Abstract
The monotypic genus Phylloporopsis is described as new to science based on Phylloporus boletinoides. This species occurs widely in eastern North America and Central America. It is reported for the first time from a neotropical montane pine woodland in the Dominican Republic. The confirmation of this newly recognised monophyletic genus is supported and molecularly confirmed by phylogenetic inference based on multiple loci (ITS, 28S, TEF1-α, and RPB1). A detailed morphological description of P. boletinoides from the Dominican Republic and Florida (USA) is provided along with colour images of fresh basidiomata in habitat, line drawings of the main anatomical features, transmitted light microscopic images of anatomical features and scanning electron microscope images of basidiospores. The taxonomic placement, ecological requirements and distribution patterns of P. boletinoides are reviewed and the relationships with phylogenetically related or morphologically similar lamellate and boletoid taxa such as Phylloporus, Phylloboletellus, Phyllobolites and Bothia are discussed.
Keywords: Boletales, lamellate boletes, molecular phylogeny, taxonomy, Xerocomoideae
INTRODUCTION
Phylloporus is a genus of lamellate fungi in the family Boletaceae that is primarily distributed throughout the tropics (Kirk et al. 2008, Neves & Halling 2010, Neves et al. 2012, Zeng et al. 2013). Singer (1945b) moved Phylloporus to the subfamily Xerocomoideae due to its Phylloporus-type hymenophoral trama and olive-brown spore print. Bresinsky & Besl (2003) synonymised Phylloporus with Xerocomus based on molecular data, though only a few taxa were sampled in that study. Recent molecular phylogenetic analyses with more extensive sampling support the monophyly of Phylloporus, showing that Xerocomus is highly polyphyletic (Binder & Hibbett 2006, Šutara 2008, Nuhn et al. 2013, Wu et al. 2014, 2016) and inferring the placement of Phylloporus in an expanded Xerocomoideae that now also includes taxa with Boletus-type hymenophoral trama and pale yellow to brown spore prints (Šutara 2008, Wu et al. 2014, 2016).
Neves & Halling (2010) estimated the genus Phylloporus to include about 70 species, but several subsequent studies have described a number of additional taxa (Neves et al. 2010, 2012, García-Jiménez 2013, Zeng et al. 2013, Ye et al. 2014, Hosen & Li 2015, 2017). Species of Phylloporus form ectomycorrhizal (ECM) associations with plants in the Casuarinaceae, Dipterocarpaceae, Fabaceae, Fagaceae, Myrtaceae, and Pinaceae (Heinemann 1951, Corner 1970, Heinemann & Rammeloo 1987, Singer 1986, Watling 2008, Neves & Halling 2010, Neves et al. 2012, García-Jiménez 2013, Zeng et al. 2013, Ye et al. 2014, Hosen & Li 2015, 2017). Five species of Phylloporus have been described from North America (Neves 2007, Neves et al. 2010).
Phylloporus boletinoides is a lamellate to subporoid bolete that was formerly described based on material collected by Harry D. Thiers from Alachua Co., northern Florida (Smith & Thiers 1964) and subsequently reported from all along the Atlantic Coast of eastern and south-eastern USA south to the Gulf Coast (Singer et al. 1990, Singer & Williams 1992, Both 1993, Bessette et al. 2000, 2016, Justice 2008), down into Central America (Ortiz-Santana et al. 2007, Neves & Halling 2010), although not reported by Ortiz-Santana et al. (2007) as occurring in the Dominican Republic. It is a putative ECM partner of several American pine trees (Pinus spp., Pinaceae), but is also found in mixed pine and oak (Quercus spp., Fagaceae) forests (Smith & Thiers 1964, Singer et al. 1990, Both 1993, Bessette et al. 2000, 2016, Neves & Halling 2010). Singer et al. (1990) re-described the type collection and placed the species in Phylloporus sect. Fibulati. The species was originally assumed to represent an intermediate taxon between Suillus and Boletinus (Smith & Thiers 1964). Singer et al. (1990) tentatively placed P. boletinoides in Phylloporus sect. Manausenses but later argued that it belonged to a new section that was never formally established (Singer & Williams 1992). As a matter of fact, this species has always been regarded as retaining an isolated position within the genus because of its peculiar morphological features with special reference to the pallid-coloured hymenophore and unusual macro-chemical reactions.
Several collections of P. boletinoides from Belize, the Dominican Republic and Florida were carefully examined using morphological and molecular approaches. Outcomes revealed strong morphological affinities with other members of Phylloporus, however, phylogenetic analysis of fungal DNA sequences from four gene regions (ITS, 28S, TEF1-α, and RPB1) indicate they are distantly related and support the recognition of Phylloporopsis as a unique and as yet monotypic generic lineage in the Boletaceae, in phylogenetic sister inference to the poroid genus Bothia and the sequestrate genus Solioccasus.
This study also widens the geographical extension of P. boletinoides to the Dominican Republic, where it is reported for the first time and is found in association with Pinus occidentalis, although several specimens have been spotted directly growing on decayed wood or even on trunks of living trees, determining some uncertainty relative to its trophic status.
MATERIALS AND METHODS
Collection site and sampling
Specimens from Belize, Dominican Republic, and the USA were examined from several public herbaria (CFMR, F, FLAS, JBSD, MICH, USF), as well as private herbaria [personal herbaria of Claudio Angelini (ANGE) and Matteo Gelardi (MG)]. Acronyms of the public herbaria follow Thiers (2018), Herbarium numbers are cited for all collections from which morphological features were examined. Author citations follow the Index Fungorum, Authors of Fungal Names (www.indexfungorum.org/authorsoffungalnames.htm).
Morphological studies
Macroscopic descriptions, macro-chemical reactions (30 % NH4OH, 30 % KOH, FeSO4·7H2O) and ecological information, such as habitat, time of fruiting and associated plant communities accompanied the detailed field notes of the fresh basidiomata. Colour terms in capital letters (e.g. Light Buff, Plate XV) are from Ridgway (1912). Microscopic anatomical features were observed and recorded from revived dried material. Sections were rehydrated in water, 5 % potassium hydroxide (KOH), or in anionic solution saturated with Congo Red. All anatomical structures were measured from preparations in anionic Congo Red. Colours and pigments were described after examination in water and 5 % KOH. Measurements were made at 1000 × using an ocular micrometer. Basidiospores were measured directly from the hymenium of mature basidiomata, dimensions are given as (minimum) average ± standard deviation (maximum), Q = length/width ratio with the extreme values in parentheses, Qm = average quotient (length/width ratio) ± standard deviation and average spore volume was approximated as a rotation ellipsoid [V = (π.L.W2)/6 ± standard deviation]. The notation [n/m/p] indicates that measurements were made on “n” randomly selected basidiospores from “m” basidiomata of “p” collections. The width of each basidium was measured at the widest part, and the length was measured from the apex (sterigmata excluded) to the basal septum. Metachromatic, cyanophilic and iodine reactions were tested by staining the basidiospores in Brilliant Cresyl blue, Cotton blue and Melzer’s reagent, respectively. Line drawings of microstructures were traced free hand based on digital photomicrographs of rehydrated material. Scanning electron micrographs have been obtained using a JEOL JSM IT300LV (High Vacuum – Low Vacuum 10/650 Pa - 0.3-30kV) Scanning Electron Microscope (SEM) operating at 10 kV. Selected air dried lamellae from the hymenophore were fixed on pin stubs using a Carbon Conductive Cement glue and then sputter-coated with carbon to a thickness of 400 Å.
DNA extraction, PCR amplification and DNA sequencing
DNA extraction and PCR amplification were performed from dried basidiomata (Table 1) or from tissues preserved in CTAB buffer as described by Alvarado et al. (2015). Primers ITS1F and ITS4 (White et al. 1990, Gardes & Bruns 1993) were used for the ITS region; primers LR0R and LR5 (Vilgalys & Hester 1990, Rehner & Samuels 1994) were used for the 28S rDNA, EF1-983F and EF1-1567R (Rehner & Buckley 2005) for the translation elongation factor 1-α (TEF1-α) gene. Amplifications of DNA-directed RNA polymerase II subunit 1 region (RPB1) were attempted using the fungal primer pair RPB1-Af/RPB1-Cr and the bolete-specific primer pair RPB1-B-F/RPB1-B-R (Wu et al. 2014) but were unsuccessful. To increase amplification success, RPB1-primers specific to the Bothia clade were developed (Table 2). A touchdown PCR was used to amplify the RPB1 region with the newly developed primer pairs RPB1-32-F/RPB1-835-R, RPB1-147-F/RPB1-1091-R. The cycle parameters were as follows: (1) 94 oC for 2 min, (2) 94 °C for 40 s, (3) 66 °C for 40 s, minus 1 °C every cycle, (4) 72 °C 90 s, (5) repeat steps 2–4 for nine additional cycles, (6) 94 °C for 45 s, (7) 56 °C for 90 s, (8) 72 °C for 90 s, (9) repeat steps 6–8 for 35 cycles, (10) 72 °C for 300 s. The PCR products were purified with the Wizard SV Gel and PCR Clean-Up System (Promega) following manufacturer’s instructions and sequenced forward and reverse by MACROGEN Inc. (Seoul, Republic of Korea).
Table 1.
Specimens and sequences used for the molecular phylogenetic analyses (i.e., the ITS dataset and the 28S + TEF1-α and 28S + RPB1 combined datasets). Sequences newly generated for this study are highlighted in bold.
| Taxon | Voucher No. | Locality | ITS | 28S | TEF1-α | RPB1 |
|---|---|---|---|---|---|---|
| Afroboletus luteolus | 00-436 | Africa | - | KF030238 | KF030397 | - |
| Afrocastellanoa ivoryana | Arora 126 | Mukuvisi, Zimbabwe | - | KX685721 | KX685715 | - |
| Australopilus palumanus | REH 6791 | Queensland, Australia | - | JX889650 | JX889691 | - |
| Austroboletus fusisporus | HKAS75207 | China | - | JX889720 | JX889718 | - |
| Austroboletus gracilis | 112/96 | MA, USA | - | DQ534624 | KF030425 | KF030358 |
| Austroboletus mutabilis | BRI AQ0795793 | Queensland, Australia | - | KP242263 | - | KP242078 |
| Austroboletus subvirens | BRI AQ0794171 | Queensland, Australia | - | KP242227 | - | KP242045 |
| Baorangia bicolor | MB07-001 | NY, USA | - | KF030246 | - | KF030370 |
| Baorangia pseudocalopus | HKAS 63607 | China | - | KY418895 | - | KJ184564 |
| HKAS 75739 | China | - | KJ184558 | - | KF030369 | |
| HKAS 75081 | Nanhua County, Yunnan Prov., China | - | KF112356 | KF112168 | - | |
| Boletus aereus | REH 8721 | Humboldt County, California, USA | - | KF030339 | - | KF030377 |
| Boletus edulis | Be3 | Bavaria, Germany | - | KF030282 | GU187682 | - |
| BD380 | Colorado, USA | - | HQ161848 | - | - | |
| Boletus pulchriceps | DS4514 | Chiricahua Mnts, AZ, USA | - | KF030261 | KF030409 | - |
| Boletus rufomaculatus | 4414 | Chestnut Ridge Park, NY, USA | - | KF030248 | KF030406 | KF030369 |
| Boletus semigastroideus | PBM3076 | Auckland, New Zealand | - | KF030352 | KF030430 | - |
| Boletus variipes var. fagicola | A.H. Smith 4249 | Cheboygan Co, Michigan, USA | - | JQ327014 | JQ327017 | - |
| Borofutus dhakanus | HKAS 73792 | Bangladesh | - | JQ928617 | JQ928575 | - |
| Bothia castanella | MB 03-053 | MA, USA | DQ867110 | DQ867117 | KF030421 | KF030382 |
| NY28003 | NY, USA | DQ867111 | - | - | - | |
| NY8669 | NY, USA | DQ867112 | - | - | - | |
| NY28002 | NY, USA | DQ867113 | - | - | - | |
| MB 03-067 | MA, USA | DQ867114 | - | - | - | |
| Bothia fujianensis | HKAS 82693 | Fujian Prov., China | KM269196 | - | - | - |
| HKAS 82694 | Fujian Prov., China | KM269195 | KM269193 | KM272860 | - | |
| Buchwaldoboletus lignicola | HKAS 76674 | Yichun, Heilongjiang Prov., China | - | KF112350 | KF112277 | KF112642 |
| Butyriboletus appendiculatus | Bap1 | Bavaria, Germany | - | AF456837 | JQ327025 | - |
| Butyriboletus roseoflavus | HKAS 54099 | China | - | JX290184 | - | KF739741 |
| Caloboletus firmus | MB 06-060 | Chestnut Ridge Park, NY, USA | - | KF030278 | KF030408 | KF030368 |
| Caloboletus inedulis | MB 06-044 | Erie Co., NY, USA | - | JQ327013 | JQ327020 | KF030362 |
| Chalciporus piperatus | MB 04-001 | Rutland State Park, MA, USA | - | DQ534648 | GU187690 | GU187453 |
| Fistulinella prunicolor | REH 9502 | Fraser Island, Queensland, Australia | - | JX889648 | JX889690 | - |
| Gymnogaster boletoides | REH 9455 | Cooloola, Queensland, Australia | - | JX889673 | JX889683 | - |
| Gyrodon lividus | Gl1 | Bavaria, Germany | - | AF098378 | GU187701 | - |
| Harrya chromapes | HKAS 50527 | Dêqên, Yunnan Prov., China | - | KF112437 | KF112270 | - |
| Heliogaster columellifer | KPM-NC 23012 | Odawara, Kanagawa Pref., Japan | - | KX685724 | KX685718 | - |
| Hourangia cheoi | HKAS 52269 | China | - | KF112385 | KF112286 | - |
| Hourangia microcarpa | HKAS 83763 (Wu1324) | China | - | KP136945 | KP136923 | - |
| Hourangia nigropunctata | HKAS 76657 | China | - | KF112388 | KF112287 | - |
| Hourangia sp. | HKAS 68178 | China | - | KF112453 | KF112301 | - |
| Imleria badia | xb2 | Bavaria, Germany | - | KF030357 | KF030422 | - |
| S-F119691 | Sweden | - | KJ806971 | KJ806969 | - | |
| Imleria obscurebrunnea | HKAS 52557 | Ning’er, Yunnan Prov., China | - | KF112374 | KF112190 | KC215225 |
| Lanmaoa angustispora | HKAS 74759 | China | - | KM605140 | - | KM605167 |
| Lanmaoa asiatica | HKAS 63592 | Heqing, Yunnan Prov., China | - | KM605142 | KM605152 | KM605163 |
| Lanmaoa carminipes | MB 06-061 | Erie Co., NY, USA | - | JQ327001 | JQ327022 | - |
| Leccinellum aff. griseum | KPM-NC 17831 | Hyogo Pref., Japan | - | JN378508 | JN378449 | - |
| Leccinellum cremeum | HKAS 90639 | China | - | - | - | KT990936 |
| Leccinellum crocipodium | KPM-NC 18041 | Yazu-cho, Tottori Pref., Japan | - | KC552053 | KC552094 | - |
| Leccinum monticola | HKAS 76669 | China | - | KF112443 | - | KF112592 |
| Leccinum scabrum | KPM-NC 17840 | Burn O’ Vat, Scotland, UK | - | JN378515 | JN378455 | - |
| Leccinum versipelle | KPM-NC 17833 | Scotland, UK | - | JN378514 | JN378454 | - |
| Neoboletus magnificus | HKAS 74939 | Baoshan, Yunnan Prov., China | - | KF112320 | KF112148 | - |
| Nigroboletus roseonigrescens | GDGM 43238 | Guangdong Prov., China | - | KT220588 | KT220595 | - |
| Octaviania decimae | KPM-NC 17763 | Mt. Hiei, Kyoto Pref., Japan | - | JN378465 | JN378409 | - |
| Octaviania kobayasii | KPM-NC 17785 | Mt. Kasuga, Nara Pref., Japan | - | JN378478 | JN378420 | - |
| Octaviania nonae | KPM-NC 17748 | Amami-oshima, Kagoshima Pref., Japan | - | JN378459 | JN378403 | - |
| Octaviania tasmanica | MEL2341996 | Tasmania, Australia | - | JN378495 | JN378436 | - |
| Octaviania yaeyamaensis | KPM-NC 17819 | Ishigaki Isl., Okinawa Pref., Japan | - | JN378491 | JN378432 | - |
| Paragyrodon sphaerosporus | MB06-066 | Iowa, USA | - | GU187593 | - | - |
| Paxillus vernalis | MB062 (CUW) | China | - | AY645059 | - | - |
| Phylloboletellus chloephorus | XAL3388 | Veracruz, Municipio Coatepec, El Grande, Mexico | - | DQ534658 | - | - |
| Phylloporopsis boletinoides | JBSD127411 | Jarabacoa, Dominican Republic | MH571675 | MH571711 | MH588312 | - |
| JBSD127412 | Jarabacoa, Dominican Republic | MH571676 | MH571712 | MH588313 | - | |
| JBSD127413 | Jarabacoa, Dominican Republic | MH571677 | MH571713 | MH588314 | - | |
| JBSD127414 | Jarabacoa, Dominican Republic | MH571678 | MH571714 | MH588315 | - | |
| FLAS-F-60407 | Putnam County, Florida, USA | MG845193 | - | - | - | |
| FLAS-F-60413 | Putnam County, Florida, USA | MG845194 | - | - | - | |
| FLAS-F-61158 | Putnam County, Florida, USA | MH211774 | - | - | - | |
| Farid 617 (USF 296126) | Tampa, Florida, USA | MG817716 | MG817715 | - | MG820263 | |
| CORT014483 | Mountain Pine Ridge, Belize | MH571679 | MH571715 | MH588316 | - | |
| CORT010991 | Kountze, Texas, USA | - | MH571716 | MH588317 | - | |
| F1118420 | Sarasota, Florida, USA | MH571680 | MH571717 | - | - | |
| Phylloporus attenuatus | HKAS 76168 (holotype) | Tangail, Bangladesh | - | NG_059569 | KR094791 | - |
| Phylloporus bellus | HKAS 56763 | Yunnan, SW China | - | JQ967196 | JQ967153 | - |
| Phylloporus brunneiceps | HKAS 56903 | Yunnan, SW China | - | JQ967198 | JQ967155 | - |
| Phylloporus catenulatus | HKAS 76157 | Bangladesh | - | KR094779 | KR094789 | - |
| Phylloporus gajari | HKAS 76158 | Gazipur, Bangladesh | - | KR231697 | KR231695 | - |
| Phylloporus imbricatus | HKAS 54647 | Yunnan, SW China | - | JQ967202 | JQ967159 | - |
| HKAS 68642 | China | - | KF112398 | KF112299 | - | |
| Phylloporus leucomycelinus | HKAS 74678 | eastern USA | - | JQ967206 | JQ967163 | - |
| Phylloporus luxiensis | HKAS 57036 | Yunnan, SW China | - | JQ967207 | JQ967164 | - |
| HKAS 75077 | China | - | KF112490 | KF112298 | KF112636 | |
| Phylloporus maculatus | HKAS 56683 | Yunnan, SW China | - | JQ967210 | JQ967167 | - |
| Phylloporus pachycystidiatus | HKAS 54540 | Yunnan, SW China | - | JQ967211 | JQ967168 | - |
| Phylloporus parvisporus | HKAS 54768 | Yunnan SW China | - | JQ967214 | JQ967171 | - |
| Phylloporus pelletieri | Pp1 | Austria | - | AF456818 | JQ327036 | KF030390 |
| Phylloporus pelletieri | Q7199c | Slovakia | - | JQ003668 | - | - |
| K 128205 | England, UK | - | JQ967215 | - | - | |
| Phylloporus rhodoxanthus | SAR 89.457 | eastern USA | - | U11925 | - | - |
| MAN075 | eastern USA | - | JQ003674 | - | - | |
| REH8714 | eastern USA | - | JQ003675 | - | - | |
| MAN099 | eastern USA | - | JQ003676 | - | - | |
| JLM1808 | eastern USA | - | JQ003688 | - | - | |
| BD374 | - | HQ161851 | - | HQ161820 | ||
| Phylloporus rubeolus | HKAS 52573 | Yunnan, SW China | - | JQ967216 | JQ967172 | - |
| Phylloporus rubrosquamosus | HKAS 54542 | Yunnan, SW China | - | JQ967217 | JQ967173 | - |
| HKAS 52552 | China | - | KF112391 | KF112289 | - | |
| Phylloporus rufescens | HKAS 59722 | Hainan, southern China | - | JQ967220 | JQ967176 | - |
| Phylloporus yunnanensis | HKAS 52225 | Yunnan, SW China | - | JQ967222 | JQ967178 | - |
| Phylloporus sp. | HKAS 74679 | Hunan, central China | - | JQ967228 | JQ967184 | - |
| HKAS 74680 | Fujian, SE China | - | JQ967229 | JQ967185 | - | |
| HKAS 74681 | Hainan, southern China | - | JQ967227 | JQ967183 | - | |
| HKAS 74682 | Yunnan, SW China | - | JQ967230 | JQ967186 | - | |
| HKAS 74683 | Yunnan, SW China | - | JQ967231 | JQ967187 | - | |
| HKAS 74684 | Fujian, SE China | - | JQ967232 | JQ967188 | - | |
| HKAS 74685 | Yunnan, SW China | - | JQ967233 | JQ967189 | - | |
| HKAS 74687 | Yunnan, SW China | - | JQ967235 | JQ967190 | - | |
| HKAS 74688 | Yunnan, SW China | - | JQ967236 | JQ967191 | - | |
| HKAS 74689 | Hainan, southern China | - | JQ967237 | JQ967192 | - | |
| Porphyrellus brunneus | REH 9527 | Fraser Island, QLD, Australia | - | JX889647 | JX889689 | - |
| Porphyrellus porphyrosporus | KPM-NC 22667 | Mt. Tarumae, Hokkaido, Japan | KX685713 | KX685722 | KX685716 | - |
| KPM-NC 25017 | Rishiri Island, Hokkaido, Japan | KX685714 | KX685723 | KX685717 | - | |
| MB 97-023 | Walhalla, Bavaria, Germany | DQ534563 | DQ534643 | GU187734 | - | |
| HKAS 76771 | Yanbian, Jilin Prov., China | - | KF112482 | KF112243 | - | |
| Porphyrellus sp. | HKAS 53366 | Sanming, Fujian Prov., China | - | KF112480 | KF112241 | - |
| HKAS 75078 | Chuxiong, Yunnan Prov., China | - | KF112481 | KF112242 | - | |
| Pseudoboletus parasiticus | Xps1 | Bavaria, Germany | - | AF050646 | KF030443 | - |
| Retiboletus fuscus | HKAS59460 | Yunnan Prov., China | - | JQ928626 | JQ928580 | - |
| Retiboletus griseus | Both sn | NY, USA | - | KF030308 | KF030414 | KF030373 |
| Rossbeevera griseovelutina | TNS-F-36989 | Hyogo, Japan | - | KC552031 | KC552076 | - |
| Rossbeevera vittatispora | MEL2128491 | NSW, Australia | - | KX685725 | KX685719 | - |
| Royoungia boletoides | Trappe 27456 | NSW, Australia | - | JX889655 | JX889696 | - |
| Rubroboletus rhodosanguineus | 4252 | Chestnut Ridge Park, NY, USA | - | KF030252 | KF030412 | - |
| Solioccasus polychromus | J. Trappe 15399 | Australia | JX888459 | - | - | - |
| REH 9417 | Queensland, Australia | - | JQ287642 | JQ287644 | - | |
| Spongiforma thailandica | DED 7873 | Thailand | - | EU685108 | KF030436 | - |
| Strobilomyces strobilaceus (as S. floccopus) | Sf1 | Bavaria, Germany | - | DQ534626 | JQ327037 | - |
| Suillellus amygdalinus | 112605ba | Mendocino Co., CA, USA | - | JQ326996 | JQ327024 | - |
| Sutorius aff. eximius | HKAS 52672 | Kunming, Yunnan Prov. CHINA | - | KF112399 | KF112207 | - |
| Sutorius eximius | REH 8594 | Jardin de Dota, Costa Rica | - | JQ327008 | JQ327027 | - |
| Turmalinea persicina | KPM-NC 18001 | Iwakura Kyoto Pref., Japan | - | KC552038 | KC552082 | - |
| Tylopilus alpinus | HKAS 55438 | China | - | KF112404 | - | KF112538 |
| Tylopilus ballouii | Osmundson 1198 | Thailand | - | EU430740 | - | EU434340 |
| REH 9467 | Fraser Island, Queensland, Australia | - | JX889676 | JX889686 | - | |
| Tylopilus felleus | AT2001011 | Uppsala, Sweden | - | JQ326993 | JQ327015 | |
| HKAS 90203 | China | - | KT990545 | - | KT990913 | |
| MCVE98230 | Italy | JF908787 | - | - | - | |
| Tylopilus ferrugineus | MB 06-053 | Erie Co., NY, USA | - | JQ326994 | JQ327016 | - |
| Tylopilus microsporus (T. neofelleus) | HKAS 59661 | Yunnan Prov., China | - | KF112450 | KF112225 | - |
| Tylopilus otsuensis | HKAS 53401 | Chenzhou, Hunan, China | - | KF112449 | KF112224 | - |
| Tylopilus plumbeoviolaceus | MB06-056 | NY, USA | - | KF030350 | KF030439 | - |
| Veloporphyrellus alpinus | HKAS 57490 | Yunnan Prov., China | - | KF112380 | KF112209 | KF112555 |
| Xerocomellus chrysenteron | Xch1 | Bavaria, Germany | - | AF050647 | KF030415 | - |
| Xerocomellus zelleri | REH 8724 | Humbolt Co., CA, USA | - | KF030271 | KF030416 | KF030366 |
| Xerocomus magniporus | HKAS 59820 | Yunnan, SW China | - | JQ678699 | JQ967195 | - |
| Xerocomus perplexus | MB00-005 | USA | - | JQ003702 | KF030438 | - |
| Xerocomus subtomentosus | KM167686 | England, U.K. | - | KC215222 | - | - |
| Xs1 | Bavaria, Germany | - | AF139716 | JQ327035 | KF030391 | |
| Zangia citrina | HKAS 52684 | China | - | HQ326941 | - | - |
| Zangia erythrocephala | HKAS 75046 | Nujiang, Yunnan, Prov., China | - | KF112414 | KF112269 | - |
| Zangia roseola | HKAS 52661 | China | - | JQ928623 | - | JQ928595 |
| Uncultured Boletaceae | clone 47C_G1_H9 | Jonathan Dickinson State Park, Hobe Sound, Florida, USA | KX899732 | - | - | - |
| clone 4C_G2_C3 | Big Lagoon Start Park, Pensacola, FL, USA | KX899785 | - | - | - |
Table 2.
Newly designed RPB1-primers specific for the Bothia clade.
| Primer name | Sequence (5′ → 3′) |
|---|---|
| RPB1-32-F | AGGCYGATATCGTGAGTCGC |
| RPB1-147-F | CTCGAGYTATCGAGGCGT |
| RPB1-835-R | ACCCTCRTCYTCRTCCTTGGG |
| RPB1-1091-R | CCATCYACYGCTATACTCGG |
Sequence alignment, data set assembly and phylogenetic analyses
The sequences obtained in this study were checked and assembled using Geneious v. 11.1.4 (Kearse et al. 2012) and compared to those available in GenBank database by using the Blastn algorithm (Altschul et al. 1990). Chromatograms were examined and manually edited for accuracy. Sequences were submitted to GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and accession numbers are reported in Table 1. Homologous sequences from vouchered specimens and from environmental samples were selected and retrieved from Halling et al. (2007), Trappe et al. (2013), Zeng et al. (2013), Zhu et al. (2015) and Orihara & Smith (2017). A general combined Maximum likelihood tree including all the Boletaceae sequences deposited in GenBank and UNITE (http://unite.ut.ee/) databases was generated to detect the phylogenetic position of our collections in the major clades of Boletaceae as circumscribed by Wu et al. (2014) (data not shown). Consequently, phylogenetic analyses were restricted to the major clade including P. boletinoides sequences and to selected genera in the Boletaceae.
Three phylogenetic analyses were performed: two large phylogenetic analysis using a 28S/TEF1-α dataset and a 28S/RPB1 dataset were focused on the generic position of P. boletinoides in the Boletaceae. According to the results by Orihara & Smith (2017), species of Paxillaceae were chosen as outgroup taxa for the combined datasets. The third phylogenetic analysis based only on an ITS dataset was restricted to the taxa closely related to P. boletinoides; Tylopilus felleus was used as outgroup taxon Alignments were generated for the ITS, 28S, TEF1-α, and RPB1 datasets with MAFFT (Katoh et al. 2002) with default conditions for gap openings and gap extension penalties. Alignments were then manually adjusted using Geneious v. 11.1.4 (Kearse et al. 2012). We estimated the best fit substitution model for each single alignment using the Bayesian information criterion (BIC) with jModelTest 2 (Darriba et al. 2012) and therefore selected the GTR+G model for all alignments. The ITS dataset was not partitioned.
Phylogenetic hypotheses were constructed with Bayesian inference (BI) and Maximum likelihood (ML) criteria. The BI was performed with MrBayes v. 3.2.6 (Ronquist et al. 2012) with one cold and three incrementally heated simultaneous Monte Carlo Markov chains (MCMC) run for 10 M generations, under the selected evolutionary model. Two simultaneous runs were performed independently. Trees were sampled every 1 000 generations, resulting in sampling of 10 001 trees per single run with the first 2 500 trees (25 %) discarded as burn-in. For the remaining trees of the two independent runs, a majority rule consensus tree showing all compatible partitions was computed to obtain estimates for Bayesian posterior probabilities (BPP).
ML analysis was performed using RAxML v. 7.3.2 (Stamatakis 2006) with 1 000 bootstrap replicates (Felsenstein 1985) and the GTRGAMMA algorithm. Support values from bootstrapping runs (MLB) were mapped on the best ML tree using the “-f a” option of RAxML and “-x 12345” as a random seed to invoke the novel rapid bootstrapping algorithm. BI and ML analyses were run on the CIPRES Science Gateway (Miller et al. 2010). Only BPP values ≥ 0.95 and MLB values ≥ 70 %, are reported in the resulting trees (Figs 1–3). Branch lengths were estimated as mean values over the sampled trees. Pairwise percent identity values (P %IV) of the ITS sequences were calculated using Geneious v. 11.1.4 (Kearse et al. 2012). Alignments and phylogenetic trees are available at TreeBASE (www.treebase.org) under ID S22978.
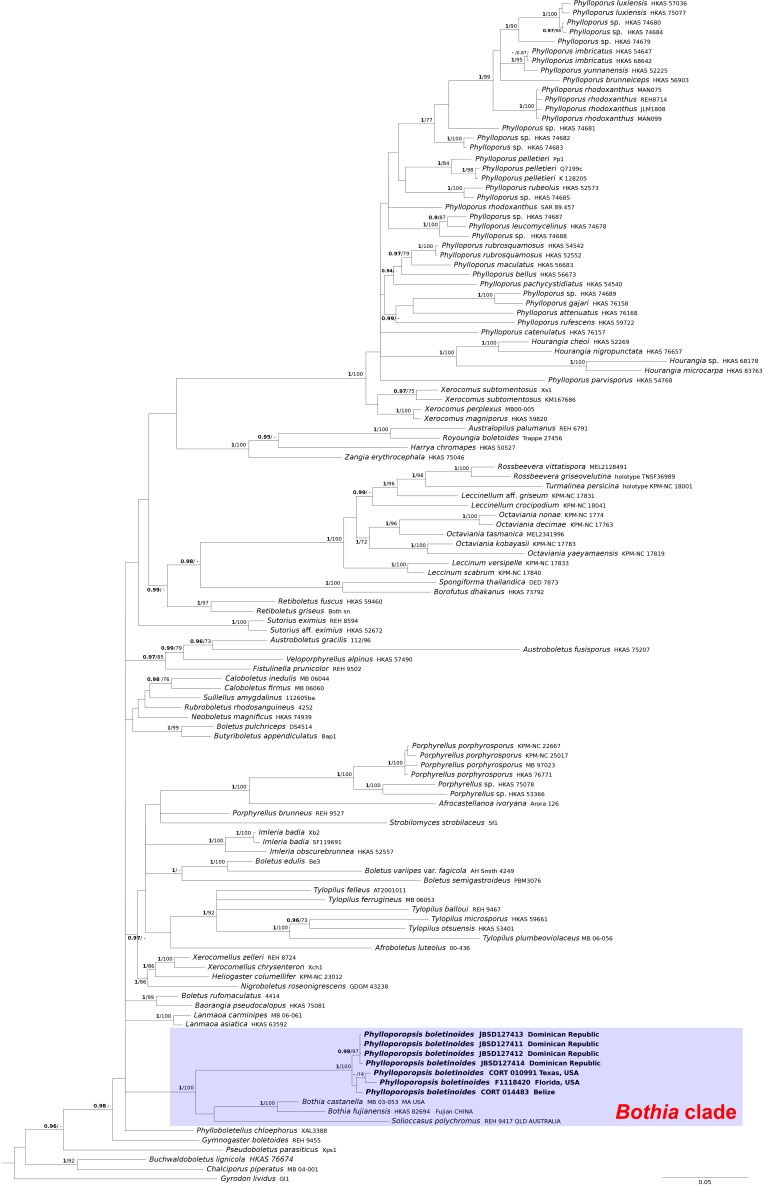
Fig. 1.
Phylogeny of the Boletaceae based on a Bayesian and Maximum likelihood inference analysis of a combined matrix of two nuclear gene regions (28S and TEF1-α). Bayesian posterior probability (BPP) values (in bold) ≥ 0.95 and Maximum likelihood bootstrap (MLB) values ≥ 70 % are shown on the branches. Newly sequenced collections are in bold.
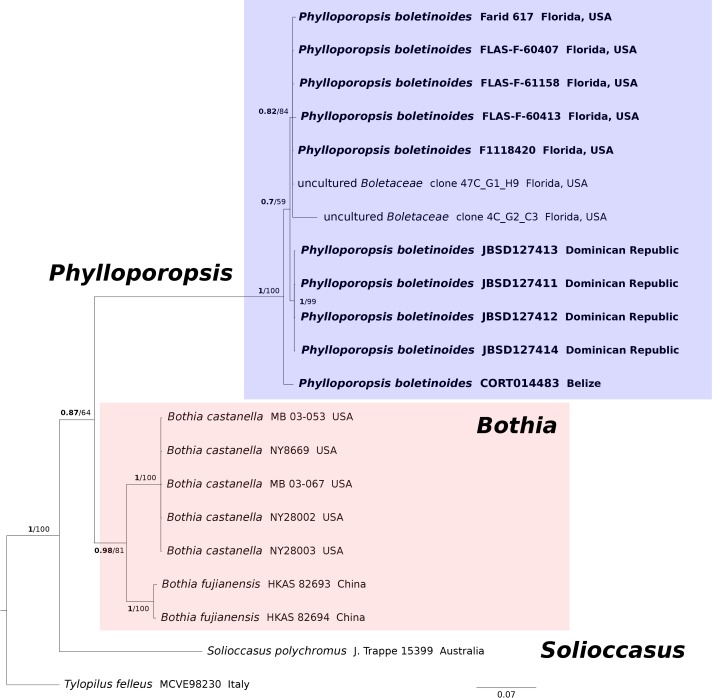
Fig. 3.
Bayesian ITS phylogeny restricted to the clade including Phylloporopsis (Bothia clade). BPP values (in bold) ≥ 0.95 and MLB values ≥ 70 % are shown on the branches. Newly sequenced collections are in bold.
RESULTS
Molecular analysis
Both Bayesian and Maximum Likelihood analyses produced comparable topologies and therefore only Bayesian trees with BPP and MLB values are shown (Figs 1–3). The combined 28S/TEF1-α and 28S/RPB1 dataset comprised 123 and 45 taxa, respectively. The ITS dataset comprised 21 taxa.
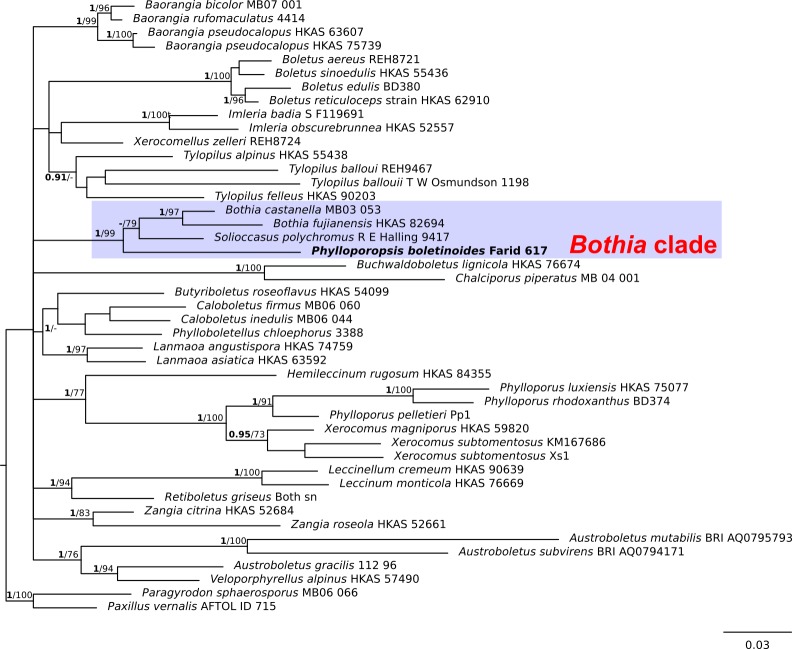
In the 28S/TEF-α dataset (Fig. 1), all the P. boletinoides collections (from Dominican Republic, Belize, Florida and Texas) form a strongly supported clade (BPP = 1.0, MLB = 100 %) which is part of a larger clade that includes Bothia and Solioccasus (BPP = 1.0, MLB = 100 %). We hereafter refer to this clade that includes Bothia, Solioccasus and P. boletinoides as the Bothia clade. Phylloporus clusters with Xerocomus and Hourangia in a strongly supported clade (BPP = 1.0, MLB = 100 %) that is distantly related to the Bothia clade. In the combined 28S/RPB1 dataset (Fig. 2), P. boletinoides was strongly supported in the Bothia clade (BPP = 1.0, MLB = 99 %). Phylloporus clusters with Xerocomus in a strongly supported clade (BPP = 1.0, MLB = 100 %). The other boletoid genus characterized by a lamellate hymenophore, Phylloboletellus, is resolved in an isolated and uncertain position within the Boletaceae (Fig. 1, 2). In the ITS analysis (Fig. 3), the newly generated sequences of P. boletinoides (BPP = 1, MLB = 100 %) are closely related to two environmental sequences: KX899732 (clone 47C_G1_H9), an environmental sequence from Jonathan Dickinson State Park, Hobe Sound, FL, USA, and KX899785 (clone 4C_G2_C3), an environmental sequence from Big Lagoon State Park, Pensacola, FL, USA. Both sequences were obtained from ectomycorrhizal samples on Pinus clausa (Sand Pine). The pairwise % identity values (P %IV) of the ITS sequences of the Phylloporopsis clade is 98 %.
Fig. 2.
Phylogeny of the Boletaceae based on a Bayesian and Maximum likelihood inference analysis of a combined matrix of two nuclear gene regions (28S and RPB1). Bayesian posterior probability (BPP) values (in bold) ≥ 0.95 and Maximum likelihood bootstrap (MLB) values ≥ 70 % are shown on the branches. Newly sequenced collections are in bold.
TAXONOMY
Phylloporopsis Angelini, A. Farid, Gelardi, M.E. Smith, Costanzo, & Vizzini, gen. nov. MycoBank MB828149.
Etymology: the generic epithet refers to the morphological affinities shared with Phylloporus.
Basidiomata pileate-stipitate with lamellate to subporoid hymenophore, epigeal, evelate, medium-small sized; pileus convex to applanate, velvety-tomentose to fibrillose; hymenophore lamellate to subporoid with anastomosing and interveined gills, strongly decurrent, beige to olive-cream or olive-buff; stipe solid to sometimes hollow at maturity, dry, pruinose to longitudinally fibrillose, reticulation absent; basal mycelium whitish to yellowish, context firm, whitish but cream-yellowish in the stipe; tissues unchangeable or turning light blue especially on hymenophore and pileus context when injured or exposed; taste mild to slightly bitter; olive-brown spore print; purplish-pink or reddish reaction with ammonia on pileus cuticle; basidiospores smooth, ellipsoid-fusiform, spore wall cyanophilic; pleuro-, cheilo- and caulocystidia present; trichodermal pileipellis; hymenophoral trama bilateral-divergent of the “Phylloporus-type”; lateral stipe stratum absent; clamp connections absent; ontogenetic development gymnocarpic. According with the phylogenetic analysis of the combined ITS, 28S, TEF1-α, and RPB1 sequences the genus is unrelated to Phylloporus and close but separated from Bothia and Solioccasus.
Typus generis: Phylloporopsis boletinoides (A.H. Smith & Thiers) Vizzini, Angelini, A. Farid, Gelardi, Costanzo, & M.E. Smith
Phylloporopsis boletinoides (A.H. Smith & Thiers) Vizzini, Angelini, A. Farid, Gelardi, Costanzo & M.E. Smith comb. nov. MycoBank MB828150. Figs 4–6.
Fig. 4.
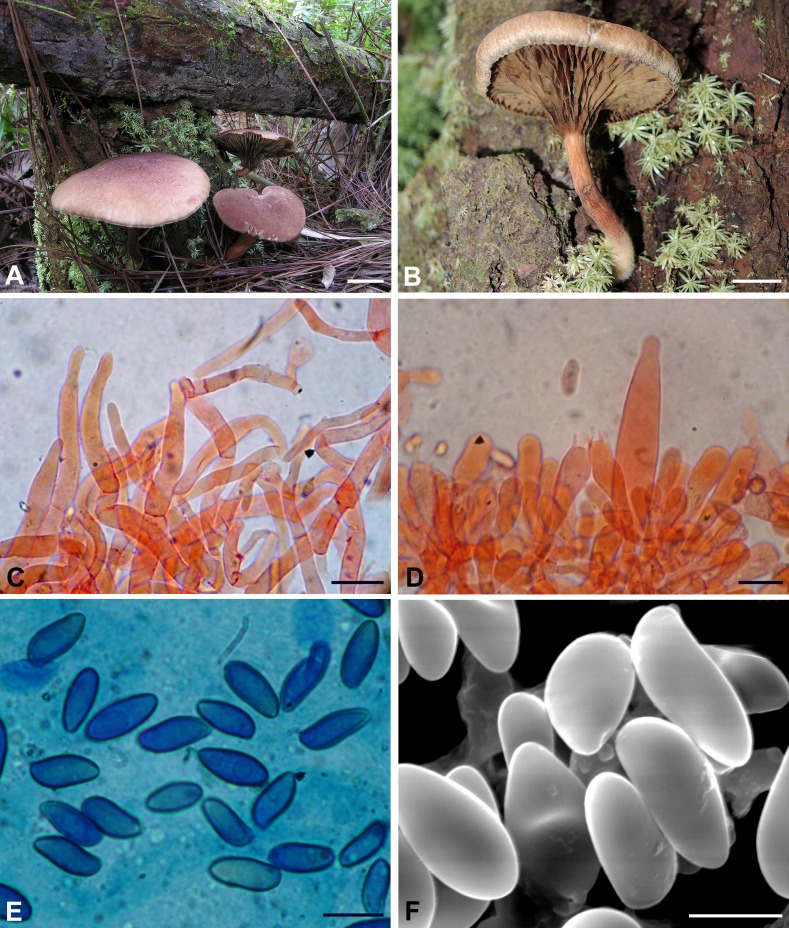
Phylloporopsis boletinoides. A–F. Fresh basidiomata (A: JBSD127411, B: JBSD127412, C: JBSD127413, D: JBSD127414, E: CORT014483, F: CORT010991). G. Detail of the lamellate hymenophore (JBSD127411). H. Detail of the context turning blue on exposure (JBSD127415). Bars = 1 cm. Pictures: A–D, G–H by C. Angelini; E–F by T.J. Baroni.
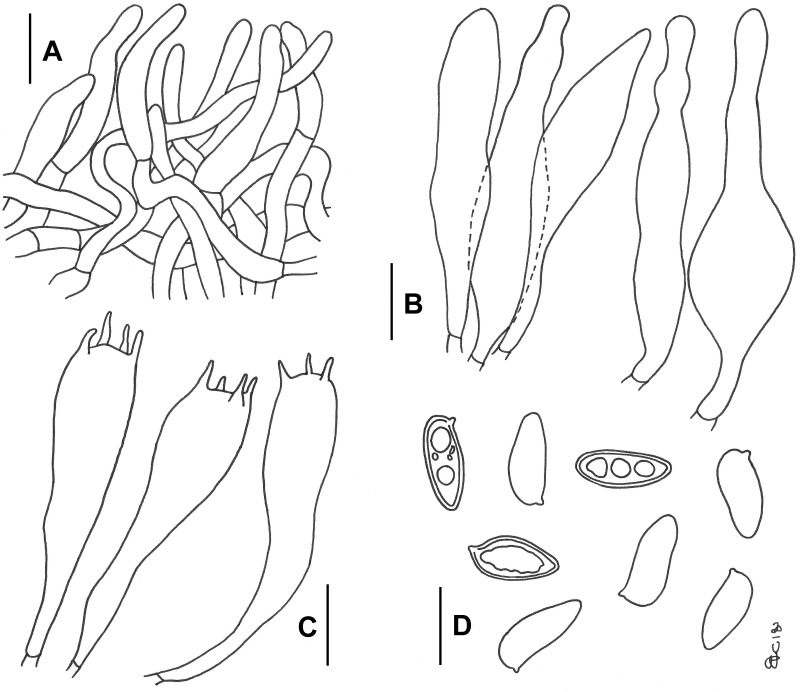
Fig. 6.
Phylloporopsis boletinoides. Drawings of the anatomical features (JBSD127412). A. Elements of the pileipellis. B. Cheilo- and pleurocystidia. C. Basidia. D. Basidiospores. Bars A–B = 20 μm; C–D = 10 μm (F. Costanzo del.).
Basionym: Phylloporus boletinoides A.H. Smith & Thiers, Contr. Monogr. North Amer. Species Suillus: 105. 1964.
Typus: USA, Florida, Alachua Co., Alachua, west side of Newnan’s Lake, east of Gainesville, solitary in deep sandy humus under pines (Pinus spp.), low hammock, 31 Jul. 1958, H.D. Thiers 4960 [MICH 11740 (holotype), SFSU 000741 (isotype)].
Basidiomata medium-small. Ontogenetic development gymnocarpic. Pileus (2.4–)2.7–7.3(–7.5) cm broad, at first hemispherical then persistently convex and finally broadly pulvinate-flattened, never depressed at centre, regularly to hardly unevenly shaped, moderate fleshy, firm at the beginning but progressively softer with age; margin steady to faintly wavy-lobed, initially involute then curved downwards and finally completely plane or even uplifted, not or only a little extending beyond the hymenophore; surface matt, dry, velvety-tomentose to finely fibrillose in all developmental stages, not cracked; cuticle at first deep orange then garnet red, carmin red, dull red, dark red to reddish-brown (Light Coral Red to Hay’s Maroon, Plate XIII; Light Corinthian Red to Prussian Red, Plate XXVII), gradually fading with age and becoming beige-ochraceous to pale brownish-pink (Light Buff, Plate XV; Pale Salmon Pink, Plate XIV), especially on the peripheral surface; slowly darkening on handling or when injured; subcuticular layer white (White, Plate LIII). Hymenophore lamellate to sub-boletinoid, gills distinctly arcuate-decurrent, somewhat distant, undulate, shorter than pileus context thickness (up to 0.5 cm high), distinctly intervenose with low anastomosing transversal or furcate veins connecting the primary gills, suggesting a subporoid appearance particularly in young specimens, concolourous edges entire (Fig. 4G); at first beige to pale cream-beige (Marguerite Yellow, Primrose Yellow, Plate XXX), in age fading to olive-cream (Light Yellowish Olive, Plate XXX; Deep Olive Buff, Plate XL; Greysh Olive, Plate XLVI) due to mature spores, staining light blue (Pale Green-Blue Grey, Plate XLVIII) on bruising or exposure. Stipe (2.5–)2.7–4.5(–5.0) × 0.4–0.7 cm, shorter than or as long as the pileus diameter at maturity, central to slightly off–centre, solid, firm, dry, straight or curved, cylindrical but slightly swollen towards the base, not rooting; surface very finely pruinose to longitudinally fibrillose, devoid of reticulum, evelate; pale orange (Light Salmon Orange, Plate II) in the upper third, concolourous with the pileus to slightly paler elsewhere (Light Coral Red to Hay’s Maroon, Plate XIII; Light Corinthian Red to Prussian Red, Plate XXVII), usually with a conspicuous whitish to pale yellowish basal tomentum (White, Plate LIII; Sea-Foam Yellow, Plate XXXI), unchangeable when pressed; rhizomorphs not observed. Context firm and tough when young, later soft textured and eventually flabby in the pileus (up to 1.1 cm thick in the central zone), a little more fibrous in the stipe, whitish in the pileus and upper third of the stipe but yellowish (Maize Yellow, Plate IV; Cream Colour, Plate XVI) in the remaining part of the stipe and increasingly deeper towards the base (Buff Yellow, Plate IV); turning light blue (Pale Green-Blue Grey, Plate XLVIII) in the pileus and the connection zone with the stipe after a couple of minutes when exposed to air (Fig. 4H), especially above the tubes and eventually fading to drab whitish, nearly unchangeable elsewhere; subhymenophoral layer whitish; exsiccate brownish. Odour indistinct. Taste mild but slightly bitter after prolonged mastication. Spore-print not obtained. Macrochemical reactions: 30 % KOH: brownish-grey to dark brown on context and hymenophore, golden yellow on pileus. 30 % NH4OH: staining purple-pink on pileus. FeSO4: slowly pale yellowish-green to olive green on context, none elsewhere.
Basidiospores [619/36/19] (8.9–)12.3 ± 1.40(–18.0) × (3.5–)5.1 ± 0.68(–8.2) μm, Q = (1.73) 1.80–3.65(–4.13), Qm = 2.4 ± 0.24, V = 231 ± 68 μm3, inequilateral, very variable in dimensions and versiform, cylindrical to fusiform or more frequently ellipsoid-fusiform to broadly ellipsoid in side view, ellipsoid to broadly ellipsoid in face view, smooth, apex rounded, with a short apiculus and usually with an indistinct to shallow suprahilar depression although in some spores the depression appears quite pronounced (Figs 5E, F and 6D), thick-walled (0.5–0.7 μm), straw yellow in water, ochraceous-yellow coloured in 5 % KOH, having one or more rarely two to three large oil droplets, occasionally pluri-guttulate when mature, inamyloid to very weakly dextrinoid, cyanophilic (Fig. 5E) and with a very faint metachromatic reaction. Basidia (27–)33–54(–58) × 8–14 μm (n = 24), long, subcylindrical, cylindrical-clavate to clavate, moderately thick-walled (0.6–0.9 μm), predominantly 4-spored but also 1-, 2- or 3-spored, usually bearing relatively long sterigmata (2–7 μm), very pale yellowish and containing straw-yellow oil guttules in water and 5 % KOH, bright yellow (inamyloid) in Melzer’s, without basal clamps (Figs 5D, 6C); basidioles subcylindrical, cylindrical-clavate to clavate, similar in size to basidia. Cheilocystidia (42–)45–108(–118) × (8–)10–22 μm (n = 26), very common, decidedly slender, projecting straight to sometimes flexuous, cylindrical-fusiform, fusiform, ventricose-fusiform to sublageniform, less frequently cylindrical to irregularly cylindrical, subclavate, sausage-like or peanut-like, rarely mucronate or subcapitate, with rounded to subacute tip, smooth, thick-walled (0.8–1.2 μm), very pale yellowish in water and 5 % KOH, bright yellow (inamyloid) in Melzer’s, orthochromatic, without epiparietal encrustations (Figs 5D, 6B). Pleurocystidia (63–)65–117 × 9–26(–28) μm (n = 29), decidedly frequent, shape, size, colour and chemical reactions similar to cheilocystidia (Fig. 6B). Pseudocystidia not recorded. Pileipellis a trichoderm consisting of moderately to strongly interwoven, elongated, filamentous and sinuous, frequently branched hyphae tending to be repent in the outermost layer and thus turning into a cutis not or only partially embedded in gelatinous matter at maturity (Figs 5C, 6A); terminal elements 25–88 × 3–14 μm, long and slender, cylindrical to cystidioid, apex rounded-obtuse to sometimes pointed, moderately thick-walled (up to 1 μm), yellowish to pale brownish-yellow in water and 5 % KOH, bright yellow to yellowish-orange (inamyloid to weakly dextrinoid) in Melzer’s, smooth; subterminal elements similar in shape, size and colour to terminal elements, all hyphae lacking encrusting pigments. Stipitipellis a layer of slender, parallel to subparallel and longitudinally running, smooth walled, adpressed hyphae, 2–5 μm wide, hyaline to pale yellowish in water and 5 % KOH; the stipe apex covered by a well-developed caulohymenial layer consisting of sterile caulobasidioles, extremely rare, 2-spored, fertile caulobasidia 54 × 10 μm, sterigmata 5 μm long (only one observed in several mounts!) and abundant projecting, fusiform, ventricose-fusiform, irregular cylindrical to subclavate or occasionally diverticulate caulocystidia similar in colour to hymenial cystidia but decidedly shorter and narrower, 30–60(–63) × 6–15 μm (n = 13), having a wall up to 0.8 μm thick. Lateral stipe stratum under the caulohymenium absent. Stipe trama composed of confusedly and densely arranged, subparallel to moderately interwoven, filamentous, smooth, hyaline to yellowish in water, inamyloid hyphae, 5–15(–20) μm broad. Hymenophoral trama bilateral divergent of the “Phylloporus-type”, with very slightly divergent to nearly subparallel and tightly arranged, non-gelatinous hyphae (lateral strata hyphae in transversal section touching or almost touching each other, 0–3 μm apart, 3–12 μm broad), hyaline to very pale yellowish in water and 5 % KOH, inamyloid in Melzer’s; lateral strata (20–)30–40(–50) μm thick, mediostratum 10–20(–30) μm thick, axially arranged, consisting of a tightly adpressed, non-gelatinous bundle of hyphae, 3–9 μm broad; in Congo Red the mediostratum is concolorous with the lateral strata. Oleiferous hyphae very common. Clamp connections absent in all tissues. Hyphal system monomitic.
Fig. 5.
Phylloporopsis boletinoides. A–B. Lignicolous basidiomata (A: JBSD127414, B: JBSD127414). C. Elements of the pileipellis (JBSD127412). D. Basidia and cheilocystidium (JBSD127411). E. Cyanophilic basidiospores under optical microscopy (JBSD127412). F. Basidiospores under SEM (JBSD127411). C–D in anionic Congo red; E in Cotton blue. Bars C–D = 20 μm; E–F = 10 μm. Pictures: A–B by C. Angelini; C–E by M. Gelardi; F by A. Vizzini.
Ecology: usually under Pinus spp., occasionally in mixed Pinus and Quercus forests, solitary to gregarious or less frequently subcaespitose, growing on soil among debris or on rotting wood but also on logs or living trunks of pine trees.
Edibility: unknown.
Materials examined: Dominican Republic, La Vega Province, Jarabacoa, 19°11′12.5″N 70°35′25.5″E, 660 m, half dozen young to middle aged specimens growing on soil under Pinus occidentalis, 21 Dec. 2013, C. Angelini (JBSD127411, ANGE120 and MG709); ibid., five mature specimens growing on soil under P. occidentalis, 22 Dec. 2013, C. Angelini (JBSD127412, ANGE121 and MG710); ibid., a single mature specimen growing on soil under P. occidentalis, 10 Jan. 2016, C. Angelini (JBSD127413, ANGE551); ibid., several basidiomata in all developmental stages growing on rotting wood and on a living trunk of P. occidentalis, 28 Nov. 2017, C. Angelini (JBSD127414, ANGE1007 and MG711); ibid., several basidiomata in all developmental stages growing on soil and on rotting wood of P. occidentalis, 23 Nov. 2017, C. Angelini (JBSD127415, ANGE1013 and MG712). Belize, Cayo District, Mountain Pine Ridge, Hidden Valley Inn property, near Lake Lolly Folly, 17°03′13.3″N 88°54′14.0″W, 565 m, seven mostly mature specimens, 7 Jan. 2002, T.J. Baroni (9195 TJB = BZ745) (CORT014483). USA, Alabama, Baldwin Co., Orange Beach, solitary in a sandy area with oaks nearby, 21 Jul. 1982, D.P. Lewis 3196 (F C0235181F); Florida, Alachua Co., W side of Newnan’s Lake, solitary in deep sandy humus under pines, low hammock, 31 Jul. 1958, H.D. Thiers 4960 (SFSU-F-000741 holotype); Austin Cary Memorial Forest, NE of Gainesville, widely scattered under Pinus sp., 11 Aug. 1985, N.S. Weber 6007 (MICH 65224); Glades Co., Fish eating Creek Wildlife Management Area, 1.2 km S of Highlands Co. line, 1.3 km E of C-731, 9 Sep. 2012, A.R. Franck 3125 (USF 273159); Hillsborough Co., Violet Cury Nature Preserve, under Quercus sp. and Pinus sp., 15 Jun. 2017, A. Farid 617 (USF 296126); Putnam Co., Ordway-Swisher Biological Station, Pinus sp. dominated habitat, 23 Oct. 2016, N. Kraisitudomsook NAT-033 (FLAS 60407); ibid., under Quercus sp. and Pinus sp., 10 Oct. 2016, M.E. Smith s.n. (FLAS 60413); ibid., 19 Oct. 2017, M.E. Smith s.n. (USF 298023); Sarasota Co., Myakka Valley Ranches, on soil near Pinus sp. and Quercus sp., 29 Dec. 1986, R.S. Williams 326 (F C0224863); Myakka Valley Ranches-residence, under Pinus sp., 12 Jan. 1991, R. Singer (F3912) (F 1118420); Mississippi, Harrison Co., National Cemetery, Biloxi, scattered under Pinus sp., 10 Sep. 1981, D. Guravich 1390 (MICH 65225); Texas, Hardin Co., N of Silsbee, Gore Cemetery, solitary in Pinus sp. woodland, 16 Aug. 1982, D.P. Lewis 3324 (F 1087286); Orange Co., Vidor, near residence, scattered on Pinus sp. stump, 5 Jul. 1976, D.P. Lewis 351 (F 1086387); ibid., solitary on well-decayed Pinus taeda stump, 13 Sep. 1979, D.P. Lewis 1982 (F 1089019); Tyler Co., near Kountze, Big Thicket National Preserve, Turkey Creek Unit, along Turkey Creek Trail, 30°31′19.0″N 94°20′44.7″W, 60 m, 6 Sep. 1996, T.J. Baroni (TJB 8172) (CORT010991).
Known distribution: Eastern and south-eastern USA (Maine, Delaware, New Jersey, Georgia, Florida, Mississippi, Alabama, Texas) (Mycology Collections Portal, MyCoPortal: http://mycoportal.org/portal/index.php. Accessed on June 29, 2018), in Central America reported from Belize and in the Greater Antilles Islands of the Caribbean from the Dominican Republic, more frequently collected in subtropical and at higher altitudes in tropical areas, western and southern limits yet to be established.
DISCUSSION
Taxonomic circumscription of P. boletinoides
There are several reliable and essential macro-morphological features that characterize P. boletinoides. The pileus is initially reddish-orange then deep red or cinnamon-brown to cocoa brown and becomes pale ochraceous-brown with age. The strongly decurrent lamellate to sub-boletinoid hymenophore showing distinctly anastomosing and interveined lamellae that are beige to olive-cream or olive-buff coloured (becoming dull ochraceous-brown when dried) and sometimes faintly bluing upon handling. The context is whitish but cream-yellowish in the lower quarter of the stipe, unchanging or turning light blue only in the pileus and the connection zone with the stipe. The pileus surface turns purplish-pink or reddish with ammonia. Anatomical traits include the ellipsoid-fusiform, cyanophilic, smooth basidiospores, trichodermal pileipellis, hymenophoral trama of the “Phylloporus-type” and absence of lateral stipe stratum (Smith & Thiers 1964, Singer et al. 1990, Bessette et al. 2000, 2016, Ortiz-Santana et al. 2007, Neves & Halling 2010, this study). The Dominican material exhibits predominant reddish hues on the pileus and stipe, larger hymenial cystidia (up to 118 × 28 μm) and slightly larger basidiospores, (11.1–)13.4 ± 1.27(–18.0) × (4.7–)5.8 ± 0.59(–8.2) μm, Q = 2.3 [136/9/5] than those reported in literature (11–13 × 5–6 μm in Smith & Thiers 1964; 10.5–14.5 × 3.5–6.5 μm, Qm = 2.7 in Singer et al. 1990; 12–14.4 × 4.8–6.8 μm, Qm = 2.4 in Ortiz-Santana et al. 2007; 10.5–11.9 × 4.2–4.9 μm, Qm = 2.4 in Neves & Halling 2010). This morphological variability likely represents a phenotypic continuum along a geographic gradient of the same species (Fig. 3).
The original species description states that the context does not change on exposure (Smith & Thiers 1964). The description was updated by Singer et al. (1990) to point out the inconsistent bluing that is present in some collections. Our observations from samples found in Alachua County, Florida, near the type locality and from Dominican collections often show evidence of this bluing. A recent treatment on the boletes from eastern North America (Bessette et al. 2016) also notes the inconsistency of the bluing context. Consequently, the occurrence of a blue oxidation in P. boletinoides is variable and consistent with Singer’s re-description of the species (Singer et al. 1990).
Data obtained from top BLASTn results on GenBank and ITS phylogenetic analysis (Fig. 3) provide molecular confirmation of the ectomycorrhizal association of P. boletinoides with Pinus. GenBank sequences KX899732 (clone 47C_G1_H9) and KX899785 (clone 4C_G2_C3) were obtained from ectomycorrhizal root samples from Pinus clausa. Florida material is typically found under stands of Pinus spp., though occasionally in mixed Pinus and Quercus forests. In the Dominican Republic P. boletinoides was found with Hispaniolan pine (Pinus occidentalis) at high elevations with no other ectomycorrhizal hosts (no endemic Fagaceae occur in the Dominican Republic), despite several specimens were collected on stumps or debris of rotting wood, sometimes on the outer bark (periderm) at the base of living trees (Figs 4D, 5A, B). The occurrence of the Belizean material of P. boletinoides on a dead tree (Ortiz-Santana et al. 2007) further confirms a lignicolous growth preference, at least at some neotropical sites. Previous studies have shown that some confirmed ECM fungi produce basidiomata in elevated positions on woody substrata (Rayner et al. 1985, Henkel et al. 2000). Examples of lignicolous growth of ECM Boletaceae have been reported from the Americas, Europe, southeast Asia and Australasia and include taxa such as those of the Boletellus ananas complex and several Tylopilus species (Singer 1945a, Corner 1972, Alessio 1985, Rayner et al. 1985, Henkel 1999, Watling 2008). This fruiting behaviour could be explained as a strategy for enhancing spore dispersal, for selective foraging of N in rotting wood, or otherwise as a strategy to preserve basidiomata from becoming water-soaked under wet conditions (Weber & Sundman 1986, Jurgensen et al. 1987, Henkel et al. 2000, 2012, Lindahl & Tunlid 2015).
Outside of the USA, P. boletinoides has a known distribution restricted to Belize and the Dominican Republic but given its ability to adapt to different climatic conditions and to form symbiosis with different pine trees, it is most likely to be expected throughout the neotropical mainland and the Caribbean where pines occur.
Taxonomic and phylogenetic relationships of Phylloporopsis to Bothia, Phylloporus, Phylloboletellus and Phyllobolites
Our phylogenetic analyses provide evidence that Phylloporopsis is a monophyletic genus sister to the poroid Bothia (typified with Boletinus castanellus) and the sequestrate Solioccasus (typified with S. polychromus) and confirm that it is not related to Phylloporus sensu stricto (Figs 1–2). Based on the available data there are no obvious shared morphological or ecological features among these three genera, with exception of the cyanophily of the spore wall (Trappe et al. 2013 and personal observations of TJB on B. castanella collections), which appears to be the sole synapomorphy known to date, though this feature has been only randomly tested and with contrasting results in the Boletaceae (Singer 1986, Watling 2008). Trappe et al. (2013) established the genus Solioccasus as a sister clade with Bothia, although they did not address the shared morphological character between the two genera. More extensive sampling is needed to elucidate the taxonomic boundaries of the Bothia clade.
Though Phylloporopsis is phylogenetically related to Bothia, the two genera are morphologically quite distinct based on the differently shaped and arranged hymenophore. In Bothia the hymenophore is truly boletinoid with compound angular and radially stretched pores that are only slightly decurrent or depressed around stipe apex and always brownish (Halling et al. 2007). Conversely, in Phylloporopsis the basic structure of the hymenophore is lamellate though anastomosing, always deeply decurrent along the stipe in all developmental stages and olive-cream to olive-buff at maturity. Moreover, the tissues stain brown in Bothia and blue in Phylloporopsis and the spore print is pale brownish-yellow in Bothia while in Phylloporopsis it has a distinct olive tinge (Singer et al. 1990, Bessette et al. 2000, 2016). The two genera are also ecologically different; Bothia is associated with Fagaceae (mostly Quercus spp.) whereas Phylloporopsis forms ECM with pine trees (see below). Finally, the geographic range of Bothia is only partially overlapping with that of Phylloporopsis, since B. castanella (= Boletinus squarrososides according to Halling et al. 2007) is only found in eastern and south-eastern North America [Peck 1900, Murrill 1909, 1914, Snell 1936 (as “B. squarrososides”), Singer 1938 (also as “Phylloporus squarrosoides”), 1945b (as “Xerocomus squarrosoides”), Coker & Beers 1943 (also as “B. squarrososides”), Snell & Dick 1958 (also as “B. squarrososides”), 1970, Smith & Thiers 1964, Both 1993, Bessette et al. 2000, 2016; Roody 2003, Watling 2008, Halling et al. 2007]. The only other known representative of the genus, viz. B. fujanensis is restricted to south-eastern China (Fujian and probably Taiwan) (Chen et al. 1997, Zeng et al. 2015). These characters are a sound basis for a clear-cut distinction between the two genera. Bothia castanella also differs from P. boletinoides in the sometimes coarsely reticulate stipe surface, smaller, ovate to broadly ellipsoid spores [(7–)8–11(–12) × (3.5–)4.5–5.5(–6) μm in B. castanella] and the presence of dark brown encrustations on pileus surface hyphae (Halling et al. 2007, Watling 2008, Bessette et al. 2000, 2016).
Our phylogenetic placement of Phylloporopsis suggests that the lamellate hymenophore has evolved at least three times in the Boletaceae from poroid ancestors, viz. in Phylloporopsis, Phylloporus and Phylloboletellus.
Phylloporopsis is morphologically similar to species of Phylloporus despite their distant phylogenetic relationship. However, Phylloporus is easily distinguished by the yellow to golden-yellow lamellate hymenophore with the bright yellow colours remaining so even after drying, by the bacillate surface ornamentation of the basidiospores under scanning electron microscope (SEM) (although a number of tropical species have smooth or finely rugulose spores) and the occurrence of clamp connections in some tropical and subtropical species (Singer 1945b, 1946 1978, 1986, Corner 1970, Heinemann & Rammeloo 1987, Šutara 2008, Watling 2008, Neves & Halling 2010, Neves et al. 2010, 2012, Zeng et al. 2013). Another noticeable distinguishing trait that was considered unifying in Phylloporus is the pileus surface that stains vivid blue to bluish-green with ammonia (Singer 1945b, 1986, Watling 2008) as opposed to the reddish to purple-pink reaction observed in Phylloporopsis. However, it should be noted that this macrochemical reaction has not been tested on species described from tropical Africa (Heinemann 1951), Malaysia (Corner 1970, 1974), China (Zeng et al. 2013, Ye at al. 2014) or Bangladesh (Hosen & Li 2015, 2017). In addition, a few taxa from tropical Central and South America, India and Thailand (e.g. P. fibulatus, P. manausensis, P. septocystidiatus, P. castanopsidis, etc.) are reported to have negative or non-bluing reaction with both NH3 and NH4OH (Singer 1986, Neves & Halling 2010, Neves et al. 2012, Pradeep et al. 2015). Accordingly, the bluing of the pileus surface with ammonia cannot be considered a synapomorphy within Phylloporus. Phylloporus is a large assemblage encompassing nearly one hundred species so far as known worldwide (comprising phylogenetic species that have not yet been formally described), it appears to be widespread being best represented throughout temperate and pantropical regions of both hemispheres and is apparently most diverse in Australasia (Corner 1970, 1974, Singer 1978, 1986, Singer & Gómez 1984, Montoya & Bandala 1991, 2011, Halling et al. 1999, Watling & Li 1999, Ortiz-Santana et al. 2007, Neves & Halling 2010, Neves et al. 2012, García-Jiménez 2013, Ye et al. 2014, Hosen & Li 2015, 2017, Pradeep et al. 2015). The neotropical P. centroamericanus is somewhat chromatically similar to P. boletinoides but diverges, apart from the generic discrepancies cited above, in the smaller size (pileus 2–3 cm broad), pileus surface tending to become areolate with age, context whitish but pinkish-brown in middle part of the stipe, white basal mycelium, encrusted hymenial cystidia and hymeniform pileipellis with 5–24 μm wide end cells (Singer & Gómez 1984, Montoya & Bandala 1991, Halling & Mueller 2005, Neves & Halling 2010). Affinities can also be found with some eastern Asian species such as P. rubiginosus and P. pachycystidiatus; the former is separated by the bright yellow to orangish-yellow lamellae, context evenly yellowish and slowly discolouring blue throughout, blue staining reaction on pileus surface with ammonia, smaller spores [9.8–11.2(–13) × 3.5–4.9 μm, Qm = 2.5], thick-walled cystidia (walls up to 2 μm wide) and the occurrence under Castanopsis and Dipterocarpus in Thailand and south-western China (Yunnan Province) (Neves et al. 2012, Ye et al. 2014), whereas the latter differs in the slightly smaller size (pileus 3–5 cm in diameter), yellow hymenophore, cream-yellowish context, whitish basal mycelium, bacillate basidiospores under SEM, thick-walled hymenial cystidia (walls 2–4 μm wide), association with Lithocarpus spp. and distribution restricted to southern and south-western China (Hainan and Yunnan Provinces) (Zeng et al. 2013, Ye et al. 2014). There appears to exist a minor affinity between P. boletinoides and the type species of the genus Phylloporus, P. pelletieri, which is separated from the former by the bright yellow to golden yellow hymenophore, bright yellow basal mycelium, unchangeable tissues, blue-green reaction with ammonia on pileus surface, bacillate basidiospores under SEM and the occurrence in temperate Europe (Pilát & Dermek 1974, Breitenbach & Kränzlin 1991, Lannoy & Estadès 2001, Ladurner & Simonini 2003, Watling & Hills 2005, Klofac 2007, Muñoz et al. 2008, Šutara 2008, Knudsen & Taylor 2012).
Another known lamellate representative in the Boletaceae is Phylloboletellus, a monotypic genus based on P. chloephorus. This taxon was described from subtropical to tropical montane forests in Argentina (Singer & Digilio 1952) but has an apparent disjunct distribution having later repeatedly been reported from Mexico (García-Jiménez et al. 1986, Singer 1988, Singer et al. 1992, Bandala et al. 2004, García-Jiménez 2013). This species, however, differs from P. boletinoides by the subumbonate pileus, yellowish basal mycelium, evenly yellow context discolouring blue on exposure, blue-green reaction with ammonia on pileus surface, hymenophoral trama of the “Boletus-type”, presence of false or incomplete clamp connections and most of all by the short, broadly ellipsoid, longitudinally winged basidiospores [(9–)9.5–11.7(–12.5) × (7.2–)8–9.5(–10.5) μm, Q = 1.2)] (Singer & Digilio 1952, Singer 1964, 1970, 1981, 1986, 1988, Horak 1968, Petersen 1974, Pegler & Young 1981, Singer et al. 1992, Bandala et al. 2004, Watling 2008). Moreover, the original material of P. chloephorus was collected under Lauraceae, Myrtaceae, Sapindaceae, Ulmaceae, etc., but the authors were unable to ascertain its nutritional mode (Singer & Digilio 1952). Later on, the species was thought to be presumably saprotrophic (Singer 1970, 1986, Watling 2008), even though Singer (1981) and subsequently Bandala et al. (2004), Binder & Hibbett (2006) and Tedersoo et al. (2010) considered it as potentially ectomycorrhizal. Phylloboletellus chloephorus var. mexicanus ad interim was informally proposed by Singer et al. (1992) to circumscribe the Mexican population but the authors themselves were not fully convinced about the separation of the two taxa and claimed that the differences of var. mexicanus with respect to the type species were nearly inconsistent, thus it has never been validated thereafter. Previous studies (Binder & Hibbett 2006, Li et al. 2011, 2014, Zeng et al. 2012) and our own observations (Figs 1–2) have yet to resolve the phylogenetic position of P. chloephorus.
It is also worth nothing here the existence of the poorly known genus Phyllobolites, which some authors have argued may belong to the Boletaceae (Kirk et al. 2008, Magnago 2014). Phyllobolites was erected based on Phyllobolites miniatus from tropical northern South America (Guyana, Suriname, Brazil) (Singer 1964, 1986, Singer et al. 1983, Henkel et al. 2012, Sulzbacher et al. 2013). This taxon has been originally assigned to the broadly conceived family Paxillaceae, along with unrelated agaricoid genera such as Omphalotus, Lampteromyces, Hygrophoropsis, Neopaxillus and Ripartites (Singer 1946, 1986). It is easily delimited by the presence of a membranous ring deriving from a partial veil, ochraceous spore print, fusiform, verrucose basidiospores, abundant pseudocystidia, ixotrichoderm to ixocutis pileipellis, hymenophoral trama subparallel to slightly divergent with gelatinized hyphae and the occurrence under cesalpinoid legumes (Rick 1906, 1961, Singer 1942, 1964, 1986, Singer et al. 1983, Watling 2008). Since it has not been possible to locate and re-examine the type specimen of P. miniatus from the Brazilian Amazon and since additional material authenticated by Rick was found by Singer to be referred to either Lentinus (Singer 1946), Tapinella or Pleurotus, the assignment of Phyllobolites to the Boletaceae (Kirk et al. 2008, Magnago 2014) or Paxillaceae (Singer 1942, 1946, 1953, 1964, 1986, Singer et al. 1983, Neves & Capelari 2007, Henkel et al. 2012) or even to the order Boletales is currently doubtful and it has been suggested that Phyllobolites might belong in the order Gomphales near the genus Linderomyces (a later synonym of Gloeocantharellus) (Watling, 2008). A modern re-description and inclusive molecular investigation is needed to resolve its phylogenetic placement.
ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to Beatriz Ortiz-Santana (CFMR), H. Thorsten Lumbsch and Wyatt Gaswick (F), and Patricia Rogers (MICH) for the loan of dried fungal materials. CA also wishes to thank Ricardo G. García, Francisco Jiménez, Brígido Peguero, Yuley E. Piñeyro and Alberto Veloz (Jardín Botánico Nacional Dr. Rafael M. Moscoso, Santo Domingo, Dominican Republic) for their interest and encouragement in studying fungi of the Dominican Republic and for their active cooperation in providing herbarium material preserved in their institution. AV is grateful to Simona Cavagna (Dipartimento di Scienze della Terra, Torino) for the technical help in taking SEM images. Mycological biodiversity research in Belize by TJB and colleagues was supported by grants from the National Science Foundation (DEB-9525902 and DEB-0103621), the National Geographic Society, and the British Mycological Society. TJB also received funding from the Faculty Research Program of the State University of New York – College at Cortland for research work in the Gulf Coast region of the USA. The authors would also like to acknowledge Scott Bingham from Arizona State University for his assistance with sequencing. Funding for MES and LSK was provided by the Florida Agricultural Experiment Station Ordway-Swisher Research grants (to MES).
REFERENCES
- Alessio CL. (1985). Boletus Dill. ex L. Fungi Europaei 2. Giovanna Biella, Saronno, Italy. [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Alvarado P, Moreno G, Vizzini A, et al. (2015). Atractosporocybe, Leucocybe and Rhizocybe, three new clitocyboid genera in the Tricholomatoid clade (Agaricales) and notes on some whitish species of Clitocybe and Lepista. Mycologia 107: 123–136. [DOI] [PubMed] [Google Scholar]
- Bandala VM, Montoya L, Jarvio D. (2004). Two interesting records of boletes found in coffee plantations in eastern Mexico. Persoonia 18: 365–380. [Google Scholar]
- Bessette AE, Roody WC, Bessette AR. (2000). North American boletes. A color guide to the fleshy pored mushrooms. Syracuse University Press, Syracuse, USA. [Google Scholar]
- Bessette AE, Roody WC, Bessette AR. (2016). Boletes of eastern North America. Syracuse University Press, Syracuse, USA. [Google Scholar]
- Binder M, Hibbett DS. (2006). Molecular systematics and biological diversification of Boletales. Mycologia 98: 971–981. [DOI] [PubMed] [Google Scholar]
- Both EE. (1993). The boletes of North America. A compendium. Buffalo Museum of Science, Buffalo, USA. [Google Scholar]
- Breitenbach J, Kränzlin F. (1991). Fungi of Switzerland. Vol. 3. Boletes and agarics 1. Mykologia, Luzern, Switzerland. [Google Scholar]
- Bresinsky A, Besl H. (2003). Beiträge zu einer Mykoflora Deutschlands - Schlüssel zur Gattungsbestimmung der Blätter-, Leisten- und Röhrenpilze mit Literaturhinweisen zur Artbestimmung. Regensburger Mykologische Schriften 11: 5–236. [Google Scholar]
- Chen C-M, Peng J-J, Yeh K-W. (1997). The boletes of Taiwan (VIII). Taiwania 42: 316–323. [Google Scholar]
- Coker WC, Beers AH. (1943). The Boletaceae of North Carolina. University of North Carolina Press, Chapel Hill, USA. [Google Scholar]
- Corner EJH. (1970). Phylloporus Quél. and Paxillus Fr. in Malaya and Borneo. Beihefte zur Nova Hedwigia 20: 793–822. [Google Scholar]
- Corner EJH. (1972). Boletus in Malaysia. The Botanic Gardens, Government Printing Office, Singapore. [Google Scholar]
- Corner EJH. (1974). Boletus and Phylloporus in Malaysia: further notes and descriptions. Gardens’ Bulletin Singapore 27: 1–16. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, et al. (2012). “jModelTest 2: more models, new heuristics and parallel computing”. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- García-Jiménez J. (2013). Diversidad de macromicetos en el estado de Tamaulipas, México. Ph.D. dissertation. Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León, Linares, Mexico. [Google Scholar]
- García-Jiménez J, Gaona G, Castillo J, et al. (1986). Nuovos registros de boletáceos en México. Revista Mexicana de Micología 2: 343–366. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for Basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Halling RE, Baroni TJ, Binder M. (2007). A new genus of Boletaceae from eastern North America. Mycologia 99: 310–316. [DOI] [PubMed] [Google Scholar]
- Halling RE, Mueller GM, Dallwitz MJ. (1999). A new Phylloporus (Basidiomycetes, Boletaceae) with a key to species in Colombia and Costa Rica. Mycotaxon 63: 63–68. [Google Scholar]
- Halling RE, Mueller GM. (2005). Common Mushrooms of the Talamanca Mountains, Costa Rica. Memoirs of the New York Botanical Garden 90. New York Botanical Garden Press, Bronx (NY), USA. [Google Scholar]
- Heinemann P. (1951). Champignons récoltés au Congo Belge par Madame M. Goossens-Fontana 1. Boletineae. Bulletin du Jardin Botanique de l’État à Bruxelles 21: 223–346. [Google Scholar]
- Heinemann P, Rammeloo J. (1987). Phylloporus (Boletineae). Flore Illustrée des Champignons d′Afrique Centrale 13: 277–309. [Google Scholar]
- Henkel TW. (1999). New taxa and distribution records of Tylopilus from Dicymbe forests of Guyana. Mycologia 91: 655–665. [Google Scholar]
- Henkel TW, Aime MC, Miller SL. (2000). Systematics of pleurotoid Russulaceae from Guyana and Japan, with notes on their ectomycorrhizal status, Mycologia 92: 1119–1132. [Google Scholar]
- Henkel TW, Aime MC, Chin MML, et al. (2012). Ectomycorrhizal fungal sporocarp diversity and discovery of new taxa in Dicymbe monodominant forests of the Guiana Shield. Biodiversity and Conservation 26: 2195–2220. [Google Scholar]
- Horak E. (1968). Synopsis generum Agaricalium (Die Gattungstypen der Agaricales). Beiträge zur Kryptogamenflora der Schweiz, Vol. 13 Waber, Bern, Switzerland. [Google Scholar]
- Hosen I, Li T-H. (2015). Phylloporus gajari, a new species of the family Boletaceae from Bangladesh. Mycoscience 56: 584–589. [Google Scholar]
- Hosen I, Li T-H. (2017). Two new species of Phylloporus from Bangladesh, with morphological and molecular evidence. Mycologia 109: 277–286. [DOI] [PubMed] [Google Scholar]
- Jurgensen MF, Larsen MJ, Graham RT, et al. (1987). Nitrogen fixation in woody residue of northern Rocky Mountain conifer forests. Canadian Journal of Forestry Research 17: 1283–1288. [Google Scholar]
- Justice J. (2008). Bolete Workshop – Studies in Boletology. Retrieved from the website: http://www.ashevillemushroomclub.com/docs/key-Justice-bolete.pdf [Google Scholar]
- Katoh K, Misawa K, Kuma K, et al. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. (eds.) (2008). Dictionary of the Fungi. 10th Edition CAB International, Wallingford, UK. [Google Scholar]
- Klofac W. (2007). Schlüssel zur Bestimmung von Frischfunden der europäischen Arten der Boletales mit röhrigem Hymenophor. Österreichische Zeitschrift für Pilzkunde 16: 187–279. [Google Scholar]
- Knudsen H, Taylor AFS. (2012). Xerocomus Quél. In: Funga Nordica – Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera (2nd ed.). Vol. 1 (Knudsen H, Vesterholt J, eds). Nordsvamp, Copenhagen: 228–233. [Google Scholar]
- Ladurner H, Simonini G. (2003). Xerocomus s.l. Fungi Europaei 8. Edizioni Candusso, Alassio, Italy. [Google Scholar]
- Lannoy G, Estadès A. (2001). Flore Mycologique d’Europe 6 - Les Bolets. Documents Mycologiques, Mém. Hors Série 6. Association d’Écologie et de Mycologie, Lille. [Google Scholar]
- Li YC, Feng B, Yang ZL. (2011). Zangia, a new genus of Boletaceae supported by molecular and morphological evidence. Fungal Diversity 49: 125–143. [Google Scholar]
- Li YC, Li F, Zeng NK, et al. (2014). A new genus Pseudoaustroboletus (Boletaceae, Boletales) from Asia as inferred from molecular and morphological data. Mycological Progress 13: 1207–1216. [Google Scholar]
- Lindahl BD, Tunlid A. (2015). Ectomycorrhizal fungi–potential organic matter decomposers, yet not saprotrophs. New Phytologist 205: 1443–1447. [DOI] [PubMed] [Google Scholar]
- Magnago AC. (2014). Taxonomia e sistemática de Boletaceae (Boletales) para o Brasil. Dissertação Pós-Graduação em Biologia de Fungos, Algas e Plantas, Universidade Federal de Santa Catarina, Florianópolis. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, LA: 1–8. [Google Scholar]
- Montoya L, Bandala VM. (1991). Studies on the genus Phylloporus in Mexico. 1. Discussion of the known species and description of a new species and a new record. Mycotaxon 41: 471–482. [Google Scholar]
- Montoya L, Bandala VM. (2011). A new Phylloporus from two relict Fagus grandifolia var. mexicana populations in a montane cloud forest. Mycotaxon 117: 9–18. [Google Scholar]
- Muñoz JA, Cadiñanos Aguirre JA, Fidalgo E. (2008). Contribución al catálogo corológico del género Xerocomus en la Peninsula Iberica. Boletín de la Sociedad Micológica de Madrid 32: 249–277. [Google Scholar]
- Murrill WA. (1909). The Boletaceae of North America – I. Mycologia 1: 4–18. [Google Scholar]
- Murrill WA. (1914). American Boletes. Privately published, New York. [Google Scholar]
- Neves MA. (2007). Toward a revision of the genus Phylloporus (Boletaceae): systematics and phylogeny of species from various parts of the world, Phd Dissertation, City University of New York Press, New York. [Google Scholar]
- Neves MA, Capelari M. (2007). A preliminary checklist of Boletales from Brazil and notes on Boletales specimens at the Instituto de Botânica (SP) Herbarium, São Paulo, SP, Brazil. Sitientibus Série Ciências Biologicas 7: 163–169. [Google Scholar]
- Neves MA, Halling RE. (2010). Study on species of Phylloporus I: Neotropics and North America. Mycologia 102: 923–943. [DOI] [PubMed] [Google Scholar]
- Neves MA, Henkel TW, Halling RE. (2010). Phylloporus colligatus sp. nov., a new gilled bolete from Guyana. Mycotaxon 111: 143–148. [Google Scholar]
- Neves MA, Binder M, Halling RE, et al. (2012). The phylogeny of selected Phylloporus species, inferred from NUC-LSU and ITS sequences, and descriptions of new species from the Old World. Fungal Diversity 55: 109–123. [Google Scholar]
- Nuhn ME, Binder M, Taylor AF, et al. (2013). Phylogenetic overview of the Boletineae. Fungal Biology 117: 479–511. [DOI] [PubMed] [Google Scholar]
- Orihara T, Smith ME. (2017). Unique phylogenetic position of the African truffle-like fungus, Octaviania ivoryana (Boletaceae, Boletales), and the proposal of a new genus, Afrocastellanoa. Mycologia 109: 323–332. [DOI] [PubMed] [Google Scholar]
- Ortiz-Santana B, Lodge DJ, Baroni TJ, et al. (2007). Boletes from Belize and the Dominican Republic. Fungal Diversity 27: 247–416. [Google Scholar]
- Peck CH. (1900). New species of fungi. Bulletin of the Torrey Botanical Club 27: 609–613. [Google Scholar]
- Pegler DN, Young TWK. (1981). A natural arrangement of the Boletales, with reference to spore morphology. Transactions of the British Mycological Society 76: 103–146. [Google Scholar]
- Petersen RH. (1974). Notes on cantharelloid fungi V. Some fungi suspected of being gomphoid. The Journal of the Elisha Mitchell Scientific Society 90: 53–54. [Google Scholar]
- Pilát A, Dermek A. (1974). Hríbovité huby. Èeskoslovenské hríbovité a sliziakovité huby (Boletaceae – Gomphidiaceae). Veda, Bratislava. [Google Scholar]
- Pradeep CK, Vrinda KB, Varghese SP, et al. (2015). A new species of Phylloporus (Agaricales, Boletaceae) from India. Phytotaxa 226: 269–274. [Google Scholar]
- Rayner ADM, Watling R, Frankland JC. (1985). Resource relations - an overview. In: Developmental biology of higher fungi (Moore D, Casselton LA, Wood DA, et al., eds). Cambridge University Press, Cambridge: 1–40. [Google Scholar]
- Rehner SA, Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. [Google Scholar]
- Rick JE. (1906). Pilze aus Rio Grande do Sul (Brazilien). Brotéria, série Botânica 5: 4–53. [Google Scholar]
- Rick JE. (1961). Basidiomycetes eubasidii in Rio Grande do Sul - Brasilia. 5. Agaricaceae. Iheringia, série Botânica 8: 296–450. [Google Scholar]
- Ridgway R. (1912). Color standards and color nomenclature. Self-published, Washington D.C. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roody WC. (2003). Mushrooms of West Virginia and the Central Appalachians. University of Kentucky Press, Lexington. [Google Scholar]
- Singer R. (1938). Sur les genres Ixocomus, Boletinus, Phylloporus, Gyrodon et Gomphidius. 2. Les Boletinus. Revue de Mycologie 3: 157–177. [Google Scholar]
- Singer R. (1942). Das System der Agaricales. II. Annales Mycologici 40: 1–132. [Google Scholar]
- Singer R. (1945a). The Boletineae of Florida with notes on extralimital species. I. The Strobilomycetaceae. Farlowia 2: 97–141. [Google Scholar]
- Singer R. (1945b). The Boletineae of Florida with notes on extralimital species. II. The Boletaceae (Gyroporoideae). Farlowia 2: 223–303. [Google Scholar]
- Singer R. (1946). The Boletineae of Florida with notes on extralimital species. IV. The lamellate families (Gomphidiaceae, Paxillaceae, and Jugasporaceae). Farlowia 2: 527–567. [Google Scholar]
- Singer R. (1953). Type Studies on Basidiomycetes. VI. Lilloa 26: 57–159. [Google Scholar]
- Singer R. (1964). Boletes and related groups in South America. Monograph of South American Basidiomycetes, especially those of the east slope of Andes and Brazil VI. The families Paxillaceae, Gomphidiaceae, Boletaceae and Strobilomycetaceae. Nova Hedwigia 7: 93–132. [Google Scholar]
- Singer R. (1970). Strobilomycetaceae (Basidiomycetes). Flora Neotropica 5. Hafner, New York. [Google Scholar]
- Singer R. (1978). Notes on bolete taxonomy – II. Persoonia 9: 421–438. [Google Scholar]
- Singer R. (1981). Notes on bolete taxonomy – III. Persoonia 11: 269–302. [Google Scholar]
- Singer R. (1986). The Agaricales in Modern Taxonomy. 4th ed Koeltz Scientific Books, Koenigstein, Germany. [Google Scholar]
- Singer R. (1988). La fitogeografia de las Boletineas (Basidiomycetes, Agaricales) en relacion a las especies Mexacanas. Revista Mexicana de Micología 4: 267–274. [Google Scholar]
- Singer R, Digilio APL. 1951 (“1952”). Pródromo de la Flora Agaricina Argentina. Lilloa 22: 5–462. [Google Scholar]
- Singer R, Araujo I, Ivory MH. (1983). Litter decomposition and ectomycorrhiza in Amazonian forests II. The Ectotrophically Mycorrizal Fungi of the Neotropical Lowlands, Especially Central Amazonia. Beihefte Nova Hedwigia 77: 1–352. [Google Scholar]
- Singer R, Gómez LD. (1984). The Basidiomycetes of Costa Rica. III. The genus Phylloporus (Boletaceae). Brenesia 22: 163–181. [Google Scholar]
- Singer R, Ovrebo CL, Halling RE. (1990). New species of Phylloporus and Tricholomopsis from Colombia, with notes on Phylloporus boletinoides. Mycologia 82: 452–459. [Google Scholar]
- Singer R, García J, Gómez LD. (1992). The Boletineae of Mexico and Central America IV. Beihefte zur Nova Hedwigia 105: 1–62. [Google Scholar]
- Singer R, Williams R. (1992). Some boletes from Florida. Mycologia 84: 724–728. [Google Scholar]
- Smith AH, Thiers HD. (1964). A contribution toward a monograph of North American species of Suillus (Boletaceae). Privately published, Ann Arbor. [Google Scholar]
- Snell WH. (1936). Notes on boletes. V. Mycologia 28: 463–475. [Google Scholar]
- Snell WH, Dick EA. (1958). Notes on boletes. X. A few miscellaneous discussions and a new subspecies. Mycologia 50: 57–65. [Google Scholar]
- Snell WH, Dick EA. (1970). The boleti of northeastern North America. SH Service Agency, J. Cramer, Lehre. [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Sulzbacher MA, Grebenc T, Jacques RJS, et al. (2013). Ectomycorrhizal fungi from southern Brazil – a literature-based review, their origin and potential hosts. Mycosphere 4: 61–95. [Google Scholar]
- Šutara J. (2008). Xerocomus s.l. in the light of the present state of knowledge. Czech Mycology 60: 29–62. [Google Scholar]
- Tedersoo L, May TW, Smith ME. (2010). Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20: 217–263. [DOI] [PubMed] [Google Scholar]
- Thiers B. (2018). (continuously updated). Index Herbariorum: a global directory of public herbaria and associated staff. New York botanical garden’s virtual herbarium; http://sweetgum.nybg.org/ih/ [Google Scholar]
- Trappe JM, Castellano MA, Halling RE, et al. (2013). Australasian sequestrate fungi 18: Solioccasus polychromus gen. & sp. nov., a richly colored, tropical to subtropical, hypogeous fungus. Mycologia 105: 888–895. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling R. (2008). A manual and source book of the boletes and their allies. Synopsis Fungorum 24. Fungiflora, Oslo. [Google Scholar]
- Watling R, Hills AE. (2005). Boletes and their allies - Boletaceae, Strobilomycetaceae, Gyroporaceae, Paxillaceae, Coniophoraceae, Gomphidiaceae (revised and enlarged edition). In: British Fungus Flora, Agarics and Boleti. Vol. 1 (Henderson DM, Watling R. eds). HMSO, Edinburgh. [Google Scholar]
- Watling R, Li T-H. (1999). Australian boletes. A preliminary survey. Royal Botanic Garden Edinburgh, Edinburgh. [Google Scholar]
- Weber A, Sundman V. (1986). Nitrogen fixation in coniferous bark litter. Plant and Soil 90: 419–425. [Google Scholar]
- White TJ, Bruns TD, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky J, et al., eds). Academic Press, San Diego: 315–322. [Google Scholar]
- Wu G, Feng B, Xu J, et al. (2014). Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity 69: 93–115. [Google Scholar]
- Wu G, Li YC, Zhu XT, et al. (2016). One hundred noteworthy boletes from China. Fungal Diversity 81: 25–188. [Google Scholar]
- Ye L, Mortimer PE, Xu J-C, et al. (2014). The Genus Phylloporus (Boletaceae, Boletales), from Mekong River Basin (Yunnan Province, China). Chiang Mai Journal of Sciences 41: 1–13. [Google Scholar]
- Zeng N-K, Cai Q, Yang ZL. (2012). Corneroboletus, a new genus to accommodate the southeastern Asian Boletus indecorus. Mycologia 104: 1420–1432. [DOI] [PubMed] [Google Scholar]
- Zeng N-K, Tang L-P, Li Y-C, et al. (2013). The genus Phylloporus (Boletaceae, Boletales) from China: morphological and multilocus DNA sequence analyses. Fungal Diversity 58: 73–101. [Google Scholar]
- Zeng N-K, Su M-S, Liang Z-Q, et al. (2015). A geographical extension of the North American genus Bothia (Boletaceae, Boletales) to East Asia with a new species B. fujianensis from China. Mycological Progress 14: 1015. [Google Scholar]
- Zhu XT, Wu G, Zhao K, et al. (2015). Hourangia, a new genus of Boletaceae to accommodate Xerocomus cheoi and its allied species. Mycological Progress 14: 37. [Google Scholar]