Abstract
Pathological interplay between the heart and kidneys is widely encountered in heart failure (HF) and is linked to worse prognosis and quality of life. Inotropes, along with diuretics and vasodilators, are a core medical response to HF but decompensated patients who need inotropic support often present with an acute worsening of renal function. The impact of inotropes on renal function is thus potentially an important influence on the choice of therapy. There is currently relatively little objective data available to guide the selection of inotrope therapy but recent direct observations on the effects of levosimendan and milrinone on glomerular filtration favour levosimendan. Other lines of evidence indicate that in acute decompensated HF levosimendan has an immediate renoprotective effect by increasing renal blood flow through preferential vasodilation of the renal afferent arterioles and increases in glomerular filtration rate: potential for renal medullary ischaemia is avoided by an offsetting increase in renal oxygen delivery. These indications of a putative reno-protective action of levosimendan support the view that this calcium-sensitizing inodilator may be preferable to dobutamine or other adrenergic inotropes in some settings by virtue of its renal effects. Additional large studies will be required, however, to clarify the renal effects of levosimendan in this and other relevant clinical situations, such as cardiac surgery.
Keywords: Acute heart failure, Renal function, Levosimendan, Glomerular filtration rate, Dobutamine, Dopamine, Milrinone, Renal blood flow
Introduction
Heart failure (HF), whether acute or chronic, is often accompanied by impairment of renal function of a greater or less degree of severity.1 Whereas ≈5% of the general population have an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, a similar reduction in eGFR is seen in some 50% of patients with acute or chronic HF (with either preserved or reduced ejection fraction).1
This deterioration in renal performance is associated with increased mortality and readmission rates,2 and with longer in-hospital and intensive care unit length of stay.3,4 Renal insufficiency evidenced by eGFR <60 mL/min/1.73 m2, when encountered in HF patients, is associated with a significantly higher mortality rate than in otherwise similar patients without renal compromise.5,6
Risk factors for worsening of renal function include a history of chronic renal insufficiency, acute coronary syndrome, arterial hypertension, diabetes mellitus, old age, and severe atherosclerosis.7 Acute decompensated heart failure (AHF) has immediate deteriorating effects on renal function due to decreased perfusion and oxygen supply to the kidneys, and diuretics given to relieve oedema increase indirectly the oxygen demands of the glomeruli. Low mean arterial pressure, low left and right ventricular ejection fractions and hyponatraemia are also implicated in worse prognosis.8
The interdependence of cardiac and renal dysfunction in HF management has led to the development of the concept of cardiorenal syndrome (CRS). This can be broadly described as a pathophysiologic interplay between the heart and kidneys whereby acute or chronic dysfunction of either organ may induce acute or chronic dysfunction of the other.9,10
It is therefore crucial to understand the renal effects of cardio- and vasoactive drugs used in HF. The effects of inotropes and inodilators on renal function in the specific setting of AHF merit particular attention, since those drugs directly affect cardiac output (CO), central venous pressure (CVP), and systemic arterial pressure. Inotropes constitute the third pharmacological pillar in the treatment of patients with decompensated HF, the other two being diuretics and vasodilators.11
Adrenergic/catecholaminergic inotropes
Exemplars of this group are dobutamine and dopamine. Dobutamine, a synthetic catecholamine, acts primarily on beta-1 adrenoceptors (and also, weakly, at beta-2 receptors). Dobutamine improves CO by reducing afterload as well as through its inotropic action.12 Higher doses may also augment urinary sodium excretion, either via altered renal (or systemic) haemodynamics or via a direct effect on the kidney.13 Effects of dobutamine on renal sympathetic activity associated with increases in renal blood flow (RBF) (11%) and glomerular filtration rate (GFR) (12%) have been described.14 Separately reported research from the same authors has described a strong relation between the effect of dobutamine (or nitroprusside) on right atrial mean pressure in patients with HF and improvement in GFR. These data may signify a mechanism by which increased atrial pressures in the setting of congestive HF can lead to increases in sympathetic activity, with subsequent adverse effects on renal function.15 Dobutamine exerts vasodilator effects on both afferent and efferent arterioles and increases RBF. However, it impairs oxygenation of the medulla, increasing the oxygen demand of kidney tissue.
These conceptual and hypothesis-generating ideas about the possible renal effects of dobutamine have to be set against tangible indications from multiple sources that use of dobutamine may be associated with adverse clinical outcomes.
Madeira et al.16 recently reported findings from a retrospective data analysis of 108 consecutive patients with AHF who required inotrope therapy with either dobutamine (29% of patients) or levosimendan (71% of patients). These groups were not fully matched for baseline inclusion criteria: the dobutamine group had lower mean blood pressure on admission, while the levosimendan group had lower average left ventricular ejection fraction (LVEF); there were, however, no significant differences in eGFR or cystatin C levels. The incidence of CRS was higher in the dobutamine group, and those patients more often had incomplete recovery of renal function at discharge. The dobutamine group also had higher in-hospital mortality; the presence or persistence of CRS and the choice of inotrope proved to be strong predictors of in-hospital death.
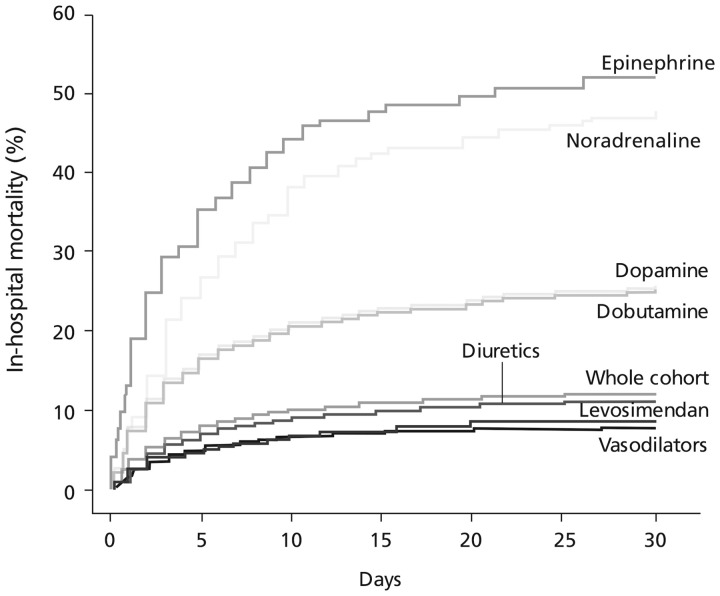
These data are supplementary to observations from the Levosimendan Infusion vs. DObutamine (LIDO) trial,17 in which the effects of dobutamine on GFR compared unfavourably with those of levosimendan in patients with severe low-output HF. Also relevant in this context is experience in the ALARM-HF registry, in which use of adrenergic inotropics, including dobutamine, was associated with substantially higher mortality than other interventions, including levosimendan (Figure 1).18 Adverse effects associated with dobutamine use can be an obstacle to its deployment: these include increased myocardial oxygen consumption, tachyarrhythmias, arrhythmogenesis, hypotension, and hypokalaemia. Dobutamine’s adrenergic mechanism of action means that it may be of reduced effectiveness in patients pre-treated with beta-blockers,19 as is now widely the case in HF, in response to guidelines recommendations.
Figure 1.
Impacts of principal i.v. vasoactive medications on in-hospital mortality (during the first 48 h) in acute heart failure patients in the ALARM-HF registry in patient subsets derived by propensity scoring methods. Based on Mebazaa et al.18
The receptor-level effects of dopamine vary with dose. When infused at rates of 3–5 μg/kg/min (i.e. those generally applied for inotropic effect), the drug’s effects are principally determined by the activation of beta-1 and beta-2 adrenergic receptors and are characterized by increased myocardial contractility, heart rate and CO. Lower doses of dopamine (≤3 μg/kg/min) act primarily at dopaminergic D1 and D2 receptors. Activation of D1 receptors causes vasodilation of both the large-conductance and small-resistance renal blood vessels;20 activation of D2 receptors indirectly has a similar effect through indirect pathways. These renal effects of dopamine may become impaired in the more advanced stages of HF due to selective loss of renal vasodilating capacity.21
A potential renoprotective effect of low-dose dopamine was identified in various small open-label studies in which the drug was combined with diuretic therapy in patients with AHF.22 This was subsequently investigated in larger controlled trials, including DAD-HF I and DAD-HF II.23,24 The DAD-I (N = 60) trial, which compared high-dose furosemide (20 mg/h) with dopamine (5 μg/kg/min) plus low-dose furosemide (5 mg/h), reported preservation of renal function but no significant differences in 60-day mortality and rehospitalization rates. In DAD-HF II (N = 161), the combination of low-dose dopamine (5 μg/kg/min/8 h) plus furosemide (5 mg/h) was well tolerated but not associated with beneficial effects beyond those attributable to low-dose furosemide alone. Moreover, the trial was terminated early due to an excess of tachycardia among patients assigned to the (dopamine + diuretic) group. Dopamine was not effective for improving renal function and was not associated with reduced mortality in this patient population.
The ROSE-AHF trial evaluated a more convincingly ‘renal’ dose of dopamine (2 μg/kg/min for 72 h) in 360 patients hospitalized for AHF and with evidence of renal dysfunction (GFR 15–60 mL/min/1.73 m2) and systolic blood pressure (SBP) ≥90 mmHg. A comparison arm was randomized to nesiritide, the recombinant form of human B-type natriuretic peptide, at a dose of 0.005 μg/kg/min/72 h. High-dose intravenous (i.v.) furosemide (2.5 times the equivalent oral outpatient dose) was administered to all patients for the first 24 h of the study.25
The findings of the ROSE-AHF trial provided no firm support for the routine use of low-dose dopamine in AHF patients with reasonably well-sustained blood pressure. The incidence of tachycardia suggested that the relatively low dose used may not in fact be renal-specific in all patients.26
SOAP investigators also demonstrated a deleterious effect of dopamine on mortality and rate of new-onset arrhythmias in cardiogenic shock patients.27
Brief reference may be made here to fenoldopam, a D1-receptor agonist, for which no strong case can be made.28
Phosphodiesterase inhibitors
These agents—exemplified by milrinone and enoximone—promote inotropy via the inhibition of phosphodiesterase (PDE) III in cardiomyocytes; they can also induce vasodilation by inhibition of PDE in vascular smooth muscle cells. As agents that act independently of beta-adrenoceptor-dependent pathways, their effects are not attenuated by beta-blockade. These effects offer theoretical advantages for renal function, with drug-induced vasodilation notionally enhancing transrenal perfusion pressure.
Lannemyr et al.29 have recently reported on the renal effects of milrinone (0.04 mg/kg i.v. bolus then infusion of 0.30–0.50 μg/kg/min depending on the haemodynamic response) in seven patients undergoing cardiac surgery who developed AHF and low cardiac performance [cardiac index (CI) <2.1 L/min/m2] shortly after weaning from cardiopulmonary bypass (CPB). A further 19 patients acted as controls (ClinicalTrials.gov identifier: NCT02405195). Observations were made before and up to 60 min after weaning from CPB.
In the control group, the filtration fraction was lower after weaning than at baseline (Δ13%; P = 0.03) and there was a trend towards decreased RBF and increased renal oxygen extraction (both P ≈ 0.06). In contrast, and also compared with baseline, milrinone administration increased RBF by 36% (P < 0.05) and renal oxygen delivery by 35% (P < 0.05), while renal vascular resistance was decreased by 29% (P < 0.05).
Relative to controls, milrinone treatment was associated with increased RBF (P = 0.007) and renal oxygen delivery (P = 0.003); renal vascular resistance (P < 0.001), filtration fraction (P < 0.05), and renal oxygen extraction (P < 0.05) all decreased. No significant changes in GFR or renal oxygen consumption were noted.
Overall, these data are consistent with the view that milrinone improved renal oxygenation. The investigators noted, however, that a prerequisite for any beneficial renal effect in this situation is the maintenance of mean arterial pressure (MAP) and renal perfusion pressure at the pre-milrinone level (by administration of noradrenaline). Without that intervention, MAP would probably have declined, counteracting any advantageous effects on renal perfusion.
The results of the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) trial of milrinone provided only limited indications of any advantageous renal effect and no demonstration that such changes could be related to wider clinical outcomes. A priori, this prospective, randomized, double-blind, placebo-controlled trial (n = 951) offered no support for the routine use of i.v. milrinone (0.5 μg/kg/min) in the treatment of patients hospitalized for an exacerbation of HF. No significant effects were seen on the outcomes of median number of days hospitalized for cardiovascular causes within 60 days after randomization, or in-hospital mortality, or the composite incidence of death or readmission. Furthermore, hypotension requiring intervention (10.7% vs. 3.2%; P < 0.001) and new atrial arrhythmias (4.6% vs. 1.5%; P = 0.04) occurred more frequently in patients who received milrinone.30
The authors of a separate retrospective analysis of OPTIME-CHF data concluded that ‘Although milrinone treatment led to a minor improvement in renal function by discharge, the 60-day death and readmission rates were similar between the milrinone and placebo groups’.31 A further retrospective interrogation of the OPTIME-CHF database concluded that clinical-event (endpoint) outcomes were worse among milrinone-treated patients who had HF of ischaemic origin than in those with non-ischaemic HF.32
Milrinone is subject to renal elimination and there is a widespread presumption towards careful dosing in situations of established renal dysfunction in order to minimize the risks of arrhythmias and hypotension. A recent review of this matter reiterated the value of milrinone in patients with advanced HF but acknowledged a lack of dedicated studies on dosage, patient selection and outcomes in situations of renal impairment.33 A correlation has been demonstrated between plasma milrinone concentration and renal function in patients with cardiac disease.34
Enoximone is mainly eliminated by the liver and this may be prima facie an advantage over milrinone in situations of renal compromise. Few detailed investigations of this aspect of i.v. enoximone have been published.35
Levosimendan and renal function
Both the pre-clinical and clinical evidence for a renal-protective action of levosimendan is suggestive rather than conclusive. The findings are not always consistent and sometimes acquire statistical significance only when considered in meta-analyses. There are, nevertheless, suggestions of a renal-protective effect of levosimendan in a range of low-output states, including patients with acutely decompensated HF and renal impairment, and in critical illness situations, cardiac surgery and heart transplantation.36–41
Timely introduction of levosimendan may prevent the development or progression of renal dysfunction through several protective mechanisms involving both the macro- and microcirculation.42 For example, levosimendan may augment renal perfusion via vasodilatation arising from its effects on adenosine triphosphate-dependent potassium (KATP) channels.
Levosimendan exerts a vasodilator effect mainly on the afferent arterioles of the kidney, increasing GFR without modifying renal oxygen consumption and extraction. Other potentially relevant mechanisms within the kidney relate to preconditioning, pleiotropic, anti-inflammatory and anti-apoptotic effects, and increased glomerular surface area.43
Pre-clinical insights on the effect(s) of levosimendan on renal function tend to be consistent within individual models but vary substantially between different models.43 In septic models of renal compromise, any beneficial effects appear to be relatable primarily to improvements in haemodynamics, whereas the organ-protective effect of the drug dominates in situations of ischaemia/reperfusion injury. The opening of mitochondrial KATP channels may be involved in these situations.
Enhanced expression of nominally protective enzymes, along with significantly (P < 0.001) elevated levels of the antioxidant glutathione and lower levels of malondialdehyde, have been reported in renal tissue in a rat model, consistent with speculation that levosimendan reduces oxidative stress in renal tissue.44
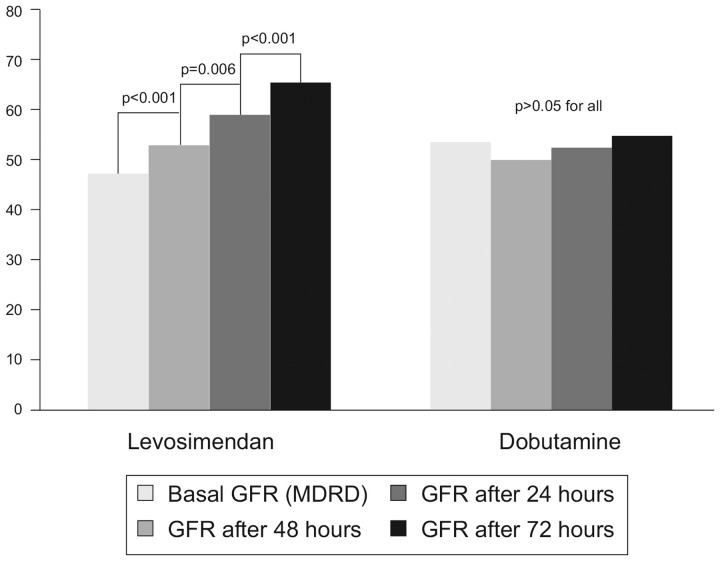
Direct investigations of the renal effects of levosimendan in human patients include observations made in 2007 by Yilmaz et al.,45 who randomized 88 patients hospitalized for the stabilization of acutely decompensated HF to levosimendan (0.1 µg/kg/min, increased to 0.2 µg/kg/min after 6 h of infusion if tolerated) or dobutamine (5 µg/kg/min for at least 6 h, with scope for later dose increases). Renal function indices, including serum creatinine, blood urea nitrogen (BUN), 24-h urinary output levels, and calculated GFR, were measured beforehand and for up to 72 h after inotrope infusion. Median baseline furosemide dosage was 60 mg/day in both groups and was kept constant in all patients during the trial.
LVEF increased by 4–5% in both treatment groups and 24-h urinary output was augmented (levosimendan: 1054 ± 441 mL at baseline to 1947 ± 870 mL at 24 h after infusion, 2535 ± 865 mL at 48 h and 1994 ± 609 mL at 72 h: all P < 0.001 vs. pre-treatment). Corresponding data for dobutamine were: 1066 ± 373 mL at baseline to 1920 ± 599 mL after 24 h (P < 0.001), 1821 ± 523 mL at 48 h (P < 0.001), and 1523 ± 295 mL at 72 h (P = 0.027). However, progressive enhancement of eGFR 24 h after treatment infusions was observed only in patients randomized to levosimendan (Figure 2). (The increase in urine output with dobutamine was not regarded as proof of a beneficial effect on renal function from dobutamine; in these substantially fluid-overload patients, diuresis might have been achieved via inotropy and enhancement of cardiac function.)
Figure 2.
Comparison of the effects of i.v. levosimendan and i.v. dobutamine on glomerular filtration rate (GFR) in 41 patients with acute heart failure. GFR (shown on vertical axis) was calculated from the Modification of Diet in Renal Disease (MDRD) formula. Data from Yilmaz et al.45
In separate later observations, Fedele et al.46 randomized 21 adult patients with acute decompensated HF, moderate renal impairment (GFR 30–60 mL/min/1.73 m2), pulmonary capillary wedge pressure (PCWP) >20 mmHg and ejection fraction <40% to i.v. levosimendan (6 µg/kg/10 min loading dose, then 0.1 µg/kg/min for 24 h) or placebo, on top of standard therapy that was maintained during the study (ClinicalTrials.gov identifier NCT00527059). An intravascular renal artery Doppler examination was performed at baseline, after levosimendan bolus and 1 h thereafter. Renal blood flow, GFR, urinary output, serum levels of cystatin C, BUN, sodium excretion, and plasma sodium were measured.
Clear and progressive increases in GFR were seen during the observation period in the levosimendan group only. By 72 h, mean GFR had increased from 38.71 ± 7.94 to 53.34 ± 14.93 mL/min/1.73 m2; various other specified indices of renal function also demonstrated significant improvements in response to levosimendan (Table 1).
Table 1.
Changes in renal function variables at 72 h vs. baseline in response to i.v. levosimendan in 21 patients with acute decompensated HF and moderate renal impairment randomized to active treatment or placebo in addition to usual standard therapies

|
From Fedele et al.46
GFR, glomerular filtration rate; BUN, blood urea nitrogen.
These responses were accompanied by significant, nominally favourable changes in CI (P = 0.029) and PCWP (P < 0.001). A significant increase in CI was apparent from 24 h, while PCWP fell promptly after the commencement of levosimendan, reaching a new, lower level at 1 h that was thereafter maintained. Both mean pulmonary artery pressure and mean renal artery pressure also decreased significantly in the levosimendan group (P < 0.05 for both at 1 h).
Correlations were identified between changes in renal function indices and alterations in several dimensions of renal haemodynamic parameters. The average RBF velocity in the levosimendan-treated patients increased from 18.71 ± 7.62 cm/s at baseline to 22.49± 0.27 cm/s after administration of the initial bolus (P = 0.04) and to 22.28 ± 9.76 cm/s at 1 h (P = 0.03). No significant alterations were noted in the placebo group. The inter-group test of statistical significance was not fulfilled for that outcome (P for interaction = 0.055) but a significant difference in favour of levosimendan was recorded for treatment effect on renal artery diameter (RAD) in comparison with placebo. In detail, mean RAD in the levosimendan group increased from 0.57 ± 0.18 cm at baseline to 0.60 ± 0.15 cm after 1 h (P = 0.002), whereas no meaningful change was observed in the placebo group [0.56 ± 0.12 cm at baseline, 0.56 ± 0.16 cm at 1 h (P = 0.55; P for interaction = 0.033)]. As a result of these changes, RBF increased in response to the initial bolus of levosimendan and during the first hour of infusion [from 301.3 ± 184.6 mL/min at baseline to 383.8 ± 198.9 mL/min at 1 h (P < 0.01; P for interaction vs. placebo = 0.037)].
These data are notable for the fact that levosimendan improved RBF before a significant effect on CI was recorded. This temporal discrepancy was interpreted as indicating that mechanisms other than enhancement of cardiac inotropy and output may contribute to the positive impact on renal function, with local vasodilation playing an important role. These findings are compatible with indications that assignment to levosimendan therapy predicted improved renal function independent of changes in left ventricular performance in patients with advanced chronic HF awaiting cardiac transplantation.47
Other relevant explorations in this area include research by Bragadottir et al.,48 who performed a prospective, randomized, placebo-controlled trial in 30 cardiac surgery patients at risk of developing post-operative acute kidney injury due to low CO syndrome. Patients were randomized to levosimendan (12 µg/kg loading dose, then 0.1 µg/kg/min; n = 15) or placebo (n = 15) commencing 4–6 h after the completion of surgery. Of note, CVP was kept constant by colloid/crystalloid infusion.
Compared with placebo, levosimendan increased CI (22%), stroke volume index (15%), and heart rate (7%) and decreased systemic vascular resistance index (21%). It also significantly increased RBF, GFR, and renal vascular resistance relative to placebo, while causing no significant changes in filtration fraction, renal oxygen consumption, or renal oxygen extraction.
These findings were regarded as compatible with the proposition that levosimendan induces preferential vasodilation of pre-glomerular resistance vessels, thereby increasing both RBF and GFR without jeopardizing renal oxygenation. This study did not include experimental measures to provide direct information on the oxygen supply/demand relationship of the renal medulla, so that the last of these conclusions should be regarded as a working hypothesis rather than a proven fact. Even so, it is in contrast to observations from the same researchers that vasopressin treatment in post-operative cardiac surgery patients increases renal oxygen extraction and may thereby compromise the renal oxygen supply/demand relationship in medullary tissue.49
Lannemyr et al.50 compared the renal effects of levosimendan and dobutamine in patients with chronic HF (LVEF < 40%) and renal impairment (GFR < 80 mL/min/1.73 m2) in a randomized, double-blind study (ClinicalTrials.gov identifier: NCT02133105). Patients (n = 32) were assigned to levosimendan (loading dose 12 μg/kg, then 0.1 μg/kg/min) or dobutamine (7.5 μg/kg/min) for 75 min. A pulmonary artery catheter was used to monitor systemic haemodynamics, and a renal vein catheter was used to measure renal plasma flow by means of para-aminohippurate clearance.
Both drugs had broadly comparable effects on systemic haemodynamics, with no statistically significant differences across a wide range of indices. Their effects on RBF were also similar and not significantly different (increase from 426 ± 197 to 518 ± 276 mL/min with levosimendan vs. increase from 397 ± 121 to 499 ± 154 mL/min with dobutamine; P = 0.732 for comparison of treatment effect in a linear mixed model). In contrast, the study drugs exerted differential effects on GFR, with an increase in the levosimendan group (from 36.5 ± 18.3 to 44.5 ± 19.0 mL/min; P < 0.5 vs. baseline) but no meaningful change in the dobutamine group (47.1 ± 14.5 mL/min at baseline vs. 47.3 ± 16.9 mL/min after treatment; P = not significant vs. baseline; P = 0.012 for inter-group comparison). Filtration fraction was unaltered in the levosimendan group but declined by an average of 17% in the dobutamine group (P = 0.045). Small increases in renal oxygen consumption were seen in both groups (levosimendan: 9.2 ± 6.3 mL/min at baseline, 10.1 ± 6.2 mL/min after treatment; dobutamine: 8.3 ± 2.6 mL/min at baseline, 8.9 ± 4.3 mL/min after treatment; P ≈ 0.8 for inter-group comparison).
Other small studies have produced indications of renal benefits from levosimendan therapy, including in patients with biventricular HF.47,51–53 However, robust indications of ‘pro-renal’ effects were not forthcoming from the SURVIVE or REVIVE trials, in both of which levosimendan was compared with dobutamine.
These various lines of investigation have recently been elegantly consolidated by Honore et al.54
In acute decompensated HF, levosimendan has an immediate renoprotective effect by increasing RBF through preferential vasodilation of the renal afferent arterioles.
In addition to increasing RBF, levosimendan increases GFR significantly. (No comparable effect is seen with dobutamine.)
An isolated increase in GFR could jeopardize oxygenation of the medulla, which is sensitive to ischaemia, but this is unlikely to occur with levosimendan, because it causes balanced increases in GFR and renal oxygen delivery.
CVP is an important predictor of renal dysfunction in HF patients. Elevated CVP will increase renal venous backward pressure and thus decrease renal perfusion pressure and impair renal function. An elevated CVP may adversely impact kidney haemodynamics and promote acute kidney injury even in the absence of volume overload.
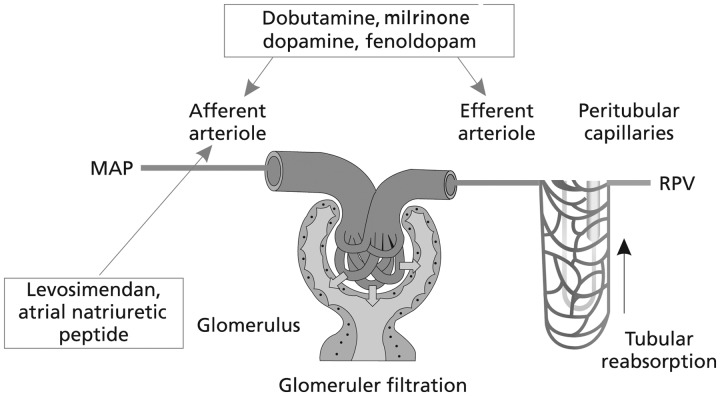
These conclusions, which we endorse, highlight that in order to be beneficial an increase in RBF has to be accompanied by an increase in GFR but not at the cost of medullary hypoxaemia; levosimendan appears to deliver this suite of requirements. We would qualify those conclusions, however, with the observation that the evidence base for beneficial renal effects of levosimendan in HF settings is both heterogeneous and methodologically variable and that the largest of the well-powered regulatory studies has produced neutral or inconclusive results on these outcomes. These considerations do not restrict us from the conclusion that levosimendan may be preferable to dobutamine or other adrenergic inotropes by way of both its renal and wider therapeutic effects in AHF, including in those patients who are at risk of developing acute kidney injury due to hypoperfusion. However, additional large studies are required to clarify the renal effects of levosimendan in this and other relevant clinical situations, such as cardiac surgery and perhaps septic shock or acute HF/cardiogenic shock complicating acute coronary syndrome.55 Pending such research, the ideas of Yilmaz et al.43 regarding differential drug effects on RBF and perfusion also remain pertinent (Figure 3).
Figure 3.
Differential effects of renal vasodilatation on preglomerular (afferent arteriole) and post-glomerular (efferent arteriole) renal vascular resistance. RBF, renal blood flow; MAP, mean arterial pressure; RPV, renal parenchymal volume; GFR, glomerular filtration rate. With a predominant afferent vasodilation: RBF↑ and GFR↑; with a predominant efferent vasodilation: RBF↑ and GFR↓; with both afferent and efferent vasodilation: RBF↑↑ and GFR↔. After Yilmaz et al.43
Conclusions
Congestion is a central clinical sign and therapeutic target in AHF patients, and a link is discernible between persistent congestion at discharge and subsequent prognosis and mortality. Eradication of clinical congestion by the time of hospital discharge may be considered a surrogate marker for the successful treatment of AHF. Inotropes can be used to augment cardiac function when there is a known low-output state in order to achieve better renal perfusion. It must be acknowledged, however, that there are little well-founded, objective data available to guide the selection or use of the various inotropes, even though this approach is quite widely used. These considerations apply to levosimendan as much as to the other agents discussed in this review, although the volume and quality of data available for levosimendan are arguably more encouraging than for some other drugs. The fact that the dataset includes direct observations of renal vascular responses and attendant functional changes provides some confidence that any views on renal-protective effects of levosimendan are grounded in testable criteria.
HF patients who might derive particular benefit from levosimendan administration include those with HF of ischaemic origins, those with well-sustained systemic blood pressure (SBP >100 mmHg) and those receiving concomitant treatment with beta-blockers. In contrast to levosimendan, dobutamine would be ineffective in patients receiving concomitant beta-blocker treatment.
Recent observations on the effects of levosimendan and milrinone on glomerular filtration differentiate these two inodilators as regards their renal effects, showing advantages of the former over the latter.29,50 Moreover, the fact that milrinone undergoes renal excretion argues for its use with informed caution in patients with renal failure.56 Other drugs currently under evaluation may be expected to expand the medical repertoire for the management or protection of renal function in AHF, although substantial additional work may be needed to provide a sufficient evidence base for their introduction as routine therapy.
Acknowledgements
The authors acknowledge Hughes associates, Oxford, UK, for assistance in editing of the manuscript.
Funding
This paper was published as part of a supplement made possible by an unrestricted educational grant by Orion Pharma.
Conflict of interest: In the past 5 years, E.Z., D.F., and J.T.P. have received honoraria for educational events organized by Orion Pharma. P.P. is a full-time employee of Orion Pharma, where levosimendan was discovered and developed.
References
- 1. Damman K, Valente MAE, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL.. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 2. Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM.. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 2006;47:1987–1996. [DOI] [PubMed] [Google Scholar]
- 3. Chittineni H, Miyawaki N, Gulipelli S, Fishbane S.. Risk for acute renal failure in patients hospitalized for decompensated congestive heart failure. Am J Nephrol 2007;27:55–62. [DOI] [PubMed] [Google Scholar]
- 4. Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ. ; COACH investigators. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Eur J Heart Fail 2009;11:847–854. [DOI] [PubMed] [Google Scholar]
- 5. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA.. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 2008;51:1268–1274. [DOI] [PubMed] [Google Scholar]
- 6. Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–580. [DOI] [PubMed] [Google Scholar]
- 7. Rafouli-Stergiou P, Parissis JT, Anastasiou-Nana M.. Inotropes for the management of acute heart failure patients with renal dysfunction. Still an option? Expert Opin Pharmacother 2012;13:2637–2647. [DOI] [PubMed] [Google Scholar]
- 8. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J; ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13:422–430. [DOI] [PubMed] [Google Scholar]
- 9. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R.. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 10. Mentz RJ, Lewis EF.. Epidemiology of cardiorenal syndrome. Heart Fail Clin 2010;6:333–346. [DOI] [PubMed] [Google Scholar]
- 11. Farmakis D, Parissis J, Karavidas A, Karvounis C, Triposkiadis F, Filippatos G, Lekakis J; Collaborators. In-hospital management of acute heart failure: practical recommendations and future perspectives. Int J Cardiol 2015;201:231–236. [DOI] [PubMed] [Google Scholar]
- 12. Sarraf M, Schrier RW.. Cardiorenal syndrome in acute heart failure syndromes. Int J Nephrol 2011;2011:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Good J, Frost G, Oakley CM, Cleland JG.. The renal effects of dopamine and dobutamine in stable chronic heart failure. Postgrad Med J 1992;68(Suppl 2):S7–S11. [PubMed] [Google Scholar]
- 14. Al-Hesayen A, Parker JD.. The effects of dobutamine on renal sympathetic activity in human heart failure. J Cardiovasc Pharmacol 2008;51:434–436. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert C, Cherney DZ, Parker AB, Mak S, Floras JS, Al-Hesayen A, Parker JD.. Hemodynamic and neurochemical determinates of renal function in chronic heart failure. Am J Physiol Regul Integr Comp Physiol 2016;310:R167–R175. [DOI] [PubMed] [Google Scholar]
- 16. Madeira M, Caetano F, Almeida I, Fernandes A, Reis L, Costa M, Gonçalves L.. Inotropes and cardiorenal syndrome in acute heart failure—a retrospective comparative analysis. Rev Port Cardiol 2017;36:619–625. [DOI] [PubMed] [Google Scholar]
- 17. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L; Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002;360:196–202. [DOI] [PubMed] [Google Scholar]
- 18. Mebazaa A, Parissis J, Porcher R, Gayat E, Nikolaou M, Boas FV, Delgado JF, Follath F.. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med 2011;37:290–301. [DOI] [PubMed] [Google Scholar]
- 19. Triposkiadis F, Dalampiras P, Kelepeshis G, Skoularigis J, Sitafidis G.. Hemodynamic effects of dobutamine in patients with an exacerbation of chronic systolic heart failure treated with low doses of carvedilol. Int J Clin Pharmacol Ther 2008;46:136–139. [DOI] [PubMed] [Google Scholar]
- 20. Elkayam U, Ng TM, Hatamizadeh P, Janmohamed M, Mehra A.. Renal vasodilatory action of dopamine in patients with heart failure: magnitude of effect and site of action. Circulation 2008;117:200–205. [DOI] [PubMed] [Google Scholar]
- 21. Bock J, Gottlieb S.. Cardiorenal syndrome. New perspectives. Circulation 2010;121:2592–2600. [DOI] [PubMed] [Google Scholar]
- 22. Cotter G, Weissgarten J, Metzkor E, Moshkovitz Y, Litinski I, Tavori U, Perry C, Zaidenstein R, Golik A.. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther 1997;62:187–193. [DOI] [PubMed] [Google Scholar]
- 23. Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, Rovithis D, Economou D, Savvatis K, Kirlidis T, Tsaknakis T, Skoularigis J, Westermann D, Tschöpe C, Triposkiadis F.. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) trial. J Card Fail 2010;16:922–930. [DOI] [PubMed] [Google Scholar]
- 24. Triposkiadis FK, Butler J, Karayannis G, Starling RC, Filippatos G, Wolski K, Parissis J, Parisis C, Rovithis D, Koutrakis K, Skoularigis J, Antoniou CK, Chrysohoou C, Pitsavos C, Stefanadis C, Nastas J, Tsaknakis T, Mantziari L, Giannakoulas G, Karvounis H, Kalogeropoulos AP, Giamouzis G.. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–121. [DOI] [PubMed] [Google Scholar]
- 25. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WHW, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Dávila-Román VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM; NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jain AK, Chen HH.. ROSE-AHF and lessons learned. Curr Heart Fail Rep 2014;11:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779–789. [DOI] [PubMed] [Google Scholar]
- 28. Bove T, Zangrillo A, Guarracino F, Alvaro G, Persi B, Maglioni E, Galdieri N, Comis M, Caramelli F, Pasero DC, Pala G, Renzini M, Conte M, Paternoster G, Martinez B, Pinelli F, Frontini M, Zucchetti MC, Pappalardo F, Amantea B, Camata A, Pisano A, Verdecchia C, Dal CE, Cariello C, Faita L, Baldassarri R, Scandroglio AM, Saleh O, Lembo R, Calabro MG, Bellomo R, Landoni G.. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA 2014;312:2244–2253. [DOI] [PubMed] [Google Scholar]
- 29. Lannemyr L, Bragadottir G, Redfors B, Ricksten SE.. Effects of milrinone on renal perfusion, filtration and oxygenation in patients with acute heart failure and low cardiac output early after cardiac surgery. J Crit Care 2020: pii:S0883-944(19)31428-5.doi: 10.016/j.jcrc.2019.12.022 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Cuffe MS, Califf RM, Adams KF Jr, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M; Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002;287:1541–1547. [DOI] [PubMed] [Google Scholar]
- 31. Klein L, Massie BM, Leimberger JD, O’Connor CM, Piña IL, Adams KF, Califf RM, Gheorghiade M; OPTIME-CHF Investigators. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF). Circ Heart Fail 2008;1:25–33. [DOI] [PubMed] [Google Scholar]
- 32. Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O’Connor CM; OPTIME-CHF Investigators. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol 2003;41:997–1003. [DOI] [PubMed] [Google Scholar]
- 33. Chong LYZ, Satya K, Kim B, Berkowitz R.. Milrinone dosing and a culture of caution in clinical practice. Cardiol Rev 2018;26:35–42. [DOI] [PubMed] [Google Scholar]
- 34. Hasei M, Uchiyama A, Nishimura M, Mashimo T, Fujino Y.. Correlation between plasma milrinone concentration and renal function in patients with cardiac disease. Acta Anaesthesiol Scand 2008;52:991–996. [DOI] [PubMed] [Google Scholar]
- 35. Berti S, Palmieri C, Ravani M, Bonini R, Iascone MR, Clerico A, Manfredi C, Iervasi G, Ferrazzi P, Biagini A.. Acute enoximone effect on systemic and renal hemodynamics in patients with heart failure. Cardiovasc Drugs Ther 1996;10:81–87. [DOI] [PubMed] [Google Scholar]
- 36. Rafouli-Stergiou P, Parissis JT, Farmakis D, Bistola V, Frogoudaki A, Vasiliadis K, Ikonomidis I, Paraskevaidis I, Kremastinos D, Filippatos G, Lekakis J.. Effects of levosimendan on markers of kidney function in patients with acutely decompensated heart failure and renal impairment. J Cardiovasc Med (Hagerstown) 2017;18:771–773. [DOI] [PubMed] [Google Scholar]
- 37. Bove T, Matteazzi A, Belletti A, Paternoster G, Saleh O, Taddeo D, Dossi R, Greco T, Bradic N, Husedzinovic I, Nigro Neto C, Lomivorotov VV, Calabrò MG.. Beneficial impact of levosimendan in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Heart Lung Vessel 2015;7:35–46. [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B.. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016;67:408–416. [DOI] [PubMed] [Google Scholar]
- 39. Balzer F, Treskatsch S, Spies C, Sander M, Kastrup M, Grubitzsch H, Wernecke KD, Braun JP.. Early administration of levosimendan is associated with improved kidney function after cardiac surgery—a retrospective analysis. J Cardiothorac Surg 2014;9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knezevic I, Poglajen G, Hrovat E, Oman A, Pintar T, Wu JC, Vrtovec B, Haddad F.. The effects of levosimendan on renal function early after heart transplantation: results from a pilot randomized trial. Clin Transplant 2014;28:1105–1111. [DOI] [PubMed] [Google Scholar]
- 41. Fedele F, Karason K, Matskeplishvili S.. Pharmacological approaches to cardio-renal syndrome: a role for the inodilator levosimendan. Eur Heart J Suppl 2017;19:C22–C28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farmakis D, Alvarez J, Gal TB, Brito D, Fedele F, Fonseca C, Gordon AC, Gotsman I, Grossini E, Guarracino F, Harjola VP, Hellman Y, Heunks L, Ivancan V, Karavidas A, Kivikko M, Lomivorotov V, Longrois D, Masip J, Metra M, Morelli A, Nikolaou M, Papp Z, Parkhomenko A, Poelzl G, Pollesello P, Ravn HB, Rex S, Riha H, Ricksten SE, Schwinger RHG, Vrtovec B, Yilmaz MB, Zielinska M, Parissis J.. Evidence for pleiotropic effects of levosimendan and their consequences on the heart and non-cardiac organs: an expert consensus opinion. Int J Cardiol 2016;222:303–312. [DOI] [PubMed] [Google Scholar]
- 43. Yilmaz MB, Grossini E, Silva Cardoso JC, Édes I, Fedele F, Pollesello P, Kivikko M, Harjola VP, Hasslacher J, Mebazaa A, Morelli A, Le Noble J, Oldner A, Oulego Erroz I, Parissis JT, Parkhomenko A, Poelzl G, Rehberg S, Ricksten SE, Rodríguez Fernández LM, Salmenperä M, Singer M, Treskatsch S, Vrtovec B, Wikström G.. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther 2013;27:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gecit İ, Kavak S, Yüksel MB, Basel H, Bektaş H, Gümrükçüoğlu HA, Meral İ, Demir H.. Effect of short-term treatment with levosimendan on oxidative stress in renal tissues of rats. Toxicol Ind Health 2014;30:47–51. [DOI] [PubMed] [Google Scholar]
- 45. Yilmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, Turgut OO, Yilmaz A, Tandogan I.. Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther 2007;21:431–435. [DOI] [PubMed] [Google Scholar]
- 46. Fedele F, Bruno N, Brasolin B, Caira C, D'Ambrosi A, Mancone M.. Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail 2014;16:281–288. [DOI] [PubMed] [Google Scholar]
- 47. Zemljic G, Bunc M, Yazdanbakhsh AP, Vrtovec B.. Levosimendan improves renal function in patients with advanced chronic heart failure awaiting cardiac transplantation. J Card Fail 2007;13:417–421. [DOI] [PubMed] [Google Scholar]
- 48. Bragadottir G, Redfors B, Ricksten SE.. Effects of levosimendan on glomerular filtration rate, renal blood flow, and renal oxygenation after cardiac surgery with cardiopulmonary bypass: a randomized placebo-controlled study. Crit Care Med 2013;41:2328–2335. [DOI] [PubMed] [Google Scholar]
- 49. Bragadottir G, Redfors B, Nygren A, Sellgren J, Ricksten SE.. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol Scand 2009;53:1052–1059. [DOI] [PubMed] [Google Scholar]
- 50. Lannemyr L, Ricksten SE, Rundqvist B, Andersson B, Bartfay SE, Ljungman C, Dahlberg P, Bergh N, Hjalmarsson C, Gilljam T, Bollano E, Karason K.. Differential effects of levosimendan and dobutamine on glomerular filtration rate in patients with heart failure and renal impairment: a randomized double-blind controlled trial. J Am Heart Assoc 2018;7:e008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silva-Cardoso J, Ferreira J, Oliveira-Soares A, Martins-de-Campos J, Fonseca C, Lousada N, Ilídio-Moreira J, Rabaçal C, Damasceno A, Amorim S, Seabra-Gomes R, Ferreira R, Abreu-Lima C; PORTLAND Investigators. Effectiveness and safety of levosimendan in clinical practice. Rev Port Cardiol 2009;28:143–154. [PubMed] [Google Scholar]
- 52. Yilmaz MB, Yontar C, Erdem A, Karadas F, Yalta K, Turgut OO, Yilmaz A, Tandogan I.. Comparative effects of levosimendan and dobutamine on right ventricular function in patients with biventricular heart failure. Heart Vessels 2009;24:16–21. [DOI] [PubMed] [Google Scholar]
- 53. Zorlu A, Yücel H, Yontar OC, Karahan O, Tandogan I, Katrancioglu N, Yilmaz MB.. Effect of levosimendan in patients with severe systolic heart failure and worsening renal function. Arq Bras Cardiol 2012;98:537–543. [DOI] [PubMed] [Google Scholar]
- 54. Honore PM, Gutierrez LB, Redant S, Kaefer K, Gallerani A, De Bels D.. How levosimendan can improve renal function? Crit Care 2019;23:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nieminen MS, Buerke M, Cohen-Solál A, Costa S, Édes I, Erlikh A, Franco F, Gibson C, Gorjup V, Guarracino F, Gustafsson F, Harjola Vp Husebye T, Karason K, Katsytadze I, Kaul S, Kivikko M, Marenzi G, Masip J, Matskeplishvili S, Mebazaa A, Møller JE, Nessler J, Nessler B, Ntalianis A, Oliva F, Pichler-Cetin E, Põder P, Recio-Mayoral A, Rex S, Rokyta R, Strasser RH, Zima E, Pollesello P.. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol 2016;218:150–157. [DOI] [PubMed] [Google Scholar]
- 56. Verbrugge FH, Grieten L, Mullens W.. Management of the cardiorenal syndrome in decompensated heart failure. Cardiorenal Med 2014;4:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]