Abstract
Background
Parkinson’s disease (PD) is a neurodegenerative disease, mostly presenting with characteristic motor symptoms. Organochlorines (OC) are a class of widely-used pesticides that have been included among the list of environmental factors incriminated in PD pathogenesis. However, most studies reporting this association are based on questionnaires, and few have reported exposure data.
Aim
To examine the relationship between OC blood concentrations and PD risk.
Methods
In the present study, we studied the concentrations of 8 OC compounds (hexachlorobenzene, heptachlor, hepachlor epoxide, c-chlordane, a-chlordane, p,p’-DDE, DDD, DDT) in 104 Greek PD patients and 110 healthy controls.
Results
All substances studied were present in at least one sample. The most frequently detected (above the level of quantification) pesticides were p,p’-DDE (n = 214, 100 % of both groups) and hexachlorobenzene, HCB (n = 189, cases 46.5 %, controls 53.5 %). Higher levels of DDE were detected among PD patients in comparison to controls by using logistic regression analysis to control for confounders [Odds Ratio, OR (95 % confidence interval, C.I.)]: 2.592,(1.29–5.21)], whilst lower levels of HCB were detect among PD patients [OR,95 %CI:0.176(0.09−0.35)].
Conclusions
Our data suggest that exposure to specific OCs is related to the risk of PD. Further studies, using real exposure data, are needed in order to confirm and extend these findings.
Abbreviations: PD, Parkinson’s disease; AD, Alzheimer’s disease; OC, organochlorines
Keywords: PD, Parkinson’s disease, organochlorines, Environmental factors
1. Introduction
Parkinson’s disease (PD) is one of the commonest neurodegenerative diseases, stemming from the progressive loss of dopaminergic neurons in the substantia nigra [1,2]. The main pathological process involves intraneuronal protein aggregations known as Lewy bodies. A-synuclein, a presynaptic nerve terminal protein, alongside ubiquitin, has been identified as the major component of Lewy bodies [3].
The symptoms are mostly motor and extrapyramidal, with resting tremor, bradykinesia, and muscle rigidity, though the clinical manifestations are not restricted to movement, with additional signs and symptoms such as olfactory dysfunction, cognitive impairment and depression being present as well [4,5]. Additionally, symptoms pertaining to psychosis, such as visual hallucinations at first and even paranoid beliefs as the disease progresses, may occasionally manifest, and antiparkinsonian drugs can lead to psychotic symptoms as well [[6], [7], [8]]. The diagnosis is mostly based on clinical criteria mainly focused around the cardinal parkinsonism symptoms, and only recently has the proper attention to non-motor manifestations been paid in the suggested diagnostic algorithms [9,10]. It has been shown, however, that up to 1 in 4 patients with a diagnosis of PD, are proven to have a different disease post-mortem, despite the growing specificity of the criteria [11].
The disease can either be familial or sporadic, while common pathways are thought to be involved in both forms [4,12]. Regarding familial PD, several gene mutations have been incriminated [13], such as mutations in the α-synuclein gene [14], though awide variety of genetic and environmental factors are considered to be implicated in thes pathogenesis of both forms, with single risk factors generally not sufficient on their own to trigger its manifestation; their interplay, however, seems to have a cumulative effect [1].
Among the environmental factors incriminated for PD, pesticides are one of the most commonly identified. Almost three decades ago, Koller et al. (1990) identified rural living and the drinking of well water as risk factors for PD [15], and so suspicion towards pesticides was raised; studies that followed identified the presence of organochlorine substances in the brains of PD patients [[16], [17], [18]]. Since then, organochlorines are most frequently associated with the risk of PD among the pesticides [[19], [20], [21], [22], [23], [24], [25]]. Research has also shown that these products can find their way towards humans in ways that surpass the limits of agriculture; for example, higher concentrations than what is allowed in the European Union have been found in cosmetic products [26].
Organochlorines (OCs) exert their pesticide activity by blocking voltage-gated sodium channels and interacting with GABA receptors, causing uncontrollable neuronal activation and spasms, which eventually lead to the death of the insects [27,28]. They are, in general, lipophilic substances with long half-lives, properties that aid in their accumulation and biomagnification in species, leading to a plethora of problems that have, in turn, led to the withdrawal of several of them from the market [26,[29], [30], [31], [32]]. Their lipophilicity also allows them to easily cross the blood-brain barrier (BBB), where they can be the triggering stimulus for subsequent neurodegeneration [5,[33], [34], [35]].
Concerning the association between exposure to OCs and PD, several studies have been conducted on the matter, as mentioned before, without reaching a consensus, although the majority hint towards a positive association of OC exposure to PD [1,25]. Most of these studies, however, rely on questionnaires and retrospective recollection of exposure of patients and controls, and are associated with inherent bias and uncertainties. Additionally, factors such as genetic and environmental interplay may be involved, since polymorphisms in a wide variety of genes have been shown to influence pesticide toxicity [36,37]. Very few studies have actually measured pesticide levels, and in particular OC exposure in a case-control study setting. Thus, the purpose of this study is to add much needed data concerning the relation between PD and exposure to specific OC compounds. We hereby present the results on a Greek PD cohort, living in a rural area of Greece, where the pesticide exposure is expected to be high. We deemed it of great importance to assess these concentrations, since defining what consists a “toxic” level is particularly hard, and incriminating substances that otherwise have benefits for a community (in this case as pesticides) could be damaging [38]. This notion of dosage is not new; since ancient times scholars have extensively discussed the ideas of moderation of exposure and of “dose-response”, ideas that have heavily affected modern toxicology, like with the concept of hormesis [39]. Therefore, accurately assessing the relationship between exposure, with measures in blood samples, and PD is of paramount importance.
2. Methods
2.1. Participants
The study participant group consisted of 104 Parkinson’s disease affected cases [male:female = 55:45, age (mean 70.75, SD: 9.4)] and 110 healthy controls [male:female = 48:62, age (mean 70.16, SD: 8.4], recruited from the Neurology Clinic of the University Hospital of Larissa, in Greece. All the participants were Greek and lived n the province of Thessaly in Central Greece. The diagnosis of PD was made by a specialist neurologist according to established diagnostic criteria (UK Parkinson’s Disease Society Brain Bank’s clinical criteria) [40]. Eligibility criterion for controls was the negative reported history related to neurological diseases. All the participants (PD cases and healthy controls) granted a written informed consent. The appropriate approval of the local Ethics Committee was obtained.
2.2. Blood sampling collection and laboratory techniques
Peripheral venous blood was extracted from each individual via venipuncture. The serum from each sample was collected after centrifugation of the blood samples. The samples were frozen and stored at −80 °C until the time of the analysis. The serum samples were analyzed in the Laboratory of Hygiene and Epidemiology, University of Thessaly, Greece and were treated according to a procedure described in great detail in a previous publication [41].
Briefly, in 0.5 mL of serum, 10 μl of Internal Standard (PCB 101 at 1 μg/mL) were added and subsequently protein precipitation was performed by adding 0.5 mL of acetonitrile, vortexing for 1 min and then centrifuging for 5 min at 15,680 rcf. The supernatant was then transferred to a 4 mL glass vial with 0.5 mL of water and 1 mL of K2HPO4 (0.1 M). The analytes of interest were obtained by Solid Phase Microextraction in Head Space mode (HS-SPME) via a Polydimethylsiloxane (PDMS), df 100 mm fiber. The fiber was exposed to the vapor at a temperature of 85 °C for half an hour. After the extraction, the fiber was promptly inserted in the injector of a Gas Chromatograph-Mass Spectrometer system (GC–MS Finnigan Trace GC Ultra/PolarisQQuadrupole Ion Trap GC/ MSn), and desorbed for 5 min at 270 °C. The analytes were separated in an ATTM-5MS 30 m, 0.25 mm column with 0.25 m film thickness of 5% phenyl–95 % methylpolysiloxane stationary phase (Alltech Associates, USA). Pure helium (99.999 %) was used as the carrier gas in the constant flow mode at 1 mL/min. The GC oven program started with an initial temperature of 100 °C, kept for 5 min, and then ramped to 160 °C with a heating rate of 15 °C/min, then again up to 300 °C at 5 °C/min, kept for 2 min, and finally cooled down to the initial temperature. The quantification of the substances was performed by internal standard method (PCB 101). The limits of detection (LOD) and limits of quantitation (LOQ) were calculated according to the ratio signal / noise ratio (S/N) as follows: LOD¼3 * S/N, LOQ¼10*S/N. The LOD (in ng/mL) were 0.03 for HCB, 0.04 for Heptachlor, 0.04 for Heptachlor Epoxide, 0.02 for c-chlordane, 0.04 for a – chlordane, 0.01 for p,p′ – DDE, 0.15 for DDD and 0.21 for DDT.
2.3. Statistical analysis
Total lipids were calculated according to the formula total lipids= (227 X Total cholesterol) + Triglycerides + 623 mg/dl [42]. Levels of organochlorine pesticides in nanograms per gram of total lipids were used as values in statistical analysis. We used the x2 test for analysis of categorical variables and Student t-test for comparing means of groups’ age. We used linear regression analysis to investigate the correlation of pesticide levels with age and disease duration. Nonparametric independent sample test of Mann-Whitney (U) was used to compare disease state with pesticide levels. Odds ratios (ORs) with 95 % confidence intervals (CIs) were estimated using logistic regression analysis, with nanograms of HCB or p,p’-DDE per gram of total lipids (high/low based on the sample’s median value), age, and sex as predictor variables for PD versus control status. Values smaller than the Level of Quantification (LOQ) were imputed with the standard value of LOQ/√2 [43]. We also created tertiles regarding the serum levels of p,p’-DDE and we estimated the effect of p,p’-DDE serum levels (predictor) on the PD, by using logistic regression. The reference group regarding the predictor was the participants with the lower serum levels p,p’-DDE, while the reference group regarding the outcome was the healthy controls. For all statistical analyses, the SPSS v.21 (IBM Corp. Released 2012.IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) was used.
3. Results
The study participants included 104 Parkinson’s disease affected cases [male/female = 1.22, age (mean 70.75, SD: 9.4)] and 110 healthy controls [male/female = 0.77, age (mean 70.16, SD: 8.4], recruited from the Neurology Clinic of the University Hospital of Larissa. The demographic characteristics of the participants are presented in Table 1. There was no significant difference in the groups in terms of age (t-test p-value = 0.626),sex (p = 0.102), history of smoking (p = 0.471), history of alcohol consumption (p = 0.736) and history of hypertension (p = 0.564). Disease duration was available for 51 out of 104 patients and had a mean duration of 8.2 years (min:2, max: 22 years, SD: 5.2).
Table 1.
Demographic Data for two study groups.
| PD group (n = 104) | Controls (n = 110) | |
|---|---|---|
| Age, mean (SD), years | 70.75 (9.4) | 70.16 (8.4) |
| Sex, M/F ratio | 1.22 | 0.77 |
| History of smoking n, (%) | 10 (9.6) | 14 (12.7) |
| History of alcohol consumption n, (%) | 18 (17.3) | 21 (19.1) |
| History of hypertension n, (%) | 68 (65.4) | 76 (69.1) |
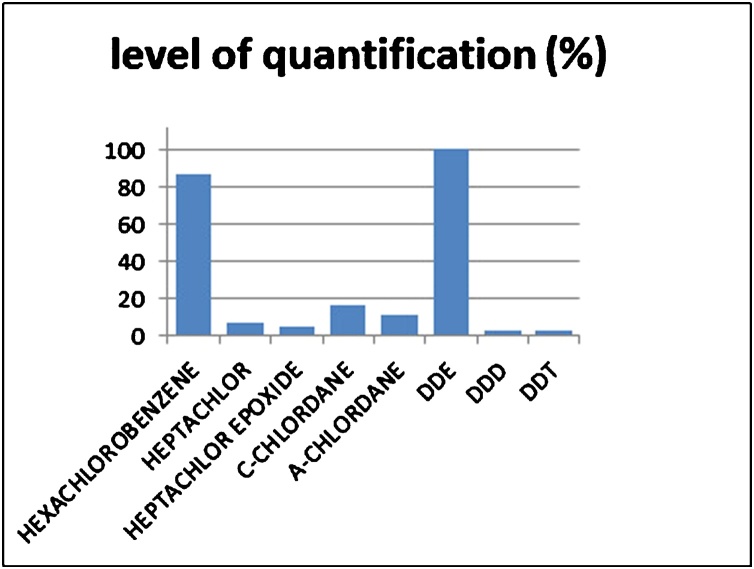
All eight organochlorine pesticides studied were present in at least one sample. The level of quantification (%) for each pesticide is depicted at Fig. 1. The most frequently detected (above the level of quantification) pesticides were p,p’-DDE (n = 214, 100 % of both groups) and hexachlorobenzene, HCB, (n = 189, cases 46.5 %, controls 53.5 %). Levels of p,p’-DDE and HCB (ng/g of total lipids) were linearly correlated with age (p value 0.006 and 0.021 respectively) as shown by the linear regression analysis. No linear correlation between levels of HCB and disease duration (n = 51 patients) was found.Non parametric mean comparison of p,p’-DDE levels between PD patients and controls showed no significant difference (Mann-Whitney U = 5060.0, p = 0.425). On the other hand, levels of HCB were significantly different between groups (Mann-Whitney U = 3527.5, p < 0.001). HCB levels were higher in the control group (median = 140, range: 10.99–1885.14 ng/g) than the PD group (median = 65.62, range: 7.47–1822.6 ng/g) (respective results are presented at Table 2).
Fig. 1.
Level of quantification (%) for each pesticide.
Table 2.
Results from testing association between p-p DDE and HCB serum levels and PD phenotypes.
| Phenotype | organochlorine pesticide |
|
|---|---|---|
| p-p DDE | HCB | |
| Age | 0.006*1 | 0.021*1 |
| Disease Duration | >0.05*1 | >0.05*1 |
| mean comparison of OCs levels between PD patients and controls | 0.425*2 | <0.001*2 |
*1 p-value for linear correlation.
*2 p-value for Mann-Whitney U.
HCB, hexachlorobenzene; PD, Parkinson’s Disease; OC organochlorines.
Higher levels of DDE were detected among PD patients in comparison to controls by using logistic regression analysis to control for confounders [Odds Ratio, OR (95 % confidence interval, C.I.)]: 2.592, (1.29–5.21)], whilst lower levels of HCB were detected among PD patients [OR, 95 %CI:0.176(0.09−0.35)].However, logistic regression comparing the difference in serum levels of p,p’-DDE in tertiles between the PD and the healthy controls, did not reach the statistically significant threshold (Table 3).
Table 3.
Results from logistic regression evaluated the association between p-p DDE serum levels and PD.
| Tertiles | p-pDDE serum levels (ng/mL) |
|||||
|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
|||||
| OR | 95 %CI | p-value | OR | 95 %CI | p-value | |
| Q1 (reference) | 1.00 | – | – | 1.00 | – | – |
| Q2 | 0.929 | 0.399−2.159 | 0.863 | 0.964 | 0.404−2.304 | 0.935 |
| Q3 | 1.090 | 0.527−2.257 | 0.663 | 1.194 | 0.538−2.649 | 0.663 |
OR, odds ratio; CI, confidence interval.
*Adjusted model for age, sex.
4. Discussion
Organochlorine pesticides have consistently been shown to be neurotoxic and to promote oxidative stress [29,30,[44], [45], [46]]. In our study, all eight studied OCs were found in at least one sample, and the most frequently detected were p,p’-DDE and hexachlorobenzene (HCB). HCB levels were found higher in the control group. Regression analysis showed a significant odds ratio for the presence of high levels of DDE to predict a diagnosis of PD versus control,whereas high levels of HCB predicted a diagnosis of control versus PD. Higher levels of DDE were detected among PD patients in comparison to controls by using logistic regression analysis to control for confounders, whilst lower levels of HCB were detected among PD patients.
P,p’-DDE is an extremely stable metabolite of dichlorodiphenyltrichloroethane (DDT) (Costa 2015). Higher levels of p,p’-DDE and DDT have been found in PD samples when compared to controls in several studies besides the present one [17,18,[47], [48], [49]]. Other studies have also linked DDT and systematic exposure to pesticides to an elevated risk of developing PD [28,50]; its extremely long half-life facilitates bioaccumulation, biomagnification and its transport through the BBB and into the brain parenchyma [29,31].
It has long been suggested that hydrophobic pesticides, such as OCs, tend to bind more strongly to a partially folded a-synuclein conformation, accelerating the fibril deposit process [51], one of the main components of PD pathology. Studies on OC compounds have also implicated a plethora of mechanisms, such as the induction of oxidative stress in dopamine cells, leading to ubiquitin–proteasome deficits and enhancement of α-synuclein aggregations [23]. Dopamine cells have also been shown to be more vulnerable to OC-induced oxidative stress [52], further highlighting the importance of these pesticides in PD pathogenesis.
Regarding DDT and p,p’-DDE in particular, Rossi et al. (2017) propose the formation of vesicular buds, released from neuronal cells upon fusion of an intermediate endocytic compartment with the plasma membrane as the mechanism via which DDT may lead to PD [34]. Upon entering the brain via their lipophilicity, they corrupt the cell membranes and give way to the formation of microvesicules and exosomes, which in turn exacerbate the pathological aggregation of proteins [53]. This may lead to the development of PD, since exosomes have been involved in a wide variety of neurodegenerative processes [54,55]. This is also condoned by the report of Richardson at el. (2009), where p,p’-DDE was the most frequently identified pesticide between PD, Alzheimer’s disease (AD) and control groups, and was found higher in patients with AD, a typical pathological-protein-aggregation disease, when compared to the other groups [20]. This is consistent with the findings of Fleming et al. (1994), where p,p’-DDT, another DDT metabolite, was found to be significantly higher in AD brain samples [18]. DDT has also long been known to cause mitochondrial dysfunction [56], a process heavily involved in PD pathogenesis as well [57], and DDT and its derivatives have been shown to promote neuronal cell apoptosis by activating MAPKs (mitogen-activated protein kinases) [58].
Hexachlorobenzene exposure has been less studied compared to DDT and p,p’-DDE, probably pertaining to the fact that it was widely banned as a fungicide several decades ago and is nowadays solely used for laboratory purposes [59]. HCB is a lipophilic compound [60] and is generally linked to diseases like porphyria and thyroid malfunction [[61], [62], [63]]. Animal models have shown that it gives rise to oxidative stress in the brain [64] and the reports of its toxicity on humans and other mammals describe manifestations similar to PD [60,[65], [66], [67]]. It was also significantly associated with several “HCB-related” diseases, PD included, in a 1999 survey of male workers in an electrochemical factory [68]and correlated to Lewy body pathology in a 2018 study, but only when combined with heptachlor epoxide [69]. Our results, however, seem to contradict what the literature has shown so far, since HBC levels were higher in the control group. At this point, the bibliographical data on humans is not particularly strong with this OC compound, and an etiological bond between HCB and PD remains to be established. For example, Weisskopf et al. (2010) also found higher concentrations in serum samples of controls than PD patients and no correlation of the substance to PD whatsoever, even when controlled for smoking, a factor known to have a protective effect in the disease [24]. Other studies have also reported similar concentrations between controls and patients, failing to show an association of HCB with PD [17,19]. Concerning the concentrations themselves, our mean concentration of 140 ng/g in controls and 65 ng/g in patients rank higher than those reported by Weisskopf et al. (2010) (0.45 ng/g for patients, 47.5 ng/g for controls) and Corrigan et al. (2000) (around 30 ng/g for both groups), but lower than Petersen et al. (2008) (around 210 ng/g for both groups). It is possible that confounding factors, such as genetic predisposition, sex or exposure to other substances, may be responsible for the correlations found, or the lack thereof and maybe even the discrepancies in the measured concentrations [1,18,25].
Another interesting topic are exposure sources, especially when one considers that fact that HCB has been banned as a fungicide in many countries, but still continues to be found in a great proportion of samples. HCB occurs as a byproduct of many chlorinated compounds, such as carbon tetrachloride and tetrachloroethylene, and it is consequently released in the environment, where it can spread into air, water, soil and other environmental compartments [70]. It can accumulate in humans, where it causes a plethora of diseases and is also linked with carcinogenesis [59]. Its absorption heavily involves the lymphatic system, and it is thus deposed in adipose tissue, where it can remain for long periods of time [71]. Therefore, its persistent detection could be explained by its durable accumulation in adipose tissues from subjects exposed before its widespread use was banned, and by the fact that it has not been wiped out from the environment, since it reappears as a byproduct in the manufacture of other compounds. On the contrary, the constant detection of DDT and its metabolite DDE seems much more logical, since these substances have not been banned and are still into widespread use due to their pesticidal capabilities that have also led countries into using them to even control populations of insects that carry diseases such as malaria (Salihovic et al. 2016) [72]. DDT gets quickly degraded into metabolites, of which p,p’-DDE is very resilient and it used to monitor DDT exposure in biological samples (UNEP,2015) (http://www.pops.int/).
Finally, in the search of PD pathogenesis, it is important to note that the interaction of several factors may be the definite trigger, and that none of those factors on its own may suffice in leading to the disease. Firstly, genes implicated in PD pathogenesis and pesticide metabolism have been extensively examined and several studies have demonstrated the synergistic effect of pesticide exposure with specific alleles [1,73]. Additionally, the male sex also seems to act as a predisposing factor and several studies reported elevated PD risk after pesticide/OC exposure only when men were concerned, or at a higher rate in men [18,22,25,68]. Lastly, rural living and occupational exposure to pollutants and pesticides are, naturally, additional exacerbating factors, as highlighted by an array of publications [1,15,18,21,22,[74], [75], [76], [77]]. Taking all of the above into consideration, it is necessary for future research to try and elucidate the complex ways that these factors intertwine with each other in order for causative associations to be made clearer and for future therapies or preventive measures to be developed.
The present study has a few limitations that need to be acknowledged. First of all, the calculation of the total lipids was based on the levels of total cholesterol and triglyceride levels, excluding the small proportion of postprandial increases observed in free cholesterol and phospholipids [78]. Their inclusion in our total lipid calculation would have given extra validity to our analysis [78]. Moreover,we imputed the values smaller than the LOQ with the standard value of LOQ/√2, a method which may have resulted in biased risk assessments [43]. Furthermore, adding potential confounding factors, such as professional exposure, individual genetic makeup and of participants to our analysis would have granted more robustness to our results [80,81]. Additionally, analysis on other OC compounds frequently encountered in PD-related bibliography, such as dieldrin or b-hexachlorocyclohexane, could have provided us with bigger insight into the relationship between OCs and PD. Also, current study was performed without measuring the possible interactions between the OCs, and also overlooking low exposures to environmental toxicants, suggesting that Real-Life Risk Simulation approach [38,82] would represent an alternative approach for future studies. Lastly, as the sample collection was made after the diagnosis of PD, a prospective cohort study would have produced more robust and accurate results, regarding the role of OCs in PD.
It is important at this point to note thatthe mechanism of action of pesticides on dopaminergic neurons extends beyond the induction of neurotoxicity (as described previously), to the deregulation of key factor genes involved in the protection and function of dopaminergic neurons. Subsequently, future endeavors could include the association of genetic alterations in genes involved in pesticide detoxification and PD pathogenesis in general, in conjunction with pesticide exposure [79].
Despite these caveats, this study considerably enriches the existing data on human studies regarding pesticide exposure and the risk of Parkinson’s disease. It also adds to the bibliography on various ethnic groups, since it consists of an ethnically homogenous cohort of Greek origin, and it will hopefully help fully clarify the relationship between pesticides and PD in the future. For that goal to be achieved, further research on these compounds is more than necessary and recommended.
Funding
This study was supported, in part, through the Hellenic General Secretariat of Research and Technology Program COOPERATION 09ΣΥΝ-12-876 (coordinator, L.S.).
CRediT authorship contribution statement
Efthimios Dardiotis: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing - review & editing. Athina-Maria Aloizou: Formal analysis, Methodology, Software, Visualization, Writing - original draft. Evagelos Sakalakis: Formal analysis, Methodology, Software, Visualization, Writing - original draft. Vasileios Siokas: Formal analysis, Methodology, Software, Visualization, Writing - original draft. Michalis Koureas: Formal analysis, Methodology, Software, Visualization, Writing - original draft. Georgia Xiromerisiou: Writing - review & editing. Efthymia Petinaki: Writing - review & editing. Martin Wilks: Writing - review & editing. Aristidis Tsatsakis: Writing - review & editing. Christos Hadjichristodoulou: Writing - review & editing. Leonidas Stefanis: Writing - review & editing. Georgios M. Hadjigeorgiou: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
References
- 1.Dardiotis E., Xiromerisiou G., Hadjichristodoulou C., Tsatsakis A.M., Wilks M.F., Hadjigeorgiou G.M. The interplay between environmental and genetic factors in Parkinson’s disease susceptibility: the evidence for pesticides. Toxicology. 2013;307:17–23. doi: 10.1016/j.tox.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Theuns J., Verstraeten A., Sleegers K., Wauters E., Gijselinck I., Smolders S., Crosiers D., Corsmit E., Elinck E., Sharma M., Kruger R., Lesage S., Brice A., Chung S.J., Kim M.J., Kim Y.J., Ross O.A., Wszolek Z.K., Rogaeva E., Xi Z., Lang A.E., Klein C., Weissbach A., Mellick G.D., Silburn P.A., Hadjigeorgiou G.M., Dardiotis E., Hattori N., Ogaki K., Tan E.K., Zhao Y., Aasly J., Valente E.M., Petrucci S., Annesi G., Quattrone A., Ferrarese C., Brighina L., Deutschlander A., Puschmann A., Nilsson C., Garraux G., LeDoux M.S., Pfeiffer R.F., Boczarska-Jedynak M., Opala G., Maraganore D.M., Engelborghs S., De Deyn P.P., Cras P., Cruts M., Van Broeckhoven C. Global investigation and meta-analysis of the C9orf72 (G4C2)n repeat in Parkinson disease. Neurology. 2014;83:1906–1913. doi: 10.1212/wnl.0000000000001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMaagd G., Philip A. Parkinson’s disease and its management: Part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T. 2015;40:504–532. [PMC free article] [PubMed] [Google Scholar]
- 4.Baltazar M.T., Dinis-Oliveira R.J., de Lourdes Bastos M., Tsatsakis A.M., Duarte J.A., Carvalho F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases--a mechanistic approach. Toxicol. Lett. 2014;230:85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Moretto A., Colosio C. The role of pesticide exposure in the genesis of Parkinson’s disease: epidemiological studies and experimental data. Toxicology. 2013;307:24–34. doi: 10.1016/j.tox.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Tsatsakis A., Docea A.O., Calina D., Tsarouhas K., Zamfira L.M., Mitrut R., Sharifi-Rad J., Kovatsi L., Siokas V., Dardiotis E., Drakoulis N., Lazopoulos G., Tsitsimpikou C., Mitsias P., Neagu M. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. J. Clin. Med. 2019;8 doi: 10.3390/jcm8091295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussbaum L., Hogea L., Calina D., Andreescu N., Gradinaru R., Ștefănescu R., Puiu M. Modern treatment approaches in psychoses. Pharmacogenetic, neuroimagistic and clinical implications. Farmacia. 2017;65:75–81. [Google Scholar]
- 8.Papapetropoulos S., Mash D.C. Psychotic symptoms in Parkinson’s disease. From description to etiology. J. Neurol. 2005;252:753–764. doi: 10.1007/s00415-005-0918-5. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatr. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 10.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., Halliday G., Goetz C.G., Gasser T., Dubois B., Chan P., Bloem B.R., Adler C.H., Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 11.Marsili L., Rizzo G., Colosimo C. Diagnostic criteria for Parkinson’s disease: from James Parkinson to the concept of prodromal disease. Front. Neurol. 2018;9:156. doi: 10.3389/fneur.2018.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dardiotis E., Tsouris Z., Mentis A.A., Siokas V., Michalopoulou A., Sokratous M., Dastamani M., Bogdanos D.P., Deretzi G., Kountouras J. H. Pylori and Parkinson’s disease: meta-analyses including clinical severity. Clin. Neurol. Neurosurg. 2018;175:16–24. doi: 10.1016/j.clineuro.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 13.Schiesling C., Kieper N., Seidel K., Kruger R. Review: familial Parkinson’s disease--genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol. Appl. Neurobiol. 2008;34:255–271. doi: 10.1111/j.1365-2990.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 14.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E.S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W.G., Lazzarini A.M., Duvoisin R.C., Di Iorio G., Golbe L.I., Nussbaum R.L. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science (New York, N.Y.) 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 15.Koller W., Vetere-Overfield B., Gray C., Alexander C., Chin T., Dolezal J., Hassanein R., Tanner C. Environmental risk factors in Parkinson’s disease. Neurology. 1990;40:1218–1221. doi: 10.1212/wnl.40.8.1218. [DOI] [PubMed] [Google Scholar]
- 16.Corrigan F.M., Murray L., Wyatt C.L., Shore R.F. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp. Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- 17.Corrigan F.M., Wienburg C.L., Shore R.F., Daniel S.E., Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J. Toxicol. Environ. Health Part A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- 18.Fleming L., Mann J.B., Bean J., Briggle T., Sanchez-Ramos J.R. Parkinson’s disease and brain levels of organochlorine pesticides. Ann. Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- 19.Petersen M.S., Halling J., Bech S., Wermuth L., Weihe P., Nielsen F., Jorgensen P.J., Budtz-Jorgensen E., Grandjean P. Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. Neurotoxicology. 2008;29:584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Richardson J.R., Shalat S.L., Buckley B., Winnik B., O’Suilleabhain P., Diaz-Arrastia R., Reisch J., German D.C. Elevated serum pesticide levels and risk of Parkinson disease. Arch. Neurol. 2009;66:870–875. doi: 10.1001/archneurol.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidler A., Hellenbrand W., Robra B.P., Vieregge P., Nischan P., Joerg J., Oertel W.H., Ulm G., Schneider E. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: a case-control study in Germany. Neurology. 1996;46:1275–1284. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- 22.Elbaz A., Clavel J., Rathouz P.J., Moisan F., Galanaud J.P., Delemotte B., Alperovitch A., Tzourio C. Professional exposure to pesticides and Parkinson disease. Ann. Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- 23.Hatcher J.M., Pennell K.D., Miller G.W. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol. Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisskopf M.G., Knekt P., O’Reilly E.J., Lyytinen J., Reunanen A., Laden F., Altshul L., Ascherio A. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74:1055–1061. doi: 10.1212/WNL.0b013e3181d76a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freire C., Koifman S. Pesticide exposure and Parkinson’s disease: epidemiological evidence of association. Neurotoxicology. 2012;33:947–971. doi: 10.1016/j.neuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Adekunle A.S., Oyedele Oyekunle J.A., Ola I.J., Obisesan O.R., Maxakato N.W. Determination of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in some personal care products in Nigeria. Toxicol. Rep. 2018;5:994–1001. doi: 10.1016/j.toxrep.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong K. Insect sodium channels and insecticide resistance. Invert. Neurosci. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeedi Saravi S.S., Dehpour A.R. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: a review. Life Sci. 2016;145:255–264. doi: 10.1016/j.lfs.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Costa L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015;131:135–148. doi: 10.1016/b978-0-444-62627-1.00009-3. [DOI] [PubMed] [Google Scholar]
- 30.Kanthasamy A.G., Kitazawa M., Kanthasamy A., Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Mansouri A., Cregut M., Abbes C., Durand M.J., Landoulsi A., Thouand G. The environmental issues of DDT pollution and bioremediation: a multidisciplinary review. Appl. Biochem. Biotechnol. 2017;181:309–339. doi: 10.1007/s12010-016-2214-5. [DOI] [PubMed] [Google Scholar]
- 32.Oyekunle J.A.O., Akindolani O.A., Sosan M.B., Adekunle A.S. Organochlorine pesticide residues in dried cocoa beans obtained from cocoa stores at Ondo and Ile-Ife, Southwestern Nigeria. Toxicol. Rep. 2017;4:151–159. doi: 10.1016/j.toxrep.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanavouras K., Tzatzarakis M.N., Mastorodemos V., Plaitakis A., Tsatsakis A.M. A case report of motor neuron disease in a patient showing significant level of DDTs, HCHs and organophosphate metabolites in hair as well as levels of hexane and toluene in blood. Toxicol. Appl. Pharmacol. 2011;256:399–404. doi: 10.1016/j.taap.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Rossi M., Scarselli M., Fasciani I., Maggio R., Giorgi F. Dichlorodiphenyltrichloroethane (DDT) induced extracellular vesicle formation: a potential role in organochlorine increased risk of Parkinson’s disease. Acta Neurobiol. Exp. (Wars) 2017;77:113–117. doi: 10.21307/ane-2017-043. [DOI] [PubMed] [Google Scholar]
- 35.Dardiotis E., Siokas V., Sokratous M., Tsouris Z., Michalopoulou A., Andravizou A., Dastamani M., Ralli S., Vinceti M., Tsatsakis A., Hadjigeorgiou G.M. Genetic polymorphisms in amyotrophic lateral sclerosis: evidence for implication in detoxification pathways of environmental toxicants. Environ. Int. 2018;116:122–135. doi: 10.1016/j.envint.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Teodoro M., Briguglio G., Fenga C., Costa C. Genetic polymorphisms as determinants of pesticide toxicity: recent advances. Toxicol. Rep. 2019;6:564–570. doi: 10.1016/j.toxrep.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dardiotis E., Aloizou A.M., Siokas V., Tsouris Z., Rikos D., Marogianni C., Aschner M., Kovatsi L., Bogdanos D.P., Tsatsakis A. Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology. 2019;411:24–31. doi: 10.1016/j.tox.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Tsatsakis A.M., Docea A.O., Calina D., Buga A.M., Zlatian O., Gutnikov S., Kostoff R.N., Aschner M. Hormetic Neurobehavioral effects of low dose toxic chemical mixtures in real-life risk simulation (RLRS) in rats. Food Chem. Toxicol. 2019;125:141–149. doi: 10.1016/j.fct.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 39.Tsatsakis A.M., Vassilopoulou L., Kovatsi L., Tsitsimpikou C., Karamanou M., Leon G., Liesivuori J., Hayes A.W., Spandidos D.A. The dose response principle from philosophy to modern toxicology: the impact of ancient philosophy and medicine in modern toxicology science. Toxicol. Rep. 2018;5:1107–1113. doi: 10.1016/j.toxrep.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibb W.R., Lees A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatr. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koureas M., Karagkouni F., Rakitskii V., Hadjichristodoulou C., Tsatsakis A., Tsakalof A. Serum levels of organochlorine pesticides in the general population of Thessaly, Greece, determined by HS-SPME GC-MS method. Environ. Res. 2016;148:318–321. doi: 10.1016/j.envres.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Bernert J.T., Turner W.E., Patterson D.G., Jr., Needham L.L. Calculation of serum "total lipid" concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere. 2007;68:824–831. doi: 10.1016/j.chemosphere.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 43.Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5:46–51. doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 44.Ortiz Martinez A., Martinez-Conde E. The neurotoxic effects of lindane at acute and subchronic dosages. Ecotoxicol. Environ. Saf. 1995;30:101–105. doi: 10.1006/eesa.1995.1011. [DOI] [PubMed] [Google Scholar]
- 45.Dick F.D. Parkinson’s disease and pesticide exposures. Br. Med. Bull. 2006;79-80:219–231. doi: 10.1093/bmb/ldl018. [DOI] [PubMed] [Google Scholar]
- 46.Akinyemi A.J., Miah M.R., Ijomone O.M., Tsatsakis A., Soares F.A.A., Tinkov A.A., Skalny A.V., Venkataramani V., Aschner M. Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: involvement of the dopamine transporter. Toxicol. Rep. 2019;6:833–840. doi: 10.1016/j.toxrep.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koldkjaer O.G., Wermuth L., Bjerregaard P. Parkinson’s disease among Inuit in Greenland: organochlorines as risk factors. Int. J. Circumpolar Health. 2004;63(Suppl 2):366–368. doi: 10.3402/ijch.v63i0.17937. [DOI] [PubMed] [Google Scholar]
- 48.Singh N.K., Banerjee B.D., Bala K., Chhillar M., Chhillar N. Gene-gene and gene-environment interaction on the risk of Parkinson’s disease. Curr. Aging Sci. 2014;7:101–109. doi: 10.2174/1874609807666140805123621. [DOI] [PubMed] [Google Scholar]
- 49.Chhillar N., Singh N.K., Banerjee B.D., Bala K., Mustafa M., Sharma D., Chhillar M. Organochlorine pesticide levels and risk of Parkinson’s disease in north Indian population. ISRN Neurol. 2013;2013:371034. doi: 10.1155/2013/371034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Mark M., Brouwer M., Kromhout H., Nijssen P., Huss A., Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ. Health Perspect. 2012;120:340–347. doi: 10.1289/ehp.1103881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uversky V.N., Li J., Fink A.L. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 52.Kitazawa M., Anantharam V., Kanthasamy A.G. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic. Biol. Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 53.Grey M., Dunning C.J., Gaspar R., Grey C., Brundin P., Sparr E., Linse S. Acceleration of alpha-synuclein aggregation by exosomes. J. Biol. Chem. 2015;290:2969–2982. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Properzi F., Ferroni E., Poleggi A., Vinci R. The regulation of exosome function in the CNS: implications for neurodegeneration. Swiss Med. 2015;145:w14204. doi: 10.4414/smw.2015.14204. [DOI] [PubMed] [Google Scholar]
- 55.Dardiotis E., Aloizou A.M., Siokas V., Patrinos G.P., Deretzi G., Mitsias P., Aschner M., Tsatsakis A. The role of MicroRNAs in patients with amyotrophic lateral sclerosis. Journal of molecular neuroscience: MN. 2018;66:617–628. doi: 10.1007/s12031-018-1204-1. [DOI] [PubMed] [Google Scholar]
- 56.Moreno A.J., Madeira V.M. Mitochondrial bioenergetics as affected by DDT. Biochim. Biophys. Acta. 1991;1060:166–174. doi: 10.1016/s0005-2728(09)91004-0. [DOI] [PubMed] [Google Scholar]
- 57.Bose A., Beal M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016;139(Suppl 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- 58.Shinomiya N., Shinomiya M. Dichlorodiphenyltrichloroethane suppresses neurite outgrowth and induces apoptosis in PC12 pheochromocytoma cells. Toxicol. Lett. 2003;137:175–183. doi: 10.1016/s0378-4274(02)00401-0. [DOI] [PubMed] [Google Scholar]
- 59.Starek-Swiechowicz B., Budziszewska B., Starek A. Hexachlorobenzene as a persistent organic pollutant: toxicity and molecular mechanism of action. Pharmacological reports: PR. 2017;69:1232–1239. doi: 10.1016/j.pharep.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Knauf V., Hobson W. Hexachlorobenzene ingestion by female rhesus monkeys: tissue distribution and clinical symptomatology. Bull. Environ. Contam. Toxicol. 1979;21:243–248. doi: 10.1007/BF01685418. [DOI] [PubMed] [Google Scholar]
- 61.Cam C.C., Nigogosyan G.G. Acquired toxic porphyria cutanea tarda due to hexachlorobenzene: report of 348 cases caused by this fungicide. JAMA. 1963;183:88–91. doi: 10.1001/jama.1963.03700020038009. [DOI] [PubMed] [Google Scholar]
- 62.Cripps D.J., Peters H.A., Gocmen A., Dogramici I. Porphyria turcica due to hexachlorobenzene: a 20 to 30 year follow-up study on 204 patients. Br. J. Dermatol. 1984;111:413–422. doi: 10.1111/j.1365-2133.1984.tb06603.x. [DOI] [PubMed] [Google Scholar]
- 63.Meeker J.D., Altshul L., Hauser R. Serum PCBs, p,p’-DDE and HCB predict thyroid hormone levels in men. Environ. Res. 2007;104:296–304. doi: 10.1016/j.envres.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song S.B., Xu Y., Zhou B.S. Effects of hexachlorobenzene on antioxidant status of liver and brain of common carp (Cyprinus carpio) Chemosphere. 2006;65:699–706. doi: 10.1016/j.chemosphere.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 65.den Tonkelaar E.M., Verschuuren H.G., Bankovska J., de Vries T., Kroes R., van Esch G.J. Hexachlorobenzene toxicity in pigs. Toxicol. Appl. Pharmacol. 1978;43:137–145. doi: 10.1016/s0041-008x(78)80038-6. [DOI] [PubMed] [Google Scholar]
- 66.Gocmen A., Peters H.A., Cripps D.J., Bryan G.T., Morris C.R. Hexachlorobenzene episode in Turkey. Biomedical and environmental sciences: BES. 1989;2:36–43. [PubMed] [Google Scholar]
- 67.Khera K.S. Tetratogenicity and dominant lethal studies on hexachlorobenzene in rats. Food Cosmet. Toxicol. 1974;12:471–477. doi: 10.1016/0015-6264(74)90060-1. [DOI] [PubMed] [Google Scholar]
- 68.Sala M., Sunyer J., Otero R., Santiago-Silva M., Ozalla D., Herrero C., To-Figueras J., Kogevinas M., Anto J.M., Camps C., Grimalt J. Health effects of chronic high exposure to hexachlorobenzene in a general population sample. Arch. Environ. Health. 1999;54:102–109. doi: 10.1080/00039899909602243. [DOI] [PubMed] [Google Scholar]
- 69.Ross G.W., Abbott R.D., Petrovitch H., Duda J.E., Tanner C.M., Zarow C., Uyehara-Lock J.H., Masaki K.H., Launer L.J., Studabaker W.B., White L.R. Association of brain heptachlor epoxide and other organochlorine compounds with lewy pathology. Movement disorders: official journal of the Movement Disorder Society. 2018 doi: 10.1002/mds.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reed L., Buchner V., Tchounwou P.B. Environmental toxicology and health effects associated with hexachlorobenzene exposure. Rev. Environ. Health. 2007;22:213–243. doi: 10.1515/reveh.2007.22.3.213. [DOI] [PubMed] [Google Scholar]
- 71.Iatropoulos M.J. Absorption, transport and organotropism of dichlorobiphenyl (DCB), dieldrin, and hexachlorobenzene (HCB) in rats. Environ. Res. 1975;10:384–389. doi: 10.1016/0013-9351(75)90033-x. [DOI] [PubMed] [Google Scholar]
- 72.Salihovic S., Ganna A., Fall T., Broeckling C.D., Prenni J.E., van Bavel B., Lind P.M., Ingelsson E., Lind L. The metabolic fingerprint of p,p’-DDE and HCB exposure in humans. Environ. Int. 2016;88:60–66. doi: 10.1016/j.envint.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 73.Longo G.S., Pinhel M.S., Sado C.L., Gregorio M.L., Amorim G.S., Florim G.S., Mazeti C.M., Martins D.P., Oliveira F.N., Tognola W.A., Nakazone M.A., Souza D.R. Exposure to pesticides and heterozygote genotype of GSTP1-Alw26I are associated to Parkinson’s disease. Arq. Neuropsiquiatr. 2013;71:446–452. doi: 10.1590/0004-282x20130060. [DOI] [PubMed] [Google Scholar]
- 74.Steenland K., Hein M.J., Cassinelli R.T., 2nd, Prince M.M., Nilsen N.B., Whelan E.A., Waters M.A., Ruder A.M., Schnorr T.M. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology (Cambridge, Mass.) 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- 75.Wan N., Lin G. Parkinson’s Disease and Pesticides Exposure: New Findings From a Comprehensive Study in Nebraska, USA. J. Rural Health. 2016;32:303–313. doi: 10.1111/jrh.12154. [DOI] [PubMed] [Google Scholar]
- 76.Ascherio A., Chen H., Weisskopf M.G., O’Reilly E., McCullough M.L., Calle E.E., Schwarzschild M.A., Thun M.J. Pesticide exposure and risk for Parkinson’s disease. Ann. Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 77.Wermuth L., Pakkenberg H., Jeune B. High age-adjusted prevalence of Parkinson’s disease among Inuits in Greenland. Neurology. 2002;58:1422–1425. doi: 10.1212/wnl.58.9.1422. [DOI] [PubMed] [Google Scholar]
- 78.Phillips D.L., Pirkle J.L., Burse V.W., Bernert J.T., Jr., Henderson L.O., Needham L.L. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 79.Ahmed H., Abushouk A.I., Gabr M., Negida A., Abdel-Daim M.M. Parkinson’s disease and pesticides: a meta-analysis of disease connection and genetic alterations. Biomed. Pharmacother. 2017;90:638–649. doi: 10.1016/j.biopha.2017.03.100. [DOI] [PubMed] [Google Scholar]
- 80.Aloizou A.M., Siokas V., Vogiatzi C., Peristeri E., Docea A.O., Petrakis D., Provatas A., Folia V., Chalkia C., Vinceti M., Wilks M., Izotov B.N., Tsatsakis A., Bogdanos D.P., Dardiotis E. Pesticides, cognitive functions and dementia: a review. Toxicol. Lett. 2020;326:31–51. doi: 10.1016/j.toxlet.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Salehi B., Calina D., Docea A.O., Koirala N., Aryal S., Lombardo D., Pasqua L., Taheri Y., Marina Salgado Castillo C., Martorell M., Martins N., Iriti M., Suleria H.A.R., Sharifi-Rad J. Curcumin's Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsatsakis A., Tyshko N.V., Docea A.O., Shestakova S.I., Sidorova Y.S., Petrov N.A., Zlatian O., Mach M., Hartung T., Tutelyan V.A. The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes - a real-life risk simulation approach. Toxicol. Lett. 2019;315:96–106. doi: 10.1016/j.toxlet.2019.07.026. [DOI] [PubMed] [Google Scholar]