Graphical abstract
Keywords: Edible insect, Insect extract with water, Gryllus bimaculatus, Oral dose toxicity, NOAEL
Highlights
-
•
Gryllus bimaculatus, edible insects, is the best alternative food source.
-
•
Evaluation of Gryllus bimaculatus extracts with water in Sprague Dawley rats for its safety.
-
•
No Observed Adverse Effect Level for Gryllus bimaculatus is considered to be 3000 mg/kg in rats.
Abstract
This study was performed to demonstrate a No Observed Adverse Effect Level (NOAEL) for an aqueous extract of Gryllus bimaculatus. According to other studies, using dried material or extract with ethanol or methanol determined a NOAEL dose of 1000 mg/kg or 5000 mg/kg in rats. Therefore, the Gryllus bimaculatus groups were administered orally at doses of 0, 1000, 2000, and 3000 mg/kg for four weeks. Two-week recovery groups were administered at doses of 0, and 3000 mg/kg. During administration and recovery period, the animals were observed for clinical signs, change of body weight, food consumption, hematology, and clinical chemistry. Rats in each group were periodically sacrificed, and organs were weighed and examined histologically. No difference arose between any of the dosage groups and the control group in clinical signs, histopathological examination, hematology, or clinical chemistry. In conclusion, 3000 mg/kg is a NOAEL dose for Gryllus bimaculatus extracts in Sprague Dawley rats.
1. Introduction
Predictions show that by 2050, the population of the world will be increased to nearly 10 billion people [1]. Because rapid population growth increases food consumption, many countries will require a higher consumption of meat and produce. Eventually commensurate shifts in output and finding alternative resources will become a necessity. In the coming food crisis, edible insects are one of the best alternatives [2]. Certain insects as a food have many benefits from the perspective of efficiency and the environment. Insects as omnivores have a high fecundity, can be multivoltine, have a low feed conversion rate, and require only a small space to inhabit and breed. In addition, insects are high in nutrition with protein, moisture, essential fatty acids (oleic, linoleic acid), and minerals (Ca, P, K, Mg, Mn, Fe, Zn, Cu) [3]. Gryllus bimaculatus, like other insects, contains many nutrients such as protein, fatty acid, and minerals [4].
G. bimaculatus has been widely used in insect genetic engineering research. RNA interference (RNAi) technology has been a useful tool to analyze gene functions such as those during regeneration and in transgenic technology [5,6]. In 2015, the EU approved the use of insects including G. bimaculatus as novel food ingredients. In 2016, Korea Food and Drug Safety classified G. bimaculatus as a normal food without restraint. Recently, water and methanol extracts from G. bimaculatus significantly decreased blood ethanol concentrations against acute hepatic damage [4]. In addition, several studies reported that G. bimaculatus has various benefits including anti-aging [7], anti-inflammation [8], anti-oxidant [9], and anti-obesity ([10]n.d) effects. Despite various efficacy reports, toxicological reports are limited to dried material and other solvent extracts [[11], [12], [13]]. The safety of water extracts has not been reported. Therefore, we present the result of a 28-day repeated oral toxicity study for G. bimaculatus aqueous extract.
2. Materials and methods
2.1. Materials
Dried G. bimaculatus (10 kg) was provided from the Agricultural Corporation Osang Kinsect (South Korea). It was extracted with distilled water for 3 h by boiling. The extracted sample was concentrated by vacuum rotary evaporator (Rotavapor, R-220SE, BUCHI, Switzerland) and vacuum dehydration with a yield of 12 % w/w. The dried sample was dissolved in distilled water and homogenized (T25 digital ULTRA-TURRAX, IKA, Germany) before administration.
2.2. Animals
Sprague Dawley rats (6 weeks old, male and female) were obtained from OrientBio Co., Ltd. (South Korea). Animals were acclimated for a week and housed in specific pathogen-free room at a standard temperature of 24.0℃±1.0℃ with humidity 50 %±10 % under a 12 h light/dark cycle. This animal study was implemented in accordance with guidelines for the testing of chemicals (Organization for Economic Cooperation and Development Guideline 407 “Repeated Dose 28-day Oral Toxicity studies of drugs” and Korean Food and Drug Administration 1998−116 “Guidelines for toxicity studies of drugs”) [14]. Naturetech Institutional Animal Care and Use Committees (IACUC) based on the Animal Protection Act approved this study (Approval No. NT 11,803).
Rats totaling 50 males and 50 females were assigned into four main groups and two recovery groups. Each main group consisted of 10 animals of both sexes, and each recovery group consisted of 5 animals of both sexes. Treatment groups were designed based on previously safety study of G. bimaculatus in rats. The G. bimaculatus extract with 70 % ethanol was reported to have a No Observed Adverse Effect Level (NOAEL) of more than 1000 mg/kg [13], and that of dried G. bimaculatus was greater than 5000 mg/kg [11] in rats. According to these previous studies, the highest dose level was set to 3000 mg/kg. The rats of our study received G. bimaculatus extracted with water (GB) at oral gavages of 0, 1000, 2000, and 3000 mg/kg. Each dose was administrated in 10 mL per body weight was given the appropriate dose. The control group (0 mg/kg) was treated with 10 mL/kg body weight.
2.3. Body weight and food consumption
The body weights of all animals were measured weekly during the study period. The measured body weights were used to calculated individual doses, which were adjusted each week to maintain the correct dosage. Weekly food consumption was recorded and calculated for each group.
2.4. Hematology
All animals were anesthetized with isoflurane (2%∼3%, Terrell isoflurane, USP, USA) and blood was collected from the abdominal aorta and into EDTA tubes (Vacutainer plus plastic EDTA blood collection tube, BD, USA). All blood samples were centrifuged at 3000 rpm for 15 min to obtain plasma within 1 h after collection. Hematological parameters included RBC (Red Blood Cell), HGB (Hemoglobin), HCT (Hematocrit), MCV (Mean corpuscular volume), MCH (Mean corpuscular hemoglobin), MCHC (Mean corpuscular hemoglobin concentration), PLT (Platelet), WBC (White Blood cell), NEU (Neutrophils), LYM (Lymphocyte), MONO (Monocyte), EOS (Eosinophils), BASO (Basophils), and LUC (Leucocyte). Whole blood for hematology analysis was transferred to KP&T (South Korea) for analysis using automatic analyzers (BS-220, Mindray, China; ADVIA 2120i, SIEMENS, Germany).
2.5. Clinical biochemistry
Blood was collected from the abdominal aorta and into serum separating tube (SSTⅡ advance plus blood collection tube, BD, USA). All blood samples had been centrifuged at 3000 rpm for 15 min to obtain serum within 1 h after collection. Clinical chemistry included ALT (Alanine aminotransferase), AST (Aspertate aminotransferase), ALP (Alkaline phosphatase), BUN (Blood urea nitrogen), Crea (Creatinine), T-Bili (Total bilirubin), TP (Total protein), Alb (Albumin), CK (Creatine kinase), T-Chol (Total cholesterol), TG (Triglyceride), GLU (Glucose), Ca (Calcium), P (Phosphorus), Na (Sodium), K (Potassium), and Cl (Chloride). Whole blood serum for blood chemistry analysis were transferred to KP&T (South Korea) for analysis using automatic analyzers (BS-220, Mindray, China; ADVIA 2120i, SIEMENS, Germany).
2.6. Necropsy
After blood sampling, animals were sacrificed by exsanguination via the abdominal aorta and then subjected to necropsy. All external and internal surfaces of all animals were observed via complete gross postmortem examinations. The following organs were weighed: heart, lung, spleen, liver, kidney, adrenals, testis or ovary, and epididymis or uterus. Relative organ weights were calculated from fasted body weights on necropsy day.
2.7. Statistical analysis
Statistical analysis was presented using Sigmaplot (version 11.0, Systat software, Inc., San Jose, CA, USA). All data were analyzed using the analysis of variance (ANOVA) and differences of each treated group compared with the control group for each sex was assessed using t-test. All data are presented as the mean ± SD (standard deviation). A value of p < 0.05 was considered significant.
3. Results
During 28 days of administration and 14 days of recovery, no death or adverse clinical signs were observed in rats who received 1000, 2000, and 3000 mg/kg GB in both sexes. Additionally, no death or clinical signs of either sex were observed during the recovery period.
3.1. Body weight and food consumption
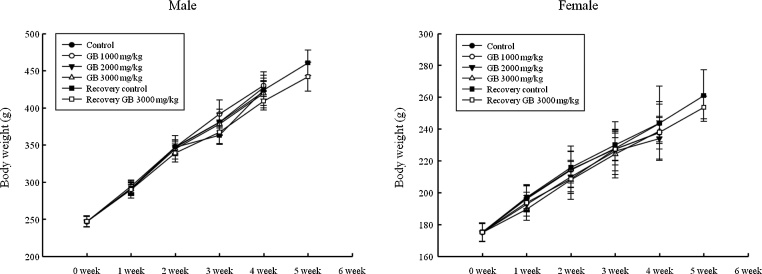
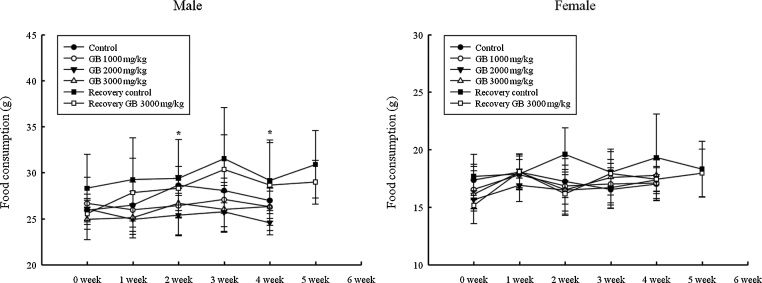
During the 28-day administration period, there was no significant change in body weight in either sex compared with the controls in the main groups (Fig. 1). Food consumption at weeks 2 and 4 was significantly decreased among male rats receiving 2000 and 3000 mg/kg GB at week 2. However, no significant difference in food consumption for the rats receiving 3000 mg/kg was observed between control and treated groups during the two-week recovery period. There was no significant difference in females for food consumption during either the administration or the recovery period when compared with the control group (Fig. 2).
Fig. 1.
Change in body weight for male and female rats treated with Gryllus bimaculatus.
Fig. 2.
Change in food consumption for male and female rats treated with Gryllus bimaculatus.
3.2. Hematology
No significant adverse changes in hematology parameters arose for either of rats (Table 1, Table 2). Decreases in RBC, MCH, and LUC were observed for in rats treated with 1000 mg/kg GB in both sexes. Male rats receiving 3000 mg/kg GB showed decreased MONO levels when compared with the control group. Female rats in the 3000 mg/kg group had decreased PLT levels. In the recovery period, the male and female rats in the 3000 mg/kg group had decreased WBC levels.
Table 1.
Hematological value of male rats treated with Gryllus bimaculatus.
| Group/dose (mg/kg) | Values | RBC(×106 cells/μL) | HGB(g/dL) | HCT(%) | RBC Indices |
PLT (×103 cells/μL) | WBC (×103 cells/μL) | WBC Differential Counting (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCV (fL) | MCH (pg) | MCHC (g/dL) | NEU | LYM | MONO | EOS | BASO | ||||||||
| Main group | G1 (0 mg/kg) | Mean | 7.65 | 14 | 42.43 | 55.48 | 18.31 | 33.02 | 1123.6 | 5.8 | 17.21 | 79.04 | 1.62 | 1.15 | 0.04 |
| SD | 0.42 | 0.68 | 2.04 | 1.09 | 0.43 | 0.46 | 147.34 | 1.43 | 5.15 | 5.19 | 0.5 | 0.47 | 0.05 | ||
| G2 (1000 mg/kg) | Mean | 7.30* | 13.8 | 41.54 | 56.96 | 18.92* | 33.22 | 1180.9 | 5.45 | 17.36 | 79.26 | 1.61 | 1.07 | 0.17 | |
| SD | 0.3 | 0.63 | 1.93 | 1.82 | 0.6 | 0.25 | 195.38 | 1.6 | 5.11 | 5.59 | 0.67 | 0.34 | 0.29 | ||
| G3 (2000 mg/kg) | Mean | 7.54 | 13.88 | 41.72 | 55.44 | 18.44 | 33.25 | 1229.4 | 5.13 | 18.53 | 77.75 | 1.54 | 1.27 | 0.11* | |
| SD | 0.42 | 0.57 | 1.81 | 1.87 | 0.72 | 0.5 | 153.54 | 1.43 | 3.6 | 3.57 | 0.41 | 0.66 | 0.06 | ||
| G4 (3000 mg/kg) | Mean | 7.42 | 14.03 | 42.14 | 56.84 | 18.91 | 33.29 | 1111.2 | 4.73 | 19.07 | 77.46 | 1.35* | 1.31 | 0.08 | |
| SD | 0.45 | 0.68 | 2.02 | 1.74 | 0.76 | 0.52 | 131.3 | 1.89 | 8.21 | 8.71 | 0.46 | 0.8 | 0.06 | ||
| Recovery group | G5 (0 mg/kg) | Mean | 7.99 | 14.52 | 44.1 | 55.24 | 18.22 | 32.98 | 1067 | 8.6 | 18.02 | 77.74 | 2.08 | 1.46 | 0.18 |
| SD | 0.41 | 0.41 | 1.61 | 1.07 | 0.59 | 0.52 | 199.71 | 1.53 | 6.17 | 6.79 | 0.33 | 0.64 | 0.11 | ||
| G6 (3000 mg/kg) | Mean | 7.71 | 13.86 | 42.26 | 54.8 | 17.98 | 32.78 | 1162.6 | 5.95* | 21.76 | 74.44 | 1.92 | 1.44 | 0.12 | |
| SD | 0.1 | 0.5 | 1.13 | 1.43 | 0.54 | 0.43 | 91.57 | 1.8 | 4.34 | 4.93 | 0.5 | 0.47 | 0.08 | ||
Means significant differences of p < 0.05 compared with the control group.
Table 2.
Hematological value of female rats treated with Gryllus bimaculatus.
| Group/dose (mg/kg) | Values | RBC (×106 cells/μL) | HGB (g/dL) | HCT (%) | RBC Indices |
PLT (×103 cells/μL) | WBC (×103 cells/μL) | WBC Differential Counting (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC V(fL) | MCH(pg) | MCHC (g/dL) | NEU | LYM | MONO | EOS | BASO | ||||||||
| Main group | G1 (0 mg/kg) | Mean | 7.28 | 13.82 | 40.89 | 56.22 | 19.00 | 33.80 | 1175.50 | 3.17 | 12.97 | 83.27 | 1.24 | 1.82 | 0.08 |
| SD | 0.19 | 0.45 | 1.11 | 1.34 | 0.49 | 0.52 | 201.29 | 1.49 | 4.86 | 5.65 | 0.41 | 1.43 | 0.09 | ||
| G2 (1000 mg/kg) | Mean | 7.26* | 13.69 | 40.44 | 55.79 | 18.91* | 33.92 | 1139.80 | 2.97 | 21.49 | 73.80 | 1.56 | 2.59 | 0.06 | |
| SD | 0.54 | 0.82 | 2.60 | 1.99 | 0.81 | 0.42 | 198.73 | 0.80 | 9.90 | 11.79 | 0.51 | 2.28 | 0.05 | ||
| G3 (2000 mg/kg) | Mean | 7.41 | 13.93 | 41.37 | 55.87 | 18.82 | 33.69 | 1046.40 | 3.81 | 16.09 | 79.68 | 1.31 | 2.17 | 0.06 | |
| SD | 0.24 | 0.49 | 1.51 | 1.34 | 0.42 | 0.33 | 175.07 | 1.22 | 4.70 | 4.73 | 0.33 | 1.24 | 0.05 | ||
| G4 (3000 mg/kg) | Mean | 7.23 | 13.70 | 40.36 | 55.78 | 18.96 | 33.95 | 899.40* | 3.42 | 20.34 | 76.15 | 1.30 | 1.61 | 0.06 | |
| SD | 0.30 | 0.53 | 1.70 | 0.70 | 0.33 | 0.51 | 315.66 | 0.87 | 11.73 | 11.83 | 0.30 | 0.81 | 0.07 | ||
| Recovery group | G5 (0 mg/kg) | Mean | 7.44 | 13.80 | 40.60 | 54.65 | 18.55 | 34.00 | 1180.00 | 3.75 | 12.75 | 83.90 | 1.55 | 1.23 | 0.10 |
| SD | 0.41 | 0.41 | 1.13 | 2.45 | 0.54 | 0.56 | 116.47 | 0.87 | 1.20 | 1.07 | 0.58 | 0.17 | 0.00 | ||
| G6 (3000 mg/kg) | Mean | 7.14 | 13.72 | 39.84 | 55.96 | 19.26 | 34.46 | 1182.60 | 2.51* | 14.70 | 81.96 | 1.38 | 1.52 | 0.04 | |
| SD | 0.49 | 0.56 | 1.19 | 2.60 | 0.55 | 0.70 | 144.45 | 0.40 | 4.82 | 5.12 | 0.37 | 0.23 | 0.05 | ||
Means significant differences at p < 0.05 compared with the control group.
3.3. Clinical biochemistry
In male rats, a significantly different increase in TP was observed at 1000 mg/kg compared with the controls (Table 5). After 28 days of treatment, male rats receiving 3000 mg/kg GB had no significant changes in the recovery period. Therefore, this test substance contains no toxicological qualities at this dose. In males, a statistically significant decrease in A/G, Na, and Cl was observed in the group with 2000 mg/kg, and decreased Crea, A/G, K in the 3000 mg/kg test group significantly. During the recovery period, male rats in the 3000 mg/kg group showed no significant differences in blood chemistry results. In female rats, statistically different increases in ALT, AST, and Na were observed in the 2000 mg/kg group, and ALT and Na in the 3000 mg/kg group when compared with the control in the main group (Table 6). However, during the recovery period, female rats in the 3000 mg/kg group had no significant differences in ALT, AST, and Na, although the Ca level significantly decreased. Decreases in A/G were observed in the 1000, and 2000 mg/kg groups and K in the 3000 mg/kg group during the recovery period.
Table 5.
Clinical chemistry of male rats treated with Gryllus bimaculatus.
| Group/dose (mg/kg) | Values | ALT (U/L) | AST (U/L) | ALP (U/L) | GGT (U/L) | LDH (U/L) | T-Bili (mg/dL) | BUN (mg/dL) | Crea (mg/dL) | TP (g/dL) | Alb (g/dL) | A/G ratio | T-Chol (mg/dL) | TG (mg/dL) | GLU (mg/dL) | Ca (mg/dL) | P (mg/dL) | Na (mmol/L) | K (mmol/L) | Cl (mmol/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maingroup | G1 (0 mg/kg) | Mean | 35.51 | 76.02 | 226.5 | 0.01 | 264.2 | 0.01 | 13.2 | 0.19 | 5.61 | 3.27 | 1.4 | 75.9 | 43 | 168.39 | 10.37 | 7.55 | 141.44 | 4.82 | 104.84 |
| SD | 3.36 | 3.59 | 36.62 | 0.02 | 87.37 | 0.01 | 1.19 | 0.03 | 0.21 | 0.09 | 0.1 | 22.54 | 15.2 | 17.42 | 0.36 | 0.26 | 1.96 | 0.24 | 2.07 | ||
| G2 (1000 mg/kg) | Mean | 36 | 76.78 | 208.2 | 0.01 | 337.9 | 0.01 | 12.81 | 0.17 | 5.86* | 3.32 | 1.32 | 77 | 42.6 | 161.69 | 10.34 | 7.23 | 139.91 | 4.64 | 103.38 | |
| SD | 5.88 | 14.18 | 39.26 | 0.01 | 190.03 | 0.01 | 1.49 | 0.04 | 0.22 | 0.18 | 0.17 | 13.24 | 24.64 | 20.92 | 0.51 | 0.52 | 2.99 | 0.43 | 2.31 | ||
| G3 (2000 mg/kg) | Mean | 32.64 | 69.81 | 215.6 | 0 | 224.5 | 0 | 12.24 | 0.16 | 5.77 | 3.24 | 1.28* | 83.2 | 39.3 | 168.82 | 10.15 | 7.19 | 138.86* | 4.6 | 102.67* | |
| SD | 4.12 | 8.86 | 40.4 | 0.01 | 77.18 | 0.01 | 1.69 | 0.03 | 0.14 | 0.1 | 0.05 | 13.47 | 9.87 | 17.3 | 0.2 | 0.52 | 1.66 | 0.23 | 1.06 | ||
| G4 (3000 mg/kg) | Mean | 33.87 | 76.53 | 240.6 | 0.03 | 256.8 | 0.01 | 12.69 | 0.14* | 5.78 | 3.23 | 1.27* | 82.6 | 38.8 | 169.33 | 10.24 | 7.73 | 139.9 | 4.51* | 103.83 | |
| SD | 3.84 | 8.43 | 41.1 | 0.08 | 104.07 | 0.01 | 1.44 | 0.04 | 0.32 | 0.13 | 0.09 | 18.03 | 24.33 | 23.85 | 0.32 | 0.87 | 1.53 | 0.31 | 1.18 | ||
| Recoverygroup | G5 (0 mg/kg) | Mean | 28.7 | 81.66 | 179.4 | 0.59 | 744.8 | 0.03 | 13.32 | 0.2 | 5.9 | 3.3 | 1.27 | 91 | 43.4 | 169.02 | 10.12 | 7.55 | 139.3 | 5 | 100.4 |
| SD | 2.46 | 8.54 | 31.6 | 0.00 | 245.56 | 0.02 | 0.8 | 0.04 | 0.21 | 0.16 | 0.1 | 16.17 | 12.74 | 20.91 | 0.23 | 0.88 | 2.32 | 0.36 | 2.31 | ||
| G6 (3000 mg/kg) | Mean | 31.76 | 106.54* | 186 | 0.00 | 1546.2 | 0.03 | 13.64 | 0.18 | 5.82 | 3.26 | 1.27 | 89.4 | 40 | 165.18 | 10.22 | 7.18 | 139.38 | 4.9 | 101.18 | |
| SD | 3.09 | 14.31 | 32.95 | 0.00 | 872.13 | 0.02 | 1.53 | 0.04 | 0.28 | 0.13 | 0.04 | 12.44 | 11.4 | 25.04 | 0.62 | 0.4 | 1.23 | 0.22 | 0.67 | ||
Means significant differences at p < 0.05 compared with the control group.
Table 6.
Clinical chemistry of female treated with Gryllus bimaculatus.
| Group/dose (mg/kg) | Values | ALT (U/L) | AST (U/L) | ALP (U/L) | GGT (U/L) | LDH (U/L) | T-Bili (mg/dL) | BUN (mg/dL) | Crea(mg/dL) | TP (g/dL) | Alb (g/dL) | A/G ratio | T-Cho l(mg/dL) | T G(mg/dL) | GL U(mg/dL) | C a(mg/dL) | P (mg/dL) | Na (mmol/L) | K (mmol/L) | Cl (mmol/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maingroup | G1 (0 mg/kg) | Mean | 28.81 | 64.44 | 111.50 | 0.02 | 215.50 | 0.03 | 15.67 | 0.28 | 6.87 | 3.95 | 1.36 | 97.60 | 34.60 | 154.29 | 10.61 | 6.30 | 138.28 | 4.10 | 103.36 |
| SD | 4.76 | 11.15 | 30.60 | 0.05 | 83.67 | 0.02 | 3.28 | 0.06 | 0.37 | 0.19 | 0.05 | 10.99 | 6.96 | 27.94 | 0.30 | 0.78 | 1.03 | 0.29 | 1.34 | ||
| G2 (1000 mg/kg) | Mean | 32.90 | 74.18 | 109.00 | 0.01 | 183.30 | 0.05 | 15.77 | 0.29 | 7.17 | 4.01 | 1.27* | 105.90 | 29.80 | 144.54 | 10.86 | 6.61 | 139.04 | 3.93 | 102.99 | |
| SD | 6.99 | 16.72 | 20.31 | 0.03 | 35.00 | 0.07 | 3.01 | 0.06 | 0.65 | 0.38 | 0.09 | 22.15 | 8.34 | 21.07 | 0.41 | 0.96 | 1.22 | 0.32 | 1.81 | ||
| G3 (2000 mg/kg) | Mean | 37.82* | 87.00* | 115.20 | 0.00 | 264.00 | 0.04 | 14.77 | 0.30 | 7.05 | 3.99 | 1.31* | 92.60 | 30.70 | 148.58 | 10.71 | 6.42 | 139.92* | 3.94 | 104.19 | |
| SD | 6.75 | 28.32 | 42.36 | 0.00 | 149.62 | 0.03 | 2.58 | 0.03 | 0.45 | 0.23 | 0.05 | 13.62 | 4.92 | 17.71 | 0.30 | 0.86 | 1.15 | 0.21 | 1.54 | ||
| G4 (3000 mg/kg) | Mean | 33.37* | 73.41 | 102.60 | 0.00 | 284.60 | 0.04 | 14.44 | 0.31 | 7.14 | 4.11 | 1.36 | 96.50 | 38.60 | 166.49 | 10.94 | 6.26 | 139.62* | 3.81* | 103.47 | |
| SD | 3.08 | 17.02 | 28.78 | 0.00 | 167.40 | 0.02 | 1.83 | 0.06 | 0.49 | 0.27 | 0.11 | 17.07 | 12.38 | 18.75 | 0.59 | 0.78 | 1.17 | 0.22 | 1.40 | ||
| Recoverygroup | G5 (0 mg/kg) | Mean | 34.94 | 89.40 | 65.00 | 0.00 | 285.40 | 0.09 | 14.24 | 0.32 | 7.60 | 4.28 | 1.29 | 115.40 | 35.80 | 159.76 | 11.30 | 5.78 | 138.76 | 4.02 | 101.20 |
| SD | 7.34 | 53.94 | 26.50 | 0.00 | 244.91 | 0.04 | 1.55 | 0.04 | 0.22 | 0.08 | 0.04 | 12.38 | 12.48 | 28.31 | 0.20 | 0.77 | 0.79 | 0.18 | 1.00 | ||
| G6 (3000 mg/kg) | Mean | 59.24 | 109.83 | 78.20 | 0.00 | 316.60 | 0.04 | 16.80 | 0.31 | 7.50 | 4.14 | 1.23 | 106.60 | 29.80 | 146.78 | 10.84* | 5.76 | 139.76 | 3.96 | 102.24 | |
| SD | 36.49 | 64.99 | 17.75 | 0.00 | 156.56 | 0.03 | 2.66 | 0.02 | 0.34 | 0.13 | 0.05 | 17.37 | 7.76 | 14.80 | 0.29 | 0.57 | 1.39 | 0.19 | 1.97 | ||
Means significant differences at p < 0.05 compared with the control group.
3.4. Organ weight
Necropsy revealed, no abnormal gross findings in either sex. Significantly, decreased spleen weights were observed for male rats in the 3000 mg/kg group (Table 3, Table 4).The absolute and relative organ weights in female rats were not significantly changed when compared with the control group. In the recovery period, no significant adverse changes in relative organ weights were observed for male and female rats.
Table 3.
Absolute organ weights of male and female rats treated with Gryllus bimaculatus.
| Sex | Group/dose (mg/kg) | Values | Heart | Lung | Spleen | Liver | Kidney | Adrenal | Testis | Epididymis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Main group | G1 (0 mg/kg) | Mean | 1.176 | 1.371 | 0.891 | 11.864 | 3.058 | 0.052 | 3.402 | 1.02 |
| SD | 0.134 | 0.135 | 0.087 | 1.031 | 0.295 | 0.009 | 0.383 | 0.09 | |||
| G2 (1000 mg/kg) | Mean | 1.114 | 1.336 | 0.831 | 12.254 | 3.07 | 0.076 | 3.446 | 1.047 | ||
| SD | 0.061 | 0.103 | 0.075 | 0.75 | 0.17 | 0.086 | 0.221 | 0.099 | |||
| G3 (2000 mg/kg) | Mean | 1.125 | 1.319 | 0.826 | 12.179 | 2.899 | 0.05 | 3.394 | 1.021 | ||
| SD | 0.084 | 0.089 | 0.081 | 1.179 | 0.261 | 0.008 | 0.411 | 0.094 | |||
| G4 (3000 mg/kg) | Mean | 1.154 | 1.349 | 0.747 * | 11.954 | 3.091 | 0.052 | 3.318 | 0.961 | ||
| SD | 0.07 | 0.066 | 0.068 | 1.012 | 0.173 | 0.01 | 0.356 | 0.084 | |||
| Recovery group | G5 (0 mg/kg) | Mean | 1.277 | 1.435 | 0.891 | 13.922 | 3.115 | 0.09 | 3.549 | 1.239 | |
| SD | 0.111 | 0.049 | 0.109 | 1.79 | 0.269 | 0.082 | 0.216 | 0.168 | |||
| G6 (3000 mg/kg) | Mean | 1.173 | 1.385 | 0.818 | 12.54 | 3.118 | 0.05 | 3.512 | 1.177 | ||
| SD | 0.073 | 0.043 | 0.115 | 0.834 | 0.07 | 0.004 | 0.34 | 0.088 | |||
| Heart | Lung | Spleen | Liver | Kidney | Adrenal | Ovary | Uterus | ||||
| Female | Main group | G1 (0 mg/kg) | Mean | 0.705 | 1.038 | 0.498 | 7.066 | 1.748 | 0.096 | 0.067 | 0.525 |
| SD | 0.08 | 0.082 | 0.088 | 0.93 | 0.121 | 0.105 | 0.021 | 0.123 | |||
| G2 (1000 mg/kg) | Mean | 0.64 | 1.024 | 0.562 | 7.189 | 1.823 | 0.064 | 0.065 | 0.525 | ||
| SD | 0.149 | 0.053 | 0.205 | 0.798 | 0.134 | 0.007 | 0.013 | 0.086 | |||
| G3 (2000 mg/kg) | Mean | 0.693 | 1.019 | 0.485 | 7.061 | 1.73 | 0.062 | 0.059 | 0.517 | ||
| SD | 0.051 | 0.082 | 0.068 | 0.598 | 0.148 | 0.007 | 0.011 | 0.052 | |||
| G4 (3000 mg/kg) | Mean | 0.727 | 1.002 | 0.468 | 7.39 | 1.847 | 0.065 | 0.06 | 0.556 | ||
| SD | 0.078 | 0.056 | 0.059 | 0.702 | 0.204 | 0.007 | 0.013 | 0.039 | |||
| Recovery group | G5 (0 mg/kg) | Mean | 0.792 | 1.102 | 0.524 | 8.317 | 1.971 | 0.072 | 0.051 | 0.548 | |
| SD | 0.057 | 0.044 | 0.028 | 1.073 | 0.18 | 0.009 | 0.009 | 0.06 | |||
| G6 (3000 mg/kg) | Mean | 0.755 | 1.055 | 0.546 | 7.618 | 1.904 | 0.074 | 0.05 | 0.601 | ||
| SD | 0.041 | 0.045 | 0.137 | 0.396 | 0.111 | 0.013 | 0.006 | 0.105 | |||
Means significant differences at p < 0.05 compared with the control group.
Table 4.
Relative organ weight of male and female rats treated with Gryllus bimaculatus.
| Sex | Group/dose (mg/kg) | Values | Heart | Lung | Spleen | Liver | Kidney | Adrenal | Testis | Epididymis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Main group | G1 (0 mg/kg) | Mean | 0.283 | 0.329 | 0.214 | 2.851 | 0.735 | 0.012 | 0.818 | 0.245 |
| SD | 0.029 | 0.026 | 0.02 | 0.186 | 0.062 | 0.002 | 0.085 | 0.021 | |||
| G2 (1000 mg/kg) | Mean | 0.265 | 0.318 | 0.198 | 2.913 | 0.73 | 0.018 | 0.82 | 0.249 | ||
| SD | 0.014 | 0.021 | 0.018 | 0.154 | 0.038 | 0.021 | 0.053 | 0.028 | |||
| G3 (2000 mg/kg) | Mean | 0.272 | 0.319 | 0.2 | 2.945 | 0.701 | 0.012 | 0.821 | 0.247 | ||
| SD | 0.016 | 0.014 | 0.014 | 0.229 | 0.045 | 0.002 | 0.087 | 0.022 | |||
| G4 (3000 mg/kg) | Mean | 0.282 | 0.329 | 0.182 * | 2.913 | 0.754 | 0.013 | 0.811 | 0.235 | ||
| SD | 0.017 | 0.015 | 0.015 | 0.19 | 0.039 | 0.002 | 0.097 | 0.023 | |||
| Recovery group | G5 (0 mg/kg) | Mean | 0.272 | 0.306 | 0.19 | 2.967 | 0.334 | 0.014 | 0.379 | 0.13 | |
| SD | 0.019 | 0.01 | 0.019 | 0.342 | 0.019 | 0.018 | 0.029 | 0.015 | |||
| G6 (3000 mg/kg) | Mean | 0.263 | 0.31 | 0.183 | 2.804 | 0.345 | 0.006 | 0.392 | 0.13 | ||
| SD | 0.02 | 0.012 | 0.023 | 0.149 | 0.011 | 0.001 | 0.04 | 0.012 | |||
| Heart | Lung | Spleen | Liver | Kidney | Adrenal | Ovary | Uterus | ||||
| Female | Main group | G1 (0 mg/kg) | Mean | 0.297 | 0.438 | 0.209 | 2.967 | 0.739 | 0.039 | 0.028 | 0.222 |
| SD | 0.02 | 0.023 | 0.027 | 0.224 | 0.067 | 0.04 | 0.007 | 0.057 | |||
| G2 (1000 mg/kg) | Mean | 0.274 | 0.439 | 0.241 | 3.08 | 0.782 | 0.027 | 0.028 | 0.225 | ||
| SD | 0.06 | 0.02 | 0.088 | 0.294 | 0.051 | 0.003 | 0.006 | 0.039 | |||
| G3 (2000 mg/kg) | Mean | 0.302 | 0.444 | 0.211 | 3.069 | 0.752 | 0.027 | 0.026 | 0.225 | ||
| SD | 0.022 | 0.035 | 0.03 | 0.182 | 0.045 | 0.003 | 0.004 | 0.022 | |||
| G4 (3000 mg/kg) | Mean | 0.309 | 0.428 | 0.2 | 3.143 | 0.784 | 0.028 | 0.026 | 0.238 | ||
| SD | 0.027 | 0.044 | 0.036 | 0.3 | 0.071 | 0.003 | 0.006 | 0.033 | |||
| Recovery group | G5 (0 mg/kg) | Mean | 0.304 | 0.424 | 0.201 | 3.187 | 0.379 | 0.014 | 0.009 | 0.21 | |
| SD | 0.019 | 0.019 | 0.005 | 0.305 | 0.013 | 0.001 | 0.002 | 0.021 | |||
| G6 (3000 mg/kg) | Mean | 0.299 | 0.417 | 0.217 | 3.011 | 0.369 | 0.015 | 0.01 | 0.237 | ||
| SD | 0.017 | 0.028 | 0.059 | 0.154 | 0.032 | 0.002 | 0.002 | 0.035 | |||
Means significant differences at p < 0.05 compared with the control group.
4. Discussion
Recently, interest has grown in using G. bimaculatus as an alternative food source. However, insufficient safety studies have been conducted for insects. The safety of G. bimaculatus has been reported in acute and chronic toxicological tests. A slight acute and chronic toxicity was found with an oral NOAEL value of > 5000 mg/kg. However, these previous studies evaluated dried material or extracts with ethanol or water/methanol or and did not include extracts performed with only water [[11], [12], [13]]. Water extract of G. bimaculatus has been reported to have a protective effect against alcoholic liver damage by attenuating inflammatory responses and apoptosis [15]. However, there is no report on the safety of G. bimaculatus extracts with water. We therefore, evaluated the safety and toxicity of G. bimaculatus extracted using only water.
No mortality occurred during administration and recovery periods for all G. bimaculatus groups. Body weight changes in both sexes were not significant at doses of 0, 1000, 2000, and 3000 mg/kg during the administration and recovery periods. Significant changes in food consumption were observed, but these differences were not correlated with body weight changes. Therefore, these changes were not attributed to G. bimaculatus. The PLT and MONO of hematological results were significantly increased when compared to control in main group, but they were within the normal range (836–1102 103/μL, 0.6 %–3.1 %) [16]. Changes in relative spleen weight did not show a dose-dependent decrease. Serum chemistry changes were associated with biomarkers for immunological reduction, with no significant change in recovery group. Therefore, the hematological and organ weight results showed no treatment-related adverse changes. However, serum chemistry during treatment was significantly increased when compared with the control in the main groups. In Table 5, A/G ratio and the levels of K were also within the normal ranges (1.1–3.4, 4.3–7.3 mEq/L), and no significant changes occurred in the recovery group [17]. The Crea level was significantly decreased, but BUN, at creatinine-related biomarker, was not significantly different in the male 3000 mg/kg main group. AST, ALT, TP, and Na levels were within normal ranges [17]. ALT increased significantly in the female 3000 mg/kg main group, but it was within the normal range (24.1–113.9 IU/L) [18]. In addition, during the recovery period, no significant change in ALT was observed in the 3000 mg/kg treated group. Therefore, the serum chemistry was determined to be unrelated to test substances.
In summary, G. bimaculatus at an oral dose of 3000 mg/kg produced no adverse effects during the study period. Therefore, the NOAEL of G. bimaculatus extracted with water is higher than 3000 mg/kg in rats.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food,Agriculture Forestry (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (2017-303) in Korea.
References
- 1.FAO . Roam.; 2017. The Future of Food and Agriculture: Trends and Challenges. [DOI] [Google Scholar]
- 2.Rumpold B.A., Schlüter O. Insect-based protein sources and their potential for human consumption : nutritional composition and processing. Anim. Front. 2015;5:20–24. doi: 10.2527/af.2015-0015. [DOI] [Google Scholar]
- 3.Rumpold B.A., Schlüter O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013;57:802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- 4.Ahn M.Y., Kim M.J., Kwon R.H., Hwang J.S., Park K.K. Gene expression profiling and inhibition of adipose tissue accumulation of G. bimaculatus extract in rats on high fat diet. Lipids Health Dis. 2015;14:1–15. doi: 10.1186/s12944-015-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura T., Mito T., Bando T., Ohuchi H., Noji S. Dissecting insect leg regeneration through RNA interference. Cell. Mol. Life Sci. 2008;65:64–72. doi: 10.1007/s00018-007-7432-0. https://www.researchgate.net/profile/Sumihare_Noji/publication/5817830_Dissecting_insect_leg_regeneration_through_RNA_interference/links/5485a2500cf283750c37305e/Dissecting-insect-leg-regeneration-through-RNA-interference.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T., Noji S., Mito T. Genome Editing in Animals. Springer; 2017. Genome editing in the cricket, Gryllus bimaculatus; pp. 219–233. https://www.researchgate.net/profile/Sumihare_Noji/publication/5817830_Dissecting_insect_leg_regeneration_through_RNA_interference/links/5485a2500cf283750c37305e/Dissecting-insect-leg-regeneration-through-RNA-interference.pdf. [DOI] [PubMed] [Google Scholar]
- 7.Ahn M.Y., Hwang J.S., Yun E.Y., Kim M.J., Park K.K. Anti-aging effect and gene expression profiling of aged rats treated with G. bimaculatus extract. Toxicol. Res. 2015;31:173–180. doi: 10.5487/TR.2015.31.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn M.Y., Han J.W., Hwang J.S., Yun E.Y., Lee B.M. Anti-inflammatory effect of glycosaminoglycan derived from gryllus bimaculatus (A type of cricket, insect) on adjuvant-treated chronic arthritis rat model. J. Toxicol. Environ. Heal. - Part A Curr. Issues. 2014;77:1332–1345. doi: 10.1080/15287394.2014.951591. [DOI] [PubMed] [Google Scholar]
- 9.Taufek N.M., Aspani F., Muin H., Raji A.A., Razak S.A., Alias Z. The effect of dietary cricket meal (Gryllus bimaculatus) on growth performance, antioxidant enzyme activities, and haematological response of African catfish (Clarias gariepinus) Fish Physiol. Biochem. 2016;42:1143–1155. doi: 10.1007/s10695-016-0204-8. [DOI] [PubMed] [Google Scholar]
- 10.Boonsin P., Boonyarat C., Plekartoke K., Reubroycharoen P., Waiwut 2018. http://www.scisoc.or.th/BMBThailand/images/BMB2018/S1-P-28.pdf
- 11.Ahn M.Y., Han J.W., Kim S.J., Hwang J.S., Yun E.Y. Thirteen-week oral dose toxicity study of G. Bimaculatus in sprague-dawley rats. Toxicol. Res. 2011;27:231–240. doi: 10.5487/TR.2011.27.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu H.Y., Lee S., Ahn K.S., Kim H.J., Lee S.S., Ko H.J., Lee J.K., Cho M.H., Ahn M.Y., Kim E.M., Lim J.H., Song K.S. Oral toxicity study and skin sensitization test of a cricket. Toxicol. Res. 2016;32:159–173. doi: 10.5487/TR.2016.32.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y., Lee S.M., Lee H.S., Lee K.H. Safety evaluation of cricket(Gryllus bimaculatus) extract in Sprague-Dawley rats. 2016;32:203–208. doi: 10.7852/ijie.2016.32.1.12. [DOI] [Google Scholar]
- 14.Toyoda K., Shibutani M., Tamura T., Koujitani T., Uneyama C., Hirose M. Repeated dose (28 days) oral toxicity study of flutamide in rats, based on the draft protocol for the `Enhanced OECD Test Guideline 407’ for screening for endocrine-disrupting chemicals. Arch. Toxicol. 2000;74:127–132. doi: 10.1007/s002040050664. [DOI] [PubMed] [Google Scholar]
- 15.Hwang B.B., Chang M.H., Lee J.H., Heo W., Kim J.K., Pan J.H., Kim Y.J., Kim J.H. The edible insect Gryllus bimaculatus protects against gut-derived inflammatory responses and liver damage in mice after acute alcohol exposure. Nutrients. 2019;11:857. doi: 10.3390/nu11040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwaki S., Nanri H., Morikawa M., Ishida M., Kato T., Takata M.S.K., Katsuki S. Background data of Crj: CD (SD) IGS rats dosed with distilled water orally for 4, 13 and 26 weeks. Biol. Ref. data CD IGS rats. 1998:64–70. [Google Scholar]
- 17.Marshall M.V., Draney D., Sevick-Muraca E.M., Olive D.M. Single-dose intravenous toxicity study of IRDye 800CW in sprague-dawley Rats. Mol. Imaging Biol. 2010;12:583–594. doi: 10.1007/s11307-010-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox J.G. Elsevier; 2015. Laboratory Animal Medicine. https://doi.org/https://books.google.co.kr/books?hl=en&lr=&id=3NGcBAAAQBAJ&oi=fnd&pg=PP1&dq=Laboratory+animal+medicine&ots=bm0wf7D38i&sig=383pJQQvwuUhzfqH1EWxYBGiXsc&redir_esc=y#v=onepage&q=Laboratory animal medicine&f=false. [Google Scholar]