Abstract
Background:
Abnormal expression of inflammatory cytokines in major depressive disorder (MDD) suggests the activation of an inflammatory process. The pattern of alterations in cytokine levels is still ambiguous. The present study aimed to evaluate interleukin-7 (IL-7) and interleukin-10 (IL-10) for their involvement in the pathophysiology of MDD and determine their relationships with depression risk.
Methods:
The study included 166 medication-free subjects: 84 MDD patients and 82 sex- and age-matched healthy controls (HCs). A qualified psychiatrist diagnosed patients and evaluated controls based on the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Hamilton depression rating scale (Ham-D) was used to measure the severity of depression in MDD patients. Serum IL-7 and IL-10 levels were measured using enzyme-linked immunosorbent assay (ELISA) kits.
Results:
Compared with HCs, the serum levels of IL-7 were significantly decreased, whereas that of IL-10 increased in MDD patients. Moreover, the severity of depression is correlated with the altered levels of IL-7 and IL-10 in MDD patients. We found a negative correlation between IL-7 and Hamilton depression rating (Ham-D) scores (r = –0.580, p < 0.05), whereas there was a positive correlation between IL-10 and Ham-D scores (r = 0.555, p < 0.05).
Conclusions:
The altered levels of serum IL-7 and IL-10 in MDD patients may represent a homeostatic mechanism that enhances the inflammatory process during depression. The alterations of these cytokine levels in MDD and their association with the severity of depression support them as promising, but there may still be controversial factors for understanding the pathophysiology of depression.
Keywords: IL-7, IL-10, interleukin-7, interleukin-10, major depressive disorder, MDD
Introduction
Major depressive disorder (MDD) is a well-known, complex mood disorder in which up to 60% of patients may encounter treatment resistance that prolongs and worsens the cases.1 This illness can become chronic or recurrent and lead to massive impairments in the ability to deal with an individual’s daily responsibilities. This issue has turned into one of the main contributors to the global burden of disease.2 Common features of MDD are sadness, apathy, irritability or indifference. It is usually associated with a change of weight, sleep patterns, motor agitation or retardation, fatigue, impaired concentration, decision making, as well as feelings of shame or guilt and thoughts of death or dying.3 Depression that begins mostly in early adulthood is likely to be recurrent. It affects women more frequently than men, and unemployed people are at high risk.4
An incomplete understanding of the nature of depression might be the reason for the less effective treatment of MDD.5 For successful treatment of depression, the first step is to recognize the problem and make a correct diagnosis. But complicated neurophysiology of MDD affects the correct diagnosis of the specific condition, prediction of clinical outcomes in patients and our ability to produce newer, highly efficacious and safe antidepressants.6 The primary health care provider is the first person responsible for the diagnosis of depression, but evidence suggests that in most cases it remains unrecognized at this level due to poor understanding of the disease mechanism.7 Since the diagnosis of depression mostly depends on statements by patients, this leads to misdiagnosis and confusion.8 Evidence suggests that factors associated with the inflammatory process, neurotransmission, neurotrophy, neuroendocrine and metabolic systems could be used to recognize the mechanism of disease for individuals suffering from depression.9 The biological markers for understanding the pathophysiology of MDD are rare, and although there are few risk assessment factors, the findings are largely inconsistent.
Some recent evidence suggest that cell-mediated immunity plays an important role in the pathogenesis of MDD.10–12 Interleukin-7 (IL-7) is a growth factor that is considered to be an immune-stimulatory substance, and a major homoeostatic cytokine.13 Interleukin-10 (IL-10), which is an anti-inflammatory cytokine, increases during neuroinflammation and may be implicated in depression.14 IL-7 and IL-10 are both assumed to play a vital role in lymphocyte development since abnormal levels of these cytokines have been found in multi-analytic screening studies in the serum of psychiatric patients.15 There are many controversial studies found for these markers. Dahl et al. reported that serum IL-7 and IL-10 levels were significantly elevated in medication-free MDD patients compared with healthy controls (HCs).16 Wiener et al. showed higher serum levels of IL-10 in MDD patients than those of HCs through a cross-sectional study.17 In contrast, other studies found decreased serum IL-7 levels in MDD patients compared with HCs.18–20 However, some authors also reported no significant difference in the production of IL-10 in MDD patients compared with HCs.21–24 The above findings regarding IL-7 and IL-10 in MDD patients highlight the need to initiate further studies in this field. However, the existence of biological markers for the understanding of the pathophysiology of depression is still unknown. Taking all the above evidence and findings together, the present study evaluated inflammatory cytokines in major depression. The aim of this study was, first, to investigate serum IL-7 and IL-10 levels in MDD patients and HCs. Second, in case of any alteration in these levels, we aimed to determine the association between the altered cytokine levels and the severity of depression. Third, our objective was to detect the involvement of cytokine levels in depression risk.
Methods
Study population
A total of 84 MDD patients and 82 sex- and age-matched HCs took part in this study. All patients were recruited from the Department of Psychiatry, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, and all HCs were taken from different parts of Dhaka. The study participants were screened by a qualified psychiatrist before entering the study. This screening was conducted in five steps: (1) a standard psychiatric interview; (2) a structured clinical interview following the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5); (3) severity assessment through the Hamilton depression rating (Ham-D) scale; (4) a detailed assessment of previous psychiatric illness; and (5) a sociodemographic status recorded using predesigned questionnaires. MDD patients suffering from depressive symptoms for at least 2 weeks or longer were included in this study. Exclusion criteria were the use of illegal drugs, alcohol or substance abuse within the past 6 months, history of heart disease, kidney or inflammatory diseases, acute or chronic infection and major somatic disorders. As antidepressant or antipsychotic drugs could interfere with serum cytokine levels, all the study participants were free of medications for at least 1 week before the examination. Written consent was taken from all study participants and the study was approved by the ethical review committee of BSMMU (approval number: BSMMU/2019/3507).
Blood sample collection and cytokine measurement
Blood samples (5 ml) were collected from the cephalic vein and serum probes were immediately centrifuged at 1000 × g for 15 min. The supernatant was aliquoted in non-absorbing 300 µl polypropylene tubes and stored at –80°C until cytokine measurement. The serum IL-7 and IL-10 levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (BosterBio, USA) according to the manufacturer’s instructions. Briefly, 100 µl of serum sample or standard solution was added to the appropriate wells of the 96-well microtiter plate. The plate was covered and incubated at room temperature for 120 min. Then, the cover was removed to discard the liquid in the wells. Then, 100 µl of the biotinylated anti-human antibody of the respective cytokine was dispensed into each well with thorough mixing. The plate was again covered with a plate sealer and incubated at room temperature for 90 min. The contents of each well were aspirated, followed by three rinses with 300 µl of wash buffer. Avidin–biotin–peroxidase complex (100 µl) was added to each well and the plate was incubated at room temperature for 40 min. The contents of each well were again aspirated, followed by five rinses with 300 µl of wash buffer. Then, 90 µl of color developing reagent tetramethylbenzidine (TMB) was added into each well and the plate was incubated in the dark for 30 min at room temperature. The reaction was stopped with the addition of 100 µl stop solution and absorbance was read at 450 nm immediately. The serum levels of IL-7 and IL-10 were expressed as pg/ml. The minimum detectable doses for serum IL-7 and IL-10 were <1 pg/ml and <0.5 pg/ml, respectively.
Statistical analysis
Data for serum IL-7 and IL-10 levels were tested for normality. The distribution pattern of the data was centrally grouped about the mean but not skewed significantly. An independent sample t-test was applied to compare between MDD patients and HCs. The serum levels of IL-7 and IL-10 were expressed as the mean ± standard error of the mean (mean ± SEM). Spearman’s correlation analysis and scatter plot graphs were applied to obtain the relationship between the analyzed cytokines and the severity of depression. Box plot graphs were used to show the changes in serum cytokines between the groups. Receiver operating characteristic (ROC) curve analysis was performed to get the diagnostic or predictive value of altered cytokines in depression. Statistical significance was set at a p-value of less than 0.05 for all analyses. Statistical analysis was done using the statistical software package SPSS version 23.0 (IBM Corp., Armonk, NY).
Results
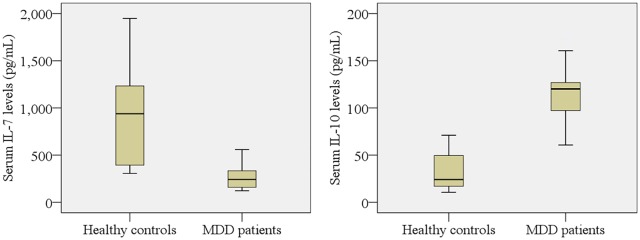
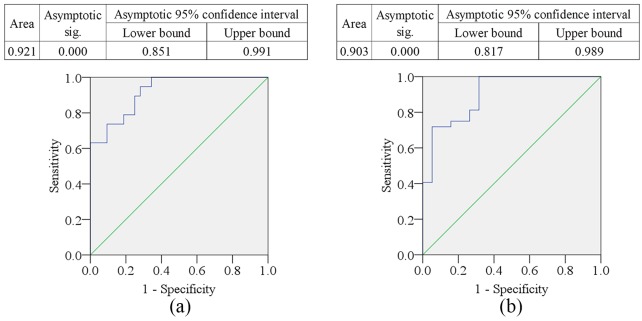
The sociodemographic profile of the study population is presented in Table 1. Age, sex, and body mass index (BMI) did not differ significantly between the MDD patients and HCs. Clinical outcomes and laboratory findings are shown in Table 2, where the patients with MDD have significantly lower levels of IL-7 (p < 0.05) and higher levels of IL-10 (p < 0.05) compared with HCs. These alterations of serum cytokine levels are displayed graphically in Figure 1. Both pro-inflammatory (IL-7) and anti-inflammatory (IL-10) cytokines were found to be higher in female patients compared with male patients. The serum IL-7 levels were negatively correlated (r = –0.580, p < 0.05) with Ham-D scores, whereas serum IL-10 levels were positively correlated (r = 0.555, p < 0.05) with Ham-D scores in MDD patients (Table 3). Moreover, the serum IL-7 and IL-10 were inversely correlated (r = –0.383, p < 0.05) in MDD patients; however, the serum IL-7 levels were positively correlated with Ham-D scores in HCs (r = 0.486, p < 0.05). Graphical illustrations of these correlations between serum cytokine levels and Ham-D scores are presented in Figure 2. The serum cytokine levels of MDD patients were not correlated with their age, education, smoking or area of residence. The ROC curves of IL-7 and IL-10 were plotted, and cut-off points were detected as 332.90 pg/ml and 44.0 pg/ml, respectively (Figure 3).
Table 1.
The sociodemographic profile of the study population.
| Parameters | MDD patients (n = 84) |
Healthy controls (n = 82) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| n | % | Mean ± SEM | n | % | Mean ± SEM | ||
| Age in years | |||||||
| 18–24 | 5 | 5.95 | 34.13 ± 1.50 | 7 | 8.54 | 32.68 ± 1.83 | 0.689 |
| 25–34 | 42 | 50.00 | 44 | 53.66 | |||
| 35–44 | 26 | 30.95 | 22 | 26.83 | |||
| 45–60 | 11 | 13.10 | 9 | 10.98 | |||
| Gender | 0.272 | ||||||
| Male | 26 | 30.95 | 35 | 42.68 | |||
| Female | 58 | 69.05 | 47 | 57.32 | |||
| Marital status | 0.741 | ||||||
| Unmarried | 37 | 44.05 | 39 | 47.56 | |||
| Married | 47 | 55.95 | 43 | 52.44 | |||
| BMI (kg/m2) | |||||||
| Below 18.5 (CED) | 8 | 9.52 | 25.18 ± 0.90 | 4 | 4.88 | 24.50 ± 0.81 | 0.575 |
| 18.5–25 (normal) | 45 | 53.57 | 48 | 58.54 | |||
| Above 25 (obesity) | 31 | 36.90 | 30 | 36.59 | |||
| Education level | 0.857 | ||||||
| Illiterate | 8 | 9.52 | 9 | 10.98 | |||
| Primary | 24 | 28.57 | 20 | 24.39 | |||
| Secondary | 39 | 46.43 | 42 | 51.22 | |||
| Graduate and above | 13 | 15.48 | 11 | 13.41 | |||
| Occupation | 0.147 | ||||||
| Service | 7 | 8.33 | 8 | 9.76 | |||
| Business | 3 | 3.57 | 4 | 4.88 | |||
| Housekeeper | 32 | 38.10 | 30 | 36.59 | |||
| Unemployed | 15 | 17.86 | 14 | 17.07 | |||
| Others | 27 | 32.14 | 26 | 31.71 | |||
| Economic impression | |||||||
| Lower | 26 | 30.95 | 30.03 ± 1.57 | 25 | 30.49 | 31.15 ± 1.63 | 0.573 |
| Middle | 45 | 53.57 | 42 | 51.22 | |||
| Higher | 13 | 15.48 | 15 | 18.29 | |||
| Area residence | 0.932 | ||||||
| Urban | 29 | 34.52 | 30 | 36.59 | |||
| Rural | 55 | 65.48 | 52 | 63.41 | |||
| Smoking history | 0.739 | ||||||
| Non-smoker | 60 | 71.43 | 60 | 73.17 | |||
| Smoker | 24 | 28.57 | 22 | 26.83 | |||
BMI, body mass index; CED, chronic energy deficiency; MDD, major depressive disorder; SEM, standard error of the mean.
Table 2.
Clinical outcomes and laboratory findings of the study population.
| Parameters | MDD patients (n = 84) |
Healthy controls (n = 82) |
p-value |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| Ham-D score | 20.15 ± 0.42 | 4.74 ± 0.65 | 0.000 |
| Serum IL-7 (pg/ml) | 265.16 ± 49.39 | 918.20 ± 233.20 | 0.001 |
| Serum IL-10 (pg/ml) | 119.2 ± 23.71 | 33.44 ± 5.53 | 0.008 |
Ham-D, 17-item Hamilton depression rating scale; IL-7, interleukin-7; IL-10, interleukin-10; MDD, major depressive disorder; SEM, standard error mean.
Significant p-values are shown in bold.
Figure 1.
Variations of serum interleukin-7 (IL-7) and interleukin-10 (IL-10) among the study population. There is a significant difference between patient and control groups at a 95% confidence interval (CI).
MDD, major depressive disorder.
Table 3.
Spearman’s correlation among various research parameters among the study population.
| MDD patients (n = 84) |
Healthy controls (n = 82) |
|||
|---|---|---|---|---|
| Correlation parameters | r | p-value | r | p-value |
| Age and IL-7 | −0.015 | 0.934 | −0.257 | 0.073 |
| BMI and IL-7 | 0.230 | 0.206 | −0.054 | 0.827 |
| Education and IL-7 | 0.050 | 0.784 | 0.322 | 0.178 |
| Income and IL-7 | 0.149 | 0.415 | 0.166 | 0.496 |
| Smoking and IL-7 | 0.083 | 0.652 | −0.361 | 0.129 |
| Ham-D and IL-7 | −0.580 | 0.000 | 0.486 | 0.035 |
| Age and IL-10 | 0.078 | 0.672 | 0.107 | 0.662 |
| BMI and IL-10 | −0.106 | 0.567 | 0.191 | 0.433 |
| Education and IL-10 | 0.053 | 0.775 | −0.068 | 0.781 |
| Income and IL-10 | −0.137 | 0.454 | 0.295 | 0.220 |
| Smoking and IL-10 | 0.038 | 0.838 | 0.176 | 0.471 |
| Ham-D and IL-10 | 0.555 | 0.001 | −0.012 | 0.960 |
| IL-7 and IL-10 | −0.383 | 0.030 | −0.051 | 0.837 |
Ham-D, Hamilton depression rating scale; IL-7, interleukin-7; IL-10, interleukin-10; MDD, major depressive disorder; r, correlation coefficient. Negative values specify the opposite correlation.
Significant p-values are shown in bold.
Figure 2.
Scatter plot of serum interleukin-7 (IL-7) and interleukin-10 (IL-10) with Hamilton depression rating (Ham-D) scores among major depressive disorder patients.
Figure 3.
Receiver operating characteristic (ROC) curves: (a) interleukin-7 (IL-7) and (b) interleukin-10 (IL-10). The cut-off point was detected as 332.90 pg/ml for IL-7 and 44.00 pg/ml for IL-10.
Discussion
In the present study, a pro-inflammatory cytokine, IL-7, was significantly lower and anti-inflammatory cytokine, IL-10, was significantly higher in MDD patients than in HCs. The major correlations were observed between altered levels of serum cytokines and the severity of depression. The inflammatory system is extremely complex and numerous biomarkers represent different aspects of this system. Several mechanisms could link cytokine-mediated immune activation to the pathophysiology of depression, including cytokine receptor activation of neurons,25 increased expression of serotonin transporters,26 kynurenine pathway activation,27 reduced neuronal growth factors,28 activation of the hypothalamic–pituitary–adrenal axis29 and alterations in the synthesis, release and reuptake of neurotransmitters.30 IL-7 is essential for lymphocyte development and survival.31 T cells and natural killer (NK) cells are important contributors to the cell-mediated immune response. Abnormal numbers and functions of T cells and NK cells have been reported in MDD patients.32 Therefore, through the regulation of T cells, which secrete lymphokines, IL-7 also participates in the cascade possibly by contributing to the hypothesized depression-related lymphokine suppression.33 Since IL-7 is a major regulator of T cell functioning, its measurement could be a potent indication of depression-related T cell alterations. Our finding that decreased serum IL-7 levels are associated with the severity of depression is consistent with past studies. Lehto et al. found that serum IL-7 levels were decreased in MDD patients compared with HCs, which supports the present study findings.18 Another study by Grosse et al. found that IL-7 levels were lowered in MDD patients compared with those of HCs.20 Gaarden et al. found lowered plasma levels of IL-7 in depressed patients compared with non-depressed controls through a cross-sectional study.19 Similar to our findings, one study revealed that serum IL-7 levels were significantly, but inversely, associated with the severity of depression among geriatric patients.34 Moreover, inflammatory processes and oxidative stress are positively correlated with the severity of drug-naïve MDD patients.23 Also, significantly positive correlations between anxiety measures and serum levels of IL-1α, IL-6, IL-8 and IFN-γ were seen.35,36
In contrast, IL-10 is elevated in response to acute immune challenge. These changes have been considered in terms of the imbalance between individual pro- and anti-inflammatory cytokines and the Th1 and Th2 imbalance in major depression.37 IL-10 increases due to inflammation induced by psychological or physiological stress.38 The elevated IL-10 may implicate a fine-tuned integrated activation linked to depression. Imbalanced interactions between different cytokines and immune pathways could be an underlying mechanism for the pathophysiology of MDD.39 Thus, theoretically, this effect may occur secondarily to an overall reduction of peripheral inflammation promoted by antidepressants. Brunoni et al. found that antidepressants significantly decreased peripheral levels of IL-10.40 However, it is difficult to determine the specific mechanisms involved. Certain cytokines may play a unique role in the pathophysiology of depression, while others could represent secondary factors not related directly to depression. In agreement with our findings, increased serum IL-10 levels and their correlation with the severity of depression were reported by some past research. One study found that antidepressant drug-naïve MDD patients had significantly elevated serum IL-10 levels compared with HCs.41 In another two recent studies, Müller et al. and Dahl et al. demonstrated the increased levels of IL-10 in MDD patients compared with HCs.16,42 Wiener et al. also found the elevated levels of serum IL-10 in MDD patients compared with HCs through a cross-sectional study.17 In addition, increased levels of IL-10 were found in drug-naïve MDD patients and the levels were normalized after antidepressant treatment.43 Moreover, a recent meta-analysis showed elevated levels of IL-10 in MDD patients compared with HCs.44 The elevated levels of serum IL-10 can be explained by the study of Iacob et al., in which the increased expression of the IL-10 gene in MDD patients was observed and the severity of depression was also positively associated with increased IL-10 expression.45
Another finding of the present study is ROC curve analysis (Figure 3), which was used to quantify the predictive performance of altered cytokine levels. Accuracy is measured by the area under the ROC curve (AUC), with ranges as follows: 0.9–1.0 = excellent, 0.8–0.9 = good, 0.7–0.8 = fair, 0.6–0.7 = poor and <0.6 = not useful.46 Several previous studies identified the altered serum levels of IL-7 and IL-10 in MDD patients, but these cannot be used to assess depression risk due to their lack of predictive performance.41,42,47 AUC of IL-7 was 0.921, which was significant (p < 0.001), and lower values were assigned as the disease state. Similarly, the AUC of IL-10 was 0.903, which was also significant (p < 0.001) where higher values were assigned as the disease state. ROC analysis showed the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) to be 89.5%, 75%, 78.6% and 87.5% for IL-7, respectively, while these were 81.3%, 73.7%, 76.0%, and 79.4% for IL-10, respectively. As MDD is a multifactorial condition predisposed by environmental and genetic aspects,48 it is essential to define biological factors for the evaluation of depression risk.
Unfortunately, the previous studies could not suggest new therapeutic approaches based on their findings of altered serum cytokine levels in MDD patients.16,19,42 In a study by Bschor et al. it was found that 30–50% of MDD patients do not respond to traditional antidepressant therapy.49 The explanation could be a lack of efficacy or intolerable side effects.50 Kopschina Feltes et al. observed that depressive patients have underlying immune deregulation that could explain the lack of therapeutic benefit from antidepressants and treatment resistance.51 The high rate of treatment resistance, the rising suicide risk in unresponsive MDD patients, together with the overwhelming economic costs to society, constitutes the basis of the search for new therapeutic agents that aim to improve the quality of life or even cure these patients.52 Furthermore, studies have demonstrated that the levels of pre-treatment biomarkers can be valuable predictors of treatment response, selection of the best treatment to be applied and indicators of possible treatment resistance to conventional antidepressant drugs.51 Evidence suggests that patients who have recovered from the depressive episode have normalized levels of pro- and anti-inflammatory cytokines after treatment.16 Based on the present study findings, we can suggest some new treatment approaches for depression. Both mind–body therapies (MBTs) and exercise are non-pharmacological augmentative strategies that can decrease stress levels and modulate immunity, increasing the quality of life of MDD patients. An increase in the pro-inflammatory cytokines is induced by laborious exercise. This release is balanced by the secretion of cytokine inhibitors (IL-1ra, sTNF-r1 and TNF-r2) and the anti-inflammatory cytokine IL-10.53 Besides MBTs, other lifestyle changes, such as dietary interventions, could have a beneficial effect on MDD. For example, nutrients like zinc and omega-3 fatty acids have been shown to have beneficial effects on depression.54,55 These nutrients may exert an affect through interaction with inflammatory pathways.56 Moreover, inhibiting the effects of cytokines with monoclonal antibodies is a promising therapeutic approach, though at present it is associated with the risk of potentially serious side effects.57 NK cells are innate immune cells that have strong cytolytic function against physiologically stressed cells.58 Grosse et al. have shown various signs of NK and T cell defects in patients with MDD.20 For a better clinical outcome, NK cell-based immunotherapeutic strategies can be applied to improve the function of NK cells. NK cell immunotherapy offers several advantages. First, the use of NK cells will bypass the need for antigen-specific T cells. Second, NK cells can secrete pro-inflammatory cytokines rapidly that can potentiate the adaptive immune response.59 Lastly, NK cells have a relatively short lifespan and are easy to isolate and manipulate. Hence, the possibility of overexpansion of transferred NK cells in the recipient’s body is minimized. Also, the metabolic reprogramming of NK cells may improve depression through cytokine therapy. For example, IL-10 is also a potent inducer of NK cell proliferation, cytotoxic function and IFN-γ production in combination with IL-18.60 Therefore, it seems that suppression of particular inflammatory cytokines may be necessary for clinical recovery from depression, and as a consequence reduction in inflammation might not happen in depressed patients who fail to respond to treatment.
Due to the limited sample size, we did not exclude participants with a smoking habit, which might interrupt cytokine analyses. Also, we did not consider food habits, physical activity or sleep patterns in this study, as these would have reduced the statistical power by decreasing sample size further. The main limitation of the present study is that our test is not specific to depression. In addition, we did not investigate the impact of antidepressant medications on our study parameters. Therefore, our findings should be treated as preliminary, and to establish them further studies with a more homogeneous and larger population is recommended in this field.
In conclusion, this study of altered serum cytokine levels in MDD patients may demonstrate a connection between levels of IL-7 and IL-10 and the severity of depression. These results, along with their predictive performance, could be used to measure the depression risk and probable therapeutic approaches.
Footnotes
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Md. Rabiul Islam  https://orcid.org/0000-0003-2820-3144
https://orcid.org/0000-0003-2820-3144
Contributor Information
Sadia Anjum, Department of Pharmacy, University of Asia Pacific, Dhaka, Bangladesh.
M. M. A. Shalahuddin Qusar, Department of Psychiatry, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Mohammad Shahriar, Department of Pharmacy, University of Asia Pacific, Dhaka, Bangladesh.
Sardar Mohammad Ashraful Islam, Department of Pharmacy, University of Asia Pacific, Dhaka, Bangladesh.
Mohiuddin Ahmed Bhuiyan, Department of Pharmacy, University of Asia Pacific, Dhaka, Bangladesh.
Md. Rabiul Islam, Department of Pharmacy, University of Asia Pacific, 74/A Green Road, Farmgate, Dhaka-1215, Bangladesh.
References
- 1. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003; 53: 649–659. [DOI] [PubMed] [Google Scholar]
- 2. Nolen-Hoeksema S, Girgus J. The emergence of gender differences in depression during adolescence. Psychol Bull 1994; 115: 424–443. [DOI] [PubMed] [Google Scholar]
- 3. Castelao CF, Kröner-Herwig B. Different trajectories of depressive symptoms in children and adolescents: predictors and differences in girls and boys. J Youth Adolesc 2013; 42: 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–223. [DOI] [PubMed] [Google Scholar]
- 5. Friedman A. Jump-starting natural resilience reverses stress susceptibility. Science 2014; 346: 555–555. [DOI] [PubMed] [Google Scholar]
- 6. Schneider B, Prvulovic D, Oertel-Knochel V, et al. Biomarkers for major depression and its delineation from neurodegenerative disorders. Prog Neuro Biol 2011; 95: 703–717. [DOI] [PubMed] [Google Scholar]
- 7. Raine R, Lewis L, Sensky T, et al. Patient determinants of mental health interventions in primary care. Br J Gen Pract 2000; 50: 620–625. [PMC free article] [PubMed] [Google Scholar]
- 8. Breslau N. Depressive symptoms, major depression, and generalized anxiety: a comparison of self-reports on CES-D and results from diagnostic interviews. Psychiatry Res 1985; 15: 219–229. [DOI] [PubMed] [Google Scholar]
- 9. Jani B, McLean G, Nicholl B, et al. Risk assessment and predicting outcomes in patients with depressive symptoms: a review of potential role of peripheral blood based biomarkers. Front Hum Neurosci 2015; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connor T, Leonard B. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci 1998; 62: 583–606. [DOI] [PubMed] [Google Scholar]
- 11. Licinio J, Wong M. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry 1999; 4: 317–327. [DOI] [PubMed] [Google Scholar]
- 12. Toben C, Baune B. An act of balance between adaptive and maladaptive immunity in depression: a role for T lymphocytes. J Neuroimmune Pharm 2015; 10: 595–609. [DOI] [PubMed] [Google Scholar]
- 13. Calzascia T, Pellegrini M, Lin A, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci 2008; 105: 2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mesquita A, Correia-Neves M, Roque S, et al. IL-10 modulates depressive-like behavior. J Psychiatr Res 2008; 43: 89–97. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz E, Guest PC, Rahmoune H, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry 2012; 17: 494–502. [DOI] [PubMed] [Google Scholar]
- 16. Dahl J, Ormstad H, Aass H, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014; 45: 77–86. [DOI] [PubMed] [Google Scholar]
- 17. Wiener C, Moreira F, Portela L, et al. Interleukin-6 and Interleukin-10 in mood disorders: a population-based study. Psychiatry Res 2019; 273: 685–689. [DOI] [PubMed] [Google Scholar]
- 18. Lehto S, Huotari A, Niskanen L, et al. Serum IL-7 and G-CSF in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 846–851. [DOI] [PubMed] [Google Scholar]
- 19. Gaarden TL, Engedal K, Benth JŠ, et al. Exploration of 27 plasma immune markers: a cross-sectional comparison of 64 old psychiatric inpatients having unipolar major depression and 18 non-depressed old persons. BMC Geriatr 2018; 18: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grosse L, Hoogenboezem T, Ambrée O, et al. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav Immun 2016; 54: 38–44. [DOI] [PubMed] [Google Scholar]
- 21. Farid HR, Jabbari AF, Talaee A, et al. Assessment of the immune system activity in Iranian patients with major depression disorder (MDD). Iran J Immunol 2007; 4: 38–43. [DOI] [PubMed] [Google Scholar]
- 22. Gazal M, Jansen K, Souza L, et al. Association of interleukin-10 levels with age of onset and duration of illness in patients with major depressive disorder. Braz J Psychiatry 2015; 37: 296–302. [DOI] [PubMed] [Google Scholar]
- 23. Rawdin BJ, Mellon SH, Dhabhar FS, et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun 2013; 31: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talarowska M, Szemraj J, Gałecki P. The role of interleukin genes in the course of depression. Open Med (Wars) 2016; 11: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 2012; 8: 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsao C, Lin Y, Chen C, et al. Cytokines and serotonin transporter in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 899–905. [DOI] [PubMed] [Google Scholar]
- 27. Myint A, Kim Y, Verkerk R, et al. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 2007; 98: 143–151. [DOI] [PubMed] [Google Scholar]
- 28. Audet M, Anisman H. Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Front Cell Neurosci 2013; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Himmerich H, Binder E, Künzel H, et al. Successful antidepressant therapy restores the disturbed interplay between TNF-α system and HPA axis. Biol Psychiatry 2006; 60: 882–888. [DOI] [PubMed] [Google Scholar]
- 30. Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol 2008; 85: 1–74. [DOI] [PubMed] [Google Scholar]
- 31. Jiang Q, Li W, Aiello F, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev 2005; 16: 513–533. [DOI] [PubMed] [Google Scholar]
- 32. Miller A. Depression and immunity: a role for T cells? Brain Behav Immun 2010; 24: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith RS. The macrophage theory of depression. Med Hypotheses 1991; 35: 298–306. [DOI] [PubMed] [Google Scholar]
- 34. Hall JR, Wiechmann A, Edwards M, et al. IL-7 and depression: the importance of gender and blood fraction. Behav Brain Res 2016; 315: 147–149. [DOI] [PubMed] [Google Scholar]
- 35. Nishuty NL, Khandoker MMH, Karmoker JR, et al. Evaluation of serum interleukin-6 and C-reactive protein levels in drug-naïve major depressive disorder patients. Cureus 2019; 11: e3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang Z, Ye G, Chen X, et al. Peripheral proinflammatory cytokines in Chinese patients with generalised anxiety disorder. J Affect Disord 2018; 225: 593–598. [DOI] [PubMed] [Google Scholar]
- 37. Henry C, Huang Y, Wynne A, et al. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav Immun 2009; 23: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roque S, Correia-Neves M, Mesquita A, et al. Interleukin-10: a key cytokine in depression? Cardiovasc Psychiatry Neurol 2009; 2009: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barnes J, Mondelli V, Pariante CM. Genetic contributions of inflammation to depression. Neuropsychopharmacology 2017; 42: 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brunoni A, Machado-Vieira R, Zarate C, et al. Cytokines plasma levels during antidepressant treatment with sertraline and transcranial direct current stimulation (tDCS): results from a factorial, randomized, controlled trial. Psychopharmacology 2013; 231: 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS One 2018; 13: e0197267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Müller N, Krause D, Barth R, et al. Childhood adversity and current stress are related to pro- and anti-inflammatory cytokines in major depression. J Affect Disord 2019; 253: 270–276. [DOI] [PubMed] [Google Scholar]
- 43. Hernández M, Mendieta D, Martínez-Fong D, et al. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. Eur Neuropsychopharmacol 2008; 18: 917–924. [DOI] [PubMed] [Google Scholar]
- 44. Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017; 135: 373–387. [DOI] [PubMed] [Google Scholar]
- 45. Iacob E, Light K, Tadler S, et al. Dysregulation of leukocyte gene expression in women with medication-refractory depression versus healthy non-depressed controls. BMC Psychiatry 2013; 13: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim JW, Lee YS, Han DH, et al. Diagnostic utility of quantitative EEG in un-medicated schizophrenia. Neurosci Lett 2015; 589: 126–131. [DOI] [PubMed] [Google Scholar]
- 47. Simon N, McNamara K, Chow C, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol 2008; 18: 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301: 386–389. [DOI] [PubMed] [Google Scholar]
- 49. Bschor T, Ising M, Erbe S, et al. Impact of citalopram on the HPA system: a study of the combined DEX/CRH test in 30 unipolar depressed patients. J Psychiatr Res 2012; 46: 111–117. [DOI] [PubMed] [Google Scholar]
- 50. Rush A, Trivedi M, Wisniewski S, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163: 1905–1917. [DOI] [PubMed] [Google Scholar]
- 51. Kopschina Feltes P, Doorduin J, Klein H, et al. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J Psychopharmacol 2017; 31: 1149–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brunello N, Alboni S, Capone G, et al. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol 2006; 21: 219–225. [DOI] [PubMed] [Google Scholar]
- 53. Ostrowski K, Rohde T, Asp S, et al. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 1999; 515: 287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Islam MR, Islam MR, Shalahuddin Qusar MMA, et al. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: a case-control study. BMC Psychiatry 2018; 18: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016; 173: 575–587. [DOI] [PubMed] [Google Scholar]
- 56. Johnson R. Feeding the beast: can microglia in the senescent brain be regulated by diet? Brain Behav Immun 2015; 43: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Toussi S, Pan N, Walters H, et al. Infections in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with tumor necrosis factor-α inhibitors: systematic review of the literature. Clin Infect Dis 2013; 57: 1318–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol 2017; 8: 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sconocchia G, Eppenberger S, Spagnoli G, et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology 2014; 3: e952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cai G, Kastelein R, Hunter C. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-γ when combined with IL-18. Eur J Immunol 1999; 29: 2658–2665. [DOI] [PubMed] [Google Scholar]