Abstract
Background
Use of genomic testing is increasing in the United States. Testing can be expensive, and not all tests and related treatments are covered by health insurance. Little is known about how often oncologists discuss costs of testing and treatment or about the factors associated with those discussions.
Methods
We identified 1220 oncologists who reported discussing genomic testing with their cancer patients from the 2017 National Survey of Precision Medicine in Cancer Treatment. Multivariable polytomous logistic regression analyses were used to assess associations between oncologist and practice characteristics and the frequency of cost discussions. All statistical tests were two-sided.
Results
Among oncologists who discussed genomic testing with patients, 50.0% reported often discussing the likely costs of testing and related treatments, 26.3% reported sometimes discussing costs, and 23.7% reported never or rarely discussing costs. In adjusted analyses, oncologists with training in genomic testing or working in practices with electronic medical record alerts for genomic tests were more likely to have cost discussions sometimes (odds ratio [OR] = 2.09, 95% confidence interval [CI] = 1.19 to 3.69) or often (OR = 2.22, 95% CI = 1.30 to 3.79), respectively, compared to rarely or never. Other factors statistically significantly associated with more frequent cost discussions included treating solid tumors (rather than only hematological cancers), using next-generation sequencing gene panel tests, having higher patient volume, and working in practices with higher percentages of patients insured by Medicaid, or self-paid or uninsured.
Conclusions
Interventions targeting modifiable oncologist and practice factors, such as training in genomic testing and use of electronic medical record alerts, may help improve cost discussions about genomic testing and related treatments.
The costs of cancer care have been rising in the United States (1–4), increasing concerns about medical financial hardship for cancer patients and their families. Many cancer survivors have difficulty paying medical bills, face high levels of financial distress, and delay or forgo medical care because of cost (5–11). Recent trends in health insurance benefit design, including increasing patient cost-sharing, with higher deductibles, copayments, and coinsurance rates (12,13), can increase financial burden even among those with health insurance. Uninsured patients can be responsible for the entire cost of cancer care.
High patient out-of-pocket costs for cancer treatment have been the subject of many discussions in the scientific literature (1,12,14–17) and popular press (18–20). In 2009, the American Society of Clinical Oncology highlighted the important role of oncologists in discussions about the expected patient out-of-pocket costs of cancer care (21,22). The Institute of Medicine later identified these discussions as an element of high-quality care (22), and cost consciousness has been proposed as a core competency for medical education (23). Although oncologists generally agree about their responsibility for cost discussions (24), these discussions are rare (24,25). Nevertheless, most cancer patients desire discussions about expected out-of-pocket costs (24), highlighting an unmet need for informed treatment decision making in cancer care.
Discussions of patient out-of-pocket costs are especially relevant when considering the increasing availability of molecularly targeted therapies for specific tumor variants. As of 2016, more than 200 targeted therapies were available in the United States and more than 2000 are in late-stage development (26). More than half are in oncology. Targeted therapies have high list prices, frequently in excess of $100 000 annually (27–29). Genomic tests to identify targetable variants can also be expensive (30) and are not always covered by health insurance. Even with health insurance coverage, cancer patients face cost-sharing for genomic testing and treatment, as high as 30% of the list price for tests and treatments (13). Discussions about expected costs of genomic testing and related treatments may inform treatment decision making and help cancer patients prepare for high expenses. However, little is known about how often oncologists discuss costs of genomic testing and related treatment with their patients or about the physician and/or practice factors associated with those discussions. In this study, we address these research gaps by analyzing data from a nationally representative survey of oncologists about their cost discussions with cancer patients and identify potentially modifiable factors associated with the frequency of cost discussions.
Methods
Data and Sample
The study sample was obtained from the 2017 National Survey of Precision Medicine in Cancer Treatment, a nationally representative survey of medical oncologists conducted between February and May 2017 (31,32). The survey was sponsored by the National Cancer Institute, National Human Genome Research Institute, and the American Cancer Society and collected information on oncologists’ sociodemographic and practice characteristics and use of genomic tests (32). Prior to fielding the survey, methodologists and clinical experts reviewed all content. Additionally, cognitive interviewing among practicing oncologists was conducted to ensure that questions were clearly worded and responses consistent with the intent of the questions. Oncologists were selected from the American Medical Association Physician Masterfile, which covers all licensed physicians in the United States. Practicing oncologists were selected using probability sampling, stratified by specialty, census region, size of metropolitan statistical area (MSA), and sex by age category. A total of 1281 practicing oncologists completed the survey via mail or online with a cooperation rate of 38.0%. We excluded oncologists who reported that they had not discussed genomic testing with patients or their families at all in the past 12 months (n = 61) and restricted our sample to the remaining 1220 oncologists who discussed genomic testing. More information about the survey design, sample weights, and analyses for nonresponse bias have been published elsewhere (31,32) and are summarized in the Supplementary Methods (available online). The survey protocol was reviewed by the Institutional Review Board (IRB) of RTI International, a nonprofit research organization. Survey data were deidentified and considered exempt by the National Institutes of Health IRB.
Measures
The measure of cost discussion frequency was based on the survey question, “In the past 12 months, when you or your staff discussed any form of genomic testing with your cancer patients or their families, how often did you discuss the likely costs of the testing and related treatment?” Response options among oncologists who discussed genomic testing within the past 12 months were never, rarely, sometimes, and often. Responses were categorized as “rarely or never,” “sometimes,” and “often.”
We selected measures of physician-, practice-, and area-level characteristics previously shown to be associated with guideline-concordant practice (33–36) or hypothesized to be associated with cost discussions. Oncologist characteristics included age, years since medical school graduation, sex, and self-reported race and ethnicity, types of tumors treated (hematological cancers only, solid tumors only, or both hematologic cancers and solid tumors), percentage of time providing patient care, medical school affiliation, training in genomic testing, and use of next-generation sequencing (NGS) gene panel tests in the past 12 months. Practice-level characteristics were MSA, geographic region, and self-reported practice type, implementation of genomic testing services within the practice (internal policies or protocols for use of genomic and biomarker testing; electronical medical record [EMR] alerts for genomic test recommendations for particular patients or drugs; genomic/molecular tumor board), patient insurance status in practice (proportion of patients insured by Medicaid, self-pay, or uninsured), and patient volume (ie, 1–99 unique patients per month or ≥100 unique patients per month).
Area-level characteristics of the county of the physician’s practice location were obtained from the 2016–2017 Area Health Resources Files (37); these included county-level mean per capita personal income, percentage of individuals ages 25 years and older with at least 4 years of college, and median gross rent. Continuous measures of physician-, practice-, and area-level characteristics were categorized based on distributions within the sample. Exact wording of survey questions and response options are listed in Supplementary Table 1 (available online).
Statistical Analyses
We calculated descriptive statistics for physician, practice, and area-level characteristics. Associations between physician-, practice-, and area-level characteristics and frequency of cost discussion were assessed using polytomous logistic regression models (38). A data-driven stagewise approach was used to identify physician-, practice-, and area-level covariates in developing parsimonious intermediate and final adjusted models. First, bivariable analyses were conducted to identify covariates statistically significantly associated with the frequency of cost discussions; those that were statistically significant at P less than .20 were included in one of three intermediate multivariable models of physician-, practice-, or area-level characteristics and cost discussions. The final multivariable model included covariates statistically significant at P less than .20 in any of the three intermediate models. Collinearity diagnostics were performed for the three intermediate and the final multivariable regression models. Statistical tests were two-sided, and statistical significance was defined as P less than .05. Analytic files were created with SAS 9.4 (SAS Institute, Cary, NC, USA) and analyses were conducted with STATA/IC 14.1 (StataCorp, College Station, TX, USA) Sample weights that accounted for the complex survey design and survey nonresponse were applied in all analyses.
Results
The majority of the 1220 oncologists who reported discussing genomic testing with patients within the past 12 months were male and non-Hispanic white, treated both hematological cancers and solid tumors, and practiced in large MSAs (Table 1). Of the oncologists, 56.2% reported that they had received training in genomic testing, 74.5% of oncologists reported using NGS in the past 12 months, and 16.6% reported that their practice has EMR alerts for genomic test recommendations.
Table 1.
Sample characteristics, National Survey of Precision Medicine in Cancer Treatment, 2017*
| Sample characteristics | No. | Weighted %† |
|---|---|---|
| Total | 1220 | 100.0 |
| Physician characteristics | ||
| Age, y | ||
| <40 | 262 | 22.0 |
| 40–49 | 371 | 30.9 |
| 50–59 | 289 | 23.5 |
| ≥60 | 298 | 23.6 |
| Years since medical school graduation | ||
| 7–14 | 293 | 25.0 |
| 15–24 | 372 | 30.9 |
| 25–34 | 272 | 21.9 |
| 35–51 | 283 | 22.2 |
| Sex | ||
| Male | 877 | 65.9 |
| Female | 343 | 34.1 |
| Race/ethnicity | ||
| White, non-Hispanic | 762 | 62.0 |
| Other | 458 | 38.0 |
| Types of tumors treated | ||
| Hematologic cancers only | 140 | 11.7 |
| Hematologic and solid | 792 | 64.4 |
| Solid tumors only | 284 | 23.9 |
| Percentage of time providing patient care | ||
| 5–75% | 368 | 31.0 |
| >76% | 852 | 69.0 |
| Affiliation with medical school or hospital | 759 | 62.7 |
| Formal training in genomic testing | 680 | 56.2 |
| Use of next-generation sequencing gene panel tests | 913 | 74.5 |
| Practice characteristics | ||
| Practice type | ||
| Solo | 52 | 4.3 |
| Single specialty | 519 | 42.0 |
| Multispecialty | 540 | 44.8 |
| Other | 103 | 8.9 |
| Located in metropolitan statistical area | ||
| Small/Medium | 179 | 14.1 |
| Large | 165 | 12.2 |
| Very large | 876 | 73.7 |
| US geographic region | ||
| Northeast | 302 | 26.5 |
| Midwest | 286 | 20.9 |
| South | 419 | 34.8 |
| West | 213 | 17.8 |
| Patient volume per month | ||
| 1–99 | 626 | 52.1 |
| ≥100 | 594 | 47.9 |
| Primary practice provides internal policies or protocols for genomic tests | 579 | 47.9 |
| Primary practice has electronic medical record alerts for genomic tests | 199 | 16.6 |
| Primary practice provides genomic and/or molecular tumor board for genomic tests | 439 | 36.2 |
| Proportion of patients insured by Medicaid≥10% or self-pay or uninsured ≥10% | 910 | 73.8 |
| Area-level characteristics | ||
| Mean per capita income | ||
| >$60 000 | 311 | 26.8 |
| $45 000–60 000 | 524 | 42.6 |
| ≤$45 000 | 385 | 30.6 |
| % persons ≥25 years with ≥4 years of college | ||
| >45 | 247 | 20.8 |
| 30–45 | 584 | 48.3 |
| ≤30 | 389 | 30.9 |
| Median gross rent | ||
| >$1000 | 467 | 40.8 |
| $850–1000 | 397 | 32.0 |
| ≤$850 | 356 | 27.3 |
Data from the 2017 National Survey of Precision Medicine in Cancer Treatment. Exact wording of survey questions and response options is listed in Supplementary Table 1 (available online).
Percentages weighted to account for complex survey design and survey nonresponse.
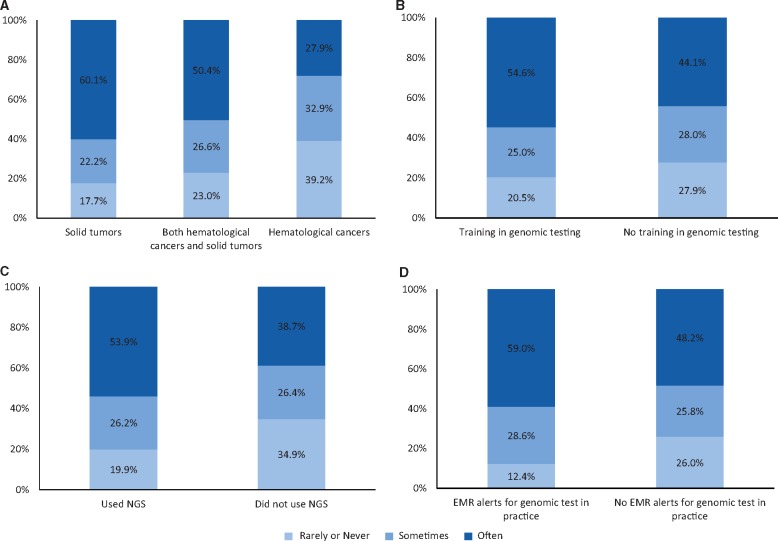
In response to the question about frequency of discussing the likely costs of testing and treatments with patients, 50.0% of oncologists reported having these discussions often; 26.3% reported sometimes; and 23.7% reported never or rarely discussing costs. The frequency of cost discussions varied by the types of tumors that oncologists treated: A total of 60.1% of those who treated only solid tumors reported often discussing costs with patients compared to 50.4% of those who treated hematological cancers and solid tumors and 27.9% of those who treated only hematological cancers (P < .001) (Figure 1A). Oncologists with formal training in genomic testing were more likely than those without this training to report discussing costs often (54.6% vs 44.1%, P = .001) (Figure 1B) as were those who used NGS tests in the past 12 months compared with those who did not (53.9% vs 38.7%, P < .001) (Figure 1C). Oncologists working in practices with EMR alerts for genomic test recommendations were more likely than those in practices without EMR alerts to report often (59.0% vs 48.2%, P < .001) discussing costs with their patients (Figure 1D).
Figure 1.
Oncologist and practice characteristics and frequency of discussions about costs of genomic testing and related treatment. A) By types of tumors treated (P < .001); (B) By training in genomic testing (P = .001); (C) by use of next-generation sequencing (NGS) gene panel tests (P < .001); (D) by whether practice has electronic medical records (EMR) with alerts for genomic tests (P < .001). Pearson χ2 test was used to calculate the P values. All statistical tested were two-sided.
Several physician characteristics were statistically significantly associated with the frequency of cost discussions in the intermediate (Supplementary Table 2, available online) and final (Table 2) multivariable models. Oncologists with more years since medical school graduation were more likely to often discuss the cost of genomic testing and related treatment with patients and their families than those who graduated less than 15 years prior to the survey. Compared with oncologists who treated only hematological cancers, those who treated both hematological cancers and solid tumors or who treated only solid tumors were more likely to often have cost discussions with their patients (odds ratio [OR] = 2.82, 95% confidence interval [CI] = 1.58 to 5.02 and OR = 4.01, 95% CI = 2.21 to 7.29, respectively). Formal training in genomic testing was associated with higher likelihood of having cost discussions often (OR = 1.74, 95% CI = 1.25 to 2.42). Oncologists who use NGS tests were more likely to have cost discussions with their patients often (OR = 1.93, 95% CI = 1.34 to 2.77) or sometimes (OR = 1.59, 95% CI = 1.07 to 2.37) instead of rarely or never.
Table 2.
Factors associated with frequency of discussions about costs of genomic testing and related treatment*
| Sample characteristics | Unadjusted (sometimes vs never or rarely) | Unadjusted (often vs never or rarely) | Adjusted† (sometimes vs never or rarely) | Adjusted† (often vs never or rarely) |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Physician characteristics | ||||
| Age, y | ||||
| <40 | Referent | Referent | — | — |
| 40–49 | 0.94 (0.60 to 1.48) | 1.27 (0.85 to 1.90) | — | — |
| 50–59 | 1.04 (0.64 to 1.67) | 1.39 (0.91 to 2.13) | — | — |
| ≥60 | 1.09 (0.67 to 1.76) | 1.54 (1.00 to 2.38) | — | — |
| Years since medical school graduation | ||||
| 7–14 | Referent | Referent | Referent | Referent |
| 15–24 | 1.07 (0.70 to 1.65) | 1.17 (0.80 to 1.72) | 1.08 (0.68 to 1.70) | 1.21 (0.78 to 1.87) |
| 25–34 | 1.17 (0.72 to 1.90) | 1.62 (1.06 to 2.49) | 1.35 (0.80 to 2.26) | 2.28 (1.40 to 3.71) |
| 35–51 | 1.14 (0.71 to 1.83) | 1.53 (1.00 to 2.33) | 1.24 (0.73 to 2.12) | 1.97 (1.19 to 3.25) |
| Sex | ||||
| Female | Referent | Referent | — | — |
| Male | 0.99 (0.69 to 1.42) | 1.07 (0.78 to 1.46) | — | — |
| Race/ethnicity | ||||
| White, non-Hispanic | Referent | Referent | — | — |
| Other | 0.89 (0.64 to 1.24) | 0.78 (0.58 to 1.05) | — | — |
| Types of tumors treated | ||||
| Hematologic cancers only | Referent | Referent | Referent | Referent |
| Hematologic cancers and solid tumors | 1.37 (0.87 to 2.17) | 3.07 (1.93 to 4.88) | 1.31 (0.75 to 2.30) | 2.82 (1.58 to 5.02) |
| Solid tumors only | 1.49 (0.85 to 2.63) | 4.77 (2.78 to 8.19) | 1.47 (0.81 to 2.69) | 4.01 (2.21 to 7.29) |
| Percentage of time providing patient care | ||||
| <76 | Referent | Referent | — | — |
| ≥76 | 1.08 (0.75 to 1.54) | 0.97 (0.71 to 1.33) | — | — |
| Affiliation with medical school or hospital | ||||
| No | Referent | Referent | — | — |
| Yes | 0.99 (0.71 to 1.40) | 0.89 (0.66 to 1.20) | — | — |
| Formal training in genomic testing | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | 1.22 (0.87 to 1.69) | 1.68 (1.26 to 2.25) | 1.15 (0.80 to 1.66) | 1.74 (1.25 to 2.42) |
| Uses next-generation sequencing gene panel tests | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | 1.74 (1.21 to 2.51) | 1.11 (0.85 to 1.45) | 1.59 (1.07 to 2.37) | 1.93 (1.34 to 2.77) |
| Practice characteristics | ||||
| Practice type | ||||
| Solo | Referent | Referent | Referent | Referent |
| Single specialty | 1.23 (0.50 to 3.03) | 0.91 (0.44 to 1.90) | 1.18 (0.43 to 3.18) | 0.75 (0.33 to 1.71) |
| Multispecialty | 1.11 (0.45 to 2.72) | 0.73 (0.35 to 1.52) | 1.11 (0.40 to 3.07) | 0.61 (0.26 to 1.41) |
| Other | 0.68 (0.24 to 1.91) | 0.49 (0.21 to 1.13) | 0.78 (0.26 to 2.37) | 0.50 (0.19 to 1.29) |
| Located in metropolitan statistical area | ||||
| Small/Medium | Referent | Referent | — | — |
| Large | 1.19 (0.63 to 2.25) | 1.00 (0.55 to 1.82) | — | — |
| Very large | 0.69 (0.43 to 1.12) | 0.75 (0.48 to 1.17) | — | — |
| US geographic region | ||||
| Northeast | Referent | Referent | Referent | Referent |
| Midwest | 1.32 (0.82 to 2.14) | 1.61 (1.05 to 2.45) | 1.25 (0.74 to 2.11) | 1.60 (0.98 to 2.62) |
| South | 1.05 (0.69 to 1.59) | 1.04 (0.72 to 1.51) | 0.93 (0.57 to 1.52) | 0.98 (0.62 to 1.53) |
| West | 1.28 (0.75 to 2.17) | 1.82 (1.16 to 2.86) | 1.28 (0.72 to 2.29) | 1.92 (1.15 to 3.21) |
| Patient volume per month | ||||
| 1–99 | Referent | Referent | Referent | Referent |
| ≥100 | 1.46 (1.05 to 2.04) | 1.79 (1.33 to 2.40) | 1.35 (0.94 to 1.92) | 1.53 (1.11 to 2.10) |
| Practice provides internal policies or protocols for genomic testing | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | 1.14 (0.82 to 1.58) | 1.35 (1.01 to 1.81) | 1.06 (0.70 to 1.59) | 1.25 (0.86 to 1.79) |
| Practice has electronic medical record alerts for genomic testing | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | 2.32 (1.38 to 3.90) | 2.56 (1.59 to 4.12) | 2.09 (1.19 to 3.69) | 2.22 (1.30 to 3.79) |
| Practice has genomic and/or molecular tumor board for genomic testing | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | 1.08 (0.76 to 1.54) | 1.31 (0.96 to 1.78) | 1.19 (0.74 to 1.90) | 1.47 (0.96 to 2.25) |
| Proportion of patients insured by Medicaid ≥ 10% or self-pay or uninsured ≥10% | ||||
| No | Referent | Referent | Referent | Referent |
| Yes | 1.70 (1.14 to 2.47) | 1.47 (1.07 to 2.02) | 1.60 (1.09 to 2.36) | 1.55 (1.09 to 2.20) |
| Area-level characteristics | ||||
| Mean per capita income | ||||
| >$60 000 | Referent | Referent | Referent | Referent |
| $45 000–60 000 | 1.43 (0.95 to 2.14) | 1.98 (1.39 to 2.82) | 1.12 (0.65 to 1.92) | 1.84 (1.09 to 3.09) |
| ≤$45 000 | 1.61 (1.05 to 2.45) | 1.59 (1.09 to 2.33) | 1.08 (0.56 to 2.07) | 1.55 (0.81 to 2.97) |
| % persons ≥25 years with ≥4 years of college | ||||
| >45 | Referent | Referent | Referent | Referent |
| 30%–45 | 1.63 (1.06 to 2.51) | 1.68 (1.16 to 2.42) | 1.57 (0.91 to 2.71) | 1.27 (0.75 to 2.16) |
| ≤30 | 2.01 (1.27 to 3.18) | 1.71 (1.14 to 2.55) | 1.96 (0.98 to 3.90) | 1.19 (0.62 to 2.28) |
| Median gross rent | ||||
| >$1000 | Referent | Referent | — | — |
| $850–1000 | 1.14 (0.77 to 1.67) | 1.07 (0.77 to 1.50) | — | — |
| ≤$850 | 1.50 (0.99 to 2.27) | 1.80 (1.24 to 2.61) | — | — |
N = 1220. Data from the National Survey of Precision Medicine in Cancer Treatment. All analyses account for complex survey design and survey nonresponse. CI = confidence interval; OR = odds ratio.
Final multivariable model included year of graduation, types of tumors treated, training in genomic testing, next-generation sequencing use, practice type, US geographic region, primary practice provides internal policies or protocols, electronical medical record alerts, practice has genomic and/or molecular tumor board for genomic testing, patient insurance status, area-level per capita income, and college education.
Several practice-level characteristics were also statistically significantly associated with the frequency of cost discussions in intermediate (Supplementary Table 2, available online) and final (Table 2) models. Oncologists with EMR alerts for genomic testing in their practice were more likely than those without alerts to have cost discussions often (OR = 2.22, 95% CI = 1.30 to 3.79) or sometimes (OR = 2.09, 95% CI = 1.19 to 3.69) instead of rarely or never. Oncologists with higher patient volume were more likely to have more frequent cost discussions than those with lower patient volume. The frequency of cost discussions also varied by the health insurance status of patients in the practice. Oncologists with a higher percentage of patients insured by Medicaid, or who were self-pay or uninsured in their practice, were more likely to discuss cost often (OR = 1.55, 95% CI = 1.09 to 2.20) or sometimes (OR = 1.60, 95% CI = 1.09 to 2.36) instead of rarely or never. Lower area-level income was also associated with greater frequency of cost discussions.
Discussion
In this study, we used data from a nationally representative survey of oncologists conducted in 2017 to assess the frequency of discussions about the costs of genomic testing and related treatments with the cancer patients in their practices. At the time of the survey, the costs of genomic testing to inform treatment ranged from $300 to more than $10 000 for available tests (30,39), and the list price of molecularly targeted therapies frequently exceeded $100 000 annually (27–29), with some prices higher than $350 000 (40). The Centers for Medicare and Medicaid Services had not yet issued a national coverage determination for genomic testing, and many private insurers did not cover genomic tests. Despite widespread attention to cost (18–20), designation of cost discussions as an important element of high-quality cancer care for all patients (14,17,21,22), and potentially high patient out-of-pocket costs, we found that only half of oncologists reported that they or their staff often discussed the costs of genomic testing and related treatment and nearly one-quarter reported never or rarely discussing costs. With rapid growth in the availability of genomic tests and targeted treatments for cancer and a large pipeline of treatments in development (26), improving provider discussions about expected out-of-pocket costs will be critical for ensuring informed patient treatment decision making and the opportunity to plan for treatment expenses and help address out-of-pocket costs by linking patients with available resources and ensuring high-quality cancer care.
We identified potentially modifiable physician- and practice-level factors associated with greater frequency of cost discussions, including oncologist training in genomic testing and EMR alerts for genomic testing within the practice. Training and alerts may reflect more attention to genomic testing and related treatment and greater familiarity with their costs. Other aspects of physician expertise in treating patients, including treating solid tumors (for which most genomic panel tests are available, therefore, physicians who treat them may be more familiar with their costs), higher patient volume, and longer time since medical school graduation were also associated with greater frequency of cost discussions. These findings are consistent with other research showing that physician expertise—measured as training, specialty, patient volume, and/or years in practice—is associated with treatment recommendations (41), as well as aspects of treatment cost-consciousness, which includes the importance of cost savings, awareness of patient out-of-pocket costs, and discussions of financial burden (42). Provider- and practice-level interventions, such as training, electronic reminders, and peer comparisons, have been shown to be effective in improving recommendations for cancer screening and other services (43–45). Better understanding of the relative influences of expertise, training, and use of EMR technology on cost-consciousness and, ultimately, patient out-of-pocket costs is needed. In addition, identification and/or adaptation of interventions to address potentially modifiable factors to increase the frequency of discussions of patient costs associated with genomic testing and related treatments is an important area for future research.
Prior research has shown that insufficient physician time, discomfort with talking about treatment costs, limited knowledge of costs, and lack of price transparency for specific treatments may be barriers to physicians engaging with patients and family members in conversations about the expected out-of-pocket and other costs of cancer care (24,46–48). Oncologists may not be the providers best suited for all discussions about the expected costs of care (49); however, they can be responsible for ensuring that these conversations take place with a member of the care team within their practice. Normalization of cost discussions with all cancer patients, regardless of health insurance coverage or apparent resources, will be necessary to avoid stigmatization as well as underidentification of medical financial hardship, which is prevalent even among those with private health insurance coverage (50,51).
Initiating a discussion about the expected out-of-pocket costs of genomic testing and related treatment is a necessary first step but is not sufficient to ensure that patients and their families can make fully informed decisions about treatment options. Less is known about the content and quality of cost discussions, which are especially important given the high costs of cancer treatment. Price transparency tools are increasingly available (52–56), and EMRs could be leveraged to provide information about prices at the point of care (57). Provider training materials and practice guides have been developed to address physician discomfort with cost-of-care discussions and limited knowledge about costs (58,59). Training materials also address aspects of discussion content beyond patient out-of-pocket costs for medical care, such as expenses for transportation to and from medical care, childcare and eldercare, housing, and food (58). Because patients may not be able to work during treatment, minimizing lost wages and maintaining access to employer-sponsored health insurance are additional topics that are increasingly recommended for informed decision making (58,60–62). Team-based approaches to cost discussions may help address barriers related to physician time. Identifying the member(s) of the care team best suited for these discussions, if not the oncologist, along with best practices for content of discussions and integrating cost conversations throughout treatment into workflow (63) will be important for future intervention research.
We also found that patient characteristics and area-level socioeconomic conditions for the practice location were associated with the frequency of cost discussions. Oncologists in practices with a higher percentage of patients with Medicaid coverage or who were self-pay or uninsured, and those practicing in areas with lower per-capita income, were more likely to report more frequent cost discussions than were oncologists with lower proportions of Medicaid or uninsured patients or who practice in higher-income areas. Although low-income and uninsured patients are most likely to experience medical financial hardship (9,11,33,51,64–68), accumulating research suggests that private health insurance coverage and higher socioeconomic status do not eliminate the risk of hardship. Even privately insured cancer survivors report problems paying medical bills, experiencing stress related to medical bills, or delaying or forgoing care because of cost (50,51,69), and nearly 30% of cancer survivors ages 18–64 years report multiple types of medical financial hardship as a result of their cancer diagnosis, treatment, or lasting effects of treatment. Thus, discussions about the expected costs of cancer care are important for all patients.
Despite the strength of being one of the first studies to address the frequency of cost discussions about genomic testing and related treatment in a large, population-based, nationally representative sample of oncologists, our study has several limitations. The survey data are cross-sectional, and we report associations between physician-, practice-, and area-level characteristics and frequency of cost discussions, rather than causality. The survey response rate was low. Although we used sample weights to adjust for survey design and nonresponse in all analyses, it is possible that responders and nonresponders differed on some characteristics. Information about provider and practice characteristics was based on self-report and is susceptible to biases related to recall and social desirability. However, given the attention to the cost of cancer care by professional societies (21,22) and the scientific and popular press (14–17), social desirability bias would suggest that our estimates overstate the frequency of cost discussions. Despite use of cognitive testing prior to fielding the survey, some questions were fairly broad (eg, training in genomic testing) and may have had varying interpretations by oncologists.
The survey question about the frequency of cost discussions did not differentiate between tests for single gene variants, gene expression, or NGS gene panels. Additionally, the survey did not include questions about whether patients were responsible for the costs of genomic testing at the oncologist’s primary institution. Anecdotal reports suggest that some manufacturers and academic institutions provide patients with financial support for genomic testing. However, it is less clear that financial support is similarly available for targeted therapies should a cancer patient be found to have a relevant variant. Finally, the survey on which this study was based was conducted in 2017, and genomic testing and targeted treatments are rapidly evolving, as are changes in insurance coverage and associated patient costs. We do not expect underlying associations between oncologist and practice factors and the frequency of cost discussions to change, however.
In conclusion, we found that physician and practice factors are associated with frequency of discussing the costs of genomic testing and related treatments by oncologists. In the context of rising costs of cancer care, interventions targeting modifiable physician and practice factors may help increase the frequency of physician-patient cost discussions, contributing to more informed patient decisions and higher-quality cancer care.
Funding
This research was conducted by employees of the Intramural Research Department of the American Cancer Society and federal employees of the National Cancer Institute and the National Institutes of Health. Specific funding was not provided for this research. The survey on which this research is based was funded by the National Institutes of Health under contract number HHSN2612010000861 to RTI International.
Notes
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The study authors do not have any conflicts of interest.
Preliminary findings were presented at the 2018 American Society of Clinical Oncology Quality Symposium in Phoenix, Arizona, the 2019 Society for Behavioral Medicine Annual Meeting in Washington, DC, and the 2019 International Health Economics Association meeting in Basel, Switzerland.
Supplementary Material
References
- 1. Bender E. Cost of cancer drugs: something has to give. Manag Care. 2018;27(5):18–22. [PubMed] [Google Scholar]
- 2. Bradley CJ, Yabroff KR, Warren JL, Zeruto C, Chawla N, Lamont EB.. Trends in the treatment of metastatic colon and rectal cancer in elderly patients. Med Care. 2016;54(5):490–497. [DOI] [PubMed] [Google Scholar]
- 3. Conti RM, Fein AJ, Bhatta SS.. National trends in spending on and use of oral oncologics, first quarter 2006 through third quarter 2011. Health Affairs (Project Hope). 2014;33(10):1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000-2014. JAMA Oncol. 2016;2(7):960–961. [DOI] [PubMed] [Google Scholar]
- 5. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR.. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2017;109(2):djw205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrera PM, Kantarjian HM, Blinder VS.. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon LG, Merollini KMD, Lowe A, Chan RJ.. A systematic review of financial toxicity among cancer survivors: we can’t pay the co-pay. Patient. 2017;10(3):295–309. [DOI] [PubMed] [Google Scholar]
- 8. Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jagsi R, Pottow JA, Griffith KA, et al. Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32(12):1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Affairs (Project Hope). 2013;32(6):1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yabroff KR, Zhao J, Zheng Z, Rai A, Han X. Medical financial hardship among cancer survivors in the United States: what do we know? What do we need to know? Cancer Epidemiol Biomarkers Prevent. 2018;27(12):1389–1397. [DOI] [PubMed] [Google Scholar]
- 12. Shankaran V, Ramsey S.. Addressing the financial burden of cancer treatment: from copay to can’t pay. JAMA Oncol. 2015;1(3):273–274. [DOI] [PubMed] [Google Scholar]
- 13. Henry J. Kaiser Family Foundation. Payments for cost sharing increasing rapidly over time. 2017. https://www.kff.org/health-costs/issue-brief/payments-for-cost-sharing-increasing-rapidly-over-time/. Accessed January 15, 2019.
- 14. Moriates C, Shah NT, Arora VM.. First, do no (financial) harm. JAMA. 2013;310(6):577–578. [DOI] [PubMed] [Google Scholar]
- 15. Zafar SY, Abernethy AP.. Financial toxicity, part I: a new name for a growing problem. Oncology (Williston Park, NY). 2013;27(2):80–81, 149. [PMC free article] [PubMed] [Google Scholar]
- 16. Khera N. Reporting and grading financial toxicity. J Clin Oncol. 2014;32(29):3337–3338. [DOI] [PubMed] [Google Scholar]
- 17. Ubel PA, Abernethy AP, Zafar SY.. Full disclosure–out-of-pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486. [DOI] [PubMed] [Google Scholar]
- 18. Howley EK. Why is cancer treatment so expensive? US News. June 20, 2018. https://health.usnews.com/health-care/patient-advice/articles/2018-06-20/why-is-cancer-treatment-so-expensive. Accessed January 15, 2019. [Google Scholar]
- 19. McGinley L. Tackling the financial toll of cancer, one patient at a time. Washington Post. April 6, 2016. https://www.washingtonpost.com/national/health-science/tackling-the-financial-toll-of-cancer-one-patient-at-a-time/2016/04/09/c8a85dd8-fb16-11e5-9140-e61d062438bb_story.html? noredirect=on&utm_term=.27fd5c36dd78. Accessed January 15, 2019. [Google Scholar]
- 20. Moore P. The high cost of cancer treatment. AARP The Magazine. June 1, 2018. https://www.aarp.org/money/credit-loans-debt/info-2018/the-high-cost-of-cancer-treatment.html. Accessed January 15, 2019. [Google Scholar]
- 21. Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27(23):3868–3874. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 23. Weinberger SE. Providing high-value, cost-conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386–388. [DOI] [PubMed] [Google Scholar]
- 24. Shih YT, Chien CR.. A review of cost communication in oncology: patient attitude, provider acceptance, and outcome assessment. Cancer. 2017;123(6):928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wollins DS, Zafar SY.. A touchy subject: can physicians improve value by discussing costs and clinical benefits with patients? Oncologist. 2016;21(10):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IQVIA. Upholding the clinical promise of precision medicine: current position and outlook (May 22, 2017). https://www.iqvia.com/institute/reports/upholding-the-clinical-promise-of-precision-medicine-current-position-and-outlook. Accessed July 19, 2019.
- 27. Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633. [DOI] [PubMed] [Google Scholar]
- 28. Kantarjian H, Patel Y.. High cancer drug prices 4 years later—progress and prospects. Cancer. 2017;123(8):1292–1297. [DOI] [PubMed] [Google Scholar]
- 29. Kantarjian H, Rajkumar SV.. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin Proc. 2015;90(4):500–504. [DOI] [PubMed] [Google Scholar]
- 30.National Cancer Institute. Tumor DNA sequencing in cancer treatment. https://www.cancer.gov/about-cancer/treatment/types/precision-medicine/tumor-dna-sequencing. Accessed January 15, 2019.
- 31. Wiant K, Geisen E, Creel D, et al. Risks and rewards of using prepaid vs. postpaid incentive checks on a survey of physicians. BMC Med Res Methodol. 2018;18(1):104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freedman AN, Klabunde CN, Wiant K, et al. Use of next-generation sequencing tests to guide cancer treatment: results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol. 2018;2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gray SW, Kim B, Sholl L, et al. Medical oncologists’ experiences in using genomic testing for lung and colorectal cancer care. J Oncol Pract. 2017;13(3):e185–e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yabroff KR, Klabunde CN, Yuan G, et al. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med. 2011;26(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yabroff KR, Saraiya M, Meissner HI, et al. Specialty differences in primary care physician reports of Papanicolaou test screening practices: a national survey, 2006 to 2007. Ann Intern Med. 2009;151(9):602–611. [DOI] [PubMed] [Google Scholar]
- 36. Lieu TA, Ray GT, Prausnitz SR, et al. Oncologist and organizational factors associated with variation in breast cancer multigene testing. Breast Cancer Res Treat. 2017;163(1):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health Resources and Services Administration. Area Health Resources File. https://data.hrsa.gov/topics/health-workforce/ahrf. Updated July 31, 2019. Accessed December 27, 2018.
- 38.Harrell FE. Ordinal logistic regression. In: Regression Modeling Strategies. Cham: Springer; 2015:311–325. [Google Scholar]
- 39.Genetic testing facilities and cost. https://www.breastcancer.org/symptoms/testing/genetic/facility_cost. Accessed January 15, 2019.
- 40. Mullin E. Gene therapy prices by eligible patients per year. MIT Technology Review. October 24, 2017. https://www.technologyreview.com/s/609197/tracking-the-cost-of-gene-therapy/. Accessed January 15, 2019. [Google Scholar]
- 41. Wilson LE, Pollack CE, Greiner MA, Dinan MA.. Association between physician characteristics and the use of 21-gene recurrence score genomic testing among Medicare beneficiaries with early-stage breast cancer, 2008-2011. Breast Cancer Res Treat. 2018;170(2):361. [DOI] [PubMed] [Google Scholar]
- 42. Resnicow K, Patel MR, McLeod MC, Katz SJ, Jagsi R.. Physician attitudes about cost consciousness for breast cancer treatment: differences by cancer sub-specialty. Breast Cancer Res Treat. 2019;173(1):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Navathe AS, Emanuel EJ.. Physician peer comparisons as a nonfinancial strategy to improve the value of care. JAMA. 2016;316(17):1759–1760. [DOI] [PubMed] [Google Scholar]
- 44. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552–1558. [DOI] [PubMed] [Google Scholar]
- 45. Johnson MJ, May CR.. Promoting professional behaviour change in healthcare: What interventions work, and why? A theory-led overview of systematic reviews. BMJ Open. 2015;5(9):e008592.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hunter WG, Zafar SY, Hesson A, et al. Discussing health care expenses in the oncology clinic: analysis of cost conversations in outpatient encounters. J Oncol Pract. 2017;13(11):e944–e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schrag D, Hanger M.. Medical oncologists’ views on communicating with patients about chemotherapy costs: a pilot survey. J Clin Oncol. 2007;25(2):233–237. [DOI] [PubMed] [Google Scholar]
- 48. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):247–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pisu M, Schoenberger YM, Herbey I, et al. Perspectives on conversations about costs of cancer care of breast cancer survivors and cancer center staff: a qualitative study. Ann Intern Med. 2019;170(suppl 9):S54–S61. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Z, Jemal A, Han X, et al. Medical financial hardship among cancer survivors in the US. Cancer. 2019;125(10):1737–1747. [DOI] [PubMed] [Google Scholar]
- 51. Yabroff KR, Dowling EC, Guy GP Jr, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34(3):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.NH Health Cost. Compare health care costs & quality of care in New Hampshire. https://nhhealthcost.nh.gov. Accessed January 10, 2019.
- 53.GoodRx. https://www.goodrx.com/. Accessed January 10, 2019.
- 54.Mayo Clinic. Cost estimator. https://costestimator.mayoclinic.org/. Accessed January 10, 2019.
- 55.Clear Health Costs. https://clearhealthcosts.com/. Accessed January 10, 2019.
- 56.Medicare.gov. Medicare hospital compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed January 15, 2019.
- 57. Miller BJ, Slota JM, Ehrenfeld JM.. Redefining the physician’s role in cost-conscious care: the potential role of the electronic health record. JAMA. 2019;322(8):721. [DOI] [PubMed] [Google Scholar]
- 58.America's Essential Hospitals. Cost of care conversations resources. https://essentialhospitals.org/cost-of-care/provider-training-modules. Accessed January 10, 2019.
- 59. Sloan CE, Ubel PA.. The 7 habits of highly effective cost-of-care conversations. Ann Intern Med. 2019;170(suppl 9): S33–S35. [DOI] [PubMed] [Google Scholar]
- 60.Robert Wood Johnson Foundation. Costs of care: getting the patient-provider conversation right. June 15, 2016. https://www.rwjf.org/en/blog/2016/06/costs_of_care_getti.html. Accessed January 15, 2019.
- 61. Bradley CJ, Brown KL, Haan M, et al. Cancer survivorship and employment: intersection of oral agents, changing workforce dynamics, and employers’ perspectives. J Natl Cancer Inst. 2018;110(12):1292–1299. [DOI] [PubMed] [Google Scholar]
- 62. de Moor JS, Alfano CM, Kent EE, et al. Recommendations for research and practice to improve work outcomes among cancer survivors. J Natl Cancer Inst. 2018;110(10):1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henrikson NB, Banegas MP, Tuzzio L, et al. Workflow requirements for cost-of-care conversations in outpatient settings providing oncology or primary care: a qualitative, human-centered design study. Ann Intern Med. 2019;170(suppl 9):S70–S78. [DOI] [PubMed] [Google Scholar]
- 64. Weaver KE, Rowland JH, Bellizzi KM, Aziz NM.. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. 2010;116(14):3493–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rim SH, Guy GP Jr, Yabroff KR, McGraw KA, Ekwueme DU.. The impact of chronic conditions on the economic burden of cancer survivorship: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16(5):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pisu M, Kenzik KM, Oster RA, et al. Economic hardship of minority and non-minority cancer survivors 1 year after diagnosis: another long-term effect of cancer? Cancer. 2015;121(8):1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wheeler SB, Spencer JC, Pinheiro LC, Carey LA, Olshan AF, Reeder-Hayes KE.. Financial impact of breast cancer in black versus white women. J Clin Oncol. 2018;36(17):1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Banegas MP, Guy GP Jr, de Moor JS, et al. For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Affairs (Project Hope). 2016;35(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.