Abstract
Acute heart failure (AHF) continues to be a substantial cause of illness and death, with in-hospital and 3-month mortality rates of 5% and 10%, respectively, and 6-month re-admission rates in excess of 50% in a range of clinical trials and registry studies; the European Society of Cardiology (ESC) Heart Failure Long-Term Registry recorded a 1-year death or rehospitalization rate of 36%. As regards the short-term treatment of AHF patients, evidence was collected in the ESC Heart Failure Long-Term Registry that intravenous (i.v.) treatments are administered heterogeneously in the critical phase, with limited reference to guideline recommendations. Moreover, recent decades have been characterized by a prolonged lack of successful innovation in this field, with a plethora of clinical trials generating neutral or inconclusive findings on long-term mortality effects from a multiplicity of short-term interventions in AHF. One of the few exceptions has been the calcium sensitizer and inodilator levosimendan, introduced 20 years ago for the treatment of acutely decompensated chronic heart failure. In the present review, we will focus on the utility of this agent in the wider context of i.v. inotropic and inodilating therapies for AHF and related pathologies.
Keywords: Acute heart failure, Advanced heart failure, Inotropes, Inodilators, Levosimendan, Cardiogenic shock, Right ventricular failure
Introduction
The use of intravenous (i.v.) inotropes to correct haemodynamic dysfunction in patients with decompensated heart failure is still a frequent feature of the medical response. The impact of these interventions on prognosis is not, however, consistently affirmative. Indeed, the data sometimes associate use of catecholamines and phosphodiesterase inhibitors with an increase in mortality risk.1,2 Proposed explanations for these observations include increased cardiomyocyte oxygen consumption in already ischaemically jeopardized myocardium, tachycardia, and an increased risk of cardiac arrhythmias.
The calcium sensitizer and potassium-channel opener levosimendan has been described over the past two decades as a safer inotropic option than traditional classes of drugs in these settings.1,2 As an inodilator that: (i) promotes contractility by augmenting the sensitivity of cardiomyocyte troponin C to ionic intracellular calcium and (ii) exerts vasodilatory and cardioprotective effects through the opening of adenosine triphosphate-dependent potassium (KATP) channels in vascular smooth muscle cells and mitochondria, levosimendan promotes inotropy via a broadly energy-neutral route. In addition, its inodilator profile combines an increase in cardiac output with vasodilating effects that include reduction of central venous pressure and pulmonary capillary wedge pressure, distinct effects on the renal vasculature and relief of hepatic congestion.3 Taken in combination with an extended duration of effect due to the formation of a long-acting metabolite designated OR-1896, this pharmacology identifies levosimendan as a notable asset for the management of acute heart failure (AHF) (Table 1).4,5
Table 1.
Comparison of key pharmacological and haemodynamic features of adrenergic drugs, the phosphodiesterase inhibitor milrinone and the calcium sensitizer levosimendan as they relate to their use as inotropes or inodilators in the treatment of heart failure4

|
The successful development and deployment of levosimendan in these indications marks it as a relative rarity in a field of cardiovascular medicine in which there is a perceived strong need for innovation but which has recorded a substantial number of setbacks, disappointments, and failures in recent decades. Some of the conceptual, procedural and technical obstacles that have frustrated developments in this area have been the subject of recent discussion papers.6,7 Summarized in the briefest terms, it may be said that future success in the development of effective therapies requires a recognition that this is ‘a heterogeneous syndrome in which functional and structural biomarkers change dynamically during disease progression in a patient-specific fashion’.8 Given the multiple possible origins of AHF, it is perhaps not surprising that in-hospital endpoints do not always correlate with long-term clinical endpoints and that so many recent clinical trials have produced disappointing results.6,7 Responses to these frustrations should include: implementation of new technologies for precision phenotyping; highly granular characterization of pathophysiological targets; and the adoption of different trial designs and concepts, favouring, for example, the platform trial over the classic twin-arm placebo-controlled trial.9
These remarks notwithstanding, levosimendan has been evaluated in several hundred clinical trials in a broad range of therapeutic settings. Experience in all those areas has been subjected to multiple meta-analyses, 31 of which have been conducted in just the past 3 years. In every instance, levosimendan has been associated with a favourable impact on the outcome(s) under consideration, although not always with a statistically significant effect.10
Against that background, we set out in this short review some views on the place and role of vasoactive drugs in the management of AHF, with an emphasis on levosimendan and some practical considerations.
Intravenous cardio-vasoactive therapies in acute heart failure
The negative effect on survival observed with various classes of traditional inotropes has already been mentioned. It must be acknowledged, however, that adverse outcomes may be related not only to their inherent limitations and dose-dependent adverse effects (e.g. increase in myocardial oxygen demand, Ca2+ overload in myocardial cells, apoptosis, etc.) but also to their inappropriate use. Registry data identify quite high rates of usage for inotropes in patients with normal or even increased systolic blood pressure.11 The correct identification of patients who are suitable candidates for inotropic or inodilator therapy is thus a cardinal consideration for their successful use.
The assessment and management of AHF have been comprehensively documented in the 2016 guidelines of the European Society of Cardiology (ESC),12 which should be regarded as the current definitive guide on that subject. Working from that source, and with insights from our own experience, we have developed a three-step guide to the use of inotropes in AHF (Figure 1).5
Figure 1.
Practical considerations guiding the use and selection of inotropes in acute heart failure. Data from Farmakis et al.5
Points for consideration in this framework include the importance of understanding causative pathophysiology in order to assign treatments appropriately. For example, AHF caused by vasoconstriction with increased venous return, raised left ventricular pressure and fluid redistribution leading to pulmonary congestion is a presentation calling primarily for vasodilators. In practice, these drugs seem to be underused13 just as inotropes appear to be overused. Inotrope therapy is properly confined to AHF arising from low cardiac output with compensatory (but excessive) neurohormonal activation and sodium and water retention manifesting as peripheral and pulmonary congestion. Those patients represent a smaller proportion of the overall population than some inotrope-prescribing data might suggest.
Within that qualifying population, however, levosimendan figures prominently in the reply to the question ‘Which inotrope is the most appropriate?’ (Figure 1). These assessments are not, of course, unrestricted endorsements for the use of levosimendan (or any other specified inotrope) but they represent our judgement of suitability based on the balance of the available evidence, as set out in the remainder of this essay.
Earlier remarks about the successful future treatment of AHF involving the correct matching of the patient’s circumstances and pathophysiology to the intervention(s) used are prefigured in several large trials of levosimendan and have helped to shape some of our views. Of note in this context is the observation that, in the sub-population of the SURVIVE study treated in Finland, levosimendan was associated with a substantially lower 180-day mortality than dobutamine (17% vs. 40%; P = 0.023).14 That significant survival benefit has been ascribed to the fact that those patients were more likely to have been treated with beta-blockers (88% vs. 52%; P < 0.0001), had their study treatment started earlier (41 ± 40 vs. 81 ± 154 h; P < 0.0001) and were more likely to have an admission diagnosis of acute myocardial infarction (MI) (39% vs. 16%; P < 0.0001).
Patients on chronic beta-blocker therapy
Patients already using beta-blockers—and this applies nowadays to a large proportion of patients with acute-on-chronic heart failure—can be expected to have better haemodynamic responses to levosimendan or milrinone than to adrenergic inotropes because both those drugs promote inotropy independently of the stimulation of beta-adrenergic receptors and the downstream cAMP pathway. Levosimendan is now considered a first-choice inotropic therapy if beta-blockade is thought to be contributing to hypotension with subsequent hypoperfusion (Class IIb, evidence level C).12,15
In the OPTIME-CHF trial in patients with decompensated ischaemic heart failure, there was evidence for an aetiology-specific deleterious impact of milrinone therapy. Specifically, milrinone-treated patients with ischaemic aetiology and without true hypoperfusion tended to have worse outcomes than placebo-treated patients on the composite outcome of death or rehospitalization at 60 days (42% vs. 36%; P = 0.01).16 In contrast, levosimendan has been associated with a consistent trend towards improved survival in various meta-analyses.17 These differences speak to the need to assign the right drug to the right patient and for the choice of intervention to be guided by pathophysiology and clinical evidence. In the instance of decompensated ischaemic heart failure, our favoured options are levosimendan and dobutamine.18
Changes in the functionality of cardiac beta-1 adrenoceptors is another aspect of heart failure that needs to be considered. Pronounced activation of the sympathetic system leads to down-regulation and desensitization of beta-adrenergic receptors and a diminished adrenoceptor-mediated contractile response in some circumstances.19 The ability of levosimendan to promote inotropy in such circumstances may be relevant to drug selection. The 2016 ESC guidelines emphasize that beta-blocker therapy should if possible be maintained during treatment of AHF. For AHF patients on beta-blockers an inotrope that acts as an agonist at beta-adrenergic receptors is not a rational choice of therapy.
Cardiogenic shock arising from acute coronary syndrome
Cardiogenic shock (CS) is a particular aspect of AHF with ominous prognosis and increased mortality. Many of these cases emerge in the context of an episode of acute coronary syndrome or acute decompensation of chronic heart failure. CS is associated with significant derangement of tissue perfusion, resulting in a vicious circle of progressive interrelated multiorgan dysfunction that can be fatal if appropriate and timely interventions are not forthcoming.
CS is identified in the 2016 ESC guidelines as a condition warranting consideration of inotrope use12 and there is, in general, widespread use of inotropes, vasopressors, and other classes of drugs.20 There is limited reliable information regarding the comparative efficacy of different agents,15,21 however, and many current practices are often empirical and pragmatic, possibly resting as much on custom and practice as on robust evidence. Broad principles of therapy may nevertheless be identified.
Firstly, successful management of CS requires rigorous (and urgent) focus on aetiology in parallel with or immediately after haemodynamic and respiratory stabilization. Haemodynamic support with inotropes and vasopressors may often take a subordinate or subsidiary role to interventions such as percutaneous coronary intervention or mechanical circulatory support with temporary ventricular assist devices or extracorporeal membrane oxygenation (ECMO), and permanent mechanical circulatory support [e.g. a left ventricular assist device (LVAD)] or heart transplantation as possible longer-term options.22
Inotropes, as a broad class, are endorsed to support the circulation of patients who are demonstrably hypotensive and/or hypoperfused despite adequate filling pressures. This is a conservative or precautionary indication that reflects concerns that adrenergic inotropes [and phosphodiesterase (PDE)-III inhibitors] may increase cellular energy demands and oxygen consumption in a situation of ischaemic compromise. These agents may also exert undesirable influences on heart rate and/or rhythm, to the detriment of patients. Because of its calcium-sensitizing action, levosimendan does not provoke similar untoward responses. Moreover, it has anti-stunning and pre-conditioning effects that may be advantageous. Data from a meta-analysis of six studies involving 1065 patients with AHF/CS documented improvements in various indices of haemodynamic function in acute coronary syndrome patients treated with i.v. levosimendan and did not identify an adverse effect on mortality.23 That finding is compatible with the survival benefit of levosimendan in the RUSSLAN trial (in patients with left ventricular failure due to an acute MI).24 Of note also in this respect is that, in the subset of patients in the SURVIVE trial who had acute MI as a cause of AHF, the absolute mortality rate among patients who received i.v. levosimendan was 4% lower than in the dobutamine-treated comparators (28% vs. 32%). That difference was not statistically significant, perhaps due to limited numbers, but is a noteworthy outcome.15
It has been proposed that levosimendan may be considered in four clinical AHF/CS scenarios based on a patient’s haemodynamic status and Killip classification.15 In the lower Killip classes, and in patients with relatively well-sustained blood pressure (systolic >110 mmHg), levosimendan may be used as monotherapy to enhance urinary output if the response to diuretics is inadequate or to overcome the effects of ongoing beta-blockade. In the more advanced stages, it should be combined with a vasopressor to augment cardiac output and support blood pressure. All i.v. inotropes and vasopressors should be used at the lowest effective dose and for the shortest possible time.
When vasopressors are used to support blood pressure, noradrenaline should be preferred to adrenaline, based on the findings of the OPTIMA-CC trial, in which adrenaline induced excessive refractory heart failure,25 and findings from the SOAP-II trial.26 Although no definitive randomized trials have been conducted which focus directly on the arrhythmogenic effect of positive inotropes, it is known from real-world experience that the beta-adrenergic drugs dopamine, dobutamine, and adrenaline have a greater propensity to trigger fatal and non-fatal arrhythmias than agents acting via alternative mechanisms, such as PDE inhibition or calcium sensitization.
Supplementing a vasopressor with an inodilator may merit consideration. This proposition is based on a propensity score-matched analysis from three observational studies by the GREAT network, and therefore requires confirmation in a suitably powered randomized controlled trial.27 Nevertheless, the scale of the benefit achieved with a (vasopressor + vasodilating inotrope) combination over a vasopressor-only intervention is notable (hazard ratio 0.66, 95% confidence interval 0.55–0.80). Subject to confirmation from controlled trials, these and related observations28,29 hint at a possible role for levosimendan in combination with noradrenaline as an alternative to dobutamine or PDE inhibitors.
These positive sentiments notwithstanding, it has to be acknowledged that there are currently no large, high-quality studies of levosimendan in CS that provide convincing evidence of survival benefit21,30,31 and that there are various obstacles to its use in that setting, notably the potential for systemic arterial hypotension. In addition, the long half-life may make it difficult to reverse vasodilatation once it has occurred.
A new randomized controlled trial (LevoHeartShock, NCT04020263) is in progress that is designed to compare the effect of levosimendan vs. placebo on top of conventional dobutamine inotropic therapy on a combined morbidity–mortality endpoint in patients with CS requiring noradrenaline pressor therapy. A guiding principle of this study, which is scheduled to recruit 610 patients, is that the early use of levosimendan, by enabling the discontinuation of dobutamine, will accelerate the resolution of signs of low cardiac output and facilitate myocardial recovery. This may be seen as an acknowledgement that at least some of any benefit derived from levosimendan may arise from the substitution of a more deleterious treatment (dobutamine).
As a further example of the differential effects of inotropes, there is evidence to suggest that patients who develop CS after cardiac surgery and require temporary extracorporeal life support and inotrope support for weaning may benefit more from levosimendan than milrinone.21 Preliminary studies, including a case series, indicate that levosimendan may facilitate weaning of patients with CS from veno-arterial ECMO support32–34 (Figure 2), thereby reducing the potential for development of pulmonary oedema. The need for high-dose conventional inotropes may also be reduced.33 These findings, while theoretically attractive, are, however, in need of thorough evaluation and essential practical aspects, such as the timing and duration of treatment and the optimal dosage, remain to be established.
Figure 2.
Kaplan–Meier analysis of the impact of addition of levosimendan therapy (vs. no levosimendan) to standard-of-care measures on survival rates for patients being weaned from intensive care veno-arterial extracorporeal membrane oxygenation. From a cohort of 150 patients, 42 of 51 were successfully weaned in the levosimendan group vs. 61 of 99 in the non-levosimendan group (P = 0.01). After Vally et al.34
In a complementary study in 64 patients with post-cardiotomy cardiac failure, use of levosimendan rather than milrinone was associated with a higher rate of successful weaning from extracorporeal life support (92% vs. 79%; P = 0.18) and a significantly reduced requirement for an intra-aortic balloon pump to aid weaning (7.7% vs. 15.40%, P = 0.008). No significant differences were recorded in inter-group noradrenaline requirements or in death rates at 28 or 180 days (35% vs. 40%; P = 0.28 and 50% vs. 44%; P = 0.80, respectively).35 Further insights into these matters may emerge from the Weanilevo trial (Interest of Levosimendan in Reducing Weaning Failures of ExtraCorporeal Life Support; NCT04158674).
Takotsubo cardiomyopathy
Catecholamines appear to play a central pathophysiological role in the pattern of temporary left ventricular dysfunction in Takotsubo cardiomyopathy. Accordingly, the use of adrenergic inotropes or PDE inhibitors should be regarded as contraindicated on the grounds that further amplification of adrenergic processes may worsen the clinical status and prognosis of patients. Owing to its non-adrenergic mode(s) of action, levosimendan has been advocated as a first-choice inotropic intervention when mechanical circulatory assist devices are not available,36,37 and it may be preferable to the introduction of beta-blockers. For the moment, however, this approach is supported primarily by the pathophysiological rationale, inferences from sophisticated preclinical models and encouraging case reports rather than outcomes data from robust clinical trials. Ivabradine may be an appropriate alternative in cases characterized by excessive sinus tachycardia.38
Right ventricular failure and/or pulmonary hypertension
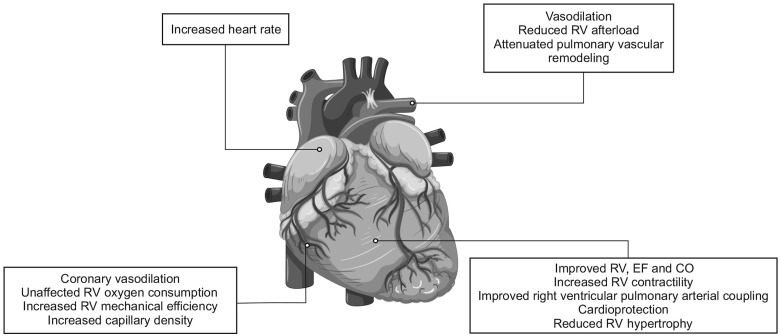
In patients with right ventricular (RV) failure and/or pulmonary hypertension (PH), we are disposed towards inodilatation with milrinone or levosimendan for inotropic support, due to their vasodilatory effects on the pulmonary vasculature (Figure 3).39,40 A material consideration is that levosimendan improves cardiac output in patients with left heart failure without increasing biventricular oxygen consumption, leading to an improvement in RV mechanical efficiency.41
Figure 3.
Outline of the main cardiovascular effects of levosimendan in pulmonary hypertension and right heart failure. CO, cardiac output; EF, ejection fraction; PH, pulmonary hypertension; RV, right ventricle. Rendition of figure from Hansen et al.48
It should be noted at the outset that this assessment applies primarily to Type 1 PH (pulmonary arterial hypertension) and Type 2 PH (PH due to left heart disease). The latter accounts for two-thirds or more of cases,42 making it by far the most significant presentation.
A recent meta-analysis of 10 studies of levosimendan in acute right heart failure identified statistically robust benefits over placebo, with reductions in systolic pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR) (P ≤ 0.003 for both), plus a non-significant trend towards lower PAP. Tricuspid annular plane systolic excursion (TAPSE) and ejection fraction (EF) were significantly augmented (P < 0.002 and P < 0.003, respectively).43
In investigations by Yilmaz et al.,44 open-label levosimendan was found to exert more favourable effects than dobutamine (also open-label) in 40 patients with acutely decompensated systolic heart failure and moderate-to-severe RV dysfunction. Both treatments improved RV ejection fraction and decreased systolic PAP, but TAPSE, 24-h urine output and creatinine all responded more favourably to levosimendan than to dobutamine. Supportive findings emerged from a separate echocardiographic study involving 62 patients with acute left heart failure who were randomized to levosimendan or dobutamine.45 Levosimendan was found to be superior to dobutamine in improving RV systolic and diastolic function and reducing systolic PAP.45
Levosimendan was found to be as effective as milrinone in improving biventricular function and reducing mean PAP and PVR in a randomized clinical trial in patients with PH (N = 40) undergoing valve replacement but was associated with a greater increase in heart rate, a decrease in systemic vascular resistance and a greater need for noradrenaline.46
Other preclinical data and observations are encouraging but require fuller investigation and scrutiny in adequately powered and well-configured randomized clinical trials.47,48 In particular, there appear to have been no studies of the effect of levosimendan on PH due to left ventricular failure with preserved EF.
Septic cardiomyopathy
A low cardiac output state emergent as septic cardiomyopathy is a relatively common phenomenon in patients with refractory septic shock.
Dobutamine has traditionally been used in this setting, but the familiar catecholaminergic effects (initiation or exacerbation of tachycardia, increased myocardial oxygen consumption) limit its usefulness.
Levosimendan, again by virtue of its non-adrenergic mode of action, can be used to support cardiac function in septic cardiomyopathy, though it has to be recognized that this is another ‘niche’ application for levosimendan, currently not well supported by clinical evidence.49–51
Pragmatic advice in this area is that it is prudent to maintain ionized calcium levels >1.2 mmol/L when delivering levosimendan. The need for increased vasopressor requirements can be mitigated by measures such as ensuring adequate volume replacement, and perhaps a lower than usual target for mean arterial pressure.52
Ventricular–aortic coupling
Effects on ventricular elastance (Ees), aortic elastance (Ea), and ventricular–aortic (V-A) coupling merit some consideration.
Optimal ventricular energy efficiency is achieved when Ees is approximately equal to Ea. In AHF with reduced EF, however, the combination of low EF and increased filling pressures shifts the left ventricular end-systolic pressure–volume relation downward and to the right: hence Ees decreases. At the same time, Ea increases as part of the normal autonomic response to compensate for the reduced stroke volume. These alterations lead to a mismatch between ventricular and aortic compliance during systole such that proportionately less energy is directed towards useful work (i.e. cardiac contractility) and more is wasted, as described in the left ventricular pressure–volume loop (PVA).53 The PVA itself correlates with myocardial oxygen consumption so these shifts in the Ees/Ea relation reflect both energetic and contractile inefficiency in an often already compromised myocardium.
Medical intervention in AHF aims to restore a more favourable Ees/Ea ratio either by (i) using vasodilators (e.g. beta-blockers, calcium-channel blockers) to reduce Ea or (ii) using i.v. inotropes to increase Ees even though several of those agents (e.g. catecholamines or PDE-III inhibitors) are associated with increased myocardial oxygen consumption, as well as adverse effects such as arrhythmias and increased mortality.54
As an inodilator with impact on both Ees and Ea, levosimendan can improve V-A coupling and enhance cardiovascular performance via a distinctive mode of action.55,56 Such effects may be relevant in several of the settings considered in this essay.
Other treatment considerations
The effects of i.v. milrinone and dobutamine have been compared directly (retrospectively) in 329 patients admitted for an acute exacerbation of congestive heart failure.57 There was no significant difference in the in-hospital mortality rate (dobutamine, 7.8%; milrinone, 10%) or clinical outcome between the two groups. Furthermore, the use of parenteral nitroglycerine and dopamine was similar in both groups. However, 109 patients (40%) in the dobutamine group required parenteral nitroprusside for haemodynamic optimization, compared with 11 (18%) in the milrinone group (P < 0.001). Dobutamine was favoured in the judgement of the researchers because of its substantially more favourable average direct cost per patient (inter-group difference ≈US$1800; P < 0.0001). Dobutamine’s potential for tachyphylaxis is at least theoretically problematic in this setting. Its association with increased mortality is discernible58 but, as noted above, may not be an absolute deterrent for some patients.
Brief examination of the possible role of inotropes as a ‘bridge to destination therapy’ is also appropriate.
Among 103 patients at Eurotransplant high-urgent status managed in a single centre in Germany, different inotropes (adrenaline, dobutamine, levosimendan, milrinone, and noradrenaline) were administered to restore haemodynamic equilibrium. Patients were weaned from inotropes as soon as possible after initial recompensation and then treated with intermittent inotropes for up to 8 weeks. During the period of observation, 14 patients died; 14 more needed an LVAD; and 87 received heart transplants. Cumulative survival rates were 75% and 67% at 3 and 12 months, respectively.59
These data are supportive of a role for intermittent inotropes as a bridge to transplant for some patients. In more than half these patients, however, additional inotropic support was necessary. Prospective trials are therefore needed to define closely the types and categories of patients most likely to benefit from this measure and the most efficacious agents and treatment protocols.
Inter alia, two pilot studies (one of them retrospective) have produced indications that pre-procedural levosimendan may be used with advantage in patients undergoing LVAD implantation, both to improve clinical outcome and survival and to aid the identification of patients at high risk of RV failure. These possibilities merit further appraisal in larger controlled trials.60,61
Conclusions
Appropriate, effective and successful use of i.v. vasoactive drugs in AHF flows from accurate appreciation of the causes and pathophysiology of decompensation. One objective of such an assessment is to avoid the inappropriate use of these agents: there are indications that inotropes may have been—and continue to be—somewhat over-prescribed in situations of acute cardiac decompensation. Inotropes (along with vasopressors) are suited to the circumstances of patients who display signs of inadequate peripheral perfusion despite adequate filling status. Levosimendan is an agent that can enhance cardiac inotropy through energy-neutral mechanisms that sidestep the traditional adrenergic/catecholaminergic pathways to enhance cardiomyocyte contractility.1 It also exerts vasodilator effects that are relevant to the relief of symptoms of haemodynamic decompensation and perhaps the perfusion of major internal organs. Due to its mode of action, levosimendan can be used in situations of chronic beta-blocker therapy.
These properties, combined with the lack of untoward effect on later survival, make levosimendan a useful alternative to conventional inotropes in various decompensation states, as reviewed in this essay and elsewhere.62–67 Once decided upon, treatment with levosimendan should be started early, to minimize the potential for end-organ damage. Bolus or loading doses should be avoided when a fast response is not needed, and infusion should be commenced at doses of 0.1–0.2 µg/kg/min and continued for 24–48 h, with monitoring for hypovolaemia and electrolyte imbalances. Noradrenaline should be available to maintain adequate perfusion pressure (broadly, a mean arterial pressure of ≥60–65 mmHg).
Acknowledgements
The authors acknowledge Hughes associates, Oxford, UK, for assistance in the editing of the manuscript.
Funding
This paper was published as part of a supplement made possible by an unrestricted educational grant by Orion Pharma.
Conflict of interest: In the past 5 years, F.G, E.Z., and J.M. have received honoraria for educational events organized by Orion Pharma. P.P. is a full-time employee of Orion Pharma, where levosimendan was discovered and developed.
References
- 1. Papp Z, Édes I, Fruhwald S, De Hert SG, Salmenperä M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikström BG, Jörgensen K, Filippatos G, Parissis JT, González MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F.. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012;159:82–87. [DOI] [PubMed] [Google Scholar]
- 2. Pollesello P, Papp Z, Papp JG.. Calcium sensitizers: what have we learned over the last 25 years? Int J Cardiol 2016;203:543–548. [DOI] [PubMed] [Google Scholar]
- 3. Farmakis D, Alvarez J, Gal TB, Brito D, Fedele F, Fonseca C, Gordon AC, Gotsman I, Grossini E, Guarracino F, Harjola VP, Hellman Y, Heunks L, Ivancan V, Karavidas A, Kivikko M, Lomivorotov V, Longrois D, Masip J, Metra M, Morelli A, Nikolaou M, Papp Z, Parkhomenko A, Poelzl G, Pollesello P, Ravn HB, Rex S, Riha H, Ricksten SE, Schwinger RHG, Vrtovec B, Yilmaz MB, Zielinska M, Parissis J.. Levosimendan beyond inotropy and acute heart failure: evidence of pleiotropic effects on the heart and other organs: an expert panel position paper. Int J Cardiol 2016;222:303–312. [DOI] [PubMed] [Google Scholar]
- 4. Bistola V, Arfaras-Melainis A, Polyzogopoulou E, Ikonomidis I, Parissis J.. Inotropes in acute heart failure: from guidelines to practical use: therapeutic options and clinical practice. Card Fail Rev 2019;5:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farmakis D, Agostoni P, Baholli L, Bautin A, Comin-Colet J, Crespo-Leiro MG, Fedele F, García-Pinilla JM, Giannakoulas G, Grigioni F, Gruchała M, Gustafsson F, Harjola VP, Hasin T, Herpain A, Iliodromitis EK, Karason K, Kivikko M, Liaudet L, Ljubas-Maček J, Marini M, Masip J, Mebazaa A, Nikolaou M, Ostadal P, Põder P, Pollesello P, Polyzogopoulou E, Pölzl G, Tschope C, Varpula M, von Lewinski D, Vrtovec B, Yilmaz MB, Zima E, Parissis J.. A pragmatic approach to the use of inotropes for the management of acute and advanced heart failure: an expert panel consensus. Int J Cardiol 2019;297:83–90. [DOI] [PubMed] [Google Scholar]
- 6. Hamo CE, Butler J, Gheorghiade M, Chioncel O.. The bumpy road to drug development for acute heart failure. Eur Heart J Suppl 2016;18:G19–G32. [Google Scholar]
- 7. Pollesello P, Ben Gal T, Bettex D, Cerny V, Comin-Colet J, Eremenko AA, Farmakis D, Fedele F, Fonseca C, Harjola VP, Herpain A, Heringlake M, Heunks L, Husebye T, Ivancan V, Karason K, Kaul S, Kubica J, Mebazaa A, Mølgaard H, Parissis J, Parkhomenko A, Põder P, Pölzl G, Vrtovec B, Yilmaz MB, Papp Z.. Short-term therapies for treatment of acute and advanced heart failure: why so few drugs available in clinical use, why even fewer in the pipeline?. J Clin Med 2019;8:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, De Keulenaer GW.. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019;40:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saville BR, Berry SM.. Efficiencies of platform clinical trials: a vision of the future. Clin Trials 2016;13:358–366. [DOI] [PubMed] [Google Scholar]
- 10. Papp Z, Agostoni P, Alvarez J, Bettex D, Bouchez S, Brito D, Černý V, Comin-Colet J, Crespo-Leiro MG, Delgado Jf, Edes I, Eremenko Aa Farmakis D, Fedele F, Fonseca C, Fruhwald S, Girardis M, Guarracino F, Harjola V-P, Heringlake M, Herpain A, Heunks LMA, Husebye T, Ivancan V, Karason K, Kaul S, Kivikko M, Kubica J, Masip J, Matskeplishvili S, Mebazaa A, Nieminen MS, Oliva F, Papp JGy, Parissis J, Parkhomenko A, Põder P, Pölzl G, Reinecke A, Ricksten S-E, Riha H, Rudiger A, Sarapohja T, Schwinger RHG, Toller W, Tritapepe L, Tschöpe C, Wikström G, von Lewinski D, Vrtovec B, Pollesello P.. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. Card Fail Rev 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC; OPTIMIZE-HF Investigators and Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006;296:2217–2226. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, Burrows N, McLean A, Vilas-Boas F, Mebazaa A.. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF). Intensive Care Med 2011;37:619–626. [DOI] [PubMed] [Google Scholar]
- 14. Kivikko M, Pollesello P, Tarvasmäki T, Sarapohja T, Nieminen MS, Harjola VP.. Effect of baseline characteristics on mortality in the SURVIVE trial on the effect of levosimendan vs dobutamine in acute heart failure: sub-analysis of the Finnish patients. Int J Cardiol 2016;215:26–31. [DOI] [PubMed] [Google Scholar]
- 15. Nieminen MS, Buerke M, Cohen-Solál A, Costa S, Édes I, Erlikh A, Franco F, Gibson C, Gorjup V, Guarracino F, Gustafsson F, Harjola VP, Husebye T, Karason K, Katsytadze I, Kaul S, Kivikko M, Marenzi G, Masip J, Matskeplishvili S, Mebazaa A, Møller JE, Nessler J, Nessler B, Ntalianis A, Oliva F, Pichler-Cetin E, Põder P, Recio-Mayoral A, Rex S, Rokyta R, Strasser RH, Zima E, Pollesello P.. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol 2016;218:150–157. [DOI] [PubMed] [Google Scholar]
- 16. Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O’Connor CM; OPTIME-CHF Investigators. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol 2003;41:997–1003. [DOI] [PubMed] [Google Scholar]
- 17. Pollesello P, Parissis J, Kivikko M, Harjola VP.. Levosimendan meta-analyses: is there a pattern in the effect on mortality? Int J Cardiol 2016;209:77–83. [DOI] [PubMed] [Google Scholar]
- 18. Greco T, Calabrò MG, Covello RD, Greco M, Pasin L, Morelli A, Landoni G, Zangrillo A.. A Bayesian network meta-analysis on the effect of inodilatory agents on mortality. Br J Anaesth 2015;114:746–756. [DOI] [PubMed] [Google Scholar]
- 19. Hamdani N, Linke WA.. Alteration of the beta-adrenergic signaling pathway in human heart failure. Curr Pharm Biotechnol 2012;13:2522–2531. [PubMed] [Google Scholar]
- 20. Tarvasmäki T, Harjola VP, Nieminen MS, Siirilä-Waris K, Tolonen J, Tolppanen H, Lassus J; FINN-AKVA Study Group. Acute heart failure with and without concomitant acute coronary syndromes: patient characteristics, management, and survival. J Card Fail 2014;20:723–730. [DOI] [PubMed] [Google Scholar]
- 21. Schumann J, Henrich EC, Strobl H, Prondzinsky R, Weiche S, Thiele H, Werdan K, Frantz S, Unverzagt S.. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev 2018;1:CD009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, Cecconi M, Choi DJ, Cohen Solal A, Christ M, Masip J, Arrigo M, Nouira S, Ojji D, Peacock F, Richards M, Sato N, Sliwa K, Spinar J, Thiele H, Yilmaz MB, Januzzi J.. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med 2016;42:147–163. [DOI] [PubMed] [Google Scholar]
- 23. Shang G, Yang X, Song D, Ti Y, Shang Y, Wang Z, Tang M, Zhang Y, Zhang W, Zhong M.. Effects of levosimendan on patients with heart failure complicating acute coronary syndrome: a meta-analysis of randomized controlled trials. Am J Cardiovasc Drugs 2017;17:453–463. [DOI] [PubMed] [Google Scholar]
- 24. Moiseyev VS, Põder P, Andrejevs N, Ruda MY, Golikov AP, Lazebnik LB, Kobalava ZD, Lehtonen LA, Laine T, Nieminen MS, Lie KI; RUSSLAN Study Investigators. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 2002;23:1422–1432. [DOI] [PubMed] [Google Scholar]
- 25. Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, Quenot J-P, Kimmoun A, Cariou A, Lassus J, Harjola V-P, Meziani F, Louis G, Rossignol P, Duarte K, Girerd N, Mebazaa A, Vignon P, Mattei M, Thivilier C, Perez P, Auchet T, Fritz C, Boisrame-Helme J, Mercier E, Garot D, Perny J, Gette S, Hammad E, Vigne C, Dargent A, Andreu P, Guiot P; Collaborators. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2018;72:173–182. [DOI] [PubMed] [Google Scholar]
- 26. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779–789. [DOI] [PubMed] [Google Scholar]
- 27. Pirracchio R, Parenica J, Resche Rigon M, Chevret S, Spinar J, Jarkovsky J, Zannad F, Alla F, Mebazaa A; GREAT network. The effectiveness of inodilators in reducing short term mortality among patient with severe cardiogenic shock: a propensity-based analysis. PLoS One 2013;8:e71659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mebazaa A, Parissis J, Porcher R, Gayat E, Nikolaou M, Boas FV, Delgado JF, Follath F.. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med 2011;37:290–301. [DOI] [PubMed] [Google Scholar]
- 29. Russ MA, Prondzinsky R, Christoph A, Schlitt A, Buerke U, Söffker G, Lemm H, Swyter M, Wegener N, Winkler M, Carter JM, Reith S, Werdan K, Buerke M.. Hemodynamic improvement following levosimendan treatment in patients with acute myocardial infarction and cardiogenic shock. Crit Care Med 2007;35:2732–2739. [DOI] [PubMed] [Google Scholar]
- 30. Landoni G, Lomivorotov VV, Alvaro G, Lobreglio R, Pisano A, Guarracino F, Calabrò MG, Grigoryev EV, Likhvantsev VV, Salgado-Filho MF, Bianchi A, Pasyuga VV, Baiocchi M, Pappalardo F, Monaco F, Boboshko VA, Abubakirov MN, Amantea B, Lembo R, Brazzi L, Verniero L, Bertini P, Scandroglio AM, Bove T, Belletti A, Michienzi MG, Shukevich DL, Zabelina TS, Bellomo R, Zangrillo A; CHEETAH Study Group. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med 2017;376:2021–2031. [DOI] [PubMed] [Google Scholar]
- 31. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: lifeline. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 32. Distelmaier K, Roth C, Schrutka L, Binder C, Steinlechner B, Heinz G, Lang IM, Maurer G, Koinig H, Niessner A, Hülsmann M, Speidl W, Goliasch G.. Beneficial effects of levosimendan on survival in patients undergoing extracorporeal membrane oxygenation after cardiovascular surgery. Br J Anaesth 2016;117:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Affronti A, di Bella I, Carino D, Ragni T.. Levosimendan may improve weaning outcomes in venoarterial ECMO patients. ASAIO J 2013;59:554–557. [DOI] [PubMed] [Google Scholar]
- 34. Vally S, Ferdynus C, Persichini R, Bouchet B, Braunberger E, Lo Pinto H, Martinet O, Vandroux D, Aujoulat T, Allyn J, Allou N.. Impact of levosimendan on weaning from peripheral venoarterial extracorporeal membrane oxygenation in intensive care unit. Ann Intensive Care 2019;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacky A, Rudiger A, Krüger B, Wilhelm MJ, Paal S, Seifert B, Spahn DR, Bettex D.. Comparison of levosimendan and milrinone for ECLS weaning in patients after cardiac surgery—A retrospective before-and-after study. J Cardiothorac Vasc Anesth 2018;32:2112–2119. [DOI] [PubMed] [Google Scholar]
- 36. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C.. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tavazzi G, Mojoli F, Iotti GA, Via G.. Does levosimendan have room in Takotsubo syndrome? JACC Heart Fail 2019;7:174. [DOI] [PubMed] [Google Scholar]
- 38. Madias JE. If channel blocker ivabradine vs. β-blockers for sinus tachycardia in patients with takotsubo syndrome. Int J Cardiol 2016;223:877–878. [DOI] [PubMed] [Google Scholar]
- 39. Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, Nyquist O, Remme WJ.. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol 2000;36:1903–1912. [DOI] [PubMed] [Google Scholar]
- 40. Fredholm M, Jörgensen K, Houltz E, Ricksten SE.. Levosimendan or milrinone for right ventricular inotropic treatment?—A secondary analysis of a randomized trial. Acta Anaesthesiol Scand 2020;64:193–201. [DOI] [PubMed] [Google Scholar]
- 41. Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, Lehikoinen P, Någren K, Lehtonen L, Voipio-Pulkki LM.. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther 2000;68:522–531. [DOI] [PubMed] [Google Scholar]
- 42. Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry JL.. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016;37:942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qiu J, Jia L, Hao Y, Huang S, Ma Y, Li X, Wang M, Mao Y.. Efficacy and safety of levosimendan in patients with acute right heart failure: a meta-analysis. Life Sci 2017;184:30–36. [DOI] [PubMed] [Google Scholar]
- 44. Yilmaz MB, Yontar C, Erdem A, Karadas F, Yalta K, Turgut OO, Yilmaz A, Tandogan I.. Comparative effects of levosimendan and dobutamine on right ventricular function in patients with biventricular heart failure. Heart Vessels 2009;24:16–21. [DOI] [PubMed] [Google Scholar]
- 45. Duygu H, Ozerkan F, Zoghi M, Nalbantgil S, Yildiz A, Akilli A, Akin M, Nazli C, Ergene O.. Effect of levosimendan on right ventricular systolic and diastolic functions in patients with ischaemic heart failure. Int J Clin Pract 2007;62:228–233. [DOI] [PubMed] [Google Scholar]
- 46. Mishra A, Kumar B, Dutta V, Arya VK, Mishra AK.. Comparative effect of levosimendan and milrinone in cardiac surgery patients with pulmonary hypertension and left ventricular dysfunction. J Cardiothor Vasc Anesth 2016;30:639–646. [DOI] [PubMed] [Google Scholar]
- 47. Tavares-Silva M, Alaa M, Leite S, Oliveira-Pinto J, Lopes L, Leite-Moreira AF, Lourenço AP.. Dose-response head-to-head comparison of inodilators dobutamine, milrinone, and levosimendan in chronic experimental pulmonary hypertension. J Cardiovasc Pharmacol Ther 2017;22:485–495. [DOI] [PubMed] [Google Scholar]
- 48. Hansen MS, Andersen A, Nielsen-Kudsk JE.. Levosimendan in pulmonary hypertension and right heart failure. Pulm Circ 2018;8:204589401870905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gordon AC, Perkins GD, Singer M, McAuley DF, Orme RM, Santhakumaran S, Mason AJ, Cross M, Al-Beidh F, Best-Lane J, Brealey D, Nutt CL, McNamee JJ, Reschreiter H, Breen A, Liu KD, Ashby D.. Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med 2016;375:1638–1648. [DOI] [PubMed] [Google Scholar]
- 50. Antcliffe DB, Santhakumaran S, Orme RML, Ward JK, Al-Beidh F, O’Dea K, Perkins GD, Singer M, McAuley DF, Mason AJ, Cross M, Ashby D, Gordon AC.. Levosimendan in septic shock in patients with biochemical evidence of cardiac dysfunction: a subgroup analysis of the LeoPARDS randomised trial. Intensive Care Med 2019;45:1392–1400. [DOI] [PubMed] [Google Scholar]
- 51. Chang W, Xie JF, Xu JY, Yang Y.. Effect of levosimendan on mortality in severe sepsis and septic shock: a meta-analysis of randomised trials. BMJ Open 2018;8:e019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nandhabalan P, Ioannou N, Meadows C, Wyncoll D.. Refractory septic shock: our pragmatic approach. Crit Care 2018;22:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walley KR. Left ventricular function: time-varying elastance and left ventricular aortic coupling. Crit Care 2016;20:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guarracino F, Baldassarri R, Pinsky MR.. Ventriculo-arterial decoupling in acutely altered hemodynamic states. Crit Care 2013;17:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guarracino F, Cariello C, Danella A, Doroni L, Lapolla F, Stefani M, Baldassarri R, Vullo C.. Effect of levosimendan on ventriculo-arterial coupling in patients with ischemic cardiomyopathy. Acta Anaesthesiol Scand 2007;51:1217–1224. [DOI] [PubMed] [Google Scholar]
- 56. Masutani S, Cheng H-J, Tachibana H, Little WC, Cheng C-P.. Levosimendan restores the positive force-frequency relation in heart failure. Am J Physiol Heart Circ Physiol 2011;301:H488–H496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamani MH, Haji SA, Starling RC, Kelly L, Albert N, Knack DL, Young JB.. Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: hemodynamic efficacy, clinical outcome, and economic impact. Am Heart J 2001;142:998–1002. [DOI] [PubMed] [Google Scholar]
- 58. Tacon CL, McCaffrey J, Delaney A.. Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensive Care Med 2012;38:359–367. [DOI] [PubMed] [Google Scholar]
- 59. Hübner T, Nickel T, Steinbeck G, Massberg S, Schramm R, Reichart B, Hagl C, Kiwi A, Weis M.. A single German center experience with intermittent inotropes for patients on the high-urgent heart transplant waiting list. Clin Res Cardiol 2015;104:929–934. [DOI] [PubMed] [Google Scholar]
- 60. Theiss HD, Grabmaier U, Kreissl N, Hagl C, Steinbeck G, Sodian R, Franz WM, Kaczmarek I.. Preconditioning with levosimendan before implantation of left ventricular assist devices. Artif Organs 2014;38:231–234. [DOI] [PubMed] [Google Scholar]
- 61. Sponga S, Ivanitskaia E, Potapov E, Krabatsch T, Hetzer R, Lehmkuhl H.. Preoperative treatment with levosimendan in candidates for mechanical circulatory support. ASAIO J 2012;58:6–11. [DOI] [PubMed] [Google Scholar]
- 62. Gustafsson F, Guarracino F, Schwinger R.. The inodilator levosimendan as a treatment for acute heart failure in various settings. Eur Heart J Suppl 2017;19:C2–C7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harjola VP, Giannakoulas G, von Lewinski D, Matskeplishvili S, Mebazaa A, Papp Z, Schwinger RHG, Pollesello P, Parissis JT.. Use of levosimendan in acute heart failure. Eur Heart J Suppl 2018;20:I2–I10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Agostoni P, Farmakis DT, García-Pinilla JM, Harjola VP, Karason KV, Lewinski D, Parissis J, Pollesello P, Pölzl G, Recio-Mayoral A, Reinecke A, Yerly P., Zima E. Hemodynamic balance in acute and advanced heart failure: an expert perspective on the role of levosimendan. Card Fail Rev 2019;5:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oliva F, Comin-Colet J, Fedele F, Fruhwald F, Gustafsson F, Kivikko M, Borbély A, Pölzl G, Tschöpe C.. Tschöpe Repetitive levosimendan treatment in the management of advanced heart failure. Eur Heart J Suppl 2018;20:I11–I20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Altenberger J, Gustafsson F, Harjola VP, Karason K, Kindgen-Milles D, Kivikko M, Malfatto G, Papp Z, Parissis J, Pollesello P, Pölzl G, Tschöpe C.. Levosimendan in acute and advanced heart failure: an appraisal of the clinical database and evaluation of its therapeutic applications. J Cardiovasc Pharmacol 2018;71:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bouchez S, Fedele F, Giannakoulas G, Gustafsson F, Harjola VP, Karason K, Kivikko M, von Lewinski D, Oliva F, Papp Z, Parissis J, Pollesello P, Pölzl G, Tschöpe C.. Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Ther 2018;32:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]