Abstract
Background:
Lower-dose cone-beam computed tomography protocols for image-guided radiotherapy may permit target localization while minimizing radiation exposure. We prospectively evaluated a lower-dose cone-beam protocol for central nervous system image-guided radiotherapy across a multinational pediatrics consortium.
Methods:
Seven institutions prospectively employed a lower-dose cone-beam computed tomography central nervous system protocol (weighted average dose 0.7 mGy) for patients ≤21 years. Treatment table shifts between setup with surface lasers versus cone-beam computed tomography were used to approximate setup accuracy, and vector magnitudes for these shifts were calculated. Setup group mean, interpatient, interinstitution, and random error were estimated, and clinical factors were compared by mixed linear modeling.
Results:
Among 96 patients, with 2179 pretreatment cone-beam computed tomography acquisitions, median age was 9 years (1-20). Setup parameters were 3.13, 3.02, 1.64, and 1.48 mm for vector magnitude group mean, interpatient, interinstitution, and random error, respectively. On multivariable analysis, there were no significant differences in mean vector magnitude by age, gender, performance status, target location, extent of resection, chemotherapy, or steroid or anesthesia use. Providers rated >99% of images as adequate or better for target localization.
Conclusions:
A lower-dose cone-beam computed tomography protocol demonstrated table shift vector magnitude that approximate clinical target volume/planning target volume expansions used in central nervous system radiotherapy. There were no significant clinical predictors of setup accuracy identified, supporting use of this lower-dose cone-beam computed tomography protocol across a diverse pediatric population with brain tumors.
Keywords: radiotherapy, image-guided, IGRT, brain neoplasms, prospective study
Background
Image-guided radiotherapy (IGRT) has become standard practice in treatment of the central nervous system (CNS) in pediatrics.1 Through the convergence of advances in medical imaging and increasingly conformal radiotherapy techniques, IGRT has been successfully employed to improve treatment accuracy and precision while allowing for shrinkage of treatment margins and dose-escalation approaches.2-6
The most commonly employed form of IGRT used in the treatment of pediatric CNS targets is cone-beam computed tomography (CBCT), generally performed daily prior to delivery of radiation. As compared to pretreatment 2-dimensional imaging such as portal and kV planar X-ray techniques, CBCT provides 3-dimensional (3D) imaging with improved soft-tissue delineation at reasonably low radiation doses.7,8
The benefits of CBCT in children must be weighed against notable risks. Although individual CBCT scans can be completed in approximately 2 minutes, the additive excess time attributable to CBCT over the course of many patients can be substantial. Monetary costs associated with CBCT vary by institution but can also be significant and prohibitive. Most importantly, although CBCT is associated with relatively low radiation dose as compared to diagnostic computed tomography (CT), individual scans using standard imaging protocols may still contribute up to approximately 30 mGy per scan9,10 and lead to substantive radiation dose over the course of several weeks of treatment. Dose attributable to IGRT is particularly important to consider in pediatrics due to concern for late effects related to both cumulative radiation exposure and distribution of radiation dose.11-13 For example, Ding and Coffey estimated the dose to normal tissues delivered by a standard CBCT protocol (125 kVp, 80 mA, 25 ms) and found that the dose was greatly dependent on patient size, with higher doses delivered to normal structures in pediatrics versus adults. Tissues with a larger atomic number were found to receive higher dose, largely attributable to the photoelectric effect at the kV range for these materials. Consequently, dose to bone was found to be 2 to 4 times higher than that to soft tissues, with an estimated 4500 to 8400 mGy to bone over 25 to 35 fraction course from CBCT.14 This may have particular singificance for treatment of pediatrics, where dose to bone may impair bone growt.
Despite these concerns, clinical predictors of setup accuracy within the pediatric population for low-dose CBCT (LD-CBCT) for IGRT are not well-defined. Yet, low-dose CBCT protocols may allow for adequate target localization while minimizing radiation exposure and subsequent risk of late toxicity. Thus, we describe a standardized LD-CBCT protocol for CNS IGRT that was prospectively employed across an international pediatrics consortium. Specifically, we analyze parameters of setup error, assess for clinical predictors of setup accuracy, and report physician satisfaction with target localization using the LD-CBCT protocol.
Methods
Study Design
Providers from 7 international institutions with dedicated pediatrics expertise participated in prospective data collection for CNS IGRT practices in patients ≤21 years, between September 2010 to August 2015. A LD-CBCT protocol was prospectively defined by the physicists and physicians from the member institutions of the consortium. The imaging system used included an X-ray volume imaging (XVI) system, an onboard kV CBCT, and a planar imaging system (Elekta, Inc, Stockholm, Sweded). The protocol employed across institutions used a partial scan, S10 collimator, no filter (or F0 filter without a bow-tie component), 183 frames (approximate), 100 kVp, and 31.5 mAs. The imaging systems in this study conform to the standards and values listed in the AAPM Medical Physics Practice Guideline #2a on quality assurance (QA) of IGRT systems.
Radiation Dose From CBCT
The methodology utilized for dose measurements followed that described in the work of Siewerdsen et al.15 This method applied the recommendations of TG 111 for16 cone-beam scans and utilized a thimble ion chamber (0.6 cm3 Farmer chamber) for point-dose measurements in a standard computed tomography dose index (CTDI) phantom. Here, we utilized the smaller 16 cm diameter “head” CTDI phantom (which is more akin to pediatric sizes and provides a more conservative measure of dose than the alternative 32 cm diameter phantom size). Measurements were made at the center of the phantom and at 4 cardinal (peripheral) positions, which were weighted into a single value as follows:
where Dc is the central measured dose, is the average of peripheral doses, and Dw is the weighted average reported in units of mGy. As described by Siewerdsen et al., the metric Dw is analogous to the standardized metric CTDIw utilized for multi-detector row computred tomography (MDCT) applications.15 An important difference is that the CTDIw conventionally utilizes a 100-mm pencil chamber, which, as described in TG 111, has conceptual inadequacies for its application to wide beam geometries such as CBCT.16
Study Population
All pediatric patients treated with conventionally fractionated photon IGRT with CBCT to CNS sites during the study period were included. Due to differences in QA protocols, patients undergoing hypofractionated stereotactic radiotherapy were excluded from this analysis. Because excessive setup variability may be expected on the first imaging session, data from imaging day 1 were not included in the analysis. This study was approved by the institutional review board (NA_00083758) at the Johns Hopkins University School of Medicine and at the participating institutions.
Radiation Planning and Patient Setup
All patients underwent planning CT (pCT), and staff radiation oncologists delineated gross target volume and applied a clinical target volume (CTV) and institution-specific planning target volume (PTV) expansions to each plan. Planning target volume expansions ranged from 3 to 5 mm. Prior to treatment, patients were immobilized with the same techniques used at the time of the pCT. Laser alignment (LA) to surface marks was performed, followed by CNS-directed CBCT scans. Cone-beam computed tomography images were registered to bone on the pCT. Automatic registration was used for 3D image registration, comparing the initial CBCT with the reference pCT. The software (XVI, Elekta, Inc, Stockhold, Sweden) supports 3 methodologies for the alignment, labeled as “seed, bone, grey value.” Bony alignment was used here. For this method, the software employed a chamfer edge matching algorithm.17 The 3D region of interest for this registration was restricted to a “clipbox,” which was manually adjusted to include the relevant visible bony anatomy for each patient.18 The relevant areas of interest would be expected to vary by clinical scenario and were determined at the treating physicians’ discretion.
Differences in positional setup with LA versus CBCT were calculated in the x (transverse), y (superior–inferior), and z (anterior–posterior) translational directions, and the treatment table was shifted accordingly to overlap the pCT and CBCT image sets. Initial alignment to laser, CBCT acquisitions, and physical table shifts were performed by radiation therapists specifically trained in CBCT technology. Prior to or following each treatment, a radiation oncologist reviewed XVI from CBCT to confirm setup.
Analysis of Setup Difference Between LA and CBCT
The difference in setup with LA alone versus with CBCT was represented by the values of the physical table shifts in the x, y, and z translational directions. These data (measured in millimeters) were entered into MOSAIQ Oncology Information System (Elekta, Inc).
Potential Predictors of Setup Accuracy
Variables considered to be potential predictors of setup accuracy were selected by consensus agreement of providers from participating institutions prior to initiating the protocol. Age, baseline Karnofsky Performance Status (KPS), gender, IGRT target location, immobilization device used, extent of resection, chemotherapy use, baseline steroid use at time of IGRT initiation, and anesthesia use during treatment were assessed as potential predictors of setup accuracy. Age was defined in years at the time of initiation of radiotherapy. Baseline KPS was rated by treating physicians on a scale from 0 to 100 in units of 10 at the time of initiation of IGRT. The location of the target for IGRT was designated as originating in the supratentorial versus infratentorial fossa by the treating physician. Targets requiring craniospinal or whole ventricular radiation were not included while analyzing for predictors of setup accuracy. Immobilization type was documented as a short versus long thermoplastic mask, with short masks reaching the chin and long masks extending to immobilize the shoulders. For patients undergoing resection, the treating physician classified the extent of resection as consistent with gross residual versus no gross residual disease based on the reports of postsurgical diagnostic imaging. For patients receiving chemotherapy, this variable was classified as being delivered prior and/or concurrent to IGRT. Baseline steroid use and anesthesia use during IGRT were recorded as dichotomous variables.
Outcome Measures
In order to evaluate the LD-CBCT protocol, we assessed setup accuracy as a function of both (1) relatively objective measures of positioning uncertainty and (2) subjective physician ratings of image quality.
Setup positioning uncertainty
As a proxy for setup accuracy with the LD-CBCT protocol, we analyzed positioning error using table shifts from setup to surface marks with LA versus CBCT. Per the literature, positional error is considered to be comprised of both systematic (interpatient) and random (inter- and intrafraction) components.19 Table shifts between expected position as delineated by surface marks and actual position at the time of CBCT permitted measurement of interpatient systematic and intrapatient random error. Similar methodology for capturing positioning error using table shifts has been described.18 Although establishing efficacy of the LD-CBCT protocol would ideally involve direct comparison of positioning error from protocol images to those rendered from standard dose CBCT for each patient, the excess radiation exposure would be prohibitive of such a direct analysis in the pediatrics population. As such, this study assumes that LD-CBCT could be considered sufficient for use in setup if table shifts from LA versus LD-CBCT are similar in magnitude to those measured from LA versus standard dose CBCT.
The vector magnitude (VM) of the table shifts was calculated, providing a 3D expression of table shifts following LD-CBCT. Vector magnitude was defined as:
where x = table shift in the transverse direction, y = table shift in the superior–inferior direction, and z = table shift in the anterior–posterior direction. The table shifts in the x, y, and z direction as well as the VM were each analyzed as a dependent variable using a linear mixed-effect (LME) model.
Of note, routine repeat CBCT was not performed after treatment. As such, there was no estimation of the intrafraction uncertainty.19
Physician ratings of image quality
The treating physicians rated a subset of CBCT for image quality relative to target localization. Ratings were performed on a Likert scale with values of excellent, adequate, inadequate, or poor for target localization, based upon their ability to render a clinical judgment using the imaging to assess anatomic alignment. An “adequate” image contained the minimum sufficient quality and information needed to render a clinical decision, whereas an “excellent” image surpassed these minimum requirements, thus permitting ease of decision-making. An “inadequate” CBCT—while perhaps not poor in quality— did not contain adequate information to readily render a clinical decision. A “poor” CBCT was inadequate in image quality and did not provide sufficient information for the clinical decision.
Data Analysis
Descriptive statistics were used to characterize patient and treatment features including mean age, median KPS and radiation dose, and frequency of categories for gender, race, immobilization device used, steroids and anesthesia requirements, and surgical and chemotherapy interventions used.
Parameters of positioning uncertainty were analyzed using mixed linear models. These models were clustered by patient and institution to account for the effects of repeated measures within the same patient and potential variations between treating institutions, respectively. Random effects for patients and institutions were assumed; no other random or fixed effects were considered. Estimates in the x, y, and z translational directions were determined using normal mixed-effect models; VMs were estimated using log-normal mixed-effect models. In these models, group mean error for each translation direction or VM was estimated as the mean (across patients) of the mean shift value for each patient over all fractions. Total variance was set equal to the sum of between-patient, between-institution, and within patient variances. Systematic interpatient and interinstitution errors were estimated by the square roots of the between-patient and between-institution model covariances, respectively. Measurable random error was estimated as the square root of model residuals, representing (within patient) interfraction uncertainty.
Potential predictors of positioning uncertainty were evaluated for the VM group mean error using a univariate log-normal mixed model, again clustered by patient and institution. Fixed effects were assumed for the potential predictor variables.
Correlation between cranial volume and patient age was tested using Spearman ρ. P value for significance was <.05. Additional statistical details of the above models are listed in Supplementary Material. All analyses were performed using SAS 9.4 (SAS Institute, North Carolina) and R 3.2.2.
Results
The IGRT characteristics for 2179 pretreatment CBCT scans from 96 patients were collected and used to estimate setup accuracy parameters. Demographic data were available for 86 patients and were used to assess for predictors of setup accuracy. Primary histologies were 47% glioma, 12% medulloblastoma, 11% ependymoma, 5% craniopharyngioma, and 25% other (including primitive neuroectodermal tumor, germinoma, Langerhans cell histiocytosis, atypical teratoid rhabdoid tumor, epidermoid tumor, and pineal parenchymal tumor). Table 1 describes patient and treatment characteristics.
Table 1.
Patient and Treatment Characteristics.
| Patient and Target Characteristics (n = 86) | |
|---|---|
| Age in years—median (range)a | 9 (1-20) |
| Gender, male—n (%)b | 32 (56%) |
| Race—n (%)c | |
| White | 36 (61%) |
| Black | 10 (17%) |
| Other | 13 (22%) |
| KPS—median (range)d | 80 (40-100) |
| Tumor location—n (%)e | |
| Supratentorial | 30 (43%) |
| Infratentorial | 19 (27%) |
| Other | 21 (30%) |
| IGRT characteristicsf | |
| Total dose—median (range)g | 54 Gy (24-59.4 Gy) |
| Immobilization device—n (%)h | |
| Short mask | 43 (68%) |
| Long mask | 20 (32%) |
| Baseline steroids use—n (%)i | |
| Yes | 38 (48%) |
| No | 41 (52%) |
| Anesthesia required—n (%)j | |
| Yes | 26 (32%) |
| No | 55 (68%) |
| Other treatment characteristics | |
| Surgical resection—n (%)k | |
| Yes | 54 (68%) |
| No | 26 (32%) |
| Extent of resection—n (%)l | |
| Gross residual disease | 17 (40%) |
| No gross residual disease | 25 (60%) |
| Chemotherapy—n (%)m | |
| Pre- or concurrent with IGRT | 41 (68%) |
| After IGRT | 15 (25%) |
| None | 5 (8%) |
Abbreviations: KPS, Karnofsky Performance Status; IGRT, image-guided radiotherapy.
a Missing data: 2. b Missing data: 29. c Missing data: 27. d Missing data: 5. e Missing data: 16. f Missing data: 6. g Missing data: 23. h Missing data: 7. i Missing data: 5. j Missing data: 6. k Missing data: 23. l Missing data: 27. m Includes sites requiring craniospinal and whole ventricle radiation.
Radiation Dose From CBCT
Peripheral doses measured ranged from 0.4 to 1 mGy, and the central dose was measured to be 0.8 mGy. The weighted average, Dw, was calculated to be 0.7 mGy. The spread of doses was consistent with that of a partial scan (see Discussion section). It should be noted as well that the peripheral dose was not uniform because a partial scan was utilized (ie, 180° plus fan), leading to the range of peripheral doses measured (0.4-1 mGy), in which the lowest dose correlates to the surface of the phantom where the X-ray beam is nonincident.
Parameters of Positioning Uncertainty
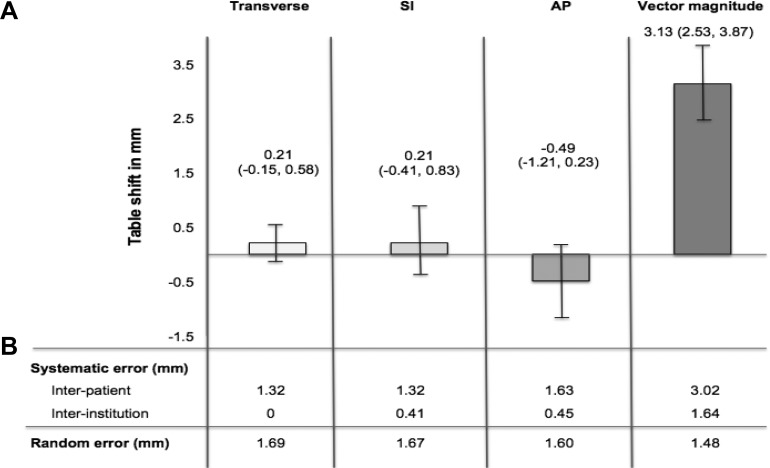
Group mean error in the translational directions estimated from the LME model was 0.21 mm (95% confidence interval [CI]: −0.15 to 0.58 mm) for transverse, 0.21 mm (95% CI: −0.41 to 0.83 mm) for superior–inferior, and −0.49 (95% CI: −1.21 to 0.23 mm) for anterior–posterior orientations. The VM group mean error was 3.13 mm (95% CI: 2.53-3.87). Interpatient error ranged from 1.32 to 1.63 mm in the translational directions and was greater than interinstitution error (range 0-0.45 mm). The VM interpatient error was also greater than VM interinstitution error at 3.02 and 1.64 mm, respectively. Interpatient error and interinstitution error accounted for 65% and 19% of variability in the model, respectively. Random error ranged from 1.60 to 1.69 mm in the translational directions, with VM of 1.48 mm. Random error accounted for 16% of variability in the model (see Figure 1).
Figure 1.
Group mean, systematic, and random error estimates between setup with laser alignment alone versus with cone-beam computed tomography (CBCT) in pediatric central nervous system (CNS) image-guided radiotherapy. A, Group mean error and 95% confidence intervals. B, Estimates of systemic and random error estimates. Table shifts between laser alignment and CBCT were measured in transverse = x, superior–inferior (SI) = y, and anterior–posterior (AP) = z translational directions. Vector magnitude (VM) was calculated as √(x 2 + y 2 + z 2).
Potential Predictors of Positioning Uncertainty
Table 2 shows the results of the univariate model for predictors of positioning uncertainty as estimated by VM. Accounting for repeated measures between individuals as well as the potential effect of different institutions, there were no statistically significant differences between VM based on age, gender, baseline KPS, target location, extent of resection, chemotherapy timing, baseline steroid use, or anesthesia requirement (all P values > .05). Immobilization device was not shown in the result because the model did not converge when this variable was included.
Table 2.
Univariate Analysis of Potential Predictors of Setup Accuracya for Pediatric CNS IGRT.
| Variablesb | VM in mm (95% CI) | P Value |
|---|---|---|
| Age | ||
| >12 years | 3.23 (2.66-3.91) | |
| ≤ 12 years | 3.26 (0.23-0.46) | .91 |
| Gender | ||
| Female | 3.38 (0.69-16.4) | .79 |
| Male | 3.29 (2.62-4.15) | |
| Baseline KPS | ||
| ≥80 | 3.00 (2.50-3.60) | .28 |
| <80 | 3.27 (2.37-4.50) | |
| Target location | ||
| Supratentorial | 3.21 (2.55-4.04) | .37 |
| Infratentorial | 2.95 (1.41-6.18) | |
| Extent of resection | ||
| Gross residual disease | 3.07 (2.34-4.02) | .49 |
| No Gross residual disease | 2.83 (1.10-7.29) | |
| Chemotherapy | ||
| Prior or concurrent with IGRT | 3.42 (2.63-4.44) | .50 |
| No prior nor concurrent IGRT | 3.10 (0.32-30.1) | |
| Baseline steroid use | ||
| Yes | 3.37 (2.90-3.91) | .22 |
| No | 3.06 (2.18-4.28) | |
| Anesthesia required | ||
| Yes | 3.08 (2.57-3.69) | .94 |
| No | 3.06 (2.38-3.95) |
Abbreviations: CBCT, cone-beam computed therapy; CI, confidence interval; CNS, central nervous system; IGRT, image-guided radiotherapy; KPS, Karnofksy Performance Status; VM, vector magnitude.
a Setup accuracy is estimated by the VM of translational treatment table shifts between setup with laser alignment alone versus with CBCT.
b Model does not converge when immobilization type was included.
Post Hoc Analysis of Cranial Volume
A total of 24 patients were evaluated for cranial volume at the Johns Hopkins University School of Medicine site. Mean age for this subgroup was 11.1 (SD 5.9, range 3-20 years), which is similar to that of the full cohort. Mean cranial volume was 12128.8 cm3 (standard deviation [SD]: 1672.4). There was no significant correlation between age and cranial volume (Spearman ρ = 0.095, P =.660). Given no significant correlation between cranial volume and age, the cranial volume variable was not selected for inclusion into statistical models.
Physician Rating of Image Quality
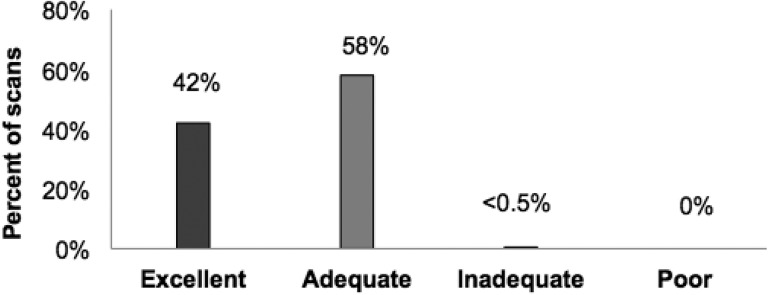
Likert scale ratings by physicians for image quality were recorded for 173 LD-CBCT scans. All images were acquired using the same CBCT protocol as defined in the study. Only 1 scan was rated as inadequate, with the remainder of the scans categorized as adequate or excellent for target localization (see Figure 2). Cone-beam computed tomography was repeated at a higher dose for the 1 scan that was rated as inadequate. Given that authors could not conclude if there was a meaningful clinical difference between “excellent” and “adequate” image quality in this patient population, no statistical comparisons were made on the basis of ratings of image quality.
Figure 2.
Physician rating of lower-dose cone-beam computed tomography (LD-CBCT) quality for tumor localization in pediatric image-guided central nervous system (CNS) radiotherapy (n = 173 LD-CBCT).
Discussion
In the present study, an LD-CBCT protocol was prospectively defined and employed for CNS radiotherapy across a consortium of 7 international institutions with pediatrics expertise. Additionally, we were able to prospectively collect measures of setup positioning uncertainty in this vulnerable population. To our knowledge, this is the first international study of its size to evaluate the feasibility of an LD-CBCT protocol in pediatrics. Additionally, it is the first to estimate parameters and assess predictors of setup accuracy with LD-CBCT for this population.
Regarding the dose from CBCT in this study, we determined that our protocol indeed fell within a lower-dose range. In a comparison study of XVI and onboard imaging CBCT protocols, Song et al. report XVI protocols to have average doses in the range of 1 to 35 mGy using a similar measurement methodology to that utilized in this article.20 The weighted average dose, Dw, from our LD-CBCT was 0.7 mGy, therefore justifying the designation of this protocol as an LD-CBCT protocol.
With respect to adequacy of the images for patient positioning, we found that measures of positional uncertainty were reasonably small and comparable or less than published reports in adults. For example, in a study comparing interfraction positioning uncertainties using 4 different thermoplastic immobilization devices in adult CNS IGRT with CBCT, Tryggestad et al. noted that mean VM for random interfraction error was 1.4 to 1.9 mm across devices.18 By comparison, our (within patient) interfraction error was similar, with a VM of 1.5 mm using the LD-CBCT. Additional studies of adult CNS and head and neck IGRT with thermoplastic mask immobilization report group mean VM ranging from 2.9 to 4.7 mm,21-23 again similar to the group mean VM in our study. Although there are likely differences in specific immobilization details, rate of anesthesia use, and KPS between the adult and pediatric populations, these data suggest that setup with LD-CBCT is similar to setup with standard-dose protocols.
Of note, the VM for group mean error in our study was estimated at approximately 3 mm. Since 3 mm was also the minimum CTV-to-PTV expansion used among consortium members, this suggests that a portion of patients evaluated would have setup within the CTV-to-PTV margins using LA alone. However, on univariate analysis, we found no significant predictors of setup accuracy (as estimated by mean VM). Therefore, we did not identify any patient demographics or treatment characteristics that would support altering IGRT practice patterns for patient subpopulations. Moreover, the 95% CIs for VM were relatively broad—for example, ranging from 1.4 to 6.2 mm for infratentorial tumors. The clinical significance of requiring a shift greater than 6 mm from initial setup with LA alone is noteworthy, particularly given proximity to critical and sensitive structures such as the brainstem in this location. In such cases, the risks associated with excess radiation dose from daily CBCT would likely weigh favorably against the risk of inadvertent exposure to adjacent normal tissue. Additionally, given the lower dose associated with the current protocol, the effects of excess dose from CBCT is likely further mitigated.
One criticism of this work may be that relative image quality and positioning uncertainty could conceivably be affected by patient size, raising the question if the current LD-CBCT protocol is indeed optimized for the target population. To address this concern, we conducted a post hoc analysis of cranial volume for a subset of patients in this study. Interestingly, the subgroup analysis did not find a significant correlation between cranial volume and age in a group of pediatrics ranging from 3 to 20 years. For comparison purposes only, pCT for 140 adults age >21 years who were treated using thermoplastic masks to brain sites at the Johns Hopkins University School of Medicine site was evaluated using the same methods for estimation of cranial volume. Among the adults, mean cranial volume was 12118.4 cm3 (SD 1486.2), with no statistical significance when compared to pediatrics by t test (P = .285; unpublished data). Corroborating the relative stability of cranial volume by age in our cohort is evidence that brain weight increases most dramatically from birth to age 3, with less substantial change thereafter.24 These data suggest that standardized LD-CBCT protocols can be safely employed for CNS-directed IGRT across all age-groups ≥3 years.
Limitations of this study include the use of translational table shifts and VM as proxies for setup accuracy associated with LA compared to CBCT. As noted in the methods, a direct comparison between LD- and standard-dose protocols was not felt to be feasible given risks of excess radiation exposure in the pediatrics population. The validity of table shifts as a proxy for setup accuracy may be supported by consistency of this measure across treating institutions and as compared to the adult population noted above. Nonetheless, we do suggest caution in interpreting results rendered from this metric, as its internal and external validity has not been thoroughly characterized. Moreover, we were unable to directly compare positioning uncertainty data with a cohort of pediatrics treated using standard-dose CBCT at the consortium sites due to heterogeneity of standard dose protocols and inconsistent documentation of table shift data off of the protocol. As such, our comparisons were limited to published results from potentially similar adult populations. Again, in order to limit unnecessary radiation dose in this population, we were unable to repeat posttreatment CBCT and were thus unable to account for intrafraction random error. Because of this, the group mean VM estimated by our study should not be misinterpreted as a margin prescription, as intrafraction error is included in most accepted margin recipes.19 Additionally, although the LD-CBCT protocol was uniform across institutions, other treatment practices may vary and skew the results. However, we found that interpatient error accounted for 65% of variability in the model, whereas interinstitution error accounted for significantly less (19% of variability). This suggests that the results had greater dependence on differences between patients than on differences between institutions. As such, these data support the use of LD-CBCT across a range of institutions—both in the United States and abroad. Finally, while our analyses suggest similar results with use of our LD-CBCT across a range of pediatrics and adults, it was out of the scope of the current study to compare various LD-CBCT dosing options to determine the optimal CBCT protocol in this setting.
Conclusions
In conclusion, our results suggest that LD-CBCT is likely adequate for target localization in pediatric CNS radiotherapy. Moreover, there are no clear patient characteristics that predict setup accuracy, suggesting that LD-CBCT may be appropriate across a wide range of patients and institutions. Given the success of the LD-CBCT protocol in pediatric CNS radiotherapy, next steps include extension of this protocol to other disease sites in pediatrics. Additionally, given similar practices for patient alignment and target localization, a study of the utility of LD-CBCT in adult CNS IGRT is also warranted. Lastly, further studies are needed to determine the threshold for the lowest possible dose required for adequate setup with CBCT and the clinical scenarios that may necessitate use of specific imaging protocols.
Supplemental Material
Supplementary_Materials_CBCT for Low-Dose Image-Guided Pediatric CNS Radiation Therapy: Final Analysis From a Prospective Low-Dose Cone-Beam CT Protocol From a Multinational Pediatrics Consortium by Sara R. Alcorn, Xian Chiong Zhou, Casey Bojechko, Rodrigo A. Rubo, Michael J. Chen, Karin Dieckmann, Ralph P. Ermoian, Eric C. Ford, Daria Kobyzeva, Shannon M. MacDonald, Todd R. McNutt, Alexey Nechesnyuk, Kristina Nilsson, Hakan Sjostrand, Koren S. Smith, Markus Stock, Erik J. Tryggestad, Rosangela C. Villar, Brian A. Winey and Stephanie A. Terezakis in Technology in Cancer Research & Treatment
Acknowledgments
We would like to thank Steven Bartolac, PhD, for his expertise and assistance during the revision of the manuscript.
Abbreviations
- CBCT
cone-beam computed therapy
- CI
confidence interval
- CNS
central nervous system
- CT
computed tomography
- CTV
clinical target volume
- IGRT
image-guided radiotherapy
- KPS
Karnofsky Performance Status
- LA
laser alignment
- LD-CBCT
low-dose cone-beam computed therapy
- LME
linear-mixed effect
- pCT
planning computed tomography
- PTV
planning target volume
- SD
standard deviation
- VM
vector magnitude
- XVI
X-ray volume imaging
- QA
quality assurance
- 3D
3-dimensional.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Statement: This study was approved by the Institutional Review Board (IRB) (NA_00083758) at the Johns Hopkins School of Medicine. The IRB granted an exemption to obtain written or oral consent for this study because the use of low-dose cone-beam computed tomography scan is considered to be a standard of care for image-guided radiation treatment.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a scientific grant awarded by Elekta Industries in support of this Pediatric research consortium.
ORCID iD: Sara R. Alcorn  https://orcid.org/0000-0003-4620-5047
https://orcid.org/0000-0003-4620-5047
Stephanie A. Terezakis  https://orcid.org/0000-0002-8125-3987
https://orcid.org/0000-0002-8125-3987
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Alcorn SR, Chen MJ, Claude L, et al. Practice patterns of photon and proton pediatric image guided radiation treatment: results from an international pediatric research consortium. Pract Radiat Oncol. 2014;4(5):336–341. doi:10.1016/j.prro.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 2. Gupta T, Narayan CA. Image-guided radiation therapy: physician′s perspectives. J Med Phys. 2012;37(4):174 doi:10.4103/0971-6203.103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verellen D, De Ridder M, Storme G. A (short) history of image-guided radiotherapy. Radiother Oncol. 2008;86(1):4–13. doi:10.1016/j.radonc.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 4. Dawson LA, Jaffray DA. Advances in image-guided radiation therapy. J Clin Oncol. 2007;25(8):938–946. doi:10.1200/JCO.2006.09.9515. [DOI] [PubMed] [Google Scholar]
- 5. Jaffray D, Kupelian P, Djemil T, Macklis RM. Review of image-guided radiation therapy. Expert Rev Anticancer Ther. 2007;7(1):89–103. doi:10.1586/14737140.7.1.89. [DOI] [PubMed] [Google Scholar]
- 6. Michalski J, Purdy JA, Gaspar L, et al. Radiation therapy oncology group. research plan 2002-2006. image-guided radiation therapy committee.Int J Radiat Oncol Biol Phys. 2001;51(3 suppl 2):60–65. doi:10.1038/358375a0. [PubMed] [Google Scholar]
- 7. Walter C, Boda-Heggemann J, Wertz H, et al. Phantom and in-vivo measurements of dose exposure by image-guided radiotherapy (IGRT): MV portal images vs. kV portal images vs. cone-beam CT. Radiother Oncol. 2007;85(3):418–423. doi:10.1016/j.radonc.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 8. Higgins J, Bezjak A, Hope A, et al. Effect of image-guidance frequency on geometric accuracy and setup margins in radiotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys. 2011;80(5):1330–1337. doi:10.1016/j.ijrobp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 9. McBain CA, Henry AM, Sykes J, et al. X-ray volumetric imaging in image-guided radiotherapy: the new standard in on-treatment imaging. Int J Radiat Oncol Biol Phys. 2006;64(2):625–634. doi:10.1016/j.ijrobp.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 10. Murphy MJ, Balter J, Balter S, et al. The management of imaging dose during image-guided radiotherapy: report of the AAPM Task Group 75. Med Phys. 2007;34(10):4041–4063. [DOI] [PubMed] [Google Scholar]
- 11. Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4(1):45–54. [PubMed] [Google Scholar]
- 12. Rodgers SP, Trevino M, Zawaski JA, Gaber MW, Leasure JL. Neurogenesis, exercise, and cognitive late effects of pediatric radiotherapy. Neural Plast. 2013;2013:698528 doi:10.1155/2013/698528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bölling T, Willich N, Ernst I. Late effects of abdominal irradiation in children: a review of the literature. Anticancer Res. 2010;30(1):227–231. [PubMed] [Google Scholar]
- 14. Ding GX, Coffey CW. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int J Radiat Oncol Biol Phys. 2009;73(2):610–617. doi:10.1016/j.ijrobp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 15. Siewerdsen JH, Uneri A, Hernandez AM, Burkett GW, Boone JM. Cone-beam CT dose and imaging performance evaluation with a modular, multipurpose phantom. Med Phys. 2020;47(2):467–479. doi:10.1002/mp.13952. [DOI] [PubMed] [Google Scholar]
- 16. Buckley JG, Wilkinson D, Malaroda A, Metcalfe P. Investigation of the radiation dose from cone-beam CT for image-guided radiotherapy: a comparison of methodologies. J Appl Clin Med Phys. 2018;19(1):174–183. doi:10.1002/acm2.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borgefors G. Hierarchical chamfer matching: a parametric edge matching algorithm. IEEE Trans Pattern Anal Mach Intell. 1988;10(6):849–865. doi:10.1109/34.9107. [Google Scholar]
- 18. Tryggestad E, Christian M, Ford E, et al. Inter- and intrafraction patient positioning uncertainties for intracranial radiotherapy: a study of four frameless, thermoplastic mask-based immobilization strategies using daily cone-beam CT. Int J Radiat Oncol Biol Phys. 2011;80(1):281–290. doi:10.1016/j.ijrobp.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 19. Van Herk M. Margins and margin recipes AAPM Summer School; 2011. [Google Scholar]
- 20. Song WY, Kamath S, Ozawa S, et al. A dose comparison study between XVI and OBI CBCT systems. Med Phys. 2008;35(2):480–486. doi:10.1118/1.2825619. [DOI] [PubMed] [Google Scholar]
- 21. Boda-Heggemann J, Walter C, Rahn A, et al. Repositioning accuracy of two different mask systems—3D revisited: comparison using true 3D/3D matching with cone-beam CT. Int J Radiat Oncol. 2006;66(5):1568–1575. doi:10.1016/j.ijrobp.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 22. Guckenberger M, Baier K, Guenther I, et al. Reliability of the bony anatomy in image-guided stereotactic radiotherapy of brain metastases. Int J Radiat Oncol. 2007;69(1):294–301. doi:10.1016/j.ijrobp.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 23. Masi L, Casamassima F, Polli C, Menichelli C, Bonucci I, Cavedon C. Cone beam CT image guidance for intracranial stereotactic treatments: comparison with a frame guided set-up. Int J Radiat Oncol. 2008;71(3):926–933. doi:10.1016/j.ijrobp.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 24. Dekaban AS, Sadowsky D. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4(4):345–356. doi:10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Materials_CBCT for Low-Dose Image-Guided Pediatric CNS Radiation Therapy: Final Analysis From a Prospective Low-Dose Cone-Beam CT Protocol From a Multinational Pediatrics Consortium by Sara R. Alcorn, Xian Chiong Zhou, Casey Bojechko, Rodrigo A. Rubo, Michael J. Chen, Karin Dieckmann, Ralph P. Ermoian, Eric C. Ford, Daria Kobyzeva, Shannon M. MacDonald, Todd R. McNutt, Alexey Nechesnyuk, Kristina Nilsson, Hakan Sjostrand, Koren S. Smith, Markus Stock, Erik J. Tryggestad, Rosangela C. Villar, Brian A. Winey and Stephanie A. Terezakis in Technology in Cancer Research & Treatment