Editor—The critical shortage of medical supplies, including personal protective equipment, during the coronavirus disease 2019 (COVID-19) pandemic has compelled clinicians to look for additional ways to protect themselves from aerosolised particles during airway management. Although a few devices with a similar goal have been described,1 , 2 limitations remain, including lack of containment and effective removal of aerosols and the need for sterilisation. We developed a negative-pressure patient isolation hood that is disposable to reduce sterilisation risks and is coupled to negative pressure generated by smoke evacuators to achieve coronavirus source control during aerosol-generating procedures (Supplementary video 1).
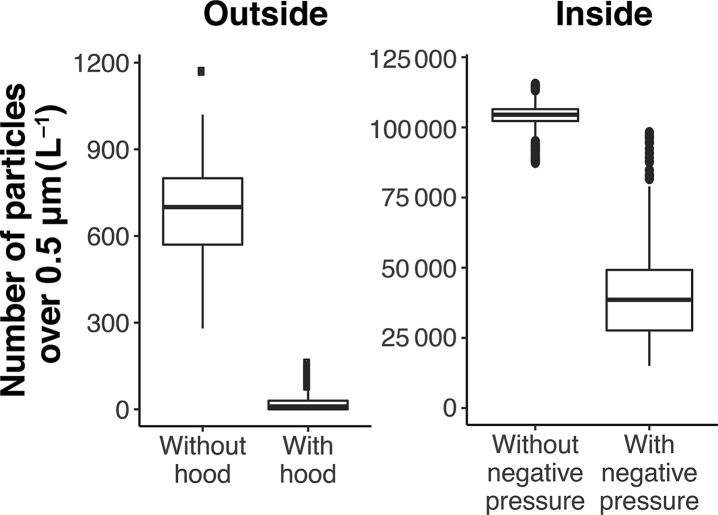
A humidifier generating supraphysiological amounts of aerosolised particles was used for testing to ensure efficacy even at extreme conditions (Supplementary Fig. 1). Most particles generated from human respiratory sources during coughing, sneezing, and talking are droplet nuclei 0.5–5.0 μm in diameter.3 A particle counter with a size detection range of 0.3–10 μm was placed inside the hood, and a second counter was placed outside of the hood at approximately the height of the clinician's head. With continuous aerosolisation, particle counts inside the hood were more than 100-fold greater than that generated by a cough.4 With the humidifier running continuously, the particle counter at the height of the clinician's head detected 700 (inter-quartile range: 570–800) L−1 aerosolised particles without the protection of a negative-pressure patient isolation hood. In contrast, the particle count was 18 (0–30) L−1 with the hood (Fig. 1 ). Particle count from inside the hood decreased by 63% when the smoke evacuator was generating 230 L min−1 of airflow, showing effective aerosol removal.
Fig 1.
Particle count outside (left-side panel) and inside (right-side panel) of the negative-pressure patient isolation hood during continuous aerosol generation. The middle horizontal line represents the median; the upper and lower borders of the box represent the upper and lower quartiles. The top and bottom horizontal lines indicate the range. Dots represent values outside of the 97.5 and 2.5 quantiles.
To compare aerosol clearance with and without negative pressure, the humidifier was turned off to simulate the end of an aerosol-generating procedure. Without negative pressure, 183 min was required for the particle count to decrease by 98%, compared with 5 min when negative pressure was applied (Supplementary Fig. 2). Whilst visual inspection correlated with the removal of large aerosolised particles (>10 μm), it was highly unreliable at determining the degree of removal of small aerosolised particles, as the hood appeared clear when particle count of particles greater than 0.5 μm was well above 200 000 L−1.
Limitations of the negative-pressure patient isolation hood device include (i) the time required for set-up, albeit a few minutes, might preclude its use in emergency situations; (ii) whilst aerosolised particles are efficiently removed by negative pressure, particles that adhere to the inner surface of the hood remain a source for contamination, so training is required to disassemble and discard the plastic drape; (iii) particles <0.3 μm in size were not measured, although smaller particles may be more susceptible to removal by negative pressure5; and (iv) the air in the hood reaches the smoke evacuator and passes through an ultra-low-particulate-air-grade filter that is rated to remove 99.999% of particles >100 μm in size. Filtered air is then recirculated back to the operating theatre. The filter has a limited plume evacuation time; thus, the need for replacement adds to the cost of using the device.
Despite these limitations, the negative-pressure patient isolation hood is expected to reduce the exposure of healthcare workers to aerosols during aerosol-generating procedures, such as tracheal intubation, extubation, and bronchoscopy, and thus may decrease the risk of viral transmission. It may also reduce the risk of cross contamination between patients in operating theatres. The utility of the hood can be broadened to most situations, in which direct patient contact is required, such as transportation, other bedside aerosol-generating procedures (e.g. tracheostomy, tracheal tube suctioning, and open circuit), and during phlebotomy. The negative-pressure patient isolation hood might also enable liberalised use of noninvasive ventilation strategies during the present pandemic in the setting of ventilator shortages. We recommend the use of particle counters to study the effectiveness of similar protective devices, as most devices have not been properly tested and may therefore provide a false sense of security and put their users at increased risk of exposure to droplets and aerosols.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Institutional and departmental sources.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.05.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Canelli R., Connor C.W., Gonzalez M., Nozari A., Ortega R. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382:1957–1958. doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matava C.T., Yu J., Denning S. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID-19. Can J Anaesth. 2020 doi: 10.1007/s12630-020-01649-w. Advance Access published on April 3 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen M.K., Ensor D.S., Sparks L.E. Airborne particle sizes and sources found in indoor air. Atmos Environ Gen Top. 1992;26:2149–2162. [Google Scholar]
- 4.Lindsley W.G., Pearce T.A., Hudnall J.B. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulkner W.B., Memarzadeh F., Riskowski G.L., Kalbasi A., Chang A.C. Effects of air exchange rate, particle size and injection place on particle concentrations within a reduced-scale room. Build Environ. 2015;92:246–255. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.