Abstract
Background
An emerging body of research suggests that noncentral nervous system cancer may negatively impact the brain apart from effects of cancer treatment. However, studies assessing cognitive function in newly diagnosed cancer patients cannot exclude selection bias and psychological effects of cancer diagnosis. To overcome these limitations, we investigated trajectories of cognitive function of patients before cancer diagnosis.
Methods
Between 1989 and 2013, a total of 2059 participants from the population-based Rotterdam Study were diagnosed with noncentral nervous system cancer. Cognitive assessments were performed every 3 to 5 years using a neuropsychological battery. The general cognitive factor was composed of individual cognitive tests to assess global cognition. Using linear mixed models, we compared change in cognitive function of cancer case patients before diagnosis with cognitive change of age-matched cancer-free control subjects (1:2). In addition, we performed sensitivity analyses by discarding assessments of control subjects 5 years before the end of follow-up to exclude effects from potential undiagnosed cancer. All statistical tests were two-sided.
Results
The Word Learning Test immediate recall declined faster among case patients than among control subjects (−0.05, 95% confidence interval = −0.09 to −0.01 vs 0.01, 95% confidence interval = −0.01 to 0.03; P for difference = .003). However, this difference was not statistically significant in sensitivity analyses. Furthermore, no statistically significant differences were observed in change of other individual cognitive tests and of the general cognitive factor.
Conclusions
In this study, we evaluated cognitive function in a large group of cancer patients prior to diagnosis, thereby excluding the psychological impact of cancer diagnosis and biased patient selection. In contrast to previous studies shortly after cancer diagnosis, we found no difference in change of cognitive function between cancer patients and control subjects.
About 20% to 30% of the patients with noncentral nervous system (non-CNS) cancer report cognitive problems following cancer diagnosis and cancer treatment that can persist into the survivorship period (1–3). Whereas most studies focused on the effects of chemotherapy on the brain, more recent evidence shows that newly diagnosed cancer patients may already perform lower than expected on cognitive tests prior to cancer treatment, including surgery (4–9). Although these patients have just been confronted with a cancer diagnosis, cognitive impairment persists after statistical correction for psychological distress and fatigue. This suggests that shared risk factors for both cancer and cognitive impairment, such as genetic susceptibility, aging, and lifestyle, could contribute to the development of cognitive impairment in cancer patients (10). Also, tumor growth itself may cause cognitive impairment, for instance, through inflammatory or vascular processes (5,11).
If the previously reported cognitive impairment in newly diagnosed cancer patients is related to shared risk factors for both cancer and cognitive impairment or to a growing, yet undiagnosed, cancer, it is conceivable that future cancer patients would already demonstrate altered cognitive function compared with cancer-free controls some time before cancer diagnosis. Based on this reasoning, it is expected that cancer patients’ cognitive function declines faster prior to cancer diagnosis than cognitive function of controls.
Understanding the origin of cognitive impairment in cancer patients is essential for prevention and treatment. We aimed to contribute to this understanding by evaluating cognitive function of cancer patients longitudinally prior to the clinical manifestation of the disease. We evaluated the longitudinal change of cognitive function to learn about the effect of shared risk factors and cancer itself as determinants of cognition. This approach is superior to a cross-sectional comparison of absolute cognition levels prior to diagnosis because it includes all available assessments. Using the unique context of a population-based cohort, we compared cognitive trajectories between individuals prior to cancer diagnosis and individuals who remained cancer-free during follow-up.
Methods
Setting
We used data from the Rotterdam Study, a Dutch population-based prospective cohort. The initial cohort (RS-I) started in 1989 with 7983 participants aged 55 years and older who reside in the district Ommoord in Rotterdam, the Netherlands. The cohort was expanded with 3011 participants in 2000 (RS-II), followed by an additional inclusion of 3392 participants aged 45 years and older in 2006 (RS-III).
Participants were interviewed at home by a trained research assistant, followed by two visits to the research facility for laboratory assessments, imaging, and physical examinations. Follow-up examinations are aimed to take place every 3 to 5 years. The design of the Rotterdam Study has been previously described in detail (12).
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus Medical Center and by the Ministry of Health, Welfare and Sport of the Netherlands. Written informed consent was obtained from all participants.
Study Population
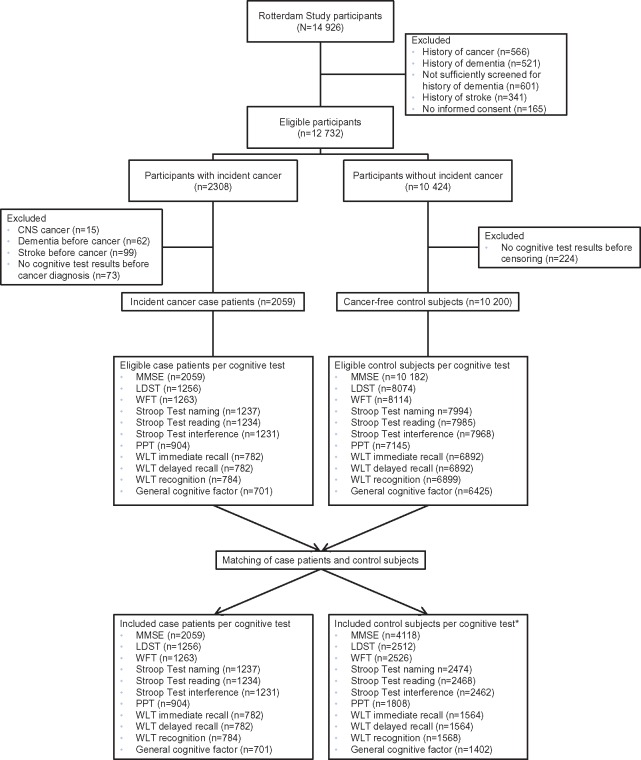
Of the 14 926 participants of the Rotterdam Study, we excluded those with a history of cancer at study entry (n = 566), prevalent dementia at study entry (n = 521), those who were insufficiently screened for prevalent dementia (n = 601), a history of stroke (n = 341), and those without informed consent to access medical records for follow-up (n = 165), leaving 12 732 eligible participants (Figure 1).
Figure 1.
Flow chart of study population, separately for cancer case patients and control subjects per cognitive test. *The number of assessments discarded after dementia or stroke diagnosis and after matching are presented in Supplementary Table 1 (available online). CNS = central nervous system; LDST = Letter-Digit Substitution Test, MMSE = Mini-Mental State Examination; PPT = Purdue Pegboard Test; WFT = Word Fluency Test; WLT = Word Learning Test.
Cases
Of the 2308 participants who were diagnosed with cancer during follow-up (between 1989 and 2013), we excluded those with primary CNS cancer (n = 15), dementia before cancer diagnosis (n = 62), stroke before cancer diagnosis (n = 99), and participants without cognitive test results prior to cancer diagnosis (n = 73), resulting in 2059 case patients.
Controls
From participants who remained cancer-free during follow-up (n = 10 424), we excluded cognitive test results after dementia or stroke diagnosis. Although this exclusion resulted in a lower number of assessments, it did not change the number of cancer-free participants, because participants with a history of dementia or stroke at study entry were already excluded (Supplementary Table 1, available online). Subsequently, we excluded participants without cognitive test results before the end of follow-up (n = 224), leaving 10 200 participants as eligible control subjects.
Matching Procedure
Each case patient was individually matched with two randomly selected cancer-free control subjects at the age of diagnosis of the case patient (index age). A participant was eligible as a control subject if he or she had at least one cognitive assessment before index age and no dementia or stroke diagnosis prior to index age. To avoid overmatching, we only matched on index age (13). Assessments of control subjects after the index age were discarded (Supplementary Table 1, available online). Matching started with the oldest case patient and was performed without replacement for each cognitive test separately.
Ascertainment of Cancer
Cancer incidence up to January 1, 2014, was based on medical records of general practitioners (including hospital discharge letters) and through linkage with Dutch Hospital Data, Netherlands Comprehensive Cancer Organization, and histology and cytopathology registries in the region. Incident cancer was defined as any primary malignant tumor, excluding nonmelanoma skin cancer. Diagnoses were coded independently by two physicians according to the International Classification of Diseases, 10th revision (14). In case of discrepancy, consensus was sought through consultation with a physician specialized in internal medicine. Date of diagnosis was based on date of biopsy (solid tumors) and laboratory assessment (hematologic tumors), or—if unavailable—date of hospital admission or discharge letter. Level of uncertainty of diagnosis was defined as follows: certain (pathology confirmed), probable (clinical diagnosis based on imaging or elevated tumor markers), and possible (suspicion based on symptoms or physical examination). Only pathology-confirmed cancers were included in the primary analysis. In sensitivity analyses, we included case patients with probable or possible cancer and excluded control subjects who had probable or possible cancer.
Cognitive Function Assessment
Cognitive function was assessed by a neuropsychological test battery during research center visits. Up to 2013, the following tests were administrated: Mini-Mental State Examination, Letter-Digit Substitution Test, Word Fluency Test, Stroop Test (reading, naming, and interference), Purdue Pegboard Test (right, left, and both hands), and 15-Word Learning Test (WLT; immediate recall, delayed recall, and recognition) (15–20).
A measure of global cognitive function was established by the general cognitive factor based on the Letter-Digit Substitution Test, Word Fluency Test , Stroop Test interference, sum-score of individual Purdue Pegboard Tests, and WLT delayed recall and was identified as the first unrotated component of a principal component analysis, which explained at least 46.1% of the total variance in individual cognitive tests (21). The general cognitive factor was only computed if all five individual tests were completed.
The total number of individuals differed per cognitive test because of different moments of implementation of cognitive tests in the examination program or because of missing data (Figure 1). All available cognitive test results prior to index age were included for analysis. An overview of the cognitive tests is provided in Supplementary Table 2 (available online).
Measurement of Covariates
During the home interview, we assessed education level (primary: primary education; lower: lower or intermediate general education, or lower vocational education; intermediate: intermediate vocational education or higher general education; or higher: higher vocational education or university), smoking status (never, current, or former), and alcohol use (yes or no). Symptoms of depression were evaluated with the Center for Epidemiologic Studies Depression scale (CES-D), which was converted to a sum-score (22). Body mass index (BMI; kg/m2) was computed from measurements of height and weight.
Statistical Analysis
Differences in characteristics between case patients and control subjects were investigated using the independent samples t test (for continuous variables with a normal distribution), the Wilcoxon signed-rank test (for continuous variables with a skewed distribution), and the χ2 test (for categorical variables). Next, we investigated the difference in cognitive change between case patients and control subjects. Each cognitive test was modeled with a two-level linear mixed model with the test result as the outcome and each observation representing one individual cognitive test result. Cognitive test results were transformed, if necessary, to reach an approximate normal distribution. When a transformation did not change the statistical significance, results were reported based on untransformed values for interpretation purposes.
Covariates were case-control status (cancer = 1 for case patients, cancer = 0 for control subjects) and time of cognitive assessments expressed as time to index age (eg, time = 0 for time at index age, time = −5 for 5 years prior to index age). An interaction term between these two variables reflects whether the change in cognitive function over time differs between case patients and control subjects. Other covariates related to both change in cognitive function (23–28) and cancer (29–31) were age at first test (continuous), sex (women or men), education level (primary, lower, intermediate, or higher), smoking status (never, current, or former), alcohol use (yes or no), CES-D sum-score (continuous), and BMI (continuous). In case of time-varying covariates—that is, smoking status, alcohol use, CES-D sum-score, and BMI—values of covariates measured closest to the date of cognitive assessment were used.
Missing data on covariates were generally between 0% and 2%, except for the CES-D sum-score, which was missing in 16% of the total study population. Missing values were replaced with mean (continuous) or mode (categorical) values of the observed data (case patients and control subjects combined). The model used is described in more detail in the Supplementary Methods (available online).
We performed separate analyses for the most frequent cancer sites (breast, prostate, colorectal, and lung) for which the matching procedure was repeated, with breast cancer case patients matched to female control subjects, and prostate cancer case patients to male control subjects only. In addition, we investigated whether change in cognitive function was different in case patients who had metastasized cancer at diagnosis compared with control subjects, excluding case patients with unknown tumor stage (n = 718 out of 2059).
To investigate the robustness of our findings, we conducted two sensitivity analyses: including case patients and excluding control subjects with probable or possible cancer (ie, cancer not confirmed by pathology) and discarding assessments of control subjects less than 5 years before the end of follow-up to minimize effects of potential undiagnosed cancer.
P values calculated with the independent samples t test, Wilcoxon signed-rank test, χ2 test, and linear mixed model were two-sided. A P value of less than .05 was considered statistically significant for the analyses to investigate differences in characteristics between case patients and control subjects and for the analyses using linear mixed models with the general cognitive factor as the primary outcome. Multiple testing for individual cognitive tests was accounted for by using the Bonferroni method so that a P value of less than .005 was considered statistically significant.
Statistical analyses were performed using SPSS (32) and the “nlme” package from R software Version 3.3.2 (33).
Results
Characteristics of Participants
At the first cognitive assessment, case patients were older than control subjects and were more often men and current smokers (Table 1). Furthermore, control subjects had a higher education compared with case patients. Mean (SD) age at cancer diagnosis was 73.8 years (8.3). Most frequently diagnosed cancer sites were prostate (31.7% among men), breast (29.2% among women), colorectal (16.0%), and lung (12.1%). Of the case patients with a known tumor stage (n = 1341), 280 had metastasized cancer at diagnosis (20.9%). More details are presented in Supplementary Table 3 (available online).
Table 1.
Characteristics of cases and their matched cancer-free controls at time of first cognitive assessment
| Characteristic | Study population |
P† | |
|---|---|---|---|
| Case patients (n = 2059) | Control subjects (n = 7403)* | ||
| Age, median (IQR), y | 64.7 (60.2–71.5) | 62.5 (58.3–70.6) | <.001 |
| Sex, No. (%) | <.001 | ||
| Women | 980 (47.6) | 4446 (60.1) | |
| Men | 1079 (52.4) | 2957 (39.9) | |
| Education, No. (%) | <.001 | ||
| Primary | 349 (16.9) | 1067 (14.4) | |
| Lower | 847 (41.1) | 3155 (42.6) | |
| Intermediate | 607 (29.5) | 2040 (27.6) | |
| Higher | 256 (12.4) | 1141 (15.4) | |
| Smoking, No. (%) | <.001 | ||
| Never | 452 (22.0) | 2149 (29.0) | |
| Current | 500 (24.3) | 1458 (19.7) | |
| Former | 1107 (53.8) | 3796 (51.3) | |
| Alcohol use, No. (%) | .99 | ||
| No | 374 (18.2) | 1344 (18.2) | |
| Yes | 1685 (81.8) | 6059 (81.8) | |
| CES-D sum-score, mean (SD) | 13.0 (4.0) | 13.8 (4.1) | <.001 |
| BMI, median (IQR), kg/m2 | 26.7 (24.5–29.2) | 27.0 (24.6–29.7) | <.001 |
| Age at time of cancer diagnosis, mean (SD), y | 73.8 (8.3) | ||
Control subjects were matched to case patients per individual cognitive test. Some control subjects were matched to case patients for different cognitive tests, whereas other control subjects were only matched to case patients for one cognitive test. The control subjects in this table represent all individual control subjects used for the different cognitive test analyses. BMI = body mass index; CES-D = Center for Epidemiol Studies Depression scale; IQR = interquartile range.
Two-sided P values were calculated using the independent samples t test (for continuous variables with a normal distribution), the Wilcoxon signed-rank test (for continuous variables with a skewed distribution), and the χ2test (for categorical variables) to investigate differences in characteristics between case patients and control subjects. A P value <.05 was considered statistically significant.
Change in Cognitive Function Prior to Cancer Diagnosis
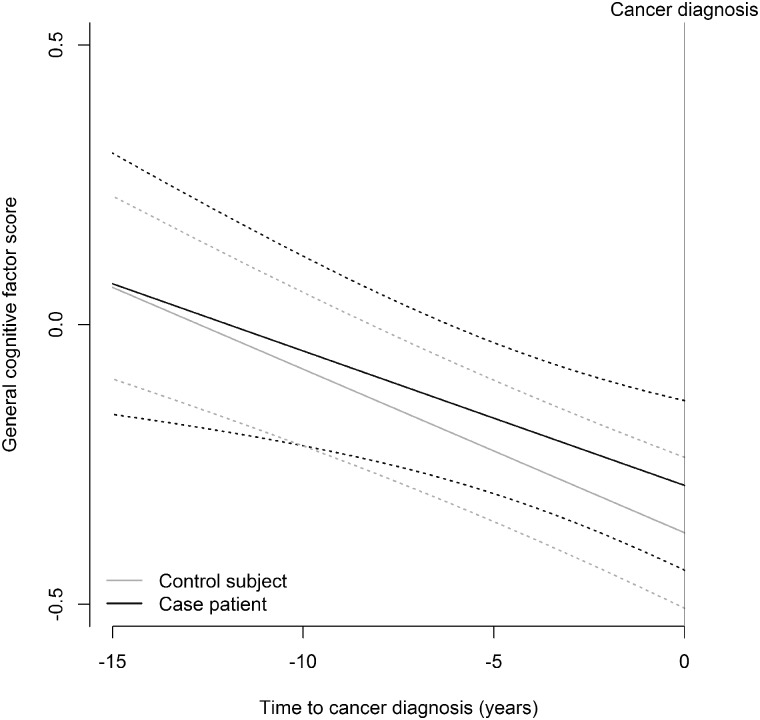
The WLT immediate recall declined among case patients by 0.05 units per year prior to index age (95% confidence interval [CI] = −0.09 to −0.01), whereas it increased by 0.01 units per year among control subjects (95% CI = −0.01 to 0.03; Pdifference =.003; Table 2). The difference was statistically significant after correction for multiple testing and corresponds to 2.4 years of age, given a decline in the WLT immediate recall of 0.25 units per 10 years (21). Although the change over time was different, there was no statistically significant difference between case patients and control subjects at index age. Furthermore, no statistically significant difference in change between case patients and control subjects was observed for the other nine individual cognitive test scores. Also, on the general cognitive factor, no statistically significant difference was found between case patients and control subjects (P = .61; Figure 2): case patients decreased by 0.02 units per year (95% CI = −0.05 to 0.00) vs 0.03 units per year among control subjects (95% CI = −0.04 to −0.02).
Table 2.
Estimated change of cognitive function by case-control status for individual cognitive tests and the general cognitive factor*
| Cognitive test | Case patients, No. | Control subjects, No. | Assessment, No. | Case patients | Control subjects | P for difference in change§ | Difference at index age‖ (95% CI) | P for difference at index age¶ |
|---|---|---|---|---|---|---|---|---|
| change/year†(95% CI) | change/year‡ (95% CI) | |||||||
| MMSE | 2059 | 4118 | 14 926 | −0.04 (−0.05 to −0.02) | −0.03 (−0.04 to −0.03) | .71 | 0.11 (−0.01 to 0.22) | .07 |
| LDST | 1256 | 2512 | 7064 | −0.29 (−0.35 to −0.23) | −0.31 (−0.33 to −0.28) | .43 | 1.07 (0.58 to 1.55) | <.001 |
| WFT | 1263 | 2526 | 7045 | −0.11 (−0.17 to −0.05) | −0.12 (−0.14 to −0.09) | .81 | 0.52 (0.08 to 0.96) | .02 |
| Stroop Test reading# | 1237 | 2474 | 6809 | 0.14 (0.09 to 0.19) | 0.11 (0.09 to 0.13) | .16 | −0.23 (−0.57 to 0.10) | .17 |
| Stroop Test naming# | 1234 | 2468 | 6839 | 0.15 (0.10 to 0.21) | 0.15 (0.13 to 0.18) | .99 | −0.68 (−1.16 to −0.20) | .006 |
| Stroop Test interference# | 1231 | 2462 | 6792 | 0.67 (0.38 to 0.95) | 0.64 (0.51 to 0.77) | .84 | −2.52 (−4.71 to −0.33) | .02 |
| PPT | 904 | 1808 | 4229 | −0.33 (−0.40 to −0.25) | −0.32 (−0.35 to −0.29) | .78 | 0.64 (0.19 to 1.09) | .005 |
| WLT immediate recall | 782 | 1564 | 3350 | −0.05 (−0.09 to −0.01) | 0.01 (−0.01 to 0.03) | .003 | −0.18 (−0.40 to 0.04) | .11 |
| WLT delayed recall | 782 | 1564 | 3395 | −0.03 (−0.09 to 0.02) | −0.02 (−0.04 to 0.01) | .56 | 0.00 (−0.29 to 0.30) | .99 |
| WLT recognition | 784 | 1568 | 3380 | −0.03 (−0.08 to 0.01) | −0.01 (−0.03 to 0.01) | .33 | −0.09 (−0.33 to 0.14) | .43 |
| General cognitive factor | 701 | 1402 | 2754 | −0.02 (−0.05 to 0.00) | −0.03 (−0.04 to −0.02) | .61 | 0.08 (−0.03 to 0.20) | .13 |
Results based on the model: for participant i and repeated measure j, assuming autocorrelation structure of the variance-covariance matrix of residuals and the general positive-definite matrix of the random part. BMI = body mass index; CES-D = Center for Epidemiologic Studies Depression scale; CI = confidence interval; LDST = Letter-Digit Substitution Test; MMSE = Mini-Mental State Examination; PPT = Purdue Pegboard Test; WFT = Word Fluency Test; WLT = Word Learning Test.
.
.
Test of using the linear mixed model, for Bonferroni adjusted conclusions about individual tests, two-sided P < .05/10 = .005 is statistically significant.
= difference between cases and controls at time = 0 (at index age).
Test of using the linear mixed model, for Bonferroni adjusted conclusions about individual tests, two-sided P < .05/10 = .005 is statistically significant.
Better performance corresponds to lower scores.
Figure 2.
Trajectories of the general cognitive factor scores reflecting global cognitive function for cases (prior to cancer diagnosis) and controls (prior to end of follow-up).
Separate analyses by cancer site revealed relatively homogeneous cognitive trajectories of both individual cognitive tests and the general cognitive factor (Table 3). Also, no statistically significant differences in cognitive change were observed for cancer case patients with metastasized disease at cancer diagnosis compared with cancer-free control subjects.
Table 3.
Estimated change of cognitive function by case-control status for individual cognitive tests and the general cognitive factor by cancer site*
| Cognitive test | Prostate cancer |
Breast cancer |
Colorectal cancer |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Change/year case patients† | Change/year control subjects‡ | P for difference in change§ | Change/year case patients† | Change/year control subjects‡ | P for difference in change§ | Change/year case patents† | Change/ year control subjects‡ | P for difference in change§ | |
| MMSE | −0.03 | −0.03 | .84 | −0.04 | −0.04 | .92 | −0.02 | −0.05 | .03 |
| LDST | −0.34 | −0.32 | .83 | −0.19 | −0.33 | .09 | −0.29 | −0.35 | .41 |
| WFT | −0.02 | −0.07 | .56 | −0.10 | −0.14 | .65 | −0.15 | −0.09 | .40 |
| Stroop Test reading‖ | 0.15 | 0.14 | .81 | 0.13 | 0.07 | .30 | 0.10 | 0.11 | .90 |
| Stroop Test naming‖ | 0.15 | 0.18 | .61 | 0.14 | 0.18 | .51 | 0.05 | 0.17 | .16 |
| Stroop Test interference‖ | 0.26 | 0.70 | .06 | 0.93 | 0.69 | .51 | 0.48 | 0.32 | .68 |
| PPT | −0.40 | −0.43 | .71 | −0.30 | −0.25 | .57 | −0.25 | −0.26 | .88 |
| WLT immediate recall | −0.06 | −0.02 | .29 | 0.03 | −0.01 | .58 | −0.11 | −0.01 | .03 |
| WLT delayed recall | 0.03 | −0.02 | .41 | 0.05 | −0.02 | .44 | −0.06 | 0.00 | .28 |
| WLT recognition | −0.07 | −0.04 | .61 | 0.06 | 0.03 | .63 | −0.07 | 0.02 | .05 |
| General cognitive factor | −0.03 | 0.02 | .12 | −0.04 | −0.02 | .41 | 0.07 | 0.06 | .74 |
| Cognitive test |
Lung cancer |
Metastasized cancer |
|||||
|---|---|---|---|---|---|---|---|
| Change/year case patients† | Change/year control subjects‡ | P for difference in change§ | Change/year case patients† | Change/year control subjects‡ | P for difference in change§ | ||
| MMSE | −0.04 | −0.03 | .49 | −0.04 | −0.04 | .86 | |
| LDST | −0.31 | −0.27 | .58 | −0.32 | −0.26 | .29 | |
| WFT | −0.10 | −0.15 | .51 | −0.07 | −0.10 | .66 | |
| Stroop Test reading‖ | 0.17 | 0.10 | .32 | 0.09 | 0.11 | .69 | |
| Stroop Test naming‖ | 0.24 | 0.11 | .06 | 0.16 | 0.14 | .71 | |
| Stroop Test interference‖ | 1.54 | 0.73 | .01 | 1.03 | 0.92 | .70 | |
| PPT | −0.32 | −0.35 | .80 | −0.33 | −0.32 | .88 | |
| WLT immediate recall | −0.07 | 0.02 | .10 | 0.06 | 0.02 | .46 | |
| WLT delayed recall | −0.08 | −0.01 | .35 | 0.07 | 0.02 | .43 | |
| WLT recognition | −0.02 | −0.03 | .83 | 0.02 | −0.00 | .64 | |
| General cognitive factor | 0.02 | 0.02 | .96 | −0.03 | −0.02 | .76 | |
|
| |||||||
Results based on the model: for participant i and repeated measure j, assuming autocorrelation structure of the variance-covariance matrix of residuals and the general positive-definite matrix of the random part. Models were run for each different cancer site separately. was not used for breast and prostate cancer. BMI = body mass index; CES-D = Center for Epidemiologic Studies Depression scale; LDST = Letter-Digit Substitution Test; MMSE = Mini-Mental State Examination; PPT = Purdue Pegboard Test; WFT = Word Fluency Test; WLT = Word Learning Test.
.
.
Test of using the linear mixed model, for Bonferroni adjusted conclusions about individual tests, two-sided P < .05/10 = .005 is statistically significant.
Better performance corresponds to lower scores.
Sensitivity Analyses
After inclusion of case patients (n = 143) and exclusion of eligible control subjects with probable or possible cancer, case patients still declined faster on the WLT immediate recall compared with control subjects (0.04 vs 0.00 units per year; P = .009). No statistically significant differences were observed for other cognitive test scores (Supplementary Table 4, available online).
After discarding assessments of control subjects less than 5 years before the end of follow-up to exclude effects of potentially undiagnosed cancer, there was no statistically significant difference in change on the WLT immediate between case patients and control subjects (P = .87; Supplementary Table 5, available online). The score of the WLT delayed recall declined by 0.04 units per year (95% CI = −0.12 to 0.04) among case patients compared with an increase by 0.03 units per year among control subjects (95% CI = −0.02 to 0.08). However, this difference was not statistically significant after correction for multiple testing (P = .04).
Discussion
This study investigated change in cognitive function among non-CNS cancer patients prior to cancer diagnosis using the unique setting of a large population-based study. There is a key need to understand the causes of cognitive impairment after non-CNS cancer. Case patients did not performed less as well over time in their ability to learn a list of words compared with cancer-free control subjects. However, this difference disappeared in sensitivity analyses when excluding the effects of potentially undiagnosed cancer. We therefore found no evidence in the current study that cognitive function changes differently over time between individuals who will be diagnosed with cancer and individuals who will remain free of cancer.
The hypothesis that cancer outside the CNS does impact the brain resulting in alterations of cognitive function has been posed by several studies that investigated cognitive function in cancer patients after diagnosis (34) and prior to any type of treatment including surgery. Five out of six studies observed cognitive impairment in patients compared with either study-specific control subjects or normative data (4–9). These observations, differentially explained by inflammation processes triggering neurotoxic cytokine responses, vascular changes, or oxidative stress (1,5,35), have been supported by preclinical studies showing that tumor-bearing, treatment-naive rodents can have impaired declarative memory (36–38). However, at this moment, we do not exactly know if and in what way processes may affect cognitive function and if specific cognitive domains may be particularly vulnerable.
How can we explain the disconnect between the current results and these previous findings? First, although most studies carried out after diagnosis and prior to subsequent treatment tried to adjust for the psychological impact of being recently confronted with a cancer diagnosis, residual confounding can still be a concern (39–41). This confounder is nonexistent in the current study. Second, recruiting patients who have been diagnosed recently with cancer can be challenging, resulting in small sample sizes and susceptibility for selection bias, whereas our study consisted of a large unselected group of both case patients and control subjects (42,43). Third, our results may not be directly comparable to previous studies because of differences in study design. In the current study, we looked at cognitive changes over time in the years preceding a cancer diagnosis, whereas the other studies only measured cognitive function once shortly after diagnosis. Also, in preclinical studies, cognitive function is assessed within a short time frame after the tumor has reached a certain size (36). Fourth, cancer patients in our study were somewhat older (mean age at diagnosis was 73.8 years) than patients in previous studies [mean age generally ranged from 48.6 to 60.5 years (4–8), only in one study was the mean age 79.8 years (9)]. It is questionable, however, whether this difference in age contributes to the discrepant results, because it would require the mechanism of cognitive impairment in cancer patients to be dependent on age.
Because we did not observe statistically significant differences in trajectories of cognitive function between cancer case patients and control subjects prior to cancer diagnosis, a strong role of shared risk factors for both cancer and cognitive impairment prevalent in our study population is less plausible. However, cancer itself could still be considered as a potential underlying cause for subtle cognitive impairment before diagnosis, because we have not evaluated cognitive function repeatedly within a short time frame, for example, in the weeks directly preceding cancer diagnosis.
This latter point is one of several limitations that we faced. Because of the design of the Rotterdam Study in which cognition is intended to be assessed every 3 to 5 years, we could not investigate cognitive function within smaller time frames directly preceding cancer diagnosis. Furthermore, by using linear mixed models, we assumed a linear change in cognitive function, which may have led to model misspecification if cognitive change is not linear. However, including time squared in the model did not improve the model fit and resulted in less power to detect differences in cognitive change. Therefore, we have chosen not to include nonlinear parameters in the model. In addition, we investigated the average cognitive trajectories by which we may not have been able to identify subgroups of patients who do have steeper cognitive declines prior to cancer diagnosis. Lastly, we did not have information about the location of metastases at time of cancer diagnosis and could therefore not exclude participants with brain metastases. However, we did not observe that cognitive function changed differently among case patients with metastasized disease than among control subjects.
In addition, our study has multiple and unique strengths. It is a population-based cohort with standardized ascertainment of cognitive function and cancer incidence, providing the opportunity to investigate change in cognitive function prior to cancer diagnosis. We studied an unselected sample of case patients and control subjects, thereby minimizing the effects of selection bias. By using linear mixed models, we were able to investigate the change in cognitive function per year. Furthermore, our study has by far the largest number of participants in this research area. Most previous studies had a sample size of 56 up to 174 patients (4–8), and even the largest study with 341 patients (9) is much smaller than the current study. This enabled us to investigate cognitive trajectories for different cancer sites. Lastly, we investigated the trajectory of the general cognitive factor in addition to the trajectories of the individual cognitive tests, because we did not have an indication for a specific cognitive domain to be affected.
In conclusion, we found no evidence that cognitive function declines differently over time among individuals who will be diagnosed with cancer prior to disease manifestation than among individuals who will remain cancer-free. Our results suggest that the role of shared risk factors for both cancer and cognitive impairment on cognitive function in cancer patients is limited. Future research needs to confirm our findings and to evaluate cognitive function within a short period before cancer diagnosis to estimate the effects of undiagnosed cancer on cognitive function more accurately.
Funding
This work was supported by the Dutch Cancer Society (grant number NKI-20157737). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam; Netherlands Organization for the Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry for Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam.
Notes
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest. We acknowledge the dedication, commitment, and contribution of inhabitants, general practitioners, and pharmacists of the Ommoord district who took part in the Rotterdam Study.
Supplementary Material
References
- 1. Ahles TA, Root JC, Ryan EL.. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012; 30(30):3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB.. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30(10):1080–1086. [DOI] [PubMed] [Google Scholar]
- 3. Janelsins MC, Kesler SR, Ahles TA, Morrow GR.. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–1913. [DOI] [PubMed] [Google Scholar]
- 5. Patel SK, Wong AL, Wong FL, et al. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. 2015;107(8):djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kesler SR, Adams M, Packer M, et al. Disrupted brain network functional dynamics and hyper-correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav. 2017;7(3):e00643.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinha P, Wong AWK, Kallogjeri D, Piccirillo JF.. Baseline cognition assessment among patients with oropharyngeal cancer using PROMIS and NIH toolbox. JAMA Otolaryngol Head Neck Surg. 2018;144(11):978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernstein LJ, Pond GR, Gan HK, et al. Pretreatment neurocognitive function and self-reported symptoms in patients with newly diagnosed head and neck cancer compared with noncancer cohort. Head Neck. 2018;40(9):2029–2042. [DOI] [PubMed] [Google Scholar]
- 9. Hshieh TT, Jung WF, Grande LJ, et al. Prevalence of cognitive impairment and association with survival among older patients with hematologic cancers. JAMA Oncol. 2018;4(5):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahles TA, Root JC.. Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol. 2018;14(1):425–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyon DE, Cohen R, Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. 2016;301:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32(9):807–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothman KJ. Modern Epidemiology. 6th ed.Philadelphia. PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 14.World Health Organization. International Statistical Classification of Diseases and Related Health Problems (10th Revision); 2010. Geneva: World Health Organization; https://icd.who.int/browse10/2010/en. Accessed February 26, 2019. [Google Scholar]
- 15. Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 16. Houx PJ, Jolles J, Vreeling FW.. Stroop interference: aging effects assessed with the Stroop color-word test. Exp Aging Res. 1993;19(3):209–224. [DOI] [PubMed] [Google Scholar]
- 17. van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J.. The letter digit substitution test: normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28(6):998–1009. [DOI] [PubMed] [Google Scholar]
- 18. Tiffin J, Asher EJ.. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32(3):234–247. [DOI] [PubMed] [Google Scholar]
- 19. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J.. Normative data for the animal, profession and letter M naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J Int Neuropsychol Soc. 2006;12(1):80–89. [DOI] [PubMed] [Google Scholar]
- 20. Bleecker ML, Bolla-Wilson K, Agnew J, Meyers DA.. Age-related sex differences in verbal memory. J Clin Psychol. 1988;44(3):403–411. [DOI] [PubMed] [Google Scholar]
- 21. Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, Ikram MA.. Patterns of cognitive function in aging: the Rotterdam study. Eur J Epidemiol. 2014;29(2):133–140. [DOI] [PubMed] [Google Scholar]
- 22. Mirza SS, de Bruijn RF, Direk N, et al. Depressive symptoms predict incident dementia during short- but not long-term follow-up period. Alzheimers Dement. 2014;10(suppl 5):S323–S329.e321. [DOI] [PubMed] [Google Scholar]
- 23. Lipnicki DM, Sachdev PS, Crawford J, et al. Risk factors for late-life cognitive decline and variation with age and sex in the Sydney memory and ageing study. PloS One. 2013;8(6):e65841.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scarmeas N, Albert SM, Manly JJ, Stern Y.. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;77(3):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anstey KJ, von Sanden C, Salim A, O’Kearney R.. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166(4):367–378. [DOI] [PubMed] [Google Scholar]
- 26. Ruitenberg A, van Swieten JC, Witteman JCM, et al. Alcohol consumption and risk of dementia: the Rotterdam study. Lancet. 2002;359(9303):281–286. [DOI] [PubMed] [Google Scholar]
- 27. Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. [DOI] [PubMed] [Google Scholar]
- 28. Kim S, Kim Y, Park SM.. Body mass index and decline of cognitive function. PLoS One. 2016;11(2):e0148908.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mouw T, Koster A, Wright ME, et al. Education and risk of cancer in a large cohort of men and women in the United States. PLoS One. 2008;3(11):e3639.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Danaei G, Vander Hoorn S, Lopez AD, Murray CJL, Ezzati M.. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–1793. [DOI] [PubMed] [Google Scholar]
- 31. Penninx B, Guralnik J, Havlik RJ, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90(24):1888–1893. [DOI] [PubMed] [Google Scholar]
- 32.IBM Corporation. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp; 2016. [Google Scholar]
- 33. Pinheiro J, Bates D, DebRoy S, Sarkar D.. nlme: Linear and Nonlinear Mixed Effects Models; 2018.
- 34. Wefel JS, Schagen SB.. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–275. [DOI] [PubMed] [Google Scholar]
- 35. Winocur G, Berman H, Nguyen M, et al. Neurobiological mechanisms of chemotherapy-induced cognitive impairment in a transgenic model of breast cancer. Neuroscience. 2018;369:51–65. [DOI] [PubMed] [Google Scholar]
- 36. Pyter LM, Cochrane SF, Ouwenga RL, Patel PN, Pineros V, Prendergast BJ.. Mammary tumors induce select cognitive impairments. Brain Behav Immun. 2010;24(6):903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walker AK, Sloan EK.. Aspirin blocks cancer-associated cognitive impairment. Brain Behav Immun. 2017;66:e21–e22. [Google Scholar]
- 38. Yang M, Kim J, Kim JS, et al. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36:147–155. [DOI] [PubMed] [Google Scholar]
- 39. Edwards B, Clarke V.. The psychological impact of a cancer diagnosis on families: the influence of family functioning and patients’ illness characteristics on depression and anxiety. Psycho-Oncology. 2004;13(8):562–576. [DOI] [PubMed] [Google Scholar]
- 40. Northouse LL. Psychological impact of the diagnosis of breast cancer on the patient and her family. J Am Med Womens Assoc (1972). 1992;47(5):161–164. [PubMed] [Google Scholar]
- 41. Goedendorp MM, Gielissen MF, Verhagen CA, Peters ME, Bleijenberg G.. Severe fatigue and related factors in cancer patients before the initiation of treatment. Br J Cancer. 2008;99(9):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wefel JS, Vardy J, Ahles T, Schagen SB.. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 43. Clapp JD, Luta G, Small BJ, et al. The impact of using different reference populations on measurement of breast cancer-related cognitive impairment rates. Arch Clin Neuropsychol. 2018;33(8):956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.