Abstract
Background
Childhood cancer survivors exposed to abdominal radiation (abdRT) are at increased risk for diabetes mellitus, but the association between risk and radiation dose and volume is unclear.
Methods
Participants included 20 762 5-year survivors of childhood cancer (4568 exposed to abdRT) and 4853 siblings. For abdRT, we estimated maximum dose to abdomen; mean doses for whole pancreas, pancreatic head, body, tail; and percent pancreas volume receiving no less than 10, 20, and 30 Gy. Relative risks (RRs) were estimated with a Poisson model using generalized estimating equations, adjusted for attained age. All statistical tests were two-sided.
Results
Survivors exposed to abdRT (median age = 31.6 years, range = 10.2–58.3 years) were 2.92-fold more likely than siblings (95% confidence interval [CI] = 2.02 to 4.23) and 1.60-times more likely than survivors not exposed to abdRT (95%CI = 1.24 to 2.05) to develop diabetes. Among survivors treated with abdRT, greater attained age (RRper 10 years = 2.11, 95% CI = 1.70 to 2.62), higher body mass index (RRBMI 30+ = 5.00, 95% CI = 3.19 to 7.83 with referenceBMI 18.5–24.9), and increasing pancreatic tail dose were associated with increased diabetes risk in a multivariable model; an interaction was identified between younger age at cancer diagnosis and pancreatic tail dose with much higher diabetes risk associated with increasing pancreatic tail dose among those diagnosed at the youngest ages (P < .001). Radiation dose and volume to other regions of the pancreas were not statistically significantly associated with risk.
Conclusions
Among survivors treated with abdRT, diabetes risk was associated with higher pancreatic tail dose, especially at younger ages. Targeted interventions are needed to improve cardiometabolic health among those at highest risk.
Because of improved therapies and supportive care for childhood cancer, 5-year survival rates now exceed 80% (1). By 2020, it is estimated that more than 500 000 survivors of childhood cancer will live in the United States (2). Improved survival, however, has led to increased recognition of survivors’ excess risk of morbidity and mortality due to treatment-related late effects (3–6). By 50 years of age, the cumulative incidence of chronic health conditions of any grade among adult survivors of childhood cancer is 99.9% (5). Survivors are at risk for a wide range of chronic conditions, including diabetes mellitus (herein, referred to as diabetes) (7,8), which subsequently increases risk for heart failure after cardiotoxic therapy in survivors (9) and, in the general population, is independently associated with increased cardiovascular and all-cause mortality (10–12). Although many chronic conditions impact survivors shortly after therapy, others, including diabetes, may not become apparent until survivors mature into adulthood.
Recent reports have noted a nearly twofold increased risk of diabetes in childhood cancer survivors with risk increasing over time (7); those exposed to abdominal radiation are at even higher risk (8,13,14). Large cohort studies have demonstrated a dose-response relationship between diabetes risk and radiation dose to the pancreatic tail, where insulin-producing β cells reside (13,14). The exact nature of this relationship, however, is contested; one large study of childhood cancer survivors demonstrated a linear dose-response relationship through doses of 20–29 Gy with subsequent plateau in risk (13), whereas another study suggested that risk continues to rise with increasing mean dose to the pancreatic tail (14). In the latter study, risk of diabetes was highest among survivors exposed to doses greater than or equal to 36 Gy to the para-aortic nodes and spleen; the authors also suggested that radiation volume, or area of the pancreas exposed to radiation, might play a role in mediating risk, but this observation has not been formally assessed.
The current analysis sought to fill this knowledge gap by investigating the association between pancreatic radiation dose-volume characteristics and risk of diabetes among childhood cancer survivors enrolled in the Childhood Cancer Survivor Study (CCSS). Additionally, we aimed to identify risk factors for the development of diabetes in the subcohort of survivors treated with abdominal radiation in an effort to lay the groundwork for risk prediction models for diabetes development after cancer therapy.
Methods
Participants
Participants include survivors enrolled in CCSS, a multi-institutional, hospital-based cohort of childhood cancer survivors with longitudinal follow-up. Eligible survivors were diagnosed with cancer between January 1, 1970, and December 31, 1999, prior to 21 years of age at 1 of 27 North American institutions and alive at least 5 years after diagnosis. The cohort methodology and study design have been described previously in detail (15,16). The protocol was approved by each of the institutional review boards at participating institutions. Participants provided informed consent prior to study participation. Participants completed a baseline survey and up to four follow-up questionnaires; questionnaires are publicly available at https://ccss.stjude.org/tools-and-documents/questionnaires.html.
For this analysis, 20 762 childhood cancer survivors were included. Any survivor also exposed to total body irradiation, an exposure associated with risk of diabetes independent of abdominal radiation (8), was excluded. A random sample of siblings of CCSS participants was selected as a comparison population and completed identical surveys. The sibling response rate was 83% for the baseline surveys.
Cancer Treatment Information
Using standardized CCSS protocols, data regarding cancer diagnosis and treatment exposures, including chemotherapy and radiotherapy, were abstracted from the medical records of all participants who provided authorization (16,17). Radiation records were centrally reviewed, and several dose-metrics were determined. The maximum tumor dose to the abdomen was determined by summing the prescribed dose from all overlapping fields within the abdomen. Pancreas-specific dose-metrics were calculated as well; each participant’s radiotherapy fields were reconstructed on a computational phantom scaled to age at time of radiotherapy. Within the phantom, the pancreas was modeled by a grid of 129 evenly spaced points, which was subdivided into the head, body, and tail substructures. For each participant’s reconstructed radiotherapy, dose to each of the 129 points was calculated. These data were used to estimate 1) mean dose to the whole pancreas, head, body, tail and 2) volume of the pancreas that received no less than 10 (V10), 20 (V20), or 30 Gy (V30) radiation (18).
Variables and Outcome of Interest
The primary outcome of interest was a self-reported history of diabetes requiring use of oral glucose-lowering agents or insulin; once individuals met these criteria, they were assumed to have the outcome in all subsequent surveys. Body mass index (BMI) was calculated from heights and weights that were self-reported by survivors and siblings at each time point when surveys were completed, using the standard formula of [weight(kilogram [kg])/(height[meters (m)])2. Obesity was defined as BMI no less than 30 kg/m2; overweight as BMI 25–29.9 kg/m2; normal weight as BMI 18.5–24.9 kg/m2; and underweight as BMI less than 18.5 kg/m2 (19,20). In addition to the baseline questionnaire, participants completed follow-up questionnaires that included information about self-reported chronic conditions and medication use, including those related to diabetes.
Statistical Analysis
Continuous variables were expressed as mean (standard deviation) or median (range). The χ2 and Wilcoxon rank sum tests were used to assess differences in covariate values between groups.
Point estimates of prevalence with 95% confidence intervals (CIs) were calculated for cross-sectional observations with repeated measurements across follow-up surveys. Cumulative incidence was not calculated because the age at onset of diabetes requiring medication was not known. We included 22 survivors for whom the data suggest diabetes may have been diagnosed prior to the primary childhood cancer diagnosis in our estimates of prevalence, in order to have comparable prevalence estimates between the survivors and siblings. Using a generalized estimating equation model assuming a Poisson distribution with a log-link function, and adjusted for attained age, relative risks comparing the prevalence of diabetes among those exposed to abdominal radiation to 1) siblings and 2) survivors without a history of abdominal radiation were estimated. Relative risk (RR) and 95% confidence interval were estimated, and P values were obtained using the Wald test.
Risk factors for diabetes among those receiving abdominal radiation were assessed using the same modeling method. The final multivariable model was built using a forward selection procedure, with all univariable risk factors with P value less than .2 considered candidates for the multivariable model. The final multivariable model included attained age (10-year increments), BMI status, mean pancreatic tail dose, and the interaction between age at diagnosis (10-year increments) and mean pancreatic tail dose. A plot showing the estimated relative risk by age at diagnosis for each radiation dose group was constructed using the linear predictors from the model to estimate the relative risk of diabetes for each radiation dose group by age, with age treated as a continuous variable. Residual plots were used to evaluate model fit.
All analyses were complete-case analyses and incorporated a weighting adjustment applied to each participant using inverse probability weighting to account for undersampling of survivors of acute lymphoblastic leukemia diagnosed from 1987 to 1999. Analyses were performed using R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
Analyses were also carried out using a second model, the linear odds ratio model (21), where the odds of diabetes were modeled as a function of dose using the form e(β0)(1+ β1Dose). Dose was modeled as a continuous variable. Departures from a linear dose-response relationship were explored by fitting an equation quadratic in dose. Departures from a constant relative risk on an additive scale were explored by fitting interaction terms looking at possible modifying effects of other factors. Beta estimates (β) and 95% confidence intervals were presented, and P values were obtained using the Wald test. This analysis was conducted in SAS for Windows version 9.4 (SAS Institute, INC., Cary, NC, USA).
A P value less than .05 was considered statistically significant, and two-sided tests of hypotheses were used throughout.
Results
Participants
A total of 20 762 childhood cancer survivors were included in this study: 4568 individuals exposed to abdominal radiation and 16 194 without exposure to abdominal radiation. These individuals were compared to 4853 siblings. Demographics of the cohort are described in Table 1. Treatment characteristics of survivors are provided in Table 2.
Table 1.
Characteristics of survivors of childhood cancer overall and by abdominal radiation exposure and siblings*
| Characteristic | Survivors exposed to abdominal RT | Survivors not exposed to abdominal RT | Siblings(n = 4853) | P† | P‡ |
|---|---|---|---|---|---|
| (n = 4568) | (n = 16 194) | ||||
| Age at cancer diagnosis, y | |||||
| Median (range) | 7.4 (0.0–21.0) | 6.6 (0.0–21.0) | N/A | <.001 | N/A |
| Mean (SD) | 8.8 (5.9) | 7.9 (5.7) | N/A | ||
| Attained age, y | <.001 | <.001 | |||
| Median (range) | 31.6 (10.2–58.3) | 28.6 (8.7–57.9) | 32.7 (0.3–60.3) | — | — |
| Mean (SD) | 31.9 (9.3) | 29.2 (8.5) | 33.0 (9.8) | — | — |
| Follow-up time, y§ | |||||
| Median (range) | 17.7 (2.3–33.9) | 16.3 (2.0–34.2) | N/A | — | — |
| Mean (SD) | 18.2 (7.1) | 16.3 (6.4) | N/A | — | — |
| Race/ethnicity | .051 | <.001 | |||
| White, non-Hispanic | 3738 (81.7) | 13 000 (79.5) | 4190 (86.3) | — | — |
| Black, non-Hispanic | 272 (5.9) | 881 (5.7) | 146 (3.0) | — | — |
| Hispanic/Latino | 116 (2.7) | 547 (3.5) | 90 (1.9) | — | — |
| Other | 334 (7.2) | 1220 (7.3) | 228 (4.7) | — | — |
| Missing | 108 (2.5) | 546 (4.1) | 199 (4.1) | — | — |
| Sex | .55 | <.001 | |||
| Female | 2137 (46.4) | 7614 (47.0) | 2529 (52.1) | — | — |
| Male | 2431 (53.6) | 8580 (53.0) | 2318 (47.8) | — | — |
| Missing | 0 (0.0) | 0 (0.0) | 6 (0.1) | — | — |
| Cancer diagnosis | <.001 | N/A | |||
| Central nervous system tumor | 998 (21.3) | 2869 (14.9) | N/A | — | — |
| Hodgkin lymphoma | 1299 (27.7) | 1390 (7.2) | N/A | — | — |
| Non-Hodgkin lymphoma | 197 (4.2) | 1496 (7.8) | N/A | — | — |
| Leukemia | 501 (13.3) | 5693 (45.3) | N/A | — | — |
| Wilms tumor | 1030 (22.0) | 880 (4.6) | N/A | — | — |
| Neuroblastoma | 333 (7.1) | 1253 (6.5) | N/A | — | — |
| Soft tissue sarcoma | 126 (2.7) | 919 (4.8) | N/A | — | — |
| Bone cancer | 84 (1.8) | 1694 (8.8) | N/A | — | — |
| Body mass index, kg/m2 | <.001 | <.001 | |||
| <18.5 | 547 (11.9) | 1393 (7.9) | 331 (6.8) | — | — |
| 18.5–24.9 | 2242 (48.6) | 7735 (47.3) | 2448 (50.4) | — | — |
| 25.0–29.9 | 989 (21.8) | 3744 (23.3) | 1205 (24.8) | — | — |
| ≥30 | 527 (11.7) | 2424 (15.9) | 716 (14.8) | — | — |
| Missing | 263 (6.0) | 898 (5.6) | 153 (3.2) | — | — |
Counts represent the actual observed number of observations present in the data, and the percentages and P values incorporate the individual sampling weights for survivors, with siblings assumed to have sampling weight of 1. N/A = not applicable; RT = radiation therapy; SD = standard deviation.
Comparison of abdominal RT and no abdominal RT was done using the χ2 test for categorical variables or the Wilcoxon rank sum test for continuous variables; all P values were two-sided.
Comparison of abdominal RT and siblings was performed using the χ2 test for categorical variables or the Wilcoxon rank sum test for continuous variables; all P values were two-sided.
Duration of follow-up starts at 5 years after diagnosis.
Table 2.
Treatment characteristics of the survivors of childhood cancer by abdominal radiation exposure (n = 20 762)
| Treatment exposure | Survivors exposed to abdominal RT | Survivors not exposed to abdominal RT | P * |
|---|---|---|---|
| (n = 4568) | (n = 16 194) | ||
| Corticosteroids | <.001 | ||
| No | 2946 (63.0) | 8090 (43.1) | — |
| Yes | 1240 (28.7) | 7237 (52.3) | — |
| Missing | 382 (8.3) | 867 (4.6) | — |
| Anthracyclines | <.001 | ||
| No | 2745 (58.6) | 8089 (44.9) | — |
| Yes | 1736 (39.4) | 7941 (54.2) | — |
| Missing | 87 (2.0) | 164 (0.9) | — |
| Alkylating agents | .17 | ||
| No | 2008 (43.5) | 7441 (45.4) | — |
| Yes | 2448 (54.0) | 8571 (53.6) | — |
| Missing | 112 (2.5) | 182 (1.0) | — |
| Cranial radiation | <.001 | ||
| No | 3002 (64.1) | 11 804 (74.2) | — |
| Yes | 1561 (35.8) | 4308 (25.3) | — |
| Missing | 5 (0.1) | 82 (0.5) | — |
| Radiation dose (Gy)† | |||
| Abdomen (maxTD) | |||
| 0.1–9.9 | 128 (3.4) | N/A | — |
| 10.0–19.9 | 982 (22.8) | N/A | — |
| 20.0–29.9 | 1 324 (28.2) | N/A | — |
| ≥30.0 | 2107 (44.9) | N/A | — |
| Missing | 27 (0.6) | N/A | — |
| Whole pancreas (mean dose) | |||
| 0.1–9.9 | 660 (16.3) | N/A | — |
| 10.0–19.9 | 1827 (39.2) | N/A | — |
| 20.0–29.9 | 1220 (26.0) | N/A | — |
| ≥30.0 | 821 (17.5) | N/A | — |
| Missing | 40 (0.9) | N/A | — |
| Head of pancreas (mean dose) | |||
| 0.1–9.9 | 492 (11.8) | N/A | — |
| 10.0–19.9 | 1480 (32.8) | N/A | — |
| 20.0–29.9 | 1369 (29.2) | N/A | — |
| ≥30.0 | 1187 (25.3) | N/A | — |
| Missing | 40 (0.9) | N/A | — |
| Body of pancreas (mean dose) | |||
| 0.1–9.9 | 552 (13.1) | N/A | — |
| 10.0–19.9 | 1408 (31.2) | N/A | — |
| 20.0–29.9 | 1363 (29.1) | N/A | — |
| ≥30.0 | 1205 (25.7) | N/A | — |
| Missing | 40 (0.9) | N/A | — |
| Tail of pancreas (mean dose) | |||
| 0.1–9.9 | 2883 (64.0) | N/A | — |
| 10.0–19.9 | 1177 (25.1) | N/A | — |
| 20.0–29.9 | 324 (6.9) | N/A | — |
| ≥30.0 | 144 (3.1) | N/A | — |
| Missing | 40 (0.9) | N/A | — |
| Whole pancreas radiation dose-volume metrics (% volume)‡ | |||
| V10 (≥10 Gy) to: | |||
| 0% of the pancreas | 211 (5.2) | N/A | — |
| 0.1–75.0% | 1908 (42.5) | N/A | — |
| 75.1–100.0% | 2409 (51.4) | N/A | — |
| Missing | 40 (0.9) | N/A | — |
| V20 (≥20 Gy) to: | |||
| 0% of the pancreas | 1317 (30.6) | N/A | — |
| 0.1–75.0% | 1627 (34.7) | N/A | — |
| 75.1–100.0% | 1584 (33.8) | N/A | — |
| Missing | 40 (0.9) | N/A | — |
| V30 (≥30 Gy) to: | |||
| 0% of the pancreas | 2745 (61.1) | N/A | — |
| 0.1–75.0% | 817 (17.4) | N/A | — |
| 75.1–100.0% | 966 (20.6) | N/A | — |
| Missing | 40 (0.9) | N/A | — |
Comparison between groups was performed using the χ2 test; all P values were two-sided. N/A = not applicable; RT = radiation therapy.
Radiation therapy dose refers to the maximum tumor dose (maxTD) from summing all of the overlapping RT fields prescribed to the abdomen and the mean dose to the whole pancreas and head, body, and tail of the pancreas.
Radiation dose-volume metrics refer to the percent of the whole pancreas that received ≥10 Gy (V10), ≥20Gy (V20), or ≥30 Gy (V30) from RT.
Risk of Diabetes Among Childhood Cancer Survivors and Siblings
Overall, 389 cases of diabetes were identified among 20 762 survivors and 53 cases in 4853 siblings (data not shown). Among 4568 survivors treated with abdominal radiation, 137 (2.3%; 95% CI = 1.9% to 2.8%) reported diabetes at a median age of 30 years (range = 3–53 years); 66 (48.2%) individuals reported use of oral hypoglycemic agents, 69 (50.4%) reported history of insulin use, 2 (1.5%) individuals reported use of both oral agents and insulin, and 3 died from diabetes-related causes. In contrast, 252 (1.2%; 95% CI = 1.0% to 1.3%) of 16 194 survivors without a history of abdominal radiation reported diabetes at a median age of 28 years (range = 0–54); 122 (48.4%) reported use of oral hypoglycemic medications, 126 (50.0%) reported use of insulin, and 8 died from diabetes-related causes. Among siblings, 53 (0.8%; 95% CI = 0.6% to 1.1%) reported diabetes at a median age of 34 years (range = 4–53); 28 (52.8%) were on oral hypoglycemic agents, 24 (45.3%) were on insulin, and 1 (1.9%) individual was on both an oral agent and insulin.
Among both survivors and siblings, diabetes prevalence increased with older attained age. For participants between the ages of 21 and 30 years, the prevalence of diabetes was 1.3% (95% CI = 1.0% to 1.9%) for survivors exposed to abdominal radiation, 0.8% (95% CI = 0.6% to 1.0%) for survivors without a history of abdominal radiation, and 0.4% (95% CI = 0.2% to 0.8%) for siblings (data not shown). In contrast, among individuals older than 40 years of age, the prevalence of diabetes was 4.6% (95% CI = 3.3% to 6.4%) for survivors exposed to abdominal radiation, 3.1% (95% CI = 2.3% to 4.1%) for survivors not so exposed, and 2.1% (95% CI = 1.4 to 3.2%) for siblings.
Survivors exposed to abdominal radiation (median age = 31.6 years, range = 10.2–58.3) were 2.92-fold more likely than siblings (95% CI = 2.02 to 4.23) and 1.60 times more likely than survivors not exposed to abdominal radiation (95% CI = 1.24 to 2.05) to develop diabetes. Table 3 summarizes the relative risks of diabetes adjusted for attained age and attained age and BMI for the whole cohort and for individual diagnostic groups. Survivors of neuroblastoma, Hodgkin lymphoma, Wilms tumor, and CNS tumors treated with abdominal radiation were at increased risk of diabetes, when compared to siblings. There was no statistically significant increased risk of diabetes in survivors without a history of abdominal radiation in each of the diagnostic groups listed above, relative to siblings. These results remained unchanged after further adjusting these analyses for race and/or ethnicity and sex (Supplementary Table 1, available online).
Table 3.
Adjusted relative risks of diabetes among childhood cancer survivors, by primary cancer diagnosis
| Diagnosis | Not adjusted for BMI |
Adjusted for BMI |
||
|---|---|---|---|---|
| Relative risk* (95% CI) | P † | Relative risk* (95% CI) | P † | |
| All patients | ||||
| Siblings | Referent | — | Referent | — |
| No abdominal RT | 1.83 (1.29 to 2.60) | .001 | 1.83 (1.29 to 2.60) | <.001 |
| Abdominal RT | 2.92 (2.02 to 4.23) | <.001 | 3.40 (2.33 to 4.95) | <.001 |
| Hodgkin lymphoma | ||||
| Siblings | Referent | — | Referent | — |
| No abdominal RT | 1.37 (0.76 to 2.47) | .30 | 1.51 (0.84 to 2.74) | .17 |
| Abdominal RT | 2.13 (1.33 to 3.42) | .002 | 2.83 (1.75 to 4.57) | <.001 |
| Neuroblastoma | ||||
| Siblings | Referent | — | Referent | — |
| No abdominal RT | 1.45 (0.62 to 3.38) | .39 | 1.48 (0.65 to 3.40) | .35 |
| Abdominal RT | 8.51 (4.27 to 16.94) | <.001 | 8.91 (4.49 to 18.67) | <.001 |
| Wilms | ||||
| Siblings | Referent | — | Referent | — |
| No abdominal RT | 0.70 (0.20 to 2.46) | .58 | 0.76 (0.21 to 2.68) | .66 |
| Abdominal RT | 3.77 (2.06 to 6.89) | <.001 | 4.10 (2.21 to 7.61) | <.001 |
| Central nervous system tumor | ||||
| Siblings | Referent | — | Referent | — |
| No abdominal RT | 1.33 (0.78 to 2.28) | .30 | 1.36 (0.79 to 2.36) | .27 |
| Abdominal RT | 3.73 (1.99 to 6.99) | <.001 | 3.67 (1.89 to 7.12) | <.001 |
| Other‡ | ||||
| Siblings | Referent | — | Referent | — |
| No abdominal RT | 2.15 (1.50 to 3.10) | <.001 | 2.14 (1.47 to 3.12) | <.001 |
| Abdominal RT | 2.61 (1.48 to 4.60) | .001 | 2.92 (1.65 to 5.15) | <.001 |
All relative risks are adjusted for attained age (continuous). BMI = body mass index; CI = confidence interval; RT = radiation therapy.
Relative risks and 95% CIs were estimated using a generalized estimation equation model with a log-link function, and P values were obtained using a two-sided Wald test.
Other category includes patients with non-Hodgkin lymphoma, soft tissue sarcoma, bone cancer, and leukemia.
Factors Associated With Diabetes Among Individuals Exposed to Abdominal Radiation
To elucidate the pathophysiology of diabetes after abdominal radiation, we performed a risk factor analysis restricted to survivors previously exposed to abdominal radiation. After adjusting for attained age, the variables statistically significantly associated with diabetes risk in univariate analysis were age at diagnosis, BMI, and pancreas tail dose. None of the volumetric parameters of interest (V10, V20, V30) were associated with diabetes risk (Supplementary Table 2, available online). Exposure to alkylating agents, anthracyclines, and corticosteroids were also not associated with increased diabetes risk among individuals exposed to abdominal radiation. Complete results of the univariate analysis are shown in Supplementary Table 2 (available online).
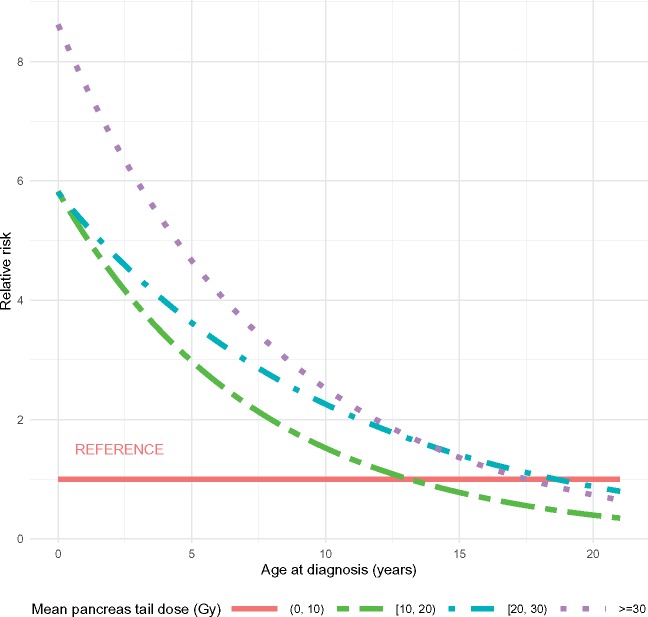
In multivariable analysis (Table 4), older attained age (RRper 10 years = 2.11, 95% CI = 1.70 to 2.62), higher BMI (RRBMI <18.5 = 0.5, 95% CI = 0.15 to 1.61; RRBMI 25–30 = 1.69, 95% CI = 1.08 to 2.65; RRBMI 30+ = 5.00, 95% CI = 3.19 to 7.83, with reference group of BMI 18.5–24.9), and higher mean pancreatic tail dose were associated with diabetes risk among those exposed to abdominal radiation. Additionally, a statistically significant interaction was noted between younger age at cancer diagnosis and pancreatic tail dose, with greater differences in the risk of diabetes noted among those diagnosed at the youngest ages (P < .001; Table 5 and Figure 1). Specifically, after adjusting for attained age and BMI, those diagnosed with cancer at younger than 10 years of age were at increased risk for diabetes development with higher mean dose to the pancreatic tail (among individuals diagnosed at age 1 year: RR10–19.9 Gy = 5.09, 95% CI = 2.18 to 11.91; RR20–29.9 Gy = 5.28, 95% CI = 1.84 to 15.18; RR30+ Gy = 7.62, 95% CI = 2.52 to 23.06; among individuals diagnosed at age 5 years: RR10–19.9 Gy = 2.98, 95% CI = 1.60 to 5.53; RR20–29.9 Gy = 3.62, 95% CI = 1.68 to 7.78; RR30+ Gy = 4.66, 95% CI = 1.96 to 11.08, both with referent group of 0.1–9.9 Gy). Those diagnosed at age 15 years or older, however, were not at statistically significantly increased risk for diabetes development at different mean pancreas tail doses, after adjusting for attained age and BMI. Full results are shown in Figure 1 and Table 5.
Table 4.
Predictors of diabetes mellitus in multivariable analysis in individuals exposed to abdominal radiation
| Characteristic | Relative risk (95% CI) | P * |
|---|---|---|
| Attained age, 10 y | 2.11 (1.70 to 2.62) | <.001 |
| Body mass index group, kg/m2 | <.001 | |
| <18.5 | 0.50 (0.15 to 1.61) | — |
| 18.5–24.9 | 1.00 (referent) | — |
| 25.0–29.9 | 1.69 (1.08 to 2.65) | — |
| ≥30 | 5.00 (3.19 to 7.83) | — |
| Pancreas tail dose, Gy† | <.001 | |
| 0.1–9.9 | 1.00 (referent) | — |
| 10.0–19.9 | 5.82 (2.33 to 14.53) | — |
| 20.0–29.9 | 5.81 (1.85 to 18.25) | — |
| ≥30 | 8.62 (2.64 to 28.14) | — |
| Interaction between age at diagnosis (10-year increments) and pancreas tail dose, Gy‡ | <.001 | |
| 0.1–9.9 | 1.28 (0.69 to 2.36) | — |
| 10.0–19.9 | 0.33 (0.19 to 0.59) | — |
| 20.0–29.9 | 0.50 (0.19 to 1.33) | — |
| ≥30 | 0.37 (0.15 to 0.92) | — |
Relative risks and 95% CIs were estimated using a generalized estimation equation model with a log-link function; P values were obtained using a two-sided Wald test. CI = confidence interval.
Pancreas tail dose refers to mean dose.
Estimates are per 10-year increases in age at diagnosis.
Table 5.
Association between radiation dose to the pancreatic tail and diabetes risk by age at cancer diagnosis among childhood cancer survivors exposed to abdominal radiation*
| Mean pancreas tail dose, Gy | 1 year | 5 years | 10 years | 15 years | 20 years |
|---|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| 0.1– 9.9 | Referent | Referent | Referent | Referent | Referent |
| 10.0–19.9 | 5.09 (2.18 to 11.91) | 2.98 (1.60 to 5.53) | 1.52 (0.96 to 2.42) | 0.78 (0.44 to 1.39) | 0.40 (0.17 to 0.94) |
| 20.0–29.9 | 5.28 (1.84 to 15.18) | 3.62 (1.68 to 7.78) | 2.26 (1.11 to 4.59) | 1.41 (0.50 to 3.95) | 0.88 (0.19 to 3.96) |
| ≥30 | 7.62 (2.52 to 23.06) | 4.66 (1.96 to 11.08) | 2.52 (1.12 to 5.64) | 1.36 (0.48 to 3.89) | 0.74 (0.17 to 3.14) |
All estimates are adjusted for attained age and body mass index. CI = confidence interval; RR = relative risk.
Figure 1.
Association between radiation dose to the pancreatic tail and diabetes risk by age at cancer diagnosis among childhood cancer survivors exposed to abdominal radiation. Shows the estimated relative risk of diabetes by age at diagnosis (as a continuous variable) for each mean pancreatic radiation dose group. Constructed using the linear predictors from the model of 11 575 survey responses among 4341 childhood cancer survivors exposed to abdominal radiation.
A linear dose-response relationship appeared to fit the data well (Supplementary Table 3, available online). We found no evidence of a quadratic relationship or of an interaction with age at diagnosis or BMI on an additive scale.
Discussion
Using the largest cohort assembled to date of childhood cancer survivors exposed to abdominal radiation for assessment of diabetes mellitus, we built on previous work establishing a dose-response relationship between radiation to the tail of the pancreas and diabetes risk (13, 14) to demonstrate a novel interaction between younger age at diagnosis and diabetes risk within the same pancreas tail dose groups and, thereby, allow for the identification of survivors at highest risk for diabetes after exposure to therapeutic abdominal radiation. These data are crucial to inform targeted screening practices and the development of interventions for survivors at highest risk for the development of diabetes over time.
Over the past two decades, the association between abdominal radiation and diabetes has been increasingly recognized. In a brief report in the Lancet in 1995, Teinturier et al. (22) first described a cohort of 121 patients treated with abdominal radiation in which 6.6% developed pancreatic diabetes, or a nonautoimmune insulinopenic form of diabetes. Concerns about a possible link between abdominal radiation and β-cell dysfunction followed in other brief reports (23,24). Subsequently, large epidemiologic analyses corroborated the link between abdominal radiation and diabetes risk (8,13,14). Using the original cohort of CCSS survivors diagnosed between 1970 and 1986, Meacham and colleagues (8) noted an increased risk of diabetes among all childhood cancer survivors compared to siblings, with risk most pronounced among those previously exposed to total body irradiation or abdominal radiation. Unlike what is observed in the general population, diabetes risk associated with radiation remained statistically significant even after accounting for BMI or sedentary lifestyle (8). Precise radiation dose estimates were not examined in that report.
Since that time, however, data from studies using radiation dosimetry have consistently shown a strong association between radiation dose to the tail of the pancreas and diabetes risk. One study of 2520 five-year childhood cancer survivors (n = 1632 treated with radiation) enrolled in the French-United Kingdom cohort demonstrated that risk of diabetes plateaued at radiation doses to the pancreatic tail greater than 20–29 Gy (13). More recently, an analysis of 2264 Hodgkin survivors in the Netherlands (41.2% diagnosed prior to age 25 years; 48% previously exposed to para-aortic radiation) (14) found that risk of diabetes increased with increasing mean dose to the pancreatic tail without an evident plateau. Para-aortic radiation with a splenic boost, which includes 90–100% of pancreatic volume, was associated with a greater risk than para-aortic radiation alone, which includes 75–85% of pancreatic volume, thus suggesting that volume of the pancreas exposed to radiation may play a role in posttreatment diabetes development. Given this suggestion, the current study sought to assess the role of pancreatic radiation volume, as well as dose, in determining diabetes risk among childhood cancer survivors. Similar to the study from the Netherlands, we found a linear dose-response relationship between mean radiation dose to the pancreatic tail and diabetes risk without evident plateau. To our surprise, however, volumetric parameters, or percentage of the pancreas exposed to different doses of radiation (V10, V20, V30), were not associated with risk in our cohort, which suggests that radiation dose to the tail of the pancreas supersedes the importance of radiation to other regions of the pancreas in mediating diabetes risk after abdominal radiation.
Importantly, given prior work showing that the risk of diabetes for children younger than age 2 years at the time of radiation therapy was greater than for older children (13), we explored the interaction between younger age at primary cancer diagnosis and pancreas tail dose. We found that individuals diagnosed at the youngest ages had a statistically significantly higher risk of diabetes within the same pancreas tail dose groups. This has important implications for risk stratification among childhood cancer survivors treated with abdominal radiation at various doses and ages, which may impact targeted screening practices and future intervention trials among those at highest risk for diabetes and future cardiometabolic disease.
It is important to note, however, that Hodgkin lymphoma survivors (14) and testicular cancer survivors (25) treated with abdominal radiation have also been found to be at risk for diabetes after abdominal radiation administered at older ages. It is unclear why this association was not apparent in our cohort but may be related to the fact that our cohort is limited to individuals diagnosed at younger than 21 years of age.
Regarding the pathophysiology of diabetes after abdominal radiation, the epidemiologic data would suggest that damage to the insulin-producing β cells concentrated in the tail of the pancreas is the key etiologic factor in development of diabetes in this population. However, the limited clinical data regarding the role of insulinopenia in the development of diabetes after abdominal radiation are inconsistent (22); moreover, more recent data suggest a role for insulin resistance rather than insulinopenia in the pathogenesis of diabetes (26). Further studies to clarify the mechanisms leading to diabetes after abdominal radiation are warranted.
Several limitations must be considered when interpreting these results. Diabetes cases were classified according to patient self-report of diabetes medication use and were not verified with a treating physician. Although it is possible that patients misreported their medication, it is more likely that some participants have undiagnosed diabetes, and thus, we may have underestimated the true number of cases of diabetes. Additionally, survivors were likely screened for diabetes more closely than their unaffected siblings because of treatment-related risks, which may have resulted in surveillance bias. Finally, because of the structure of the surveys, physical activity questions were only included on some questionnaires and were thus not explored as a predictor of diabetes in this analysis.
In this study, we described the risk of diabetes in 20 762 long-term childhood cancer survivors of whom 4568 had a history of abdominal radiation. Among those treated with abdominal radiation, we found a linear dose-response relationship between diabetes risk and higher pancreatic tail dose, but not with other dosimetric or volumetric variables. Younger age at cancer diagnosis was a key risk factor in determining risk and should be factored into risk-based screening practices for this cohort. Additional research is needed to clarify the pathophysiology of diabetes in order to design evidence-based interventions to decrease overall cardiometabolic risk in survivors after cancer therapy.
Funding
This work was supported by grants from the National Cancer Institute (CA55727, G. T. Armstrong, Principal Investigator), the CCSS Career Development Award, and the National Center for Advancing Translational Sciences (KL2 TR000458). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities.
Notes
All the co-authors declare that there is no conflict of interest in relation to the work described.
GTA and LLR provided financial support. RMH, SAS, and REW provided all radiation-related data. DNF, CAS, CSM, PH, JW, WML, GTA, and LLR obtained and assembled data. CSM, PH, JC, JW, and WML analyzed these data. DNF, CAS, CSM, PH, GTA, LLR, and KCO were responsible for the preparation and writing of the report. All authors conceived and designed the study, interpreted the data, contributed to the report, and approved the final version of the report.
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. Parts of this manuscript have been previously presented at the American Society of Clinical Oncology, June 2, 2018, Chicago, Illinois.
Supplementary Material
References
- 1. Noone AM HN, Krapcho M, Miller D, et al. (eds). SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 5. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mostoufi-Moab S, Seidel K, Leisenring WM, et al. Endocrine abnormalities in aging survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(27):3240–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the Childhood Cancer Survivor Study. Arch Intern Med. 2009;169(15):1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carnethon MR, Biggs ML, Barzilay J, et al. Diabetes and coronary heart disease as risk factors for mortality in older adults. Am J Med. 2010;123(6):556.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–1778. [DOI] [PubMed] [Google Scholar]
- 12. Wei M, Gaskill SP, Haffner SM, et al. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21(7):1167–1172. [DOI] [PubMed] [Google Scholar]
- 13. de Vathaire F, El-Fayech C, Ben Ayed FF, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. Lancet Oncol. 2012;13(10):1002–1010. [DOI] [PubMed] [Google Scholar]
- 14. van Nimwegen FA, Schaapveld M, Janus CP, et al. Risk of diabetes mellitus in long-term survivors of Hodgkin lymphoma. J Clin Oncol. 2014;32(29):3257–3263. [DOI] [PubMed] [Google Scholar]
- 15. Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. [DOI] [PubMed] [Google Scholar]
- 17. Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1):141–157. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National Center for Health Statistics. CDC growth charts. United States; May 30, 2000. https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed August 1, 2019.
- 20.Division of Nutrition PA, and Obesity, National Center for Chronic Disease Prevention and Health Promotion; Centers for Disease Control and Prevention. Defining Adult Overweight and Obesity; 2016. https://www.cdc.gov/obesity/adult/defining.html. Accessed August 1, 2019.
- 21. Richardson DB, Kaufman JS.. Estimation of the relative excess risk due to interaction and associated confidence bounds. Am J Epidemiol. 2009;169(6):756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teinturier C, Tournade M-F, Caillat-Zucman S, et al. Diabetes mellitus after abdominal radiation therapy. Lancet. 1995;346(8975):633–634. [DOI] [PubMed] [Google Scholar]
- 23. Cicognani A, Cacciari E, Mancini AF, et al. Abnormal insulin response to glucose following treatment for Wilms' tumor in childhood. Eur J Pediatr. 1997;156(5):371–375. [DOI] [PubMed] [Google Scholar]
- 24. Hawkins MM, Robertson CM, Edge JA, et al. Is risk of diabetes mellitus increased after abdominal radiotherapy? Lancet. 1996;347(9000):538–540. [PubMed] [Google Scholar]
- 25. Groot HJ, Gietema JA, Aleman BMP, et al. Risk of diabetes after para-aortic radiation for testicular cancer. Br J Cancer. 2018;119(7):901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedman DN, Hilden P, Moskowitz CS, et al. Insulin and glucose homeostasis in childhood cancer survivors treated with abdominal radiation: a pilot study. Pediatr Blood Cancer. 2018;65(11):e27304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.