Abstract
Background:
Functional respiratory imaging (FRI) is a quantitative postprocessing imaging technique used to assess changes in the respiratory system. Using FRI, we characterized the effects of the long-acting muscarinic antagonist (LAMA), glycopyrrolate metered dose inhaler (GP MDI), and the long-acting β2-agonist (LABA), formoterol fumarate metered dose inhaler (FF MDI), on airway volume and resistance in patients with moderate-to-severe chronic obstructive pulmonary disease.
Methods:
Patients in this phase IIIb, randomized, double-blind crossover study received twice-daily GP MDI (18 μg) and FF MDI (9.6 μg). Primary endpoints were specific (i.e. corrected for lobar volume) image-based airway volume (siVaw) and specific image-based airway resistance (siRaw), measured using FRI. Secondary and other endpoints included additional FRI, spirometry, and body plethysmography parameters. Postdose efficacy assessments were performed within 60–150 min of dosing on day 15.
Results:
A total of 23 patients were randomized and 19 completed both treatment periods. GP MDI and FF MDI both achieved significant improvements from baseline to day 15 in siVaw [11% (p = 0.0187) and 23% (p < 0.0001) increases, respectively] and siRaw [25% (p = 0.0219) and 44% (p < 0.0001) reductions, respectively]. Although, on average, improvements were larger for FF MDI than GP MDI, some individuals displayed greater responses with each of the two treatments. These within-patient differences increased with airway generation number. Spirometry and body plethysmography endpoints showed significant improvements from baseline in inspiratory capacity for both treatments, and numeric improvements for other endpoints.
Conclusion:
Both GP MDI and FF MDI significantly improved siRaw and siVaw at day 15 versus baseline. FRI endpoints demonstrated increased sensitivity relative to spirometry and body plethysmography in detecting differences between treatments in a small number of patients. Intra-patient differences in treatment response between the LAMA and the LABA provide further support for the benefit of dual bronchodilator therapies.
ClinicalTrials.gov registration number:
The reviews of this paper are available via the supplemental material section.
Keywords: formoterol fumarate dihydrate, functional respiratory imaging, glycopyrronium, long-acting β2-agonist, long-acting muscarinic antagonist
Introduction
The cornerstone of pharmacologic maintenance therapy for chronic obstructive pulmonary disease (COPD) is treatment with inhaled bronchodilators, which can improve lung function, reduce airway obstruction, and decrease the risk of future exacerbations.1 For patients with COPD who are at low risk of exacerbation, the current global initiative for chronic obstructive lung disease (GOLD) report recommends initial treatment with a single bronchodilator, with no preference for either a long-acting muscarinic antagonist (LAMA) or a long-acting β2-agonist (LABA).1 For those who are at high risk of exacerbation, but have a low symptom burden, a LAMA is preferred. The addition of a second bronchodilator is recommended for patients whose symptoms are inadequately controlled by monotherapy as LAMAs and LABAs can have a synergistic effect when administered together by enabling bronchodilation through separate receptor pathways.2–4
A number of fixed-dose combinations providing both a LAMA and LABA in a single inhaler are now available.5–9 These include a glycopyrrolate/formoterol fumarate metered dose inhaler (GFF MDI), formulated using co-suspension delivery technology, which is approved for the treatment of COPD in several markets including the USA, the EU and, since June 2019, Japan.9–12 Glycopyrrolate (a LAMA) and formoterol fumarate (a LABA) are both well characterized and widely available as monotherapies.13–16 Co-suspension delivery technology is a formulation technique for MDIs that provides consistent dose delivery, which is associated with drug distribution throughout the lung.10,17,18
In patients with COPD, the severity of airflow limitation, measured as forced expiratory volume in 1 s (FEV1), has traditionally been used to assess and guide treatment,19 and is still the gold standard for diagnosing COPD.1 However, FEV1 does not fully capture the complexity of COPD,20 and nonspirometric methods can help to evaluate the extent and characteristics of the disease, as well as the impact of pharmacologic treatments.21 Functional respiratory imaging (FRI), a quantitative postprocessing technology based on computed tomography (CT) images, can be used to assess regional changes in the respiratory system22 and may be more sensitive than FEV1 in evaluating the bronchodilating effect of COPD medications.23–25
FRI previously demonstrated that GFF MDI significantly improved airway volume and resistance versus placebo in patients with COPD (ClinicalTrials.gov identifier: NCT02643082).26 Here, we present a follow-up study that used FRI to characterize the effects of the monocomponents of GFF MDI, i.e. glycopyrrolate (GP MDI) and formoterol fumarate (FF MDI), also formulated using co-suspension delivery technology, in patients with moderate-to-severe COPD (ClinicalTrials.gov identifier: NCT02937584). FRI assessments were complemented by spirometry and body plethysmography measures to further characterize airflow limitation and lung hyperinflation, and to assess consistency between FRI and traditional lung-function parameters.
Methods
Study design
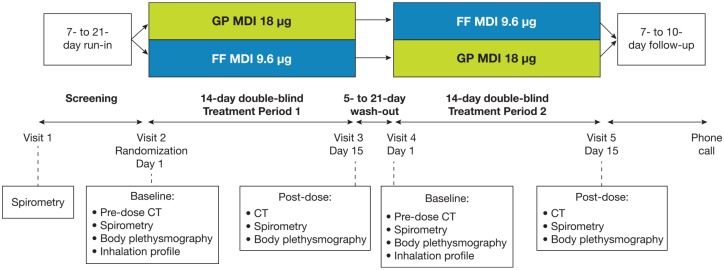
This randomized, double-blind, two-period crossover study assessed the effects of GP MDI 18 μg and FF MDI 9.6 μg (both administered as two twice-daily inhalations) on FRI parameters and pulmonary function after a 2-week dosing period in patients with moderate-to-severe COPD (Figure 1). Doses are expressed as glycopyrrolate 18 µg and formoterol fumarate 9.6 µg, equivalent to glycopyrronium 14.4 µg and formoterol fumarate dihydrate 10 µg, respectively.
Figure 1.
Study design. Patients received ipratropium bromide 34 μg four times daily during the screening and washout periods. All postdose assessments were performed within 150 min of dosing on day 15 (±5 days), with high-resolution CT scans (to assess FRI parameters) initiated 90 ± 30 min after dosing, followed by spirometry, then body plethysmography.
CT, computed tomography; FF, formoterol fumarate; FRI, functional respiratory imaging; GP, glycopyrrolate; MDI, metered dose inhaler.
Following a 7–21-day run-in period, patients were randomized into one of two treatment sequences: GP MDI followed by FF MDI, or FF MDI followed by GP MDI (Figure 1). Patients received approximately 2 weeks of treatment with each study drug, separated by a washout period of 5–21 days. High-resolution CT scans, spirometry, and body plethysmography were performed at day 1 (baseline) and day 15 of each treatment period.
All patients provided written informed consent prior to any study-specific procedures. The study protocol was approved by the Antwerp University Hospital Ethics Committee (approval number 16/39/391), and the study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines and applicable regulatory requirements.
Study participants
Patients were current or former smokers, 40–80 years of age, with ⩾10 pack-years of cigarette smoking, and an established history of COPD, as defined by American Thoracic Society/European Respiratory Society criteria.27 Patients had moderate-to-severe COPD, with an FEV1/forced vital capacity ratio of <0.70 and a postbronchodilator FEV1 >30% and <80% predicted at visit 1.
Key exclusion criteria were respiratory conditions other than COPD (including asthma), or significant diseases which, in the investigator’s opinion, could put the patient at risk or influence the results. Patients with poorly controlled COPD (defined as acute worsening of COPD that required treatment with oral corticosteroids or antibiotics within 6 weeks of screening or during the run-in period) were excluded.
Assessments
The primary FRI endpoints were specific (i.e. corrected for lobar volume) image-based airway volume (siVaw) and resistance (siRaw). Image-based airway volume (iVaw) and resistance (iRaw) were secondary FRI endpoints. Secondary lung function endpoints were FEV1 (measured using spirometry) and functional residual capacity (FRC) (measured by body plethysmography). Inspiratory capacity (IC) (assessed via spirometry) was an additional endpoint.
All endpoints were based on postdose assessments performed within 150 min of dosing on day 15 (±5 days), with high-resolution CT scans (to assess FRI parameters) initiated 90 ± 30 min after dosing, followed by spirometry, then body plethysmography.
Details of the FRI methodology have been published previously.22,26 Analysis of mass of deposited particles was performed as previously described by De Backer et al.28 Data were generated within each of the five lobes of the lung for all parameters, and iVaw was also generated for each airway generation. Adverse events (AEs) were monitored throughout the study.
Statistical analyses
The intent-to-treat (ITT) population was defined as all patients who were randomized to treatment. All patients in the ITT population who received ⩾1 dose of study drug were included in the safety population.
For FRI parameters, data were generated within each of the five lobes of the lung. Across-lobe summaries provided an average of available lobe-level data. The primary efficacy analyses of siVaw and siRaw comprised a within-treatment comparison of day 1 and day 15 using a paired t-test for each primary endpoint and for each treatment. As a supportive secondary analysis, the day 15 value for each parameter in each period was analyzed using a linear mixed-effect model to compare treatments. A multilevel by-lobe model was used to incorporate the repeated measurements from the lobes for each patient, including fixed effects for period, treatment, lobe, and treatment by lobe interaction. The model did not include treatment sequence unless that term was determined to be important (p < 0.10). Lobe was included as a random effect within each patient. Data were logarithmically transformed before analysis with treatment effect estimates then exponentiated and presented as ratios.
Analyses of secondary FRI endpoints were similar to the primary endpoint analysis. iVaw was also analyzed using generation-level data (within segment within lobe), based upon the total across all segments for a given generation number. This by-generation model included the same covariates as the by-lobe model, except that lobe was substituted with generation. A typical airway model included 5–10 generations, depending mainly on the disease state of the patient. To address this, alternative analyses of by-generation data were conducted with untransformed or log-transformed data. In the untransformed analysis, missing generations were imputed with zero and in the log-transformed analysis they were imputed by the smallest observed value of iVaw. As the number of generations visible on a CT scan can also vary over time, a patient’s airway generations were trimmed so that the generations were the same across their visits. For the analysis of iVaw, an untrimmed analysis was undertaken in addition to a trimmed analysis; only the trimmed iVaw values were used for the derivation of other FRI parameters.
For spirometry and body plethysmography parameters, paired t-tests were used for within-treatment comparisons of day 1 and day 15. For comparisons between treatments, the change from baseline to day 15 for each endpoint was analyzed using a linear mixed-effect model including patient-average baseline value as a continuous covariate and treatment and period as fixed effects. Spirometry endpoints were not log-transformed.
For the primary efficacy endpoints, Hochberg’s step-up procedure was used as multiplicity adjustment. Hochberg’s procedure was applied once for siVaw and siRaw for GP MDI and then applied separately again for the same endpoints for FF MDI. No correction was performed for the secondary and other efficacy endpoints or for between-treatment comparisons, and all were interpreted in terms of nominal significance at a 5% level.
Results
Study population
A total of 23 patients were randomized and received at least one dose of study drug, and were included in the ITT and safety populations. Nineteen patients (82.6%) completed both treatment periods; 4 patients (17.4%) discontinued early due to COPD exacerbations and did not complete any postdose assessments, and therefore could not be included in efficacy analyses. Across both treatment periods, 20 patients received treatment with GP MDI (87.0%) and 22 received treatment with FF MDI (95.7%).
Most patients in the study were men (73.9%) and the mean age was 64.6 years; 52.2% were current smokers and the mean COPD duration was 9.2 years (Table 1). At screening, 69.6% of patients had moderate COPD and the remaining 30.4% had severe COPD: the overall mean (standard deviation) postbronchodilator FEV1 was 57.3% (11.4) of predicted normal (Table 1).
Table 1.
Baseline demographics and clinical characteristics (ITT population).
| All patients (N = 23) | |
|---|---|
| Mean age (SD), years | 64.6 (9.6) |
| Male, n (%) | 17 (73.9) |
| White, n (%) | 23 (100.0) |
| Mean BMI (SD), kg/m2 | 28.8 (4.5) |
| Current smoker, n (%) | 12 (52.2) |
| Median pack-years smoked (range) | 43.0 (18.8–142.5) |
| COPD severity, n (%) | |
| Moderate | 16 (69.6) |
| Severe | 7 (30.4) |
| Mean total CAT score (SD) | 18.3 (4.9) |
| Postbronchodilator FEV1 at screening, % predicted (SD) | 57.3 (11.4) |
| ⩾1 moderate or severe COPD exacerbation in the past year, n (%) | 4 (17.4%) |
BMI, body mass index; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; ITT, intent-to-treat; SD, standard deviation.
FRI
Both GP MDI and FF MDI achieved statistically significant improvements from baseline to day 15 in the primary endpoints of siVaw [11% (p = 0.0187) and 23% (p < 0.0001) increases, respectively] and siRaw [25% (p = 0.0219) and 44% (p < 0.0001) reductions, respectively] (Table 2).
Table 2.
Comparison to baseline for primary and secondary efficacy endpoints at day 15 (ITT population).
| GP MDI 18 µg (N = 20a) | FF MDI 9.6 µg (N = 22a) | |
|---|---|---|
| Primary FRI endpoints b | ||
| siVaw at TLC | ||
| Geometric mean, ml/L | 1.33 | 1.42 |
| Ratio to baseline (95% CI) | 1.11 (1.02, 1.22)* | 1.23 (1.14, 1.33)*** |
| siRaw at TLC | ||
| Geometric mean, kPa·s | 0.15 | 0.12 |
| Ratio to baseline (95% CI) | 0.75 (0.59, 0.95)* | 0.56 (0.44, 0.71)*** |
| Secondary endpoints | ||
| FRIb | ||
| Trimmed iVaw at TLC | ||
| Geometric mean, ml | 1.71 | 1.80 |
| Ratio to baseline (95% CI) | 1.12 (1.01, 1.24)† | 1.21 (1.12, 1.31)††† |
| Untrimmed iVaw at TLC | ||
| Geometric mean, ml | 2.08 | 2.25 |
| Ratio to baseline (95% CI) | 1.14 (1.01, 1.29)† | 1.26 (1.09, 1.45)† |
| iRaw at TLC | ||
| Geometric mean, kPa·s/L | 0.13 | 0.10 |
| Ratio to baseline (95% CI) | 0.76 (0.59, 0.97)† | 0.55 (0.41, 0.72)†† |
| Spirometry | ||
| FEV1, mL | ||
| Mean (SD) | 1689 (564) | 1744 (627) |
| Change from baseline (95% CI) | 65 (–28, 158) | 151 (10, 292)† |
| IC, mlc | ||
| Mean (SD) | 2525 (805) | 2507 (814) |
| Change from baseline (95% CI) | 164 (26, 302)† | 148 (20, 275)† |
| Body plethysmography | ||
| FRC | ||
| Mean, L (SD) | 4.94 (1.25) | 4.91 (1.58) |
| Ratio to baseline (95% CI) | 0.98 (0.89, 1.07) | 0.94 (0.83, 1.06) |
Statistically significant, p < 0.05; ***statistically significant, p < 0.0001. †Nominally significant, p < 0.05; ††nominally significant, p < 0.001; †††nominally significant, p < 0.0001.
Number of patients in the ITT population with evaluable data = 19 for all endpoints except for IC for FF MDI (n = 18).
Model includes patient-level data (lobes averaged for each patient prior to analysis); p values are derived from paired t-test, and CIs are based on the t-distribution.
Other endpoint.
CI, confidence interval; FEV1, forced expiratory volume in 1 s; FF, formoterol fumarate; FRC, functional residual capacity; FRI, functional respiratory imaging; GP, glycopyrrolate; IC, inspiratory capacity; iRaw, image-based airway resistance; ITT, intent-to-treat; iVaw, image-based airway volume; MDI, metered dose inhaler; SD, standard deviation; siRaw, specific image-based airway resistance; siVaw, specific image-based airway volume; TLC, total lung capacity.
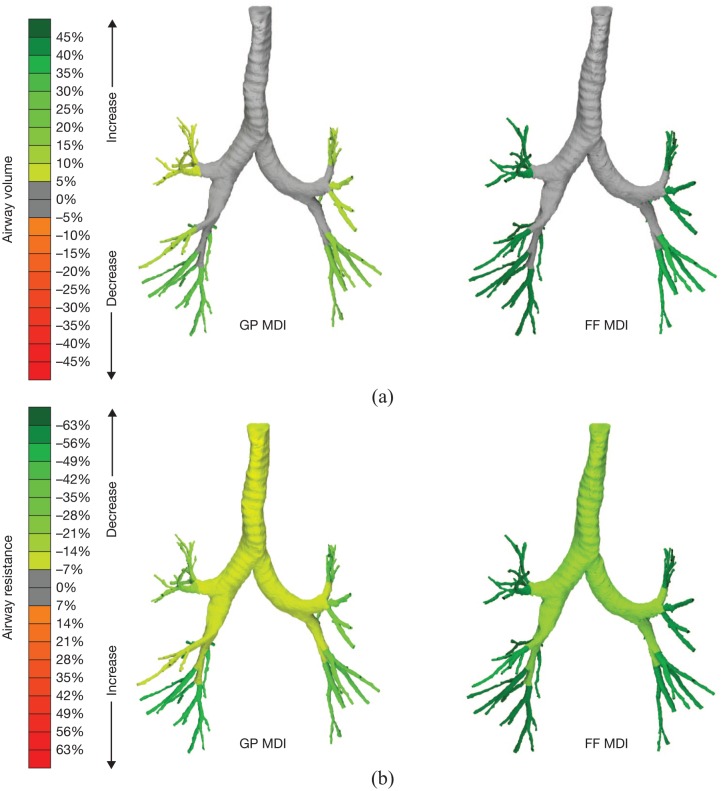
Representative images from one patient are shown in Figure 2. Although there were some individual patients for whom GP MDI had a greater benefit than FF MDI, on average greater benefits were seen for the FF MDI treatment. siVaw was ~6% smaller and siRaw was 24% larger with GP MDI versus FF MDI (p = 0.0027 and p = 0.0023, respectively) (Table 3).
Figure 2.
Images for co-primary FRI endpoints of siVaw (a) and siRaw (b) on day 15 at TLC from one representative patient.
FF, formoterol fumarate; FRI, functional respiratory imaging; GP, glycopyrrolate; MDI, metered dose inhaler; siRaw, specific image-based airway resistance; siVaw, specific image-based airway volume; TLC, total lung capacity.
Table 3.
Comparison between treatments for primary and secondary efficacy endpoints at day 15 (ITT population).
| GP MDI 18 µg (N = 20a) | FF MDI 9.6 µg (N = 22a) | |
|---|---|---|
| Primary FRI endpoints b | ||
| siVaw at TLC | ||
| Geometric LSM, ml/L (95% CI) | 1.27 (1.02, 1.59) | 1.35 (1.09, 1.68) |
| LSM ratio, GP MDI versus FF MDI | 0.94 (0.91, 0.98)†† | |
| siRaw at TLC | ||
| Geometric LSM, kPa·s (95% CI) | 0.13 (0.10, 0.16) | 0.10 (0.08, 0.13) |
| LSM ratio, GP MDI versus FF MDI | 1.24 (1.08, 1.42)†† | |
| Secondary endpoints | ||
| FRI | ||
| Trimmed iVaw at TLC | ||
| Geometric LSM, ml (95% CI) | 1.55 (1.21, 2.00) | 1.64 (1.27, 2.11) |
| LSM ratio, GP MDI versus FF MDI | 0.95 (0.91, 0.99)†† | |
| Untrimmed iVaw at TLC | ||
| Geometric LSM, ml (95% CI) | 1.90 (1.50, 2.39) | 2.07 (1.64, 2.61) |
| LSM ratio, GP MDI versus FF MDI | 0.92 (0.86, 0.98)†† | |
| iRaw at TLC | ||
| Geometric LSM, kPa·s/L (95% CI) | 0.10 (0.08, 0.13) | 0.08 (0.07, 0.11) |
| LSM ratio, GP MDI versus FF MDI | 1.23 (1.08, 1.40)†† | |
| Spirometry | ||
| FEV1, ml | ||
| LSM change from baseline (95% CI) | 82 (–30, 194) | 134 (22, 246) |
| LSM difference, GP MDI versus FF MDI | –52 (–173, 70) | |
| IC, ml | ||
| LSM change from baseline (95% CI)c | 182 (64, 299) | 148 (27, 270) |
| LSM difference, GP MDI versus FF MDI | 33 (–130, 197) | |
| Body plethysmography | ||
| FRC (95% CI) | ||
| Geometric LSM ratio to baseline, L | 0.96 (0.88, 1.06) | 0.95 (0.86, 1.04) |
| LSM ratio, GP MDI versus FF MDI | 1.02 (0.92, 1.13) | |
Nominally significant, p < 0.01.
Number of patients in the ITT population with evaluable data = 19 for all endpoints except IC for FF MDI (n = 18).
Model includes lobe-level data (number of lobes = 95); data here represent the average across-lobe values.
Other endpoint.
CI, confidence interval; FEV1, forced expiratory volume in 1 s; FF, formoterol fumarate; FRC, functional residual capacity; FRI, functional respiratory imaging; GP, glycopyrrolate; IC, inspiratory capacity; iRaw, image-based airway resistance; ITT, intent-to-treat; iVaw, image-based airway volume; LSM, least squares mean; MDI, metered dose inhaler; siRaw, specific image-based airway resistance; siVaw, specific image-based airway volume; TLC, total lung capacity.
For individual subjects, increases in siVaw at day 15 relative to baseline were generally consistent across lobes. The difference in airway volume findings between GP MDI and FF MDI was consistent across lobes (3–7% difference), but some variation was seen for resistance (4–62% difference between GP MDI and FF MDI, with the greatest difference in the right middle lobe; data not shown).
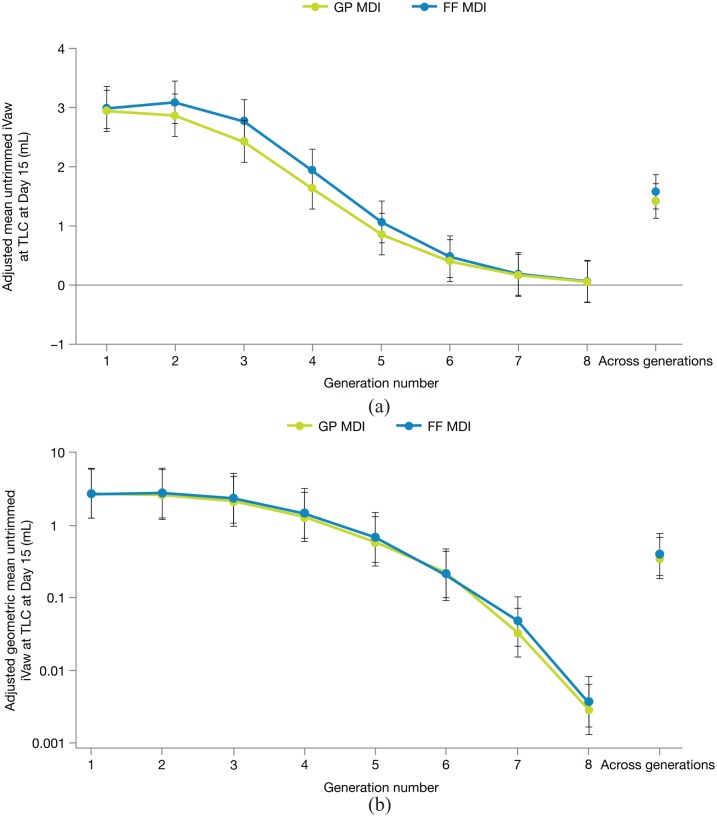
Significant improvements from baseline with GP MDI and FF MDI were also demonstrated for the secondary endpoints of iVaw (trimmed and untrimmed) and iRaw, supporting the conclusions of the primary analysis (Tables 2 and 3). Changes in airway volume were generally consistent across lobes for both treatments; however, the untransformed analysis of untrimmed iVaw by airway generation showed a nominally significant interaction between treatment and generation (p = 0.0469), indicating potential differences in the effects of GP MDI and FF MDI by generation (Figure 3(a)). The interaction was quantitative rather than qualitative, in that the absolute magnitude of the difference between GP MDI and FF MDI varied by generation, but the direction of effect remained consistent, with slightly greater improvements in untrimmed iVaw with FF MDI compared with GP MDI at each generation. In the transformed analysis (Figure 3(b)), examining relative effects, the interaction between treatment and generation was not significant (p = 0.9615). In general, a similar shape of response curve by generation was observed for GP MDI and FF MDI in both the untransformed (Figure 3(a)) and transformed (Figure 3(b)) analyses.
Figure 3.
Untransformed (a) and transformed (b) analyses of untrimmed iVaw by airway generation on day 15 (ITT population). Error bars denote 95% confidence intervals. Data from repeated measures models.
(a) LSM difference across generations: –0.157 (–0.0220, –0.095); p < 0.0001. Interaction p = 0.0469. (b) LSM difference across generations: 0.894 (0.752, 1.062); p = 0.2012. Interaction p = 0.9615.
FF, formoterol fumarate; GP, glycopyrrolate; ITT, intent-to-treat; iVaw, image-based airway volume; LSM, least squares mean; MDI, metered dose inhaler; TLC, total lung capacity.
Given the crossover design, the difference in the effects of GP MDI and FF MDI could be investigated within the same individual. Differences between GP MDI and FF MDI in untrimmed iVaw at day 15 tended to increase with airway generation number for a given patient (Figure 4). This finding applied for both the patients with a greater response to FF MDI (ratio <1 in Figure 4) and those with a greater response to GP MDI (ratio >1 in Figure 4).
Figure 4.
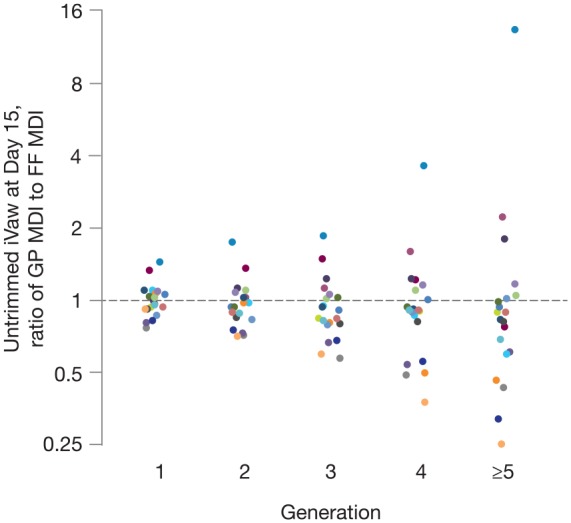
Ratio of GP MDI:FF MDI for day 15 untrimmed iVaw at TLC by individual generations (ITT population). Ratios <1 favor FF MDI; ratios >1 favor GP MDI. Colors represent different patients. Y-axis presented in the log scale.
FF, formoterol fumarate; GP, glycopyrrolate; ITT, intent-to-treat; iVaw, image-based airway volume; MDI, metered dose inhaler; TLC, total lung capacity.
In the mass of deposited particles simulation, 36.0% of the labeled glycopyrronium and 33.8% of the labeled formoterol fumarate were estimated to reach the lobes. In the by-generation analyses, mass deposition was greatest at generation 2 and then declined steadily for both glycopyrronium and formoterol fumarate.
Spirometry and body plethysmography
In the within-treatment analysis, there was a nominally significant improvement from baseline in postdose FEV1 on day 15 for FF MDI (mean change from baseline: 151 ml; p = 0.0375), and a numerical improvement for GP MDI (mean change from baseline: 65 ml; p = 0.1582) (Table 2). Both GP MDI and FF MDI demonstrated nominally significant improvements from baseline in IC (mean changes from baseline of 164 ml and 148 ml, respectively; p = 0.0228 and p = 0.0263) (Table 2). Numeric improvements from baseline in FRC were observed with both GP MDI (2.2% reduction; geometric mean ratio to baseline: 0.98; p = 0.6176) and FF MDI (6.2% reduction; geometric mean ratio to baseline: 0.94; p = 0.2699) (Table 2).
In the between-treatment comparisons, there were no statistically significant differences between GP MDI and FF MDI in any spirometry or plethysmography endpoints (Table 3).
Safety
Overall, 12 patients (52.2%) experienced at least 1 treatment-emergent AE (TEAE) during the study; 5 patients (25.0%) experienced ⩾1 TEAE while receiving GP MDI and 8 patients (36.4%) experienced ⩾1 TEAE while receiving FF MDI. The most commonly reported TEAEs were COPD (4 patients; 17.4%) and influenza (3 patients; 13.0%). All 4 patients who experienced a COPD TEAE (exacerbation) were withdrawn from the study (1 patient while receiving GP MDI, 3 while receiving FF MDI). Three TEAE events reported by 2 patients were considered to be related to the study drug: of these, one occurred during treatment with FF MDI (COPD exacerbation) and two occurred during treatment with GP MDI (exertional dyspnea and increased bronchial secretion). Two patients experienced a serious AE during the study: aortic aneurysm (while receiving FF MDI) and a recurrent case of non-Hodgkin’s lymphoma (during follow-up, after receiving GP MDI); neither were considered to be related to the treatment. Safety findings were consistent with the known safety profile of GP MDI and FF MDI and the study population of patients with moderate-to-severe COPD.
Discussion
In this phase IIIb FRI study, treatment with a LAMA (GP MDI) or LABA (FF MDI) increased specific airway volume and decreased specific airway resistance after 2 weeks of treatment in patients with moderate-to-severe COPD. Overall, the magnitude of improvement in FRI parameters with single long-acting bronchodilators (LAMA or LABA) in this study was generally comparable with previous studies of inhaled corticosteroid/LABA combinations.23,24 As expected, the improvements seen with GP MDI and FF MDI were smaller than those observed previously with the LAMA/LABA combination GFF MDI (75% increase in siVaw and 71% decrease in siRaw, both versus placebo MDI).26 The magnitude of improvement from baseline observed with GFF MDI (50% increase in siVaw and 64% decrease in siRaw; data on file) was also comparable with the sum of the improvements seen with GP MDI and FF MDI in the current study, confirming the benefit of combining both bronchodilators. Spirometry and body plethysmography findings were generally consistent with the FRI results; however, the image-based endpoints were more sensitive in detecting significant differences between the two treatments compared with traditional lung-function assessments, which were underpowered for distinguishing between the two active treatments in this number of patients. As with the FRI endpoints, improvements in FEV1 and IC observed with single bronchodilators in the current study were considerably smaller than those previously reported for GFF MDI (increases versus placebo of 443 ml and 454 ml, respectively).26
While there were some individual patients for whom each of the treatments had greater benefit, on average there were larger improvements from baseline in the FF MDI group versus the GP MDI group for the FRI parameters as well as FEV1. The greater response with FF MDI compared with GP MDI may reflect the faster onset of action of formoterol, given the timing of the postdose CT and pulmonary function assessments (between 1 h and 2.5 h postdosing). The difference between FF MDI and GP MDI in FEV1 in the current study was similar to that observed at 1 h and 2 h postdosing in previous 12-h lung-function studies, whereas the treatments were comparable at later time points in these studies.29,30 The finding that some patients responded better to the LAMA, while others responded better to the LABA, suggests the potential benefit of commencing therapy with a dual bronchodilator to minimize the possibility of an inadequate response to monotherapy.
FRI also detected a quantitative interaction between treatment and airway generation in the analysis of untrimmed iVaw with the absolute magnitude of the treatment difference between FF MDI and GP MDI varying by generation. However, a similar shape of response curve by generation was observed for both treatments, with no clear difference in the relative effects of treatment by generation. Regardless of whether a patient had a better response to FF MDI or GP MDI, within-patient treatment differences for untrimmed iVaw tended to increase with airway generation number, although it should be noted that this finding was based only on the first five airway generations, which were consistently quantifiable. It could be that LAMA and LABA receptors are unequally distributed in some patients, since some individuals have demonstrated greater response to a LAMA, whilst others demonstrated greater response to a LABA mainly, but not exclusively, from the fourth generation onwards (Figure 4).
In a previous FRI study of GFF MDI, improvements in siVaw and siRaw were strongly correlated with the change from baseline in FEV1,26 suggesting that, for dual long-acting bronchodilators with a large magnitude of effect on lung function, FRI endpoints can provide similar information to spirometric testing, with the added benefit of providing region-specific data. In the current study, we observed significant improvements from baseline for both the LAMA and LABA treatments (as well as nominally significant treatment differences) with FRI, but generally did not have sufficient statistical power to demonstrate significant differences with spirometry or body plethysmography. This finding is in agreement with previous studies showing that FRI analyses provide increased sensitivity and allow for improved detection of treatment differences in a small number of patients compared with traditional lung-function endpoints.23–25 We also used FRI to estimate the mass deposition of glycopyrronium and formoterol from GP MDI and FF MDI. For both components, approximately 35% of the total delivered dose was deposited in the lungs. The deposited amount was consistent with the 38% lung deposition found in a study of GFF MDI using gamma scintigraphy.18
No new or unexpected safety findings were observed in this study. The AEs reported were consistent with the known safety profiles of GP MDI and FF MDI formulated using co-suspension delivery technology31–33 and the study population of patients with moderate-to-severe COPD.34
In conclusion, treatment with a LAMA (GP MDI) or a LABA (FF MDI) increased airway volume and decreased airway resistance in patients with moderate-to-severe COPD. The results for these image-based endpoints were generally consistent with traditional lung-function assessments, while FRI also showed increased sensitivity relative to spirometry and body plethysmography in detecting differences between treatments in a small number of patients. As expected, the improvements seen with GP MDI and FF MDI in FRI endpoints were smaller than those observed in a previous study with the LAMA/LABA combination GFF MDI.26 It is important to note that some patients responded better to either the LAMA or the LABA. This heterogeneous response to a LAMA versus a LABA further justifies the benefit of including them both in combination therapies.
Supplemental Material
Supplemental material, Author_Response_1 for Functional respiratory imaging assessment of glycopyrrolate and formoterol fumarate metered dose inhalers formulated using co-suspension delivery technology in patients with COPD by Wilfried De Backer, Jan De Backer, Ilse Verlinden, Glenn Leemans, Cedric Van Holsbeke, Benjamin Mignot, Martin Jenkins, Dianne Griffis, Stefan Ivanov, Jane Fitzpatrick, Earl St Rose, Ubaldo J. Martin and Colin Reisner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Functional respiratory imaging assessment of glycopyrrolate and formoterol fumarate metered dose inhalers formulated using co-suspension delivery technology in patients with COPD by Wilfried De Backer, Jan De Backer, Ilse Verlinden, Glenn Leemans, Cedric Van Holsbeke, Benjamin Mignot, Martin Jenkins, Dianne Griffis, Stefan Ivanov, Jane Fitzpatrick, Earl St Rose, Ubaldo J. Martin and Colin Reisner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Functional respiratory imaging assessment of glycopyrrolate and formoterol fumarate metered dose inhalers formulated using co-suspension delivery technology in patients with COPD by Wilfried De Backer, Jan De Backer, Ilse Verlinden, Glenn Leemans, Cedric Van Holsbeke, Benjamin Mignot, Martin Jenkins, Dianne Griffis, Stefan Ivanov, Jane Fitzpatrick, Earl St Rose, Ubaldo J. Martin and Colin Reisner in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors thank all the patients and their families and the team of investigators involved in the study. The authors also thank FLUIDDA and Wim Vos for their valuable contributions to the study. Medical writing support, under the direction of the authors, was provided by Julia King, PhD, of CMC Connect, McCann Health Medical Communications, which was funded by AstraZeneca, Gaithersburg, USA in accordance with Good Publication Practice (GPP3) guidelines.35
Footnotes
Conflict of interest statement: WDB has no real or perceived conflicts of interest that relate to this manuscript. His department has received grants from AstraZeneca, Chiesi, and GlaxoSmithKline. JDB is the Chief Executive Officer and founder of FLUIDDA, and holds shares in the company. CVH and BM are employees of FLUIDDA, and GL and IV are former employees of FLUIDDA. MJ, DG, SI, JF, ESR, UJM, and CR are employees of AstraZeneca.
Data availability: Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported by AstraZeneca.
Supplementary material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Wilfried De Backer, University of Antwerp, Campus Drie Eiken, Universiteitsplein 1, Wilrijk, 2610 Antwerp, Belgium.
Jan De Backer, FLUIDDA, Inc, Los Angeles, CA, USA.
Ilse Verlinden, Formerly of FLUIDDA NV, Kontich, Belgium.
Glenn Leemans, Formerly of FLUIDDA NV, Kontich, Belgium.
Cedric Van Holsbeke, FLUIDDA NV, Kontich, Belgium.
Benjamin Mignot, FLUIDDA NV, Kontich, Belgium.
Martin Jenkins, AstraZeneca, Cambridge, UK.
Dianne Griffis, AstraZeneca, Durham, NC, USA.
Stefan Ivanov, AstraZeneca, Gothenburg, Sweden.
Jane Fitzpatrick, AstraZeneca, Morristown, NJ, USA.
Earl St Rose, AstraZeneca, Morristown, NJ, USA.
Ubaldo J. Martin, AstraZeneca, Gaithersburg, MD, USA
Colin Reisner, AstraZeneca, Morristown, NJ, USA.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease. 2019. Report: Global strategy for the diagnosis, management and prevention of COPD, https://goldcopd.org (2019, accessed 28 June 2019). [DOI] [PubMed]
- 2. Calzetta L, Matera MG, Rogliani P, et al. Dual LABA/LAMA bronchodilators in chronic obstructive pulmonary disease: why, when, and how. Expert Rev Respir Med 2018; 12: 261–264. [DOI] [PubMed] [Google Scholar]
- 3. Cazzola M, Calzetta L, Ora J, et al. Searching for the synergistic effect between aclidinium and formoterol: from bench to bedside. Respir Med 2015; 109: 1305–1311. [DOI] [PubMed] [Google Scholar]
- 4. Cazzola M, Calzetta L, Page CP, et al. Pharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on human isolated bronchi. Eur J Pharmacol 2014; 745: 135–143. [DOI] [PubMed] [Google Scholar]
- 5. AstraZeneca AB. Duaklir Genuair summary of product characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003745/WC500178413.pdf (2017, accessed 11 April 2019).
- 6. Boehringer Ingelheim International GmbH. Spiolto Respimat summary of product characteristics, https://www.medicines.org.uk/emc/medicine/30495 (2019, accessed 11 April 2019).
- 7. GlaxoSmithKline (Ireland) Limited. Anoro Ellipta summary of product characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002751/WC500168424.pdf (2019, accessed 11 April 2019).
- 8. Novartis Europharm Limited. Ultibro Breezhaler summary of product characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002679/WC500151255.pdf (2017, accessed 11 April 2019).
- 9. AstraZeneca AB. Bevespi Aerosphere summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/bevespi-aerosphere-epar-product-information_en.pdf (2018, accessed 13 June 2019).
- 10. Ferguson GT, Hickey AJ, Dwivedi S. Co-suspension delivery technology in pressurized metered-dose inhalers for multi-drug dosing in the treatment of respiratory diseases. Respir Med 2018; 134: 16–23. [DOI] [PubMed] [Google Scholar]
- 11. AstraZeneca Pharmaceuticals LP. Bevespi Aerosphere™ prescribing information, http://www.azpicentral.com/bevespi/bevespi_pi.pdf (2019, accessed 2 July 2019).
- 12. AstraZeneca. Bevespi Aerosphere approved by the Japanese Ministry of Health, Labour and Welfare for patients with chronic obstructive pulmonary disease. https://www.astrazeneca.com/media-centre/press-releases/2019/bevespi-aerosphere-approved-by-the-japanese-ministry-of-health-labour-and-welfare-for-patients-with-chronic-obstructive-pulmonary-disease-19062019.html (2019, accessed 2 July 2019).
- 13. Novartis Europharm Limited. Seebri Breezhaler summary of product characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002430/WC500133769.pdf (2017, accessed 12 February 2018).
- 14. Novartis Pharmaceuticals UK Ltd. Foradil® summary of product characteristics, https://www.medicines.org.uk/emc/medicine/1286 (2016, accessed 2 May 2018).
- 15. Campbell M, Eliraz A, Johansson G, et al. Formoterol for maintenance and as-needed treatment of chronic obstructive pulmonary disease. Respir Med 2005; 99: 1511–1520. [DOI] [PubMed] [Google Scholar]
- 16. Tashkin DP, Gross NJ. Inhaled glycopyrrolate for the treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2018; 13: 1873–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doty A, Schroeder J, Vang K, et al. Drug delivery from an innovative LAMA/LABA co-suspension delivery technology fixed-dose combination MDI: evidence of consistency, robustness, and reliability. AAPS PharmSciTech 2018; 19: 837–844. [DOI] [PubMed] [Google Scholar]
- 18. Taylor G, Warren S, Dwivedi S, et al. Gamma scintigraphic pulmonary deposition study of glycopyrronium/formoterol metered dose inhaler formulated using co-suspension delivery technology. Eur J Pharm Sci 2018; 111: 450–457. [DOI] [PubMed] [Google Scholar]
- 19. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532–555. [DOI] [PubMed] [Google Scholar]
- 20. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petousi N, Talbot NP, Pavord I, et al. Measuring lung function in airways diseases: current and emerging techniques. Thorax 2019; 74: 797–805. [DOI] [PubMed] [Google Scholar]
- 22. Hajian B, De Backer J, Vos W, et al. Functional respiratory imaging (FRI) for optimizing therapy development and patient care. Expert Rev Respir Med 2016; 10: 193–206. [DOI] [PubMed] [Google Scholar]
- 23. De Backer J, Vos W, Vinchurkar S, et al. The effects of extrafine beclometasone/formoterol (BDP/F) on lung function, dyspnea, hyperinflation, and airway geometry in COPD patients: novel insight using functional respiratory imaging. J Aerosol Med Pulm Drug Deliv 2015; 28: 88–99. [DOI] [PubMed] [Google Scholar]
- 24. De Backer LA, Vos W, De Backer J, et al. The acute effect of budesonide/formoterol in COPD: a multi-slice computed tomography and lung function study. Eur Respir J 2012; 40: 298–305. [DOI] [PubMed] [Google Scholar]
- 25. De Backer LA, Vos WG, Salgado R, et al. Functional imaging using computer methods to compare the effect of salbutamol and ipratropium bromide in patient-specific airway models of COPD. Int J Chron Obstruct Pulmon Dis 2011; 6: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Backer W, De Backer J, Vos W, et al. A randomized study using functional respiratory imaging to characterize bronchodilator effects of glycopyrrolate/formoterol fumarate delivered by a metered dose inhaler using co-suspension delivery technology in patients with COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 2673–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Celli BR, MacNee W. and ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946. [DOI] [PubMed] [Google Scholar]
- 28. De Backer JW, Vos WG, Vinchurkar SC, et al. Validation of computational fluid dynamics in CT-based airway models with SPECT/CT. Radiology 2010; 257: 854–862. [DOI] [PubMed] [Google Scholar]
- 29. Tashkin DP, Martinez FJ, Rodriguez-Roisin R, et al. A multicenter, randomized, double-blind dose-ranging study of glycopyrrolate/formoterol fumarate fixed-dose combination metered dose inhaler compared to the monocomponents and open-label tiotropium dry powder inhaler in patients with moderate-to-severe COPD. Respir Med 2016; 120: 16–24. [DOI] [PubMed] [Google Scholar]
- 30. Reisner C, Pearle J, Kerwin EM, et al. Efficacy and safety of four doses of glycopyrrolate/formoterol fumarate delivered via a metered dose inhaler compared with the monocomponents in patients with moderate-to-severe COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanania NA, Tashkin DP, Kerwin EM, et al. Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-Suspension™ Delivery Technology in patients with chronic obstructive pulmonary disease. Respir Med 2017; 126: 105–115. [DOI] [PubMed] [Google Scholar]
- 32. Lipworth BJ, Collier DJ, Gon Y, et al. Improved lung function and patient-reported outcomes with co-suspension delivery technology glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a randomized Phase III study conducted in Asia, Europe, and the USA. Int J Chron Obstruct Pulmon Dis 2018; 13: 2969–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest 2017; 151: 340–357. [DOI] [PubMed] [Google Scholar]
- 34. Chatila WM, Thomashow BM, Minai OA, et al. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015; 163: 461–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Functional respiratory imaging assessment of glycopyrrolate and formoterol fumarate metered dose inhalers formulated using co-suspension delivery technology in patients with COPD by Wilfried De Backer, Jan De Backer, Ilse Verlinden, Glenn Leemans, Cedric Van Holsbeke, Benjamin Mignot, Martin Jenkins, Dianne Griffis, Stefan Ivanov, Jane Fitzpatrick, Earl St Rose, Ubaldo J. Martin and Colin Reisner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Functional respiratory imaging assessment of glycopyrrolate and formoterol fumarate metered dose inhalers formulated using co-suspension delivery technology in patients with COPD by Wilfried De Backer, Jan De Backer, Ilse Verlinden, Glenn Leemans, Cedric Van Holsbeke, Benjamin Mignot, Martin Jenkins, Dianne Griffis, Stefan Ivanov, Jane Fitzpatrick, Earl St Rose, Ubaldo J. Martin and Colin Reisner in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Functional respiratory imaging assessment of glycopyrrolate and formoterol fumarate metered dose inhalers formulated using co-suspension delivery technology in patients with COPD by Wilfried De Backer, Jan De Backer, Ilse Verlinden, Glenn Leemans, Cedric Van Holsbeke, Benjamin Mignot, Martin Jenkins, Dianne Griffis, Stefan Ivanov, Jane Fitzpatrick, Earl St Rose, Ubaldo J. Martin and Colin Reisner in Therapeutic Advances in Respiratory Disease