Vittadini C. (1831). Monographia Tuberacearum. Mediolani. P. 30. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds): 315–322. San Diego: Academic Press. [Google Scholar]