Abstract
Objectives
To determine factors associated with interindividual variability in tenofovir diphosphate (TFV-DP) concentrations in dried blood spots (DBSs) among persons living with HIV (PLWH).
Methods
PLWH who were at least 18 years old and taking tenofovir disoproxil fumarate-containing ART were prospectively recruited and enrolled from a clinical cohort and followed longitudinally (up to three visits over 48 weeks). With log-transformed TFV-DP concentrations in DBSs as the outcome, mixed-model regression analyses were used to assess associations between self-reported 3 month ART adherence, race and other clinical covariates (gender, age, BMI, CD4+ T cell count, estimated glomerular filtration rate, haematocrit, duration on current ART and anchor drug class) on TFV-DP in DBSs.
Results
Five hundred and twenty-seven participants (1150 person-visits) were analysed. Adjusting for race and other clinical covariates, every 10% increase in self-reported 3 month ART adherence was associated with an average TFV-DP concentration increase in DBSs of 28% (95% CI: 24%–32%; P < 0.0001). In the same model, female participants had 20% (95% CI: 3%–40%; P = 0.02) higher TFV-DP concentrations in DBSs, compared with male participants, and every 1 kg/m2 increase in BMI was associated with a decrease in TFV-DP concentration in DBSs by 2% (95% CI: −3% to −1%; P < 0.0001).
Conclusions
Individual patient characteristics were predictive of TFV-DP concentration in DBSs in PLWH receiving tenofovir disoproxil fumarate-based ART. Future research to incorporate these predictors into the interpretation of this ART adherence biomarker, and to establish whether these associations extend to PLWH taking tenofovir alafenamide-containing ART, is needed.
Introduction
Despite being the main predictor of ART success in persons living with HIV (PLWH), accurately quantifying ART adherence remains a major challenge in HIV care.1,2 Several of the methods that are currently used in clinical practice to assess ART adherence—including self-reporting, pill counts and pharmacy refills—have limitations that usually lead to an overestimation of ART adherence. Similarly, while HIV viral suppression has been traditionally used as a surrogate for ART adherence, the potency of modern ART regimens allows for missed doses, making an undetectable HIV viral load (VL) an imperfect measure of ART adherence.3 Specifically, imperfect ART adherence in the setting of viral suppression has been associated with increased inflammatory markers in PLWH.4
More recently, pharmacological measures of ART adherence that quantify drug concentrations in body fluids, such as plasma and urine, or tissue matrices such as hair and dried blood spots (DBSs), have emerged as informative methods to objectively measure ART adherence. While many of these measures have been associated with virological outcomes, one of their main limitations has been pharmacokinetic variability (driven by multiple factors, including biological, behavioural, social, etc.) that can lead to misinterpretation of ART adherence. Thus, understanding the biological factors that influence these measures may help refine interpretations for clinical practice.
Tenofovir diphosphate (TFV-DP), the phosphorylated anabolite of the NRTI tenofovir, can be quantified in RBCs using DBSs and is a measure of cumulative ART adherence and exposure to tenofovir-based regimens (both to tenofovir disoproxil fumarate and tenofovir alafenamide) given its long intracellular half-life (∼17–20 days) in RBCs.5–7 TFV-DP has been used to quantify ART adherence in PLWH taking ART8–12 and individuals at risk of HIV infection taking pre-exposure prophylaxis.13,14 In PLWH, this ART adherence measure is strongly associated with viral suppression8,9 and is predictive of future viraemia.11 However, while certain individual characteristics have been associated with differences in the concentrations of TFV-DP in DBSs,8 the factors that influence the interindividual variability of this ART adherence biomarker are poorly understood.
To address this gap, we evaluated the clinical, demographic and behavioural characteristics that predict the concentrations of TFV-DP in DBSs in a clinical cohort of PLWH taking tenofovir disoproxil fumarate-based ART.
Methods
Study design and participants
Study participants were PLWH who were prospectively recruited and enrolled at the time of their regular clinical visits at the University of Colorado Hospital (UCH) Infectious Disease Group Practice (IDGP). Eligible participants were at least 18 years old and taking tenofovir disoproxil fumarate-containing ART. No comorbidity exclusions limited enrolment. Each participant completed up to three study visits over 48 weeks and a concurrent blood draw for HIV VL was obtained at each visit as part of their standard of care. Self-reported 3 month ART adherence was also collected at each visit, along with 4–6 mL of whole blood in one EDTA tube for DBS analysis. Time between study visits was variable between participants, but was at least 14 days in order to account for the half-life of TFV-DP in DBSs.8 Enrolment began in June 2014 and the last follow-up visit was completed in July 2017.
Sampling and assay strategy and quantification of TFV-DP in DBSs
Our sampling strategy was a longitudinal extension of a case–control study, where participants who were viraemic (>20 copies/mL) at any study visit acted as cases and those suppressed at all of their study visits acted as controls, as previously described.3,8,11,15,16 Intracellular TFV-DP in DBSs was quantified using previously validated methods.5,7 For each sample, five 25 μL spots of whole blood were pipetted onto a Whatman 903 Protein Saver card. DBS cards were dried at room temperature for at least 2 hours, and up to overnight, before being stored at −80°C until analysis. To quantify TFV-DP, a 3 mm punch was assayed via LC-MS/MS using previously validated methods.17
Self-reported 3 month ART adherence
Using a validated visual analogue scale (VAS),18 participants were asked to self-report their ART adherence over the preceding 3 months. The VAS ranged from 0% to 100%, with tick marks at 10% intervals. If a participant’s response was ambiguous (i.e. between two major tick marks), they were asked to write the specific percentage of ART adherence within that period.
HIV VL
HIV VL was assessed at the UCH Clinical Laboratory with the Roche Cobas 6800 HIV-1 quantitative PCR test. The UCH Clinical Laboratory is certified by the Clinical Laboratory Improvement Amendment of 1988 (CLIA) and has a linear detection range of 20–10 000 000 copies/mL.8
Statistical analyses
TFV-DP concentrations were log-transformed to address right-skewness and data are presented as percentage changes with 95% CI. Mixed-model analyses were used to account for repeated measures. Restricted maximum likelihood was used to assist in identification of the ideal covariance structure. Multiple covariance structures and model fit were compared using the Akaike Information Criterion. Once the covariance structure was chosen, the model was rerun using the maximum-likelihood method in order to gain precision of fixed-effects estimates. Beyond self-reported 3 month ART adherence and self-reported race, the following covariates (selected a priori based on our previous observations)8 were included in our models: age, gender, BMI, estimated glomerular filtration rate [eGFR, using the Modification of Diet in Renal Disease (MDRD) equation], haematocrit (HCT), absolute CD4+ T cell count, ART anchor drug class and duration on current ART. Two groups were used to evaluate associations with TFV-DP. The first group included data from all participants with analysed TFV-DP in DBSs regardless of HIV VL; the second group included just those participants with analysed TFV-DP in DBSs who were virologically suppressed (HIV VL <20 copies/mL) at all of their study visits. All hypothesis tests assumed a significance level of 0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Figures were created using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA).
Ethics statement
This study was approved by the Colorado Multiple Institutional Review Board (COMIRB, protocol 13-2104) and was registered with ClinicalTrials.gov (NCT02012621). Written informed consent was obtained from all participants prior to any study-related procedures.
Results
Demographics and clinical characteristics
Of the 807 participants enrolled, a total of 532 participants had available concentrations of TFV-DP in DBSs and were included in the full group. Of these, 303 participants were included in the suppressed-only group (demographic characteristics of both groups are shown in Table 1). Most participants in both the full and suppressed groups were white (57% and 59%, respectively), male (86% and 83%, respectively), had a BMI between 18.5 and 25 kg/m2 (42% and 40%, respectively), had CD4+ T cell counts >500 cells/mm3 (59% and 70%, respectively) and had been on their current ART for >6 months (82% and 89%, respectively). Median (IQR) self-reported 3 month ART adherence was very high in both the full [98% (90%–100%)] and suppressed [99% (92%–100%)] groups.
Table 1.
Demographic characteristics of the study population
| Variable | Full dataset (N = 532) | Suppressed dataset (N = 303) |
|---|---|---|
| Race, n (%) | ||
| black | 101 (19) | 55 (18) |
| white | 305 (57) | 178 (59) |
| Hispanic | 101 (19) | 56 (19) |
| other | 25 (5) | 14 (5) |
| Gender, n (%) | ||
| male | 456 (86) | 252 (83) |
| female | 76 (14) | 51 (17) |
| Age (years), median (IQR) | 46 (36–52) | 47 (37–53) |
| BMI (kg/m2), n (%) | ||
| <18.5 | 21 (4) | 13 (4) |
| 18.5–25 | 225 (42) | 121 (40) |
| >25–30 | 175 (33) | 105 (35) |
| >30 | 109 (21) | 62 (21) |
| CD4+ T cell count (cells/mm3), n (%) | ||
| <200 | 59 (11) | 11 (4) |
| 200–350 | 79 (15) | 43 (14) |
| >350–500 | 78 (15) | 40 (13) |
| >500 | 316 (59) | 209 (70) |
| eGFR (mL/min/1.73 m2), median (IQR) | 87 (74–102) | 85 (73–101) |
| HCT (%), median (IQR) | 45 (42–47) | 45 (42–47) |
| Duration of current ART, months, n (%) | ||
| <1 | 21 (4) | 4 (1) |
| 1–3 | 44 (8) | 18 (6) |
| >3–6 | 31 (6) | 13 (4) |
| >6 | 436 (82) | 268 (89) |
| Anchor drug class, n (%) | ||
| NNRTI | 141 (27) | 104 (34) |
| b/PI | 132 (25) | 69 (23) |
| INSTI | 192 (36) | 100 (33) |
| multiclass | 67 (13) | 30 (10) |
| PK booster, n (%) | ||
| no | 259 (49) | 174 (57) |
| yes | 273 (51) | 129 (43) |
| HIV VL (copies/mL), median (IQR) | 132 (43–699) | — |
| Self-reported 3 month adherence (%),a median (IQR) | 98 (90–100) | 99 (92–100) |
PK, pharmacokinetic.
A dash represents NA as all participants in the suppressed cohort had an HIV VL <20 copies/mL.
Self-reported 3 month adherence was available from 492 participants in the full dataset and 293 participants in the suppressed dataset.
Predictors of TFV-DP concentration in the full cohort
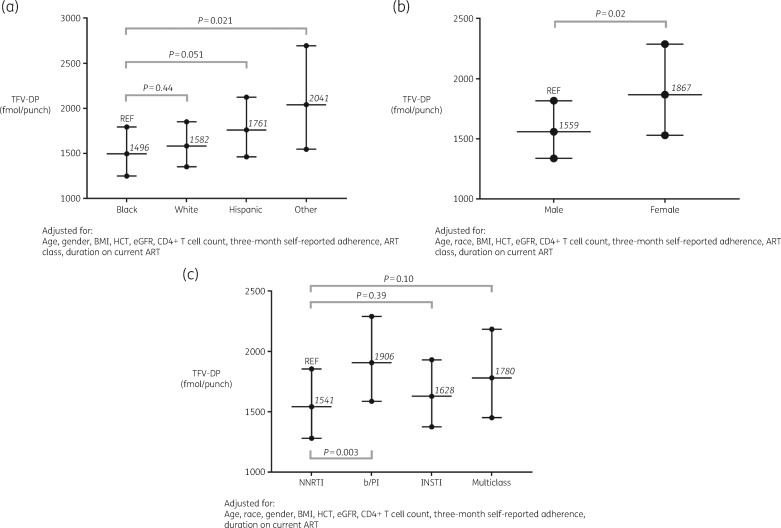
Data from 527 of 532 participants (99%) and 1150 of 1199 person-visits (96%) were included in the mixed-model analysis of the full group dataset. Participants and visits were excluded if covariate values were missing. Table 2 shows the percentage differences in TFV-DP concentration according to each variable in the mixed model. Self-reported 3 month ART adherence was a powerful predictor in the model, where every 10% increase was associated with 28% higher TFV-DP concentration (95% CI: 24%–32%; P < 0.0001). While not statistically significant, when compared with black participants, TFV-DP concentration was an average of 6% higher in white participants (95% CI: −8% to 22%; P = 0.44) and 18% higher in Hispanic participants (95% CI: 0%–39%; P = 0.051). Participants of other races (American Indian/Alaskan Native, Asian, Native Hawaiian/Pacific Islander or unreported) had an average of 36% higher TFV-DP concentration compared with black participants (95% CI: 5%–77%; P = 0.02). Female participants had an average of 20% higher TFV-DP concentration than male participants (95% CI: 3%–40%; P = 0.02). BMI was a strong predictor of TFV-DP concentration, as every 1 kg/m2 increase was associated with a 2% decrease in TFV-DP concentration (95% CI: −3% to −1%; P < 0.0001). Similarly, for every 10 mL/min/1.73 m2 increase in eGFR, TFV-DP concentration decreased by 6% (95% CI: −8% to −4%; P < 0.0001). Every 1% increase in HCT led to a 3% increase in TFV-DP concentration (95% CI: 1%–4%; P < 0.0001). Participants on boosted PI (b/PI)-based ART regimens had 24% higher TFV-DP concentrations compared with those on NNRTI-based regimens (95% CI: 7%–43%; P = 0.003). Figure 1 shows adjusted TFV-DP concentration [geometric mean (GM), 95% CI] according to (a) race, (b) gender and (c) anchor drug class. In order to produce these estimated TFV-DP concentrations, continuous covariates were set to their mean values from the full cohort (age = 45 years, BMI = 26 kg/m2, HCT = 44%, eGFR = 89 mL/min/1.73 m2, CD4+ T cell count = 618 cells/mm3 and self-reported 3 month ART adherence = 92%).
Table 2.
Percentage differences in TFV-DP concentration for full cohort of PLWH taking tenofovir disoproxil fumarate-based ART
| Variable | Percentage difference in TFV-DP concentration (95% CI) | P value |
|---|---|---|
| Race | ||
| black | REF | REF |
| white | 6 (−8 to 22) | 0.437 |
| Hispanic | 18 (0–39) | 0.051 |
| other | 36 (5–77) | 0.021 |
| Gender | ||
| male | REF | REF |
| female | 20 (3–40) | 0.021 |
| Age (every 1 year) | 0 (0–1) | 0.105 |
| BMI (every 1 kg/m2) | −2 (−3 to −1) | <0.0001 |
| CD4+ T cell count (every 50 cells/mm3) | 1 (0–2) | 0.086 |
| eGFR (every 10 mL/min/1.73 m2) | −6 (−8 to −4) | <0.0001 |
| HCT (every 1%) | 3 (1–4) | <0.0001 |
| Duration of current ART, months | ||
| <1 | REF | REF |
| 1–3 | 6 (−34 to 71) | 0.802 |
| >3–6 | 30 (−17 to 104) | 0.247 |
| >6 | 15 (−25 to 78) | 0.522 |
| Anchor drug class | ||
| NNRTI | REF | REF |
| b/PI | 24 (7–43) | 0.003 |
| INSTI | 6 (−7 to 20) | 0.390 |
| multiclass | 15 (−3 to 37) | 0.101 |
| Self-reported 3 month adherence (every 10%) | 28 (24–32) | <0.0001 |
REF, reference.
Figure 1.
Adjusted TFV-DP concentrations by race (a), gender (b) and anchor drug class (c) in the full cohort. Values shown are GM and 95% CI. Covariates were set to mean values: (i) age = 45 years; (ii) BMI = 26 kg/m2; (iii) HCT = 44%; (iv) eGFR = 89 mL/min/1.73 m2; (v) CD4+ T cell count = 618 cells/mm3; and (vi) self-reported 3 month adherence = 92%. REF, reference.
Predictors of TFV-DP concentration in virologically suppressed participants
After reviewing model diagnostics from the full group dataset, we performed an additional analysis focusing only on the participants who were virologically suppressed (HIV VL <20 copies/mL) at all of their study visits. This was intended to minimize variability due to discrepancies between self-reported ART adherence and TFV-DP concentrations (i.e. to minimize the influence of ART adherence over-reporting).19 Data from 300 of 303 participants (99%) and 615 of 627 person-visits (98%) were included in the mixed-model analysis of the suppressed group. Participants and visits were excluded if covariate values were missing. Table 3 shows the percentage differences in TFV-DP concentration according to each variable in the mixed model.
Table 3.
Percentage differences in TFV-DP concentration for the cohort of virologically suppressed (HIV VL <20 copies/mL) PLWH taking tenofovir disoproxil fumarate-based ART
| Variable | Percentage difference in TFV-DP concentration (95% CI) | P value |
|---|---|---|
| Race | ||
| black | REF | REF |
| white | 14 (0–29) | 0.054 |
| Hispanic | 22 (5–42) | 0.011 |
| other | 26 (−2 to 60) | 0.069 |
| Gender | ||
| male | REF | REF |
| female | 36 (19–56) | <0.0001 |
| Age (every 1 year) | 0 (0–1) | 0.083 |
| BMI (every 1 kg/m2) | −2 (−3 to −2) | <0.0001 |
| CD4+ T cell count (every 50 cells/mm3) | 0 (0–1) | 0.892 |
| eGFR (every 10 mL/min/1.73 m2) | −3 (−4 to −1) | 0.005 |
| HCT (every 1%) | 1 (0–2) | 0.009 |
| Duration of current ART, months | ||
| <1 | REF | REF |
| 1–3 | 34 (−15 to 114) | 0.211 |
| >3–6 | 37 (−12 to 111) | 0.159 |
| >6 | 42 (−8 to 118) | 0.109 |
| Anchor drug class | ||
| NNRTI | REF | REF |
| b/PI | 18 (4–34) | 0.009 |
| INSTI | 16 (4–30) | 0.007 |
| multiclass | 24 (5–47) | 0.012 |
| Self-reported 3 month adherence (every 10%) | 9 (5–13) | <0.0001 |
REF, reference.
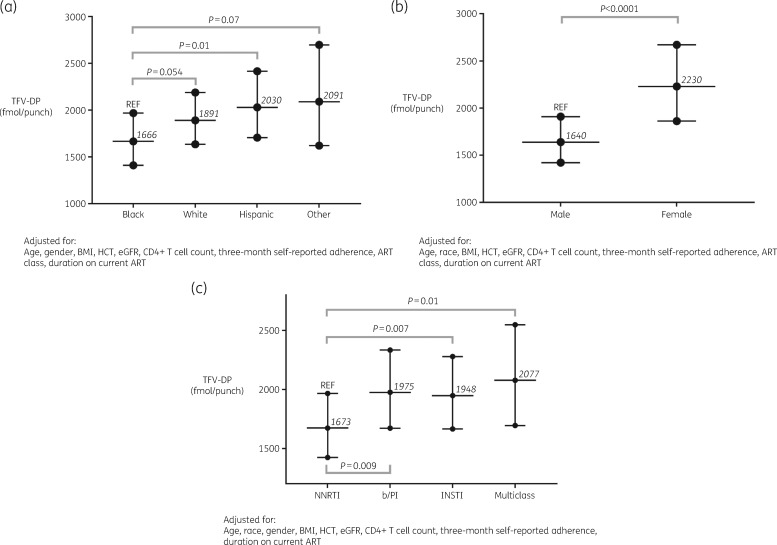
Self-reported 3 month ART adherence remained a strong predictor of TFV-DP concentration, with every 10% increase being associated with a 9% increase in TFV-DP concentration (95% CI: 5%–13%; P < 0.0001). Compared with black participants, white participants had an average of 14% (95% CI: 0%–29%; P = 0.054), Hispanic participants 22% (95% CI: 5%–42%; P = 0.01) and other races 26% (95% CI: −2% to 60%; P = 0.07) higher TFV-DP concentrations. Similar to what was observed in the full model, female participants had 36% higher TFV-DP concentrations than male participants (95% CI: 19%–56%; P < 0.0001); an increase of 1 kg/m2 in BMI was associated with a 2% decrease in TFV-DP concentration (95% CI: −3% to −2%; P < 0.0001); every 10 mL/min/1.73 m2 increase in eGFR resulted in a 3% decrease in TFV-DP (95% CI: −4% to −1%; P = 0.005) and an increase of 1% in HCT was associated with a 1% increase in TFV-DP (95% CI: 0%–2%; P = 0.009). All participants on NNRTI-based ART had lower TFV-DP concentrations in DBSs compared with all other anchor drug classes. Specifically, participants on b/PI-based ART had 18% (95% CI: 4%–34%, P = 0.009) higher TFV-DP concentrations, while participants on integrase strand transfer inhibitor (INSTI)-based ART had 16% (95% CI: 4%–30%; P = 0.007) higher and those on multiclass-based ART had 24% (95% CI: 5%–47%; P = 0.012) higher TFV-DP concentrations in DBSs compared with those on NNRTIs, respectively. A subanalysis comparing TFV-DP concentrations at baseline among virologically suppressed participants on INSTI-based ART showed no significant difference between boosted (elvitegravir/cobicistat) versus unboosted (raltegravir or dolutegravir) regimens (data not shown). Figure 2 shows adjusted TFV-DP concentrations (GM, 95% CI) according to (a) race, (b) gender and (c) anchor drug class. In order to produce these estimated TFV-DP concentrations, continuous covariates were set to their mean values from the suppressed cohort (age = 46 years, BMI = 27 kg/m2, HCT = 45%, eGFR = 88 mL/min/1.73 m2, CD4+ T cell count = 694 cells/mm3 and self-reported 3 month ART adherence = 95%).
Figure 2.
Adjusted TFV-DP concentrations by race (a), gender (b) and anchor drug class (c) in the suppressed cohort. Values shown are GM and 95% CI. Covariates were set to mean values: (i) age = 46 years; (ii) BMI = 27 kg/m2; (iii) HCT = 45%; (iv) eGFR = 88 mL/min/1.73 m2; (v) CD4+ T cell count = 694 cells/mm3; and (vi) self-reported 3 month adherence = 95%. REF, reference.
Discussion
In this study we determined the influence of several demographic, behavioural and clinical characteristics on the concentrations of TFV-DP in DBSs in a clinical cohort of PLWH receiving tenofovir disoproxil fumarate. In the full model, significant associations were observed in drug concentrations according to self-reported ART adherence, race (black versus other), gender, BMI, HCT, eGFR and concomitant ART type (b/PI versus NNRTI). Most of these covariates retained significance in the suppressed model, while race and concomitant ART became significant (e.g. race became significant for Hispanic versus black participants, and all other concomitant ART classes were associated with higher TFV-DP concentrations than NNRTIs), possibly by reducing the effect of ART adherence in this subset. Our findings for self-reported ART adherence, gender, BMI, HCT and eGFR allowed us to calculate custom estimates of TFV-DP concentration. For instance, using the full model, the GM TFV-DP concentration was 1541 (95% CI: 1280–1854) fmol/punch for a 45-year-old participant on NNRTI-based ART, with BMI = 26 kg/m2, HCT = 44%, eGFR = 89 mL/min/1.73 m2, CD4+ T cell count = 618 and self-reported 3 month ART adherence = 92%.
As previously observed in PLWH and healthy volunteers,7,8 male participants, black participants and PLWH taking NNRTI-based ART had lower TFV-DP concentrations and this anabolite was inversely related to eGFR and BMI. We also established the contribution of ART adherence (by self-reporting) on TFV-DP concentration, both in PLWH overall and in those who were virologically suppressed. The association between self-reported ART adherence and TFV-DP concentration established in this study extends our previous findings in healthy volunteers, where the drug concentrations were determined for different tenofovir disoproxil fumarate adherence categories in the setting of directly observed therapy.7 Given the inability of randomizing PLWH to variable levels of ART adherence, our results provide useful information on what would be the expected changes in TFV-DP concentration when assessing a specific behaviour (i.e. ART adherence) in the clinic. For example, if a clinician were to know the average concentration of TFV-DP in the DBSs from a patient that reports 80% ART adherence, this could trigger an informed provider–patient discussion aimed at improving ART adherence to reach a goal concentration associated with higher odds of viral suppression8 or lower odds of future viraemia.11 In this context, the TFV-DP concentration in a DBS would translate into a measure of behaviour and pharmacokinetics that can be simultaneously assessed in one single test, quantifying both ART adherence and exposure.
The gender differences in TFV-DP concentration observed in our cohort are consistent with prior observations in a directly observed therapy study of tenofovir disoproxil fumarate/emtricitabine in healthy volunteers, where women had 17% higher concentrations compared with men.7 Higher TFV-DP concentrations in DBSs in women living with HIV were also observed previously in this cohort at the enrolment visit,8 but these results have now been extended using a repeated-measures analysis. While the mechanisms behind these findings remain unknown, they are consistent with previous studies on NRTIs—including tenofovir—in PBMCs and other cell lines.20–23 However, other studies of TFV-DP in PBMCs have not observed gender differences,24 suggesting more research is needed to settle this issue. These differences could also be driven by lower BMI and slower GFR in women,25 although we adjusted for these variables in our analyses. Similarly, recent studies have proposed that sex hormones could differentially affect kinase activity for specific nucleoside analogues,23,26,27 although these results have only been derived from in vitro analyses.
The influence of race and ethnicity on TFV-DP concentration in DBSs is also an important finding worth additional discussion. While statistical significance was borderline in this study, lower concentrations of TFV-DP in DBSs have been previously observed in black PLWH8–10,12 and a trend towards lower concentrations was observed in healthy volunteers.7 These findings are opposite to previous studies for other NRTIs, where higher intracellular concentrations of zidovudine triphosphate and lamivudine triphosphate in PBMCs were observed in African Americans.28 Another study found no difference in TFV-DP in PBMCs by race.24 The reasons behind these findings remain unclear, but among them are the possible influence of pharmacogenetic differences that can have an impact on the intracellular disposition of phosphorylated NRTI anabolites, such as the differential expression of MDR protein 4 (MRP4),29,30 nucleoside transporters (e.g. ENT-1)31 and/or phosphorylating kinases.32 Interestingly, these racial differences can also be observed within gender, as lower TFV-DP concentrations in DBSs were previously observed in two cohorts of African women living with HIV (median TFV-DP concentration of 961 and 931 fmol/punch, respectively)9,12 versus women with HIV living in the USA (median TFV-DP concentration of 1874 fmol/punch),10 a finding potentially driven by the presence of white and Hispanic women, and women taking b/PIs, in the US cohort. However, the concentrations of TFV-DP in black women in the USA were still higher than those in African women, suggesting that ART adherence differences and/or additional factors (e.g. dietary influences, social determinants of health, etc.) could explain these findings.
The influence of HCT on TFV-DP concentration in DBSs is expected as the assay measures TFV-DP concentration in RBCs.33–35 However, in previous validation studies among HIV-negative volunteers, an HCT of 42 was associated with 11.9 million RBCs per 3 mm DBS punch and this estimate was acceptable (<13% different) for the HCT range of 35% to 50%.17 Conversely, in one study among PLWH taking tenofovir disoproxil fumarate, an association with HCT was observed.36 A possible explanation of the discrepancies regarding the association of HCT with TFV-DP in PLWH versus healthy volunteers is the wider range of HCT observed in our cohort (28%–56% versus 35%–49%7), which is reflective of a population with chronic diseases and comorbidities.
Other clinical characteristics such as BMI, eGFR and the type of ART were also predictive of TFV-DP concentration in DBSs, particularly in the subset of participants who were virologically suppressed. While these findings were not unexpected, based on previous reports of tenofovir in plasma37 and hair,38 and of TFV-DP in PBMCs21 and DBSs,36 they suggest that—even when ART adherence is sufficient to maintain viral suppression—several biological characteristics can influence drug exposure, which could potentially impact viral outcomes.8,11 For instance, BMI and eGFR had a similar effect on the concentrations of TFV-DP (i.e. ∼2%–3% per unit change). This inverse association with BMI raises the concern about whether significant weight gain, now a matter of concern for PLWH taking INSTI-based ART,39 could adversely impact tenofovir exposure independently of a change in ART adherence and could reduce pharmacokinetic forgiveness and increase the odds of future viraemia.11 Conversely, a decrease in eGFR leading to a higher TFV-DP concentration could be associated with a higher rate of adverse events and/or additional unrecognized drug toxicity, raising questions about whether TFV-DP in DBSs could be a biomarker to help predict adverse events or if dose modification should be entertained, particularly since generic formulations of tenofovir disoproxil fumarate have become available and this drug is part of the NRTI backbone for the international rollout of modern ART.40 Finally, the marked differences in concentrations of TFV-DP in PLWH being treated with NNRTI-based versus b/PI-based or INSTI-based regimens raise the concern about whether specific patient characteristics that also are associated with lower drug concentrations (i.e. black race, high BMI and male gender) should play a role in the initial selection of ART in this population.
Our study has several strengths that should be outlined. Among them are the large and diverse sample size obtained from a ‘real-life’ clinical cohort, the robust repeated-measures analysis of longitudinal data and the comprehensive set of demographic and clinical variables assessed. Limitations include the non-directly observed dosing and the potential for overestimation of self-reported ART adherence,1 although our secondary analysis in the subset of participants who were virologically suppressed confirmed that our findings are applicable even in PLWH with relatively high ART adherence. In addition, our assay strategy restricted the number of participants in whom drug concentrations were quantified, although our previous analyses have not demonstrated a significant influence of this approach.8,11,15 Furthermore, we did not investigate the influence of potential drug–drug interactions on TFV-DP concentrations, as our cohort did not capture detailed data on concomitant medications. Finally, our population was restricted to PLWH taking tenofovir disoproxil fumarate, which has been almost universally replaced by tenofovir alafenamide in clinical practice. However, a recent study in healthy volunteers established the benchmark concentrations of TFV-DP in DBSs during directly observed tenofovir alafenamide/emtricitabine therapy,6 providing the foundation to extend its application in PLWH in the near future.
In conclusion, we demonstrated that demographic, biological and behavioural characteristics were associated with the variability of TFV-DP concentrations in DBSs in PLWH taking tenofovir disoproxil fumarate. Prospective studies are needed to determine how much more precision is gained in ART adherence interpretations when these covariates are accounted for. We hypothesize that understanding the individual influence that each of these factors exerts on this ART adherence biomarker will facilitate interpretation of drug concentrations in clinical practice and research studies. Further research to account for these patient-related factors in PLWH taking tenofovir alafenamide is also required.
Acknowledgements
We would like to thank all study participants and personnel at the Colorado Antiviral Pharmacology Laboratory for their invaluable assistance and support of this study. We would also like to thank the director of the UCH-HIV programme (Steven Johnson, MD), the medical assistants (Nancy Olague, Brittany Limon, Ariel Cates, Maureen Sullivan and Missy Sorrell) and the nursing staff (Joslyn Axinn, Jackie Deavers and Ann Czyz) at the UCH-IDGP for their invaluable contributions and support of this study.
Funding
This work was supported by the National Institutes of Health (K23 AI104315 to J.R.C.M.; R01 AI122298 to P.L.A.).
Transparency declarations
The lead author affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. P.L.A. accepted consulting fees from Gilead Sciences. P.L.A. receives support (paid to their institution) for investigator-initiated studies from Gilead Sciences. All other authors: none to declare.
References
- 1. Castillo-Mancilla JR, Haberer JE.. Adherence measurements in HIV: new advancements in pharmacologic methods and real-time monitoring. Curr HIV/AIDS Rep 2018; 15: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nachega JB, Marconi VC, van Zyl GU. et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets 2011; 11: 167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castillo-Mancilla J, Coyle R, Coleman S. et al. Cascade of ART adherence in virologically-suppressed persons living with HIV. AIDS Res Hum Retroviruses 2019; doi:10.1089/AID.2019.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castillo-Mancilla JR, Brown TT, Erlandson KM. et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016; 63: 1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castillo-Mancilla JR, Zheng JH, Rower JE. et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29: 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yager J, Castillo-Mancilla JR, Ibrahim ME. et al. Tenofovir diphosphate in dried blood spots following escalating TAF/FTC dosing. CROI2019, Seattle, WA, USA, 2019. Abstract 463.
- 7. Anderson PL, Liu AY, Castillo-Mancilla JR. et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62: e01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castillo-Mancilla JR, Morrow M, Coyle RP. et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2019; 68: 1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phillips TK, Sinxadi P, Abrams EJ. et al. A comparison of plasma efavirenz and tenofovir, dried blood spot tenofovir-diphosphate, and self-reported adherence to predict virologic suppression among South African women. J Acquir Immune Defic Syndr 2019; 81: 311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castillo-Mancilla JR, Searls K, Caraway P. et al. Short communication: tenofovir diphosphate in dried blood spots as an objective measure of adherence in HIV-infected women. AIDS Res Hum Retroviruses 2015; 31: 428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrow M, MaWhinney S, Coyle RP. et al. Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. J Infect Dis 2019; 220: 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warne P, Robbins R, Anderson P. et al. Utility of dried blood spot-derived ARV biomarkers as an objective measure of treatment adherence in South Africa. IAPAC Adherence, Miami, FL, USA, 2015. Abstract 241.
- 13. Liu AY, Cohen SE, Vittinghoff E. et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grant RM, Anderson PL, McMahan V. et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14: 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coyle RP, Schneck CD, Morrow M. et al. Engagement in mental health care is associated with higher cumulative drug exposure and adherence to antiretroviral therapy. AIDS Behav 2019; 23: 3493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frasca K, Morrow M, Coyle RP. et al. Emtricitabine triphosphate in dried blood spots is a predictor of viral suppression in HIV infection and reflects short-term adherence to antiretroviral therapy. J Antimicrob Chemother 2019; 74: 1395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng JH, Rower C, McAllister K. et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giordano TP, Guzman D, Clark R. et al. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials 2004; 5: 74–9. [DOI] [PubMed] [Google Scholar]
- 19. Simoni JM, Kurth AE, Pearson CR. et al. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006; 10: 227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson PL, Kakuda TN, Kawle S. et al. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 2003; 17: 2159–68. [DOI] [PubMed] [Google Scholar]
- 21. Lahiri CD, Tao S, Jiang Y. et al. Impact of protease inhibitors on intracellular concentration of tenofovir-diphosphate among HIV-1 infected patients. AIDS 2015; 29: 1113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pruvost A, Negredo E, Théodoro F. et al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother 2009; 53: 1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James AM, King JR, Ofotokun I. et al. Uptake of tenofovir and emtricitabine into non-monocytic female genital tract cells with and without hormonal contraceptives. J Exp Pharmacol 2013; 5: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seifert SM, Chen X, Meditz AL. et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady-state. AIDS Res Hum Retroviruses 2016; 32: 981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ofotokun I, Chuck SK, Hitti JE.. Antiretroviral pharmacokinetic profile: a review of sex differences. Gend Med 2007; 4: 106–19. [DOI] [PubMed] [Google Scholar]
- 26. Anderson PL, King T, Zheng J-H. et al. Cytokine and sex hormone effects on zidovudine-and lamivudine-triphosphate concentrations in vitro. J Antimicrob Chemother 2008; 62: 738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia M, Cabezas J, Perez-Gonzalez M.. Effects of oestradiol, testosterone and medroxyprogesterone on subcellular fraction marker enzyme activities from rat liver and brain. Comp Biochem Physiol B 1985; 80: 347–54. [DOI] [PubMed] [Google Scholar]
- 28. Rower JE, Meditz A, Gardner EM. et al. Effect of HIV-1 infection and sex on the cellular pharmacology of the antiretroviral drugs zidovudine and lamivudine. Antimicrob Agents Chemother 2012; 56: 3011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson PL, Lamba J, Aquilante CL. et al. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr 2006; 42: 441–9. [DOI] [PubMed] [Google Scholar]
- 30. Abla N, Chinn LW, Nakamura T. et al. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther 2008; 325: 859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seifert SM, Chen X, Clayton CW. et al. Transporter genetics and TFV-DP/FTC-TP cellular pharmacology in vivo. Twenty-Third Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2016. Abstract 445.
- 32. Lade JM, To EE, Hendrix CW. et al. Discovery of genetic variants of the kinases that activate tenofovir in a compartment-specific manner. EBioMedicine 2015; 2: 1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Capiau S, Wilk LS, De Kesel PM. et al. Correction for the hematocrit bias in dried blood spot analysis using a nondestructive, single-wavelength reflectance-based hematocrit prediction method. Anal Chem 2018; 90: 1795–804. [DOI] [PubMed] [Google Scholar]
- 34. Wilhelm AJ, den Burger JC, Swart EL.. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet 2014; 53: 961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Velghe S, Delahaye L, Stove CP.. Is the hematocrit still an issue in quantitative dried blood spot analysis? J Pharm Biomed Anal 2019; 163: 188–96. [DOI] [PubMed] [Google Scholar]
- 36. Seifert SM, Castillo-Mancilla JR, Erlandson K. et al. Brief report: adherence biomarker measurements in older and younger HIV-infected adults receiving tenofovir-based therapy. J Acquir Immune Defic Syndr 2018; 77: 295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cattaneo D, Minisci D, Baldelli S. et al. Effect of cobicistat on tenofovir disoproxil fumarate (TDF): what is true for TAF may also be true for TDF. J Acquir Immune Defic Syndr 2018; 77: 86–92. [DOI] [PubMed] [Google Scholar]
- 38. Gandhi M, Gandhi RT, Stefanescu A. et al. Cumulative antiretroviral exposure measured in hair is not associated with measures of HIV persistence or inflammation among individuals on suppressive ART. J Infect Dis 2018; 218: 234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bourgi K, Rebeiro PF, Turner M. et al. Greater weight gain in treatment naïve persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2019; doi:10.1093/cid/ciz407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorward J, Hamers RL.. Dolutegravir in sub-Saharan Africa: context is crucial. Lancet HIV 2019; 6: e72–3. [DOI] [PubMed] [Google Scholar]