Abstract
Background:
Tibial-sided graft fixation is thought to be critical for the success of anterior cruciate ligament (ACL) reconstruction. Nevertheless, little is known about the graft force after fixation during the first 24 hours after surgery or the influence of screw diameter and length during this time.
Purpose:
To investigate the force, over the course of 24 hours, in soft tissue grafts secured with a tibial interference screw and to evaluate the effect of different screw diameters (7, 8, and 9 mm) and lengths (25 and 30 mm) on the force in these grafts.
Study Design:
Controlled laboratory study.
Methods:
Quadruple-strand flexor tendon grafts were fixed with bioabsorbable interference screws in 60 porcine tibiae. Grafts were pretensioned at 80 N over 10 minutes, and screws were inserted outside-in while a preload force of 80 N was applied. Different screw lengths (25 and 30 mm) and diameters (7, 8, and 9 mm), resulting in 6 groups with 10 specimens each, were tested. After release of the preload, graft force was recorded over 24 hours.
Results:
A significant decrease in graft force progressed in all groups over the 24-hour period. In total, a median loss of 75 N (IQR, 68-79 N) compared with the initial loading force was observed. Compared with the loading force of 80 N, this corresponded to a median loss of 91%. No significant differences in the remaining graft force could be found among the 6 different screw length and diameter groups after 10 minutes, 100 minutes, or 24 hours.
Conclusion:
Graft force in soft tissue grafts secured with a tibial interference screw decreased substantially over the first 24 hours after fixation. Neither the screw diameter nor the screw length affected the decrease in graft force. This raises substantial questions regarding the remaining fixation strength in vivo.
Clinical Relevance:
It should not be expected that ACL reconstruction can mechanically restabilize an injured knee as would an intact ACL. Reconstructed knees should be protected from mechanical overload in the early postoperative period.
Keywords: ACL, biomechanical testing, hamstring graft, interference screw, tibial fixation
In anterior cruciate ligament (ACL) reconstruction, fixation of the graft to the bone tunnel represents a weak point of the ACL construct, especially in the immediate postoperative period.16,29,47 Bioabsorbable interference screw (BIS) fixation is a commonly used method for securing grafts to tibial bone tunnels.4,12,16,25,41 Tibial fixation is considered to be the “weak link” in the immediate postoperative period due to the relatively low bone density and the angle at which force is applied to the graft attachment.6,31 Decreasing graft force due to fixation failure equate with a loss of fixation strength. This may result in postoperative knee laxity and limited clinical success.11 Therefore, knowledge about the intra-articular graft force in the early postoperative phase is important for a secure graft fixation.
Biomechanical studies have shown significant decreases in the initially set loading force of the grafts despite adequate pretensioning.41 The reported decrease in graft force varies according to the time of the measurement (15 minutes, 30 minutes, 60 minutes, and 4 hours after fixation) and the type of graft used (isolated double-strand semitendinosus and gracilis grafts, bone–patellar tendon–bone grafts, and quadrupled hamstring ACL grafts). The decrease in graft force ranges between 49% and 78% over a maximum of 4 hours.10,14,15,22,41 To our knowledge, mechanical evaluations of the ACL fixation strength over longer periods have not been published thus far. The early postoperative period after fixation is known to be a vulnerable phase. Regarding the time-dependent viscoelastic behavior of soft tissue grafts, a 24-hour postfixation measurement would be a reasonable amount.54
The effects of different screw diameters7,24,28,33,38,39,50,60 and lengths5,19,20,24,49,52,53,60 on tibial ACL fixation have been evaluated, with controversial results, but only at time zero and without analysis of the postfixation resting period. Thus, little is known about the influence of these parameters during the first 24 hours after fixation, which is a critical phase. Especially in the early postoperative period, patients are in danger of uncontrolled knee movements during awakening from anaesthesia due to reoval of the endotracheal tube or exaggerated postoperative pain.
According to the current literature, it is not clear whether the graft fixation strength reaches a plateau at a certain level after the initial decline or whether it continues to decrease. Furthermore, it is unclear whether the diameter or the length of the interference screw has any effect on this progression.
Therefore, the purpose of this mechanical study was 2-fold. The first specific aim was to determine the development of intra-articular graft fixation strength over 24 hours. The hypothesis was that after the initial loss of intra-articular graft force in the first 4 hours, there would be no further decrease over the first 24 hours after interference screw fixation.
The second aim of this study was to assess the influence of different screw diameters and screw lengths on the graft fixation strength in the first 24 hours after interference screw fixation. The hypothesis was that larger screw diameters and screw lengths would show significantly higher intra-articular graft force after 24 hours. The present study conducted graft force (N), not tension (N/m2), measurements.
Methods
A total of 60 fresh porcine cadaveric limbs were obtained from a local butcher. Superficial flexor digitorum tendons were initially harvested from the porcine cadavers and cleared of adherent muscle fibers and surrounding soft tissues. Subsequently, all soft tissue was removed from the tibia. All tendons and tibiae were packed in moist saline dressings and frozen at –20°C in sealed plastic bags. At 24 hours before testing, the tendons and tibiae were thawed at room temperature.36,45,61 All specimens were kept moist with 0.9% saline during the specimen preparation, fixation, and biomechanical testing procedures. The tendons were shortened to a length of 240 mm, and the ends were sutured using a whipstitch technique, analogous to the technique used in human ACL reconstruction. Then, the tendons were folded 2 times into 4 strands, resulting in a graft length of 60 mm. The grafts were trimmed to a diameter of 8 mm. A sizing tube with 0.5-mm increments was used to check the diameter of the prepared grafts.
Tibiae were embedded in custom-made metal tubes through use of the 2-component plastic Memecryl (Bauer Handels GmbH). The fibula and interosseous membrane remained connected to the tibia to stabilize the bone complex and ease orientation. After the Memecryl hardened, a guide pin for the tibial tunnel was placed using a drill guide (Acufex Drill Director; Smith & Nephew) set at 65°. The aperture of the tibial drill tunnel was positioned at the center of the native ACL insertion. The bone tunnel started extra-articularly at the anteromedial tibial cortex, 1.4 cm medial to the tibial tubercle.37 The tunnel length was set at approximately 40 mm.8,63 The tunnel was drilled with increasing drill-bit diameters (6- and 7-mm diameter) until it was expanded to match the 8-mm diameter of the quadrupled soft tissue graft. The bone tunnel and graft diameter were set equally following the recommendations of Steenlage et al.56 Subsequently, overlapping soft tissue on the tibial tunnel was safely removed for easy passage of the graft. The embedded tibia was securely mounted to the custom-made mechanical testing machine to apply the initial tension force parallel to the long axis of the graft and bone tunnel (Figure 1).
Figure 1.
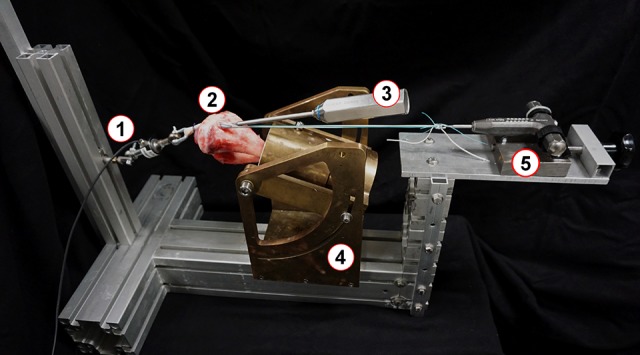
Experimental setting: (1) force sensor, (2) embedded tibia with graft, (3) screwdriver during screw insertion, (4) custom-made mechanical testing machine, (5) pretensioning device.
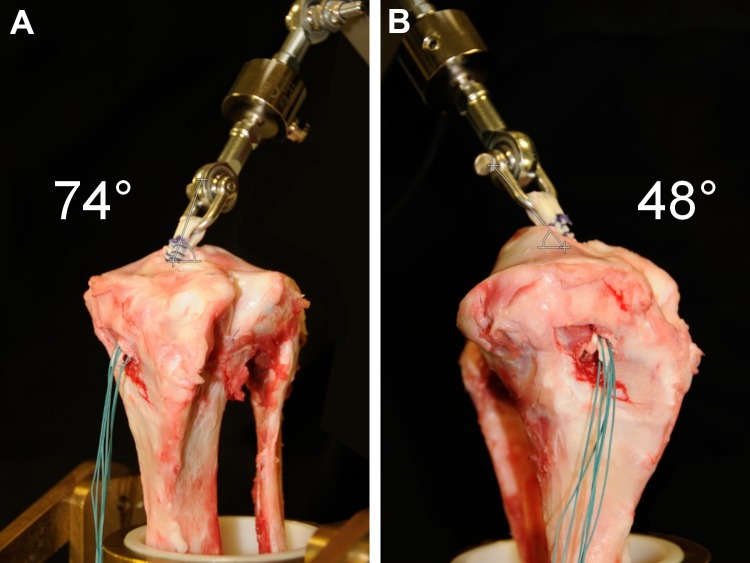
Before biomechanical testing, each quadruple-strand hamstring graft was pretensioned for 10 minutes starting at 80 N to simulate tensioning on a graft board. Directly after preconditioning, the looped end of each graft was placed through the bone tunnel with 30 mm protruding from the proximal aperture of the tunnel. Therefore, the 6-cm hamstring graft was divided into an intra-articular portion of 30 mm and a tibial tunnel portion of 30 mm.8,49,65 The looped part of the graft was attached to a shackle bolt and connected to the force sensor. Intra-articularly, the graft was positioned at the angle of the physiological anteromedial bundle of the ACL (47.9° sagittal, 73.8° coronal to tibial level) (Figure 2).55
Figure 2.
Diversion of anterior cruciate ligament graft (A) coronally and (B) sagittally to tibial level.
After graft insertion, at the distal end of the graft, an initial load of 80 N was applied along the long axis of the bone tunnel as recommended in previous studies.27,64 A bioabsorbable interference screw (BioactIF Osteotrans; Richard Wolf GmbH) was manually inserted in an outside-in fashion anterior to the tendon according to the manufacturer’s recommendations. Samples were randomly assigned to 1 of 6 groups through use of randomization.com to evaluate the influence of the different screw lengths (25 and 30 mm) and screw diameters (7, 8, and 9 mm) on the graft force over 24 hours. In each group, 10 specimens were tested (Table 1). During screw insertion, the initial load of 80 N was maintained.27,64
Table 1.
Study Groups
| Bioabsorbable Interference Screw Diameter | |||
|---|---|---|---|
| 7 mm (Graft Diameter – 1 mm) |
8 mm (Graft Diameter) |
9 mm (Graft Diameter + 1 mm) |
|
| Bioabsorbable interference screw length | |||
| 25 mm | 10 specimens | 10 specimens | 10 specimens |
| 30 mm | 10 specimens | 10 specimens | 10 specimens |
Immediately after screw insertion, the sutures applying this initial load were cut. Intra-articular force in newtons was recorded from application of the initial load before screw insertion and up to 24 hours after suture cutting. Data were recorded at 5 Hz using the data acquisition system spider3 (HBM) and measuring software Catman 4.5 (HBM). To reduce the extent of data and still register the expected massive loss of force in the first minutes after fixation, data recording proceeded in 2 phases. Phase 1 consisted of continuous data acquisition for 10 minutes. In phase 2, the measuring occurred periodically for 60 seconds at 10-minute intervals. Specimens were kept moist with 0.9% saline during the entire testing period with a specially designed moisturization device (Figure 3).
Figure 3.
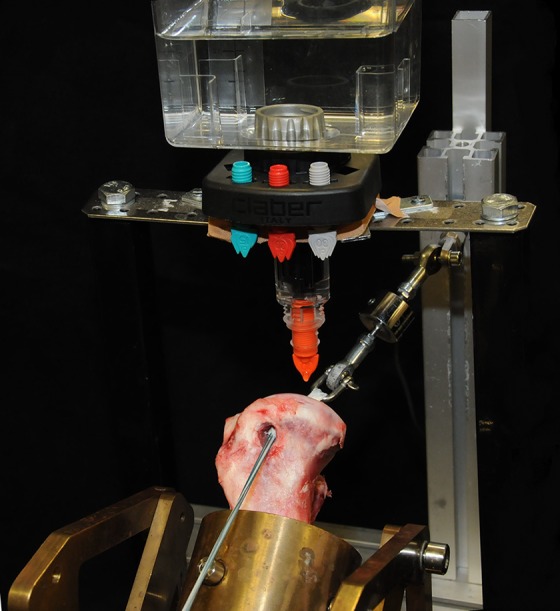
Moisturization device.
For statistical analyses, the recorded forces for the different screw lengths and diameters were compared with each other at 5 time points:
Before application of the initial loading tension
At the moment of cutting the sutures
After 10 minutes
After 4 hours
After 24 hours
Time points were chosen to adequately evaluate the graft behavior during screw insertion (time points 1 and 2), such as during the well-studied early phase (10 minutes), the maximum measurement period published so far (4 hours), and after a full 24-hour period.
The D’Agostino-Pearson omnibus normality test was used to verify the non-Gaussian distribution of all data. To determine whether there was a proceeding decrease in tension loss after the first 100 minutes, we used the nonparametric Wilcoxon matched-pairs signed rank test. When assessing the differences between the groups at the relevant time points, we analyzed the data using the Friedman nonparametric 2-way analysis of variance and the Tukey multiple comparisons test as a post hoc test.
Data are presented in box plots showing the median, the interquartile range (IQR) (25%-75%), and the 5th and 95th percentiles as whiskers. All statistical analyses were performed using GraphPad Prism version 6.01 (GraphPad Software). A P value < .05 was considered significant in 2-group comparisons and adjusted in multiple-group comparisons.
Results
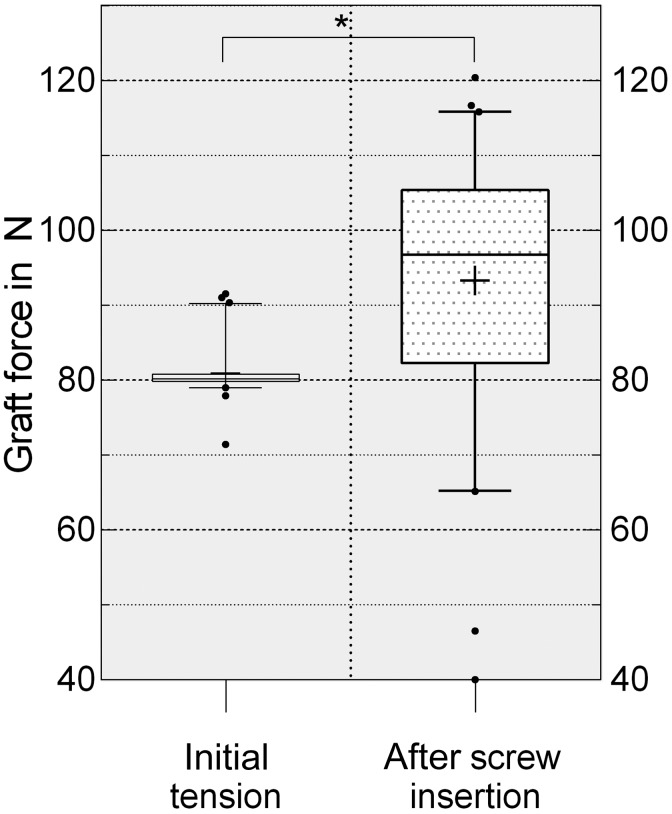
A significant increase in graft force was seen after interference screw insertion (Figure 4). The initially applied load of 80 N increased by a median of 14 N (IQR, 2-25 N) (P < .0001). We found no significant differences in graft force after screw insertion between the groups when comparing screw length and diameter.
Figure 4.
Gain of intra-articular graft force through interference screw insertion presented as box plots with the median, interquartile range (25%-75%), and whiskers (5%-95%). *Statistically significant (P < .0001).
The initially applied load showed a significant decrease of a median of 79 N (IQR, 73-86 N) in all groups in the first 4 hours after fixation. The decrease in graft force significantly progressed in all groups up to 24 hours after fixation by a median of 49% (IQR, 40%-63%) compared with the force at 4 hours (Table 2).
Table 2.
Graft Forcea
| Screw Group, mm | Before Screw Insertion | Immediately After Screw Insertion | 10 Minutes After Screw Insertion | 4 Hours After Screw Insertion | 24 Hours After Screw Insertion |
|---|---|---|---|---|---|
| 7 × 25 | 80.1 | 89.4 | 32.5 | 13.2 | 6.7 |
| (79.9-80.8) | (81.3-96.8) | (19.6-38.4) | (7.7-17.0) | (2.2-8.7) | |
| 7 × 30 | 80.4 | 98.6 | 37.4 | 16.4 | 7.5 |
| (79.7-82.9) | (83.2-103.1) | (28.3-39.4) | (11.6-18.7) | (4.8-9.3) | |
| 8 × 25 | 80.5 | 106.6 | 40.0 | 18.2 | 9.3 |
| (79.9-82.5) | (85.4-112.5) | (30.4-44.8) | (11.9-23.0) | (5.3-14.0) | |
| 8 × 30 | 79.9 | 96.3 | 31.5 | 13.2 | 4.9 |
| (79.3-80.6) | (83.6-111.3) | (22.3-52.4) | (7.8-27.6) | (1.3-16.1) | |
| 9 × 25 | 80.1 | 94.3 | 37.5 | 16.3 | 6.6 |
| (79.4-80.4) | (74.3-104.9) | (23.2-42.6) | (9.8-19.9) | (3.5-10.1) | |
| 9 × 30 | 80.3 | 97.9 | 38.9 | 18.7 | 8.3 |
| (79.8-81.1) | (79.9-106.3) | (29.9-52.3) | (12.7-28.0) | (5.0-15.6) |
aGraft force is expressed in newtons as median (25%-75% interquartile range).
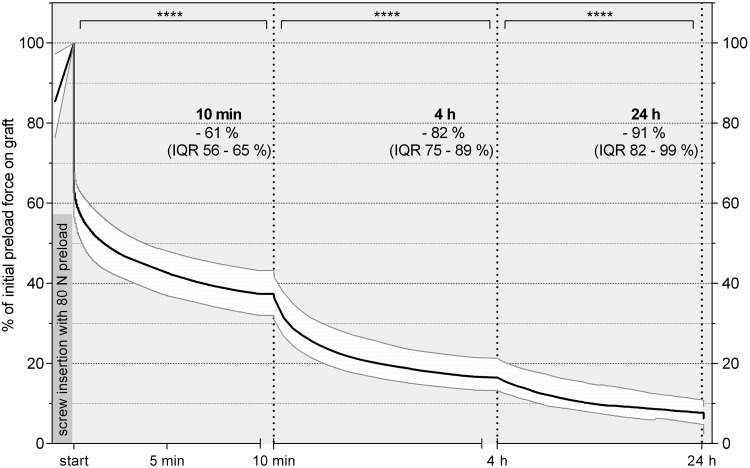
In total, over the 24 hours after interference screw fixation, we observed a median loss of 75 N compared with the initial loading force of 80 N. This corresponded with a median loss of 91% and a total percentage change between 89% (9 × 30-mm screw group) and 94% (8 × 30-mm screw group) compared with the initial loading force over the 24 hours after fixation (Figure 5).
Figure 5.
Percentage decrease in graft force for all groups over the first 24 hours after fixation presented as a curve with the median line and interquartile range (IQR) (25%-75%). ****Statistically significant (P < .0001). Initial preload force after screw insertion was considered to be 100% graft force.
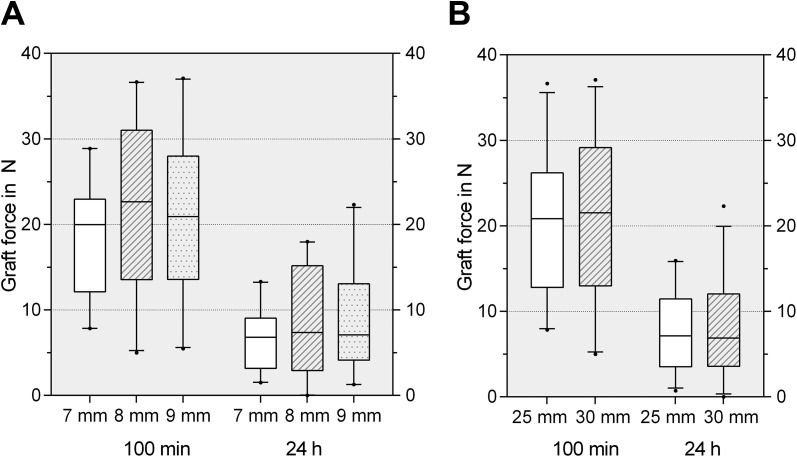
Screw diameter and length did not influence the remaining graft force at the investigated time points. No significant differences were seen among the 6 groups after 10 minutes, 100 minutes, or 24 hours. The results of the force measurements for the different screw designs are demonstrated in Table 2. Comparing screws 25 mm and 30 mm in length, we found no significant differences in the remaining graft force. No significant differences were seen in the remaining graft force among screws 7 mm, 8 mm, or 9 mm in diameter (Figure 6).
Figure 6.
(A) The remaining graft force after 100 minutes and 24 hours for different screw diameters presented as box plots with the median, interquartile range (25%-75%), and whiskers (5%-95%). No significant differences among the groups at the same time points could be observed. (B) The remaining graft force after 100 minutes and 24 hours for different screw lengths presented as box plots with the median, interquartile range (25%-75%), and whiskers (5%-95%). No significant differences among the groups at the same time points could be observed.
Discussion
The most important finding of this study was that the intra-articular graft force continuously decreased over the initial 24 hours when tibial interference screw fixation of soft tissue grafts was used. The measured 91% median decrease occurred irrespective of screw diameter and length.
In the current literature, biomechanical studies have shown significant decreases of as much as 78% of the initially set load force of hamstring ACL grafts fixed with interference screws. Studies investigating this progress have been limited to a time period between 15 minutes and 4 hours.10,14,15,23,41 In contrast, the present study demonstrated further progression in the loss of the initial applied fixation load. Despite numerous previous studies describing a relevant decrease in graft force in a relatively short time, the amount of loss after 24 hours, as observed in the present study, was remarkable. The results of this study with a comparatively long measurement period appear to be clinically meaningful for the early postoperative rehabilitation phase after ACL reconstruction.
The impressive early loss of intra-articular graft force may have occurred because of (1) a failure at the bone, graft, and screw interface (slippage effect) or (2) an elongation of the graft.41 With regard to the inferior effect of the screw that was used, a loss of graft tension due to elongation and not a loss of fixation strength due to poor fixation appears to have the major effect on the measured force after 24 hours.
Regarding the decrease in graft force in the first minutes after fixation, careful consideration should be taken in the process of applying backup fixation methods. The results of this study indicated that if backup fixation is used, the device should be attached quickly after screw insertion to minimize decreased graft force.48
The measured 91% decrease in intra-articular graft force may have indicated frequent mild knee laxity in the early postoperative phase after ACL reconstruction. This is consistent with earlier in vivo sheep59 and human patient measurements.35 However, clinical studies have reported good long-term functional scores and knee stability with the use of interference screw fixation.32,46,57
Currently, surgeons use a range of initial tensioning methods during surgery for optimal restoration of knee joint stability. It is commonly assumed that insufficient graft tension results in increased anteroposterior laxity of the tibiofemoral joint and that excessive graft tension limits the range of motion. This might lead to an overconstrained knee and negatively affect knee joint kinematics, leading to a higher risk of cartilage degeneration in the long term.2 Existing evidence has suggested that there may be a relatively wide range of safe and acceptable ACL graft tensions for achieving optimal outcomes.2,64 Regarding the observed early postoperative loss of fixation strength and generally excellent clinical results, the need for graft tensioning and maintaining loads around 80 N on the graft may be overstated.
In this study, a preconditioning protocol similar to that used in clinical practice was applied. Despite numerous studies describing the positive effects of tendon preconditioning, there is still no consensus on an optimal treatment protocol and no evidence for beneficial clinical outcomes of specific protocols. Several studies indicated reduced knee laxity by objective measurements of the outcomes when using a pretensioning protocol with static loading of approximately 80 N.3,17,26,64 Therefore, Arneja et al3 and Yasuda et al64 suggested the application of 80-N pretensioning force during ACL reconstruction. The methodical procedure in the current study followed these recommendations.
Previous studies have demonstrated improved graft fixation strength for serial tunnel dilatation when soft tissue grafts and interference screw fixation were used.9,51 Similar biomechanical experiments using porcine specimens have revealed no differences in maximum load-to-failure tests.21,40 Differences in tunnel diameter, graft size, screw diameter, or screw materials may precipitate the discrepancy in these findings.21 The aim of the present study was to assess the influence of different screw diameters while keeping the diameter of bone tunnel and tendon graft constant. Therefore, no tunnel dilatation was performed.
The current data suggest that the remaining force on the graft after 24 hours approached a limit. More research needs to be conducted to further determine the development of fixation strength beyond the 24-hour period. To improve simulation of the human knee after ACL surgery, a temperature increase should be considered, as it might trigger an additional decrease in intra-articular graft force.10,15
Furthermore, this study assessed the influence of different screw diameters and lengths on intra-articular graft force. However, no significant differences were found. Consequently, in this experimental setting, different screw diameters and lengths did not influence the intra-articular graft force. This result was obtained by use of a single fixation device and single graft type. Additional fixation devices or graft types may have varying outcomes. The design of this study focused on soft tissue grafts as well as tibial-sided interference screw fixation, because it is commonly used for ACL reconstruction.18,30
This study has some limitations. Despite continuous moistening of the graft and the related bone region, the study design could not mimic the in vivo condition. The use of porcine material for biomechanical testing is widely accepted because its mechanical behavior and characteristics are similar to those of human tendons and bone.17,34,43,62 Nevertheless, the translation of the results of such studies into clinical practice is controversial. Porcine flexor digitorum tendons have similar biomechanical properties, such as stiffness and high failure loads, compared with human hamstring tendons, and the elongation of porcine samples has been shown to be comparable with that of human hamstring tendons.13,43 Considering that a maximum load-to-failure test was not performed, it seems reasonable to use porcine tendons because of the easy acquisition and the high comparability among cadavers.
The porcine tibia is commonly used for biomechanical evaluations because of its similar size, shape, and bone quality to the young human tibia.1,44,58 Given the higher trabecular bone density, the porcine tibia can underestimate graft slippage and overestimate the failure load of the soft tissue graft in ACL reconstruction.31,42 This may have affected the comparison of different BIS diameters and lengths.
Furthermore, this study focused on tibial ACL fixation with BIS. When different fixation devices (titanium interference screws, combination screw and sheath) or supplementary fixation (spiked washers, staple) is used, an overall decrease of graft force might be different from this experimental setting. Backup fixation methods could reduce the failure at the bone, graft, and screw interface (slippage effect), resulting in a less extensive decrease of graft force. Additional loss of graft force may occur with the femoral fixation device. In this experimental setting, only the tibial side was evaluated.
Conclusion
Graft force in soft tissue grafts secured with a tibial interference screw decreased substantially during the first 24 hours after fixation. Neither the screw diameter nor the screw length affected the decrease in graft force. This finding raises substantial questions regarding the remaining fixation strength in vivo. Thus, it should not be expected that ACL reconstruction can mechanically restabilize an injured knee as would an intact ACL.
Acknowledgment
The authors thank the Richard Wolf Company, which provided the screws used in this study, and the Charité–University Medicine Berlin for funding this study by an internal research fund.
Footnotes
Final revision submitted January 2, 2020; accepted January 22, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: The screws used in this study were provided by the Richard Wolf GmbH. S.K. has received speaking fees from Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663–670. [DOI] [PubMed] [Google Scholar]
- 2. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6(suppl 1):S2–S12. [DOI] [PubMed] [Google Scholar]
- 3. Arneja S, McConkey MO, Mulpuri K, et al. Graft tensioning in anterior cruciate ligament reconstruction: a systematic review of randomized controlled trials. Arthroscopy. 2009;25(2):200–207. [DOI] [PubMed] [Google Scholar]
- 4. Barber FA, Dockery WD, Hrnack SA. Long-term degradation of a poly-lactide co-glycolide/beta-tricalcium phosphate biocomposite interference screw. Arthroscopy. 2011;27(5):637–643. [DOI] [PubMed] [Google Scholar]
- 5. Black KP, Saunders MM, Stube KC, Moulton MJ, Jacobs CR. Effects of interference fit screw length on tibial tunnel fixation for anterior cruciate ligament reconstruction. Am J Sports Med. 2000;28(6):846–849. [DOI] [PubMed] [Google Scholar]
- 6. Brand J, Jr, Weiler A, Caborn DN, Brown CH, Jr, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28(5):761–774. [DOI] [PubMed] [Google Scholar]
- 7. Brown CH, Jr, Hecker AT, Hipp JA, Myers ER, Hayes WC. The biomechanics of interference screw fixation of patellar tendon anterior cruciate ligament grafts. Am J Sports Med. 1993;21(6):880–886. [DOI] [PubMed] [Google Scholar]
- 8. Caborn DN, Nyland J, Selby J, Tetik O. Biomechanical testing of hamstring graft tibial tunnel fixation with bioabsorbable interference screws. Arthroscopy. 2003;19(9):991–996. [DOI] [PubMed] [Google Scholar]
- 9. Cain EL, Phillips BB, Charlebois SJ, Azar FM. Effect of tibial tunnel dilation on pullout strength of semitendinosus-gracilis graft in anterior cruciate ligament reconstruction. Orthopedics. 2005;28(8):779–783. [DOI] [PubMed] [Google Scholar]
- 10. Ciccone WJ II, Bratton DR, Weinstein DM, Elias JJ. Viscoelasticity and temperature variations decrease tension and stiffness of hamstring tendon grafts following anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2006;88(5):1071–1078. [DOI] [PubMed] [Google Scholar]
- 11. Dargel J, Gotter M, Mader K, Pennig D, Koebke J, Schmidt-Wiethoff R. Biomechanics of the anterior cruciate ligament and implications for surgical reconstruction. Strategies Trauma Limb Reconstr. 2007;2(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Debieux P, Franciozi CE, Lenza M, et al. Bioabsorbable versus metallic interference screws for graft fixation in anterior cruciate ligament reconstruction. Cochrane Database Syst Rev. 2016;(7):CD009772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domnick C, Wieskotter B, Raschke MJ, et al. Evaluation of biomechanical properties: are porcine flexor tendons and bovine extensor tendons eligible surrogates for human tendons in in vitro studies? Arch Orthop Trauma Surg. 2016;136(10):1465–1471. [DOI] [PubMed] [Google Scholar]
- 14. Elias JJ, Kilambi S, Ciccone WJ II. Tension level during preconditioning influences hamstring tendon graft properties. Am J Sports Med. 2009;37(2):334–338. [DOI] [PubMed] [Google Scholar]
- 15. Elias JJ, Rai SP, Ciccone WJ II. In vitro comparison of tension and stiffness between hamstring tendon and patella tendon grafts. J Orthop Res. 2008;26(11):1506–1511. [DOI] [PubMed] [Google Scholar]
- 16. Ettinger M, Schumacher D, Calliess T, et al. The biomechanics of biodegradable versus titanium interference screw fixation for anterior cruciate ligament augmentation and reconstruction. Int Orthop. 2014;38(12):2499–2503. [DOI] [PubMed] [Google Scholar]
- 17. Figueroa D, Calvo R, Vaisman A, Meleán P, Figueroa F. Effect of tendon tensioning: an in vitro study in porcine extensor tendons. Knee. 2010;17(3):245–248. [DOI] [PubMed] [Google Scholar]
- 18. Grassi A, Carulli C, Innocenti M, Mosca M, Zaffagnini S, Bait C. New trends in anterior cruciate ligament reconstruction: a systematic review of national surveys of the last 5 years. Joints. 2018;6(3):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harvey AR, Thomas NP, Amis AA. The effect of screw length and position on fixation of four-stranded hamstring grafts for anterior cruciate ligament reconstruction. Knee. 2003;10(1):97–102. [DOI] [PubMed] [Google Scholar]
- 20. Herrera A, Martinez F, Iglesias D, Cegonino J, Ibarz E, Gracia L. Fixation strength of biocomposite wedge interference screw in ACL reconstruction: effect of screw length and tunnel/screw ratio: A controlled laboratory study. BMC Musculoskelet Disord. 2010;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higano M, Tachibana Y, Sakaguchi K, Goto T, Oda H. Effects of tunnel dilation and interference screw position on the biomechanical properties of tendon graft fixation for anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(11):1804–1810. [DOI] [PubMed] [Google Scholar]
- 22. Hoher J, Offerhaus C, Steenlage E, Weiler A, Scheffler S. Impact of tendon suturing on the interference fixation strength of quadrupled hamstring tendon grafts. Arch Orthop Trauma Surg. 2013;133(9):1309–1314. [DOI] [PubMed] [Google Scholar]
- 23. Hoher J, Scheffler SU, Withrow JD, et al. Mechanical behavior of two hamstring graft constructs for reconstruction of the anterior cruciate ligament. J Orthop Res. 2000;18(3):456–461. [DOI] [PubMed] [Google Scholar]
- 24. Hulstyn M, Fadale PD, Abate J, Walsh WR. Biomechanical evaluation of interference screw fixation in a bovine patellar bone-tendon-bone autograft complex for anterior cruciate ligament reconstruction. Arthroscopy. 1993;9(4):417–424. [DOI] [PubMed] [Google Scholar]
- 25. Jagodzinski M, Geiges B, von Falck C, et al. Biodegradable screw versus a press-fit bone plug fixation for hamstring anterior cruciate ligament reconstruction: a prospective randomized study. Am J Sports Med. 2010;38(3):501–508. [DOI] [PubMed] [Google Scholar]
- 26. Jisa KA, Williams BT, Jaglowski JR, Turnbull TL, LaPrade RF, Wijdicks CA. Lack of consensus regarding pretensioning and preconditioning protocols for soft tissue graft reconstruction of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2884–2891. [DOI] [PubMed] [Google Scholar]
- 27. Kirwan GW, Bourke MG, Chipchase L, Dalton PA, Russell TG. Initial graft tension and the effect on postoperative patient functional outcomes in anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(5):934–941. [DOI] [PubMed] [Google Scholar]
- 28. Kohn D, Rose C. Primary stability of interference screw fixation: influence of screw diameter and insertion torque. Am J Sports Med. 1994;22(3):334–338. [DOI] [PubMed] [Google Scholar]
- 29. Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15(3):225–229. [DOI] [PubMed] [Google Scholar]
- 30. Lee YHD, Kuroda R, Chan KM. Anterior cruciate ligament reconstruction: a 2015 global perspective of the Magellan Society. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2015;2(4):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35–43. [DOI] [PubMed] [Google Scholar]
- 32. Maletis GB, Cameron SL, Tengan JJ, Burchette RJ. A prospective randomized study of anterior cruciate ligament reconstruction: a comparison of patellar tendon and quadruple-strand semitendinosus/gracilis tendons fixed with bioabsorbable interference screws. Am J Sports Med. 2007;35(3):384–394. [DOI] [PubMed] [Google Scholar]
- 33. Micucci CJ, Frank DA, Kompel J, Muffly M, Demeo PJ, Altman GT. The effect of interference screw diameter on fixation of soft-tissue grafts in anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(8):1105–1110. [DOI] [PubMed] [Google Scholar]
- 34. Miyata K, Yasuda K, Kondo E, Nakano H, Kimura S, Hara N. Biomechanical comparisons of anterior cruciate ligament: reconstruction procedures with flexor tendon graft. J Orthop Sci. 2000;5(6):585–592. [DOI] [PubMed] [Google Scholar]
- 35. Moewis P, Duda GN, Jung T, et al. The restoration of passive rotational tibio-femoral laxity after anterior cruciate ligament reconstruction. PLoS One. 2016;11(7):e0159600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moon DK, Woo SL, Takakura Y, Gabriel MT, Abramowitch SD. The effects of refreezing on the viscoelastic and tensile properties of ligaments. J Biomech. 2006;39(6):1153–1157. [DOI] [PubMed] [Google Scholar]
- 37. Morgan CD, Kalman VR, Grawl DM. Definitive landmarks for reproducible tibial tunnel placement in anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(3):275–288. [DOI] [PubMed] [Google Scholar]
- 38. Morris MW, Williams JL, Thake AJ, Lang Y, Brown JN. Optimal screw diameter for interference fixation in a bone tunnel: a porcine model. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):486–489. [DOI] [PubMed] [Google Scholar]
- 39. Namkoong S, Heywood CS, Bravman JT, Ieyasa K, Kummer FJ, Meislin RJ. The effect of interference screw diameter on soft tissue graft fixation. Bull Hosp Jt Dis. 2006;63(3-4):153–155. [PubMed] [Google Scholar]
- 40. Nurmi JT, Kannus P, Sievänen H, Järvelä T, Järvinen M, Järvinen TL. Interference screw fixation of soft tissue grafts in anterior cruciate ligament reconstruction, part 1: effect of tunnel compaction by serial dilators versus extraction drilling on the initial fixation strength. Am J Sports Med. 2004;32(2):411–417. [DOI] [PubMed] [Google Scholar]
- 41. Nurmi JT, Kannus P, Sievänen H, Järvelä T, Järvinen M, Järvinen TLN. Interference screw fixation of soft tissue grafts in anterior cruciate ligament reconstruction, part 2: effect of preconditioning on graft tension during and after screw insertion. Am J Sports Med. 2004;32(2):418–424. [DOI] [PubMed] [Google Scholar]
- 42. Nurmi JT, Sievänen H, Kannus P, Järvinen M, Järvinen TL. Porcine tibia is a poor substitute for human cadaver tibia for evaluating interference screw fixation. Am J Sports Med. 2004;32(3):765–771. [DOI] [PubMed] [Google Scholar]
- 43. Omar M, Dratzidis A, Klintschar M, Kwisda S, Krettek C, Ettinger M. Are porcine flexor digitorum profundus tendons suitable graft substitutes for human hamstring tendons in biomechanical in vitro-studies? Arch Orthop Trauma Surg. 2016;136(5):681–686. [DOI] [PubMed] [Google Scholar]
- 44. Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10. [DOI] [PubMed] [Google Scholar]
- 45. Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ. Effects of freezing and freeze-drying on the biomechanical properties of rat bone. J Orthop Res. 1984;1(4):405–411. [DOI] [PubMed] [Google Scholar]
- 46. Prodromos CC, Joyce BT, Shi K, Keller BL. A meta-analysis of stability after anterior cruciate ligament reconstruction as a function of hamstring versus patellar tendon graft and fixation type. Arthroscopy. 2005;21(10):1202. [DOI] [PubMed] [Google Scholar]
- 47. Saccomanni B. Graft fixation alternatives in anterior cruciate ligament reconstruction. Musculoskelet Surg. 2011;95(3):183–191. [DOI] [PubMed] [Google Scholar]
- 48. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RFG, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304–315. [DOI] [PubMed] [Google Scholar]
- 49. Selby JB, Johnson DL, Hester P, Caborn DN. Effect of screw length on bioabsorbable interference screw fixation in a tibial bone tunnel. Am J Sports Med. 2001;29(5):614–619. [DOI] [PubMed] [Google Scholar]
- 50. Shapiro JD, Jackson DW, Aberman HM, Lee TQ, Simon TM. Comparison of pullout strength for seven- and nine-millimeter diameter interference screw size as used in anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):596–599. [DOI] [PubMed] [Google Scholar]
- 51. Sorensen OG, Larsen K, Jakobsen BW, et al. Serial dilation reduces graft slippage compared to extraction drilling in anterior cruciate ligament reconstruction: a randomized controlled trial using radiostereometric analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):347–354. [DOI] [PubMed] [Google Scholar]
- 52. Stadelmaier DM, Lowe WR, Ilahi OA, Noble PC, Kohl HW III. Cyclic pull-out strength of hamstring tendon graft fixation with soft tissue interference screws: influence of screw length. Am J Sports Med. 1999;27(6):778–783. [DOI] [PubMed] [Google Scholar]
- 53. Stalder M, Farshad M, Snedeker JG, Meyer DC. Interference screws should be shorter than the hamstring tendon graft in the bone tunnel for best fixation. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):584–588. [DOI] [PubMed] [Google Scholar]
- 54. Starke C, Winkelmann U, Gluge S, Becker R, Lohmann CH, Winkelmann U. Interference screw fixation of free tendon grafts: significant time-dependent decrease of the initial contact forces. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2353–2358. [DOI] [PubMed] [Google Scholar]
- 55. Steckel H, Vadala G, Davis D, Musahl V, Fu FH. 3-T MR imaging of partial ACL tears: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1066–1071. [DOI] [PubMed] [Google Scholar]
- 56. Steenlage E, Brand JC, Jr, Johnson DL, Caborn DN. Correlation of bone tunnel diameter with quadrupled hamstring graft fixation strength using a biodegradable interference screw. Arthroscopy. 2002;18(8):901–907. [DOI] [PubMed] [Google Scholar]
- 57. Stener S, Ejerhed L, Sernert N, Laxdal G, Rostgard-Christensen L, Kartus J. A long-term, prospective, randomized study comparing biodegradable and metal interference screws in anterior cruciate ligament reconstruction surgery: radiographic results and clinical outcome. Am J Sports Med. 2010;38(8):1598–1605. [DOI] [PubMed] [Google Scholar]
- 58. Wancket LM. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet Pathol. 2015;52(5):842–850. [DOI] [PubMed] [Google Scholar]
- 59. Weiler A, Forster C, Hunt P, et al. The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):881–891. [DOI] [PubMed] [Google Scholar]
- 60. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Sudkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356–359. [DOI] [PubMed] [Google Scholar]
- 61. Woo SL, Orlando CA, Camp JF, Akeson WH. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech. 1986;19(5):399–404. [DOI] [PubMed] [Google Scholar]
- 62. Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng. 1998;26(3):345–352. [DOI] [PubMed] [Google Scholar]
- 63. Yang DL, Cheon SH, Oh CW, Kyung HS. A comparison of the fixation strengths provided by different intraosseous tendon lengths during anterior cruciate ligament reconstruction: a biomechanical study in a porcine tibial model. Clin Orthop Surg. 2014;6(2):173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yasuda K, Tsujino J, Tanabe Y, Kaneda K. Effects of initial graft tension on clinical outcome after anterior cruciate ligament reconstruction: autogenous doubled hamstring tendons connected in series with polyester tapes. Am J Sports Med. 1997;25(1):99–106. [DOI] [PubMed] [Google Scholar]
- 65. Yoo JC, Ahn JH, Kim JH, et al. Biomechanical testing of hybrid hamstring graft tibial fixation in anterior cruciate ligament reconstruction. Knee. 2006;13(6):455–459. [DOI] [PubMed] [Google Scholar]