Abstract
Statins can decrease hepatocellular carcinoma (HCC) occurrence, but the magnitude and the predictors of these effects remain unclear. This meta-analysis provides a pooled estimate of the impact of statin use on HCC occurrence. Pooled effects were calculated using a random-effects model by means of the DerSimonian and Laird test. Primary endpoint was the time-dependent correlation between statin use and HCC incidence expressed as hazard ratio (HR), both crude and adjusted. The crude and adjusted odds ratios (OR) for HCC occurrence between statin users and non-users were analyzed. Twenty-five studies with 1,925,964 patients were included. Crude OR for HCC incidence was 0.59 (95% CI: 0.47–0.74), confirmed in adjusted analysis (OR: 0.74, 95% CI: 0.70–0.78). Adjusted HR was 0.73 (95% CI: 0.69–0.76). This effect was more pronounced in HBV patients (HR: 0.46, 95% CI: 0.36–0.60) and with a cumulative daily dose beyond 365 (HR: 0.27, 95% CI: 0.11–0.67). Lipophilic statins were associated with reduced HCC incidence (HR: 0.49, 95% CI: 0.39–0.62). Atorvastatin determined the greater magnitude of effect (HR: 0.43, 95% CI: 0.28–0.65). This meta-analysis demonstrates the beneficial chemopreventive effect of statins against HCC occurrence. This effect is dose-dependent and more pronounced with lipophilic statins.
Keywords: cirrhosis, HCC, cancer, hazard ratio, survival
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most commonly occurring type of cancer and the leading cause of mortality in cirrhotic patients [1]. Despite the recent improvement in diagnosis and screening programs in cirrhotics, a great number of patients are still diagnosed in the advanced stage, thus being unsuitable to curative treatments, such as surgery, orthotopic liver transplantation, or radiofrequency ablation [2,3,4]. Therefore, the identification of prognostic factors for HCC occurrence is of paramount importance in order to decrease the burden of this disease, in particular in high-risk populations, such as cirrhotic or chronic hepatitis B virus (HBV) patients.
Three-hydroxy-3-methyl-glutaryl-coenzyme A (HMG co-A) reductase inhibitors (statins) are effective and commonly used worldwide as a treatment for dyslipidemia, and increasing evidence shows that statins also have anti-inflammatory and anti-oncogenic effects [5,6].
A previous meta-analysis, based on 10 studies, shed light on the beneficial role of statins in preventing HCC occurrence [7]; however, the relatively low number of studies included did not allow subgroup analyses based on the specific agent administered (type of statin) or to correlate the anticancer effect with the dose.
In the last years, several cohort and case–control studies have been published in the field, hence the need to update the previous data in order to better define the eventual chemopreventive role of statins in hepato-oncology.
The aim of this meta-analysis was to provide a pooled estimate of the impact of statin use on HCC occurrence. Primary endpoint was defined as the time-dependent correlation between statin use and HCC incidence (in terms of both crude and adjusted hazard ratio [HR] for several baseline variables). Secondary outcome was the correlation between the overall incidence of HCC and statin administration expressed in terms of the odds ratio (OR), again both crude and adjusted.
2. Results
2.1. Characteristics of Included Studies
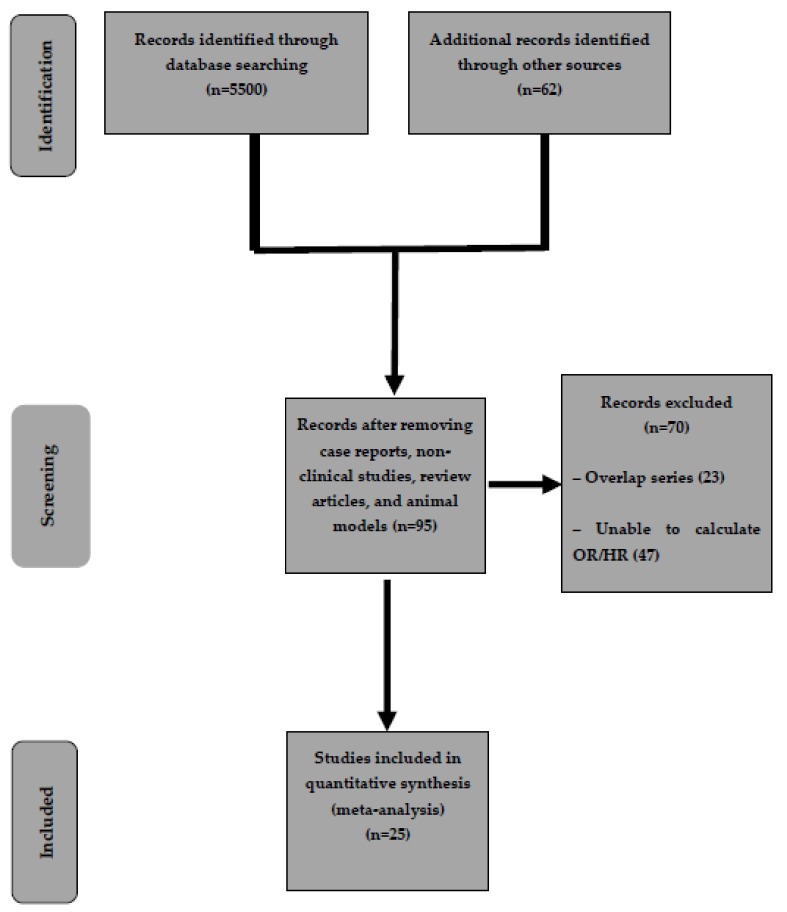
As shown in Figure 1, out of 5562 studies initially identified, after preliminary exclusion of manuscripts not fulfilling inclusion criteria, 95 potentially relevant articles were examined. Among these studies, 23 overlap series and 47 studies not reporting OR or HR (or data useful for their calculation) were further excluded.
Figure 1.
Flow chart of the search strategy conducted in this meta-analysis.
Finally, 25 studies reporting 21,576 cases of HCC in 1,925,964 patients were included in the meta-analysis [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
The main characteristics of the included studies are reported in Table 1.
Table 1.
Characteristics of included studies.
| Study, Year | Design, Period | Country | Sample Size | Age | Men, n (%) | Liver Disease Etiology (HBV/HCV/alcohol/NASH) | Follow-Up Period | Statin Use Period or Dose | Outcome Measure | Variables Adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|
| Björkhem-Bergman, 2014 [8] | Case–control, July 2006 to December 2010 | Swedish Cancer Register | HCC group: 3994 patients (of which 687 statin users) Control (non-HCC), 19970 patients (of which 3598 statin users) |
Mean age NR | 52% | NR | 4 years | At least 9 months | OR, aOR | Age, sex, diabetes, education, other drugs, liver disease etiology |
| ERCHIVES: Butt, 2015 [9] | Retrospective cohort, between 2002 and 2013 | USA | Statin users: 3347 Non-users: 3901 |
Statin users: 53 (Non-statin users: 52 | Statin Users: 3226 (96.4%) Non-users: 3702 (94.9%) |
HCV all patients | 10 years | Mean (IQR) months: Statin users: 31.7 (13.3–58.5) | HR, aHR | Baseline FIB-4 |
| Chang, 2017 [10] | Nested case–control (propensity score matching retrospective study), 1 January 2000 to 31 December 2013 | Taiwan NHIRD database (Taiwan’s National Health Insurance) | Statin users: 675 Non-users: 675 |
56.5±11.2 57.5 (14.1) |
Statin users: 492 (73%) Non-users: 476 (71%) |
313 (46%)/146 (22%)/219 (32%), 292 (43%)/152 (23%)/231 (34%) | Statin users: 5.5 years (3.5) Non-users: 5.4 years (3.6) |
Patient with cDDDs >28 were considered as statin user | aHR | Age, sex, diabetes, comorbidities, other drugs, liver disease etiology |
| Chen, 2014 [11] | Propensity score matching retrospective cohort, 1 January 2000 to 31 December 2008 | Taiwan’s National Health Insurance (NHI) Research Database (NHIRD) | Statin users: 8861 Statin + metformin: 5152 Non-users: 53037 |
Mean NR | Statin users: 4869 (54.95%) Statin + metformin: 2650 (51.44%) Non-users: 30726 (57.93%) |
All patients HBV | 9 years | Patients who used statins for <28 cDDDs were defined as statin non-users | aHR | Age, sex, comorbidities, other drugs |
| El-Serag, 2009 [12] | Nested case–control, 1997–2002 | Department of Veterans Affairs (VA) National Databases, USA | HCC group: 1303 patients of which 447 statin users Control (non-HCC): 5212 patients of which 2766 non-users |
HCC group: 72 years Control (non-HCC): 72 years |
HCC group: 1286 (99%) Control (non-HCC): 5144(99%) |
HCC group: 25 (1.9%)/192 (14.7%)/215 (16.5%)/ Control (non-HCC): 11 (0.2%)/93 (1.8%)/60 (1.2%) |
5 years | Statin use defined as >3 prescriptions | OR, aOR | Etiology of liver disease, cirrhosis, race, other drugs |
| Friedman, 2016 [13] | Case–control, 1 January 1996 to 30 June 2014 | Kaiser Permanente Northern California, USA | HCC group: 2877 patients of which 701 statin users Control group (non-HCC): 142850 patients of which 44953 statin users |
NR | NR | NR | 18 years | NR | aOR | Liver disease etiology, comorbidities, other drugs, BMI |
| German, 2019 [14] | Case–control, 2002–2016 | Wisconsin (USA) | HCC group: 34 patients of which 6 statin users Control group (non-HCC): 68 patients of which 34 statin users |
HCC group: 68.9±11.4 Control group (non-HCC): 69.4±7.5 |
HCC group: 22 Control group (non-HCC): 44 |
NAFLD all patients | 14 years | NR | OR, aOR | Age, sex, other drugs |
| Goh, 2019 [15] | Retrospective cohort, January 2008 to December 2012 | Single institution in Seoul, Republic of Korea | Statin users: 713 Non-users: 7000 |
Statin users: 50 (44–56) Non-users: 47 (39–54) |
Statin users: 482 (67.6%) Non-users: 4624 (66.1%) |
HBV (all patients) | 7.2 years (0.5–9.7) | cDDD >28 was considered as statin use | HR, aHR | Age, sex, liver cirrhosis, comorbidities, viral level, other drugs, liver function tests |
| Hsiang, 2015 [16] | Propensity score matching retrospective cohort, January 2000 to December 2012 | Hospital Authority (HA) registry database, Hong Kong | Statin users: 1176 Non-users: 52337 |
Statin users: 58.7±12.4 Non-users: 58.9±12.9 |
NR | HBV (all patients) | Statin users: 1.6 years (0.7–3.9) Non-users: 2.6 years (1–5.1) |
cDDD: 291.5 | aHR | – |
| Kaplan, 2019 [17] | Propensity score matching retrospective cohort, 1 January 2008 through 30 June 2016 | Veterans’ Health Administration | Statin users: 21921 Non-users: 51023 |
Statin users: 64 (60–69) Non-users: 63 (58–68) |
Statin users: 21373 (97.5%) Non-users: 12602 (98%) |
Statin users: NR/2457 (11.2%)/8471 (38.6%)/5158 (23.5%) Non-users: NR/2065 (14.9%)/4876 (35.2%)/2159 (15.6%) |
Statin users: 900 days (478–1546) Non-users: 1970 days (1234–2736) |
270 days (0–827) | aHR | Race, liver disease etiology, liver function tests, cirrhosis, comorbidities, BMI |
| Kim, 2018 [18] | Nested case–control study, 2002–2013 | National Health Insurance Service Physical Health Examination in the Republicof Korea. | HCC group: 1642 patients of which 111 statin users Non-HCC group: 8210 patients of which 1047 statin users | HCC group: 61.8±9.2 Non-HCC group: 61.8±9.2 |
HCC group: 1372 (83.6%) Non-HCC group: 6860 (83.6%) |
HCC group: 755 (46%)/NR/277 (16.9%)/NR Non-HCC group: 232 (2.8%)/NR/418 (5.1%)/NR |
NR | OR, aOR | Comorbidities, cirrhosis, BMI, other drugs, household income level | |
| King, 2013 [19] | Prospective cohort | USA | 136178 | NR | NR | NR | >20 years | aHR | Age, BMI, comorbidities, other drugs | |
| Lai, 2013 [20] | Case–Control study, 2000–2009 | Taiwan National Health Insurance program | HCC group: 3480 patients of which 255 statin users Non-HCC: 13920 patients of which 1635 statin users | HCC group: 62.7±13.4 Non-HCC: 62.2±13.7 |
HCC group: 2525 (72.6%) Non-HCC: 10100 (72.6%) |
HCC group: 1295 (37.2%)/1005 (28.9%)/66 (1.90%)/72 (2.07%) Non-HCC: 424 (3.05%)/274 (1.97%)/75 (0.54%)/86 (0.62%) |
9 years | HCC group: 16.7 months Non-HCC: 18.6 months |
OR, aOR | Age, sex, comorbidities, cirrhosis, etiology of liver disease, other drugs |
| McGlynn, 2014 [21] | Nested case–control, between 1999 and 2010 | Population of the Health Alliance Plan HMO of the Henry FordHealth System (HFHS), a single integrated health system. USA | HCC group: 94 patients of which 25 statin users Non-HCC group: 468 patients of which 233 statin users |
Mean NR | HCC group: 70 (74.47%) Non-HCC group: 348 (74.36%) |
HCC group: 1 (1.06%)/46 (48.94%)/24 (25.53%)/NR Non-HCC group: 1 (0.21%)/8 (1.71%)/4 (0.85%)/NR |
NR |
≤2 years: HCC group: 13 Control group: 105 > 2 years use of statin: HCC group: 12 Control: 128 |
OR, aOR | Race, etiology of liver disease, comorbidities |
| McGlynn, 2015 [22] | Nested case–control, 1988 and 2011 | UK’s Clinical Practice Research Datalink (CPRD). | HCC group: 1195 patients of which 302 statin users Non-HCC group: 4640 patients of which 1242 statin users |
HCC group: 97.2±12.1 Non-HCC group: 67±12.1 |
HCC group: 856 (71.6%) Non-HCC group: 3322 (71.6%) |
HCC group: 74 (6.2%)/189 (15.8%)/170 (14.2%) Non-HCC group: 23/(0.5%)/189 (4%)/9 (0.2%) |
NR |
Cumulative dose: <8120) HCC: 168 (14.1%) Control: 642 (13.2%) >(21 281 HCC: 152 (12.7%) Control: 649 (14%) |
OR, aOR | BMI, etiology of liver disease, comorbidities, other drugs used |
| Mohanty, 2016 [23] | Propensity score matching retrospective cohort, January 1996 through December 2009 | Veteran Affairs Clinical Case Registry, which contains nationwide data from veterans infected with the HCV | Statin users: 685 Non-users: 685 |
Statin users: 56 (52–59) Non-users: 56 (52–60) |
Statin users: 677 (98.8%) Non-users: 671 (97.9%) |
All had HCV and compensated cirrhosis | NR | NR | HR | – |
| Simon, 2019 [24] | Propensity score matching cohort study, 2005–2013 | Swedish registers | Statin users: 16668 Non-users: 8334 |
Statin users: 47.3±11 Non-users: 47.5±13.7 |
Statin users: 65.2% Non-users: 65.6% |
Statin users: 1540 (23.5%)/5014/6554 (76.5%)/NR Non-users: 1953 (23.4%)/6381/76.6% |
8 years | NR | aHR | Age, sex, duration of viral infection, cirrhosis, comorbidities, other drugs used |
| Tran, 2019 [25] | Nested case–control, 1999-2011 | Scottish Primary Care Clinical Informatics Unit (PCCIU) database. | HCC group: 434 patients of which 111 statin users Non-HCC group: 2103 patients of which 571 statin users |
Mean NR | HCC group: 292 (67.3%) Non-HCC group: 1412 (67.1%) |
NR | NR | HCC group: 4.88 years (3.1–7.29) Non-HCC group: 4.83 years (3.1–7.24) |
OR, aOR | Age, sex, obesity, comorbidities, other drugs used, alcohol |
| Tran, 2019 (II) [25] | Prospective cohort | UK Biobank | Statin users: 395301 Non-users: 76550 |
Mean NR | NR | NR | NR | NR | OR | Age, sex, body mass index, alcohol, comorbidities, other drugs used |
| Tsan, 2012 [27] | Retrospective cohort, 1997–2008 | Taiwan National Health Insurance Research Database |
Statin users: 2785 Non-users: 30628 |
Statin users: 34.7 (26.6–43.8) Non-users: 46.3 (38.9–55.3) |
Statin users: 1590 (57.1%) Non-users: 17852 (58.3%) |
All patients have HBV | NR | 28–90 cDDD: 933 (33.5%) 91–356 cDDD: 1279 (45.9%) >365 cDDDs: 573 (20.6%) |
HR, aHR | Age, sex, income, diabetes, and liver cirrhosis |
| Tsan, 2013 [26] | Retrospective cohort, 1 January 1999 to 31 December 2010 | Taiwan National Health Insurance Research Database | Statin users: 35023 Non-users: 225841 |
Statin users: 53.9 (45.4–62.1) Non-users: 49.8 (38.9–62) |
Statin users: 14973 (42.8%) Non-users: 113290 (50.2%) |
All patients had HCV | Statin users: 12 years (12.0–12.0) Non-users: 12 years (10.9–12) |
179.6 CDD (80.0–414.7) | HR, aHR | Age, sex, urbanization, income, liver cirrhosis, and diabetes |
| Sato, 2006 [28] | RCT 28 September 1991 and 31 March 1995 |
Japan | Statin users: 179 Non-users: 84 |
NR | NR | NR | NR | All patients used pravastatin | OR, aOR | – |
| Marelli, 2011 [29] | Retrospective cohort, propensity score matching, 1990–2009 | General Electric Centricity electronic medical records database | Statin users: 45857 Non-users: 45857 |
Statin users: 64.2±10.44 Non-users: 64.19±9.45 |
Statin users: 23953 (52.23%) Non-users: 24106 (52.57%) |
Viral 28 (0.06%) |
Statin users: 8.43 years Non-users: 8.43 years |
NR | OR, aOR | – |
| Friis, 2005 [30] | Population-based cohort study, 1989–2002 | The Prescription Database of North Jutland County and the Danish Cancer Registry | Statin users: 12251 Non-users: 322503 |
Statin users: 60.7 Non-users: 53.9 |
6935 (57%) 707 (56%) |
NR | 3.3 years (0–14) | Number of statin prescriptions: 2–4: 2392 (20%) 5–9: 2516 (21%) 10–19: 3282 (27%) 20+: 4061 (33%) |
aOR | Age, gender, other drugs used |
| Matsushita, 2010 [31] | Individual patient meta-analysis of RCT | Multicenter | Statin users: 7375 Non-users: 6349 |
Statin users: 57.9±8.3 Non-users: 57.1±8.7 |
Statin users: 47.4% Non-users: 49.5% |
NR | 5.3 years | All patients used pravastatin | OR, aOR | – |
| Emberson, 2012 [32] | Individual patient meta-analysis from RCTs | International | Statin users: 67258 Non-users: 67279 |
63 | 46675 (27%) | NR | 4.9 years | NR | OR, aOR | – |
Data are reported as mean (standard deviation or interquartile range) or absolute number (percentage). Abbreviations: aHR, Adjusted hazard ratio; aOR, Adjusted odds ratio; cDDD, Cumulative defined daily dose; HCC, Hepatocellular carcinoma; HCV: Hepatitis C virus; HR, Hazard ratio; OR, Odds ratio; NR, Not reported; RCT, Randomized controlled trial.
The recruitment period ranged from 1988 to 2018. Nine studies [8,10,12,13,14,18,20,22] were retrospective case–control, twelve were cohort studies [9,11,15,16,17,19,23,24,26,27,29,30], one study was a randomized controlled trial (RCT) [28], and two studies that were included as RCTs represented individual patient data analysis of patients enrolled in prospective controlled trials of cholesterol in heart disease [31,32]. The study by Tran et al. [25] included two different stages, a nested case–control and a prospective cohort drawn from two different populations, hence they were analyzed separately.
Five studies [10,11,20,26,27] were conducted in the same population (Taiwan National Health Insurance and Research Database) but they reported different outcomes or data from different subgroups, therefore were considered separately in different analyses. Nine studies were conducted in Asia [10,11,15,16,18,20,26,27,28], and the remaining studies were conducted in western countries. The two aforementioned individual patient data analyses concerned multicenter RCTs [31,32].
Baseline clinical and demographical characteristics were well balanced between statin users and the control group. Variables adjusted for were mainly age, sex, and etiology of the underlying liver disease; other comorbidities; and the use of other medications.
Quality was deemed mainly high with five observational studies assessed as low-quality articles [13,16,19,23,29].
Details on methodological characteristics and quality of included articles are shown in Table S1.
2.2. Risk of HCC
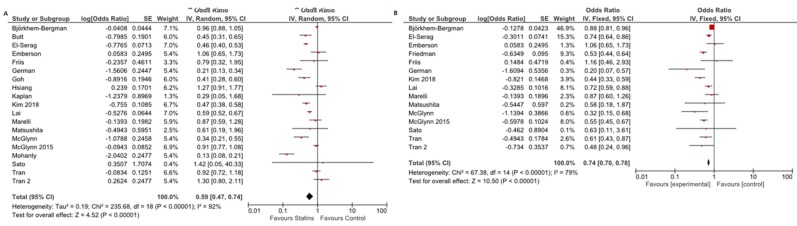
As reported in Figure 2A, overall crude OR for HCC incidence was 0.59 (95% CI: 0.47–0.74), thus highlighting a significant protective role of statins against HCC occurrence (p < 0.001). Of note, high evidence of heterogeneity was observed (I2 = 92%).
Figure 2.
Odds ratio for HCC occurrence in the comparison between statin users and non-users: (A) crude odds ratio; and (B) adjusted odds ratio.
Adjusted analysis, considering the aforementioned baseline confounders, confirmed the anti-oncogenic effect of statins, with a reported adjusted OR (aOR) as high as 0.74 (95% CI: 0.70–0.78). Heterogeneity slightly decreased to 79% in adjusted analysis (Figure 2B).
Crude HR was reported only in two studies [17,29], confirming the above reported results in favor of statin use (HR: 0.42, 95% CI: 0.39–0.45), with moderate evidence of heterogeneity (I2=37%; Figure 3A). As described in Figure 3B, when adjusting for several clinical and demographical parameters, the HR slightly increased but remained under the significance threshold (adjusted [aHR]: 0.73, 95% CI: 0.69–0.76; I2=96%).
Figure 3.
Hazard ratio for HCC occurrence in the comparison between statin users and non-users. (A) Crude HR. (B) Adjusted HR.
There was no evidence of publication bias (data not shown).
2.3. Subgroup Analysis
The aHR for HCC occurrence was confirmed as significantly in favor of statins in HBV patients (0.46, 95% CI: 0.36–0.60; I2 = 0%) while only a non-significant benefit was observed in HCV patients, although this result should be interpreted with caution due to the low number of studies and the high heterogeneity (Table 2).
Table 2.
Subgroup analysis for adjusted hazard ratio concerning hepatocellular carcinoma occurrence.
| Variable | Subgroup | Studies (n) | Summary Estimate (95% CI) | Within-Group Heterogeneity (I2) |
|---|---|---|---|---|
| Etiology of liver disease | HBV | 2 | 0.46 (0.36–0.60) | 0% |
| HCV | 2 | 0.68 (0.30–1.55) | 66% | |
| Diabetes | Yes | 5 | 0.52 (0.46–0.58) | 0% |
| No | 4 | 0.43 (0.31–0.61) | 58% | |
| Cumulative defined daily dose | ≤365 | 3 | 0.51 (0.30–0.88) | 78% |
| >365 | 3 | 0.27 (0.11–0.67) | 81% | |
| Molecule | Lipophilic | 2 | 0.49 (0.39–0.62) | 19% |
| Hydrophilic | 2 | 0.73 (0.40–1.34) | 83% | |
| Simvastatin | 2 | 0.69 (0.42–1.15) | 55% | |
| Atorvastatin | 2 | 0.43 (0.28–0.65) | 17% | |
| Fluvastatin | 2 | 1.02 (0.08–13.25) | 83% | |
| Pravastatin | 1 | 0.80 (0.46–1.39) | NA | |
| Rosuvastatin | 2 | 0.53 (0.04–6.38) | 86% |
Abbreviation: CI, Confidence interval; HBV, Hepatitis B virus; HCC, Hepatocellular carcinoma; HCV, Hepatitis C virus.
Statins were proved to be effective in both diabetic and non-diabetic patients, while the magnitude of the chemopreventive effect was found to be linearly correlated to the dose, with an aHR of 0.51 (95% CI: 0.30–0.88) and 0.27 (95% CI: 0.11–0.67) in patients administered a cumulative defined daily dose (cDDD) below or beyond 365, respectively (Table 2).
Lipophilic statins (atorvastatin, lovastatin, and simvastatin) were associated with significantly reduced HCC (aHR: 0.49, 95% CI: 0.39–0.62, I2 = 19%) incidence while an association between hydrophilic statins (pravastatin, rosuvastatin, and fluvastatin) and reduced risk for HCC was not found (aHR: 0.73, 95% CI: 0 0.40–1.34) (Table 2).
Analysis conducted according to single agents found a significant beneficial effect only with atorvastatin (aHR: 0.43, 95% CI: 0.28–0.65, I2=17%), although further studies are warranted to provide definitive results in this regard.
2.4. Sensitivity Analysis
The results of several sensitivity analyses are reported in Table 3.
Table 3.
Sensitivity analysis of the main diagnostic outcome (odds ratio for hepatocellular carcinoma occurrence) performed based on: (a) study design (observational versus RCT); (b) study location (Asia versus western); and (c) study quality (high versus low).
| Variable | Subgroup | Studies (n) | Summary Estimate (95% CI) | Within-Group Heterogeneity (I2) |
|---|---|---|---|---|
| Study design | Observational | 16 | 0.52 (0.41–0.73) | 87% |
| RCT | 3 | 0.98 (0.76–1.32) | 45% | |
| Study location | Asia | 8 | 0.51 (0.43–0.65) | 44% |
| Western | 8 | 0.59 (0.45–0.81) | 34% | |
| Study quality | High | 13 | 0.54 (0.44–0.89) | 39.4% |
| Low | 3 | 0.57 (0.41–0.98) | 55% | |
| Abbreviations: CI, Confidence Interval; RCT, Randomized-Controlled Trial | ||||
The findings of main analysis were confirmed in sensitivity analysis performed according to study quality (high versus low), design (RCT versus observational), and location (Asia versus western).
The only exception was the sub-analysis restricted to RCTs, where an OR around 1 was observed; of note, two out of three studies included as RCTs represented individual patient data analysis of patients enrolled in prospective controlled trials of cholesterol in heart disease (hence, non-cirrhotic) [31,32] where the incidence of HCC was very low.
Heterogeneity in the sensitivity analysis was mainly moderate.
3. Discussion
HCC is one of the most common cancer types and the leading cause of tumor-related deaths in cirrhotic patients [1].
Statins have been shown to consistently reduce liver fibrosis progression, mainly due to their immunomodulatory effects [33], to mitigate portal hypertension, and to upregulate transcription factors that exert vasoprotective effects in the liver and inhibit stellate cells, thus potentially further decreasing fibrosis [34]. On the other hand, the enthusiasm towards statins has been tempered by fears about their safety profile in cirrhotic patients because of the risk of dose-dependent hepatic injury [35].
A previous meta-analysis found a 37% decreased risk of HCC occurrence in statin users [7] but a specific analysis aiming to identify higher-risk settings was unfeasible due to the low number of included studies.
Through a meta-analysis of 25 studies, we made several key observations. First, we found a 26% decreased incidence of HCC in patients treated with statins, after adjustment for several variables. When considering a time-dependent outcome, such as HR, not influenced by the imbalance in follow-up length between the studies, we confirmed a 27% decreased incidence of HCC when adjusting for several clinical and demographical parameters.
Second, this effect was more pronounced and consistent in HBV patients (56% decrease in HCC incidence) and it was found to be linearly correlated to the dose, with a 73% decreased HCC risk in patients administered a cDDD beyond 365.
Third, as already observed in previous individual studies [24,27], lipophilic statins (atorvastatin, lovastatin, and simvastatin) were associated with significantly reduced HCC (51% compared to 27% in hydrophilic statin users). Among several agents tested, the more pronounced chemopreventive effect was observed with atorvastatin (57% reduction in HCC occurrence), although further studies are warranted to provide definitive results in this regard.
This preventive effect of statins is likely to be independent of its lipid-lowering effects, because lipid-lowering agents other than statins were not associated with reduction in the risk of HCC in previous reports [26,30].
The chemopreventive effect of statins in Asian populations, mainly affected by HBV hepatitis/cirrhosis, is well recognized and it was strongly confirmed in our analysis. In fact, HBV genome integration determines several DNA modifications and microdeletions that can target cancer-relevant genes, potentially providing hepatocytes with a growth advantage [36]. Statins, by inhibiting the mevalonate pathway, can prevent potential detrimental effects of these growth signaling proteins [37]. Statins also exert pro-apoptotic effects by activating several caspases and decreasing Bcl-2 [37]. Moreover, statins inhibit the activation of the proteasome pathway, limiting the breakdown of some molecules with growth-inhibitory effects, such as p21 and p27 [37].
On the other hand, the anti-oncogenic effect of statins in HCV patients is less evident, probably due to modification of metabolic syndrome, insulin-mediated cell proliferation, and obesity-associated inflammation [33,38]. A clear relationship between statin use and HCC incidence in HCV patients was not found in our meta-analysis, but this result might be due to the low number of studies specifically evaluating this subset of patients.
One of the novel findings in our study is the clear dose-dependent effect of statins in decreasing HCC occurrence, with 365 cDDDs as the cut-off to observe the highest preventive effects, as already reported in previous individual studies [28]. Several biological properties of statins, such as anti-angiogenetic or anti-fibrotic effects, were found to be strictly associated to the dose used in in vivo studies [39,40].
The greater chemoprotective effect of lipophobic statins, clearly outlined by our analysis, is likely due to greater lipid solubility and membrane permeability which enhance their pharmacological effects [41]. Further studies are needed to confirm these findings.
Of note, sensitivity analysis restricted to RCTs did not show a significant preventive benefit with statins although the result should be interpreted with caution due to the low number of RCTs included. It is evident that the limited number of RCTs was insufficient to detect a significant effect of statins, in particular considering that these RCTs were conducted in non-cirrhotic patients, hence with a very low HCC incidence.
There are some limitations to our study. First, the limited number of studies in many subgroups does not allow a strong comparison between statins users and non-users in several subsets of patients, in particular concerning single pharmacological agents or based on the use of specific antiviral treatments. Second, several comparisons were weakened by the high heterogeneity. We performed different sensitivity analyses that confirmed the main results and, noteworthy, the heterogeneity decreased when several subgroups were considered separately. To take into account the potential baseline confounders, we considered in our primary analysis the aHR. However, even if the heterogeneity decreased, it remained significant, probably none of the studies adjusted for the same confounders. Other eventual source of heterogeneity could be represented by the different populations enrolled in the included studies, with different risk of HCC occurrence and probably uneven screening campaigns in the different geographic areas. Finally, most of the included studies were retrospective series, hence prone to selection bias.
In conclusion, despite these weaknesses, our meta-analysis demonstrates the beneficial chemopreventive effect of statins against HCC occurrence. This effect is dose-dependent and more pronounced with lipophilic statins.
Further studies are warranted to confirm these results and to identify the exact setting where this anti-oncogenic effect could be enhanced.
4. Methods
4.1. Inclusion and Exclusion Criteria
Only studies meeting the following criteria were included: (1) RCTs or observational studies recruiting >10 patients with clear exposure to statin therapy; (2) studies published in English; (3) articles reporting HCC occurrence; and (4) studies reporting OR, HR, or data useful for their calculation. Case reports, non-clinical studies, review articles, and animal models were excluded.
4.2. Search Strategy
Figure 1 reports the search strategy followed in the meta-analysis.
Bibliographic research was conducted on PubMed, EMBASE, Cochrane Library, and Google Scholar including all studies fulfilling inclusion criteria published until December 2019.
The search was conducted by two study investigators (AF and MAA) independently and keywords used were “HMG-CoA reductase inhibitor(s),” “statin(s),” “atorvastatin,” “fluvastatin,” “lovastatin,” “pravastatin,” “rosuvastatin,” or “simvastatin” combined with “liver cancer” or “neoplasm(s).”
Relevant reviews and meta-analyses on the use of statins and HCC occurrence were examined for potential suitable studies. Authors of included studies were contacted to obtain full text or further information when needed.
The quality of included studies was assessed by two authors independently (AF and MAA) according to the Cochrane Collaboration’s tool for assessing the risk of bias [42] for RCTs and the Newcastle–Ottawa scale [43] for non-randomized studies. Disagreements were solved by discussion and following a third opinion (RS).
4.3. Statistical Analysis
Primary endpoint of the current meta-analysis was the comparison of HCC occurrence between statin users and non-statin users. Data of HCC occurrence were compared through a random-effects model based on the DerSimonian and Laird test, and summary estimates were expressed in terms of both HR and OR along with their relevant 95% CIs.
To partially obviate the bias due to the different follow-up length among the studies and, within each study, between the two treatment arms and to consider not only the number of events but also their timing and the follow-up of censored patients, HRs were considered in the analysis when reported in the included studies.
Two separate analyses were conducted for crude and adjusted summary estimates (both OR and HR) and aHR was considered the primary endpoint in the meta-analysis.
Chi-square and I² tests were used for across studies comparison of the percentage of variability attributable to heterogeneity beyond chance. p < 0.10 for chi-square test and I² <20% were interpreted as low-level heterogeneity.
Probability of publication bias was assessed using funnel plots and with Begg and Mazumdar’s test.
Sensitivity analysis was conducted according to the quality of included studies (high versus low), location of the studies (Asia versus western), and study design (RCT versus observational).
A subgroup analysis based on several statin molecule and class (lipophilic versus hydrophilic), etiology of liver disease, presence of diabetes, and cumulative defined daily dose (cDDD: ≤365 versus >365) was performed.
All statistical analyses were conducted using RevMan 5.3 software (the Cochrane Collaboration, Oxford, UK). For all calculations a two-tailed p-value of <0.05 was considered statistically significant.
5. Conclusions
Our meta-analysis demonstrates the beneficial chemopreventive effect of statins against HCC occurrence. This effect is dose-dependent and more pronounced with lipophilic statins. Further studies are warranted to confirm these results and to identify the exact setting where this anti-oncogenic effect could be enhanced.
Acknowledgments
Editorial assistance was provided by Sara di Nunzio and Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by internal funds.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/4/874/s1, Table S1: Risk of bias assessment and quality of included studies.
Author Contributions
Conceptualization, A.F., S.S.; Methodology (data collection), A.F. and M.A.A.E.A.; Statistical analysis, A.F., S.P., M.M., L.G., R.S.; Writing—original draft preparation, A.F., review and editing, M.A.A.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
None of the authors have any relevant financial disclosures.
References
- 1.El-Serag H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R., Zhu A.X., Murad M.H., Marrero J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 4.Rognoni C., Ciani O., Sommariva S., Facciorusso A., Tarricone R., Bhoori S., Mazzaferro V. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: systematic review and meta-analyses. Oncotarget. 2016;7:72343–72355. doi: 10.18632/oncotarget.11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G.M., Zhao J., Li B., Zhang X.F., Ma J.X., Ma X.L., Liu J. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: A systemic review and meta-analysis of 15 randomized controlled trials. Autoimmun. Rev. 2018;17:215–225. doi: 10.1016/j.autrev.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Jain M.K., Ridker P.M. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 7.Singh S., Singh P.P., Singh A.G., Murad M.H., Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkhem-Bergman L., Backheden M., Soderberg Lofdal K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer-results from a nationwide case-control study in Sweden. Pharmacoepidemiol. Drug Saf. 2014;23:1101–1106. doi: 10.1002/pds.3685. [DOI] [PubMed] [Google Scholar]
- 9.Butt A.A., Yan P., Bonilla H., Abou-Samra A.B., Shaikh O.S., Simon T.G., Chung R.T., Rogal S.S. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: Results from ERCHIVES. Hepatology. 2015;62:365–374. doi: 10.1002/hep.27835. [DOI] [PubMed] [Google Scholar]
- 10.Chang F.M., Wang Y.P., Lang H.C., Tsai C.F., Hou M.C., Lee F.Y., Lu C.L. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896–907. doi: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- 11.Chen C.I., Kuan C.F., Fang Y.A., Liu S.H., Liu J.C., Wu L.L., Chang C.J., Yang H.C., Hwang J., Miser J.S., et al. Cancer risk in HBV patients with statin and metformin use: a population-based cohort study. Medicine (Baltimore) 2015;94:e462. doi: 10.1097/MD.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Serag H.B., Johnson M.L., Hachem C., Morgana R.O. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136:1601–1608. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman G.D., Achacoso N., Fireman B., Habel L.A. Statins and reduced risk of liver cancer: evidence for confounding. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.German M.N., Lutz M.K., Pickhardt P.J., Bruce R.J., Said A. Statin use is protective against hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a case-control study. J. Clin. Gastroenterol. 2019;17 doi: 10.1097/MCG.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 15.Goh M.J., Sinn D.H., Kim S., Woo S.Y., Cho H., Kang W., Gwak G.Y., Paik Y.H., Choi M.S., Lee J.H., et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology. 2019;25 doi: 10.1002/hep.30973. [DOI] [PubMed] [Google Scholar]
- 16.Hsiang J.C., Wong G.L., Tse Y.K., Wong V.W., Yip T.C., Chan H.L. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J. Hepatol. 2015;63:1190–1197. doi: 10.1016/j.jhep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan D.E., Serper M.A., Mehta R., Fox R., John B., Aytaman A., Baytarian M., Hunt K., Albrecht J., Njei B., et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology. 2019;156:1693–1706. doi: 10.1053/j.gastro.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Kim G., Jang S.Y., Nam C.M., Kang E.S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J. Hepatol. 2018;68:476–484. doi: 10.1016/j.jhep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 19.King L., Khalili H., Huang E., Chung R., Chan A. Statins are associated with a reduced risk of liver cancer: data from a large US prospective cohort study. Hepatology. 2013;58:1216A. doi: 10.1002/hep.26883. [DOI] [Google Scholar]
- 20.Lai S.W., Liao K.F., Lai H.C., Muo C.H., Sung F.C., Chen P.C. Statin use and risk of hepatocellular carcinoma. Eur. J. Epidemiol. 2013;28:485–492. doi: 10.1007/s10654-013-9806-y. [DOI] [PubMed] [Google Scholar]
- 21.McGlynn K.A., Divine G.W., Sahasrabuddhe V.V., Engel L.S., VanSlooten A., Wells K., Yood M.U., Alford S.H. Statin use and risk of hepatocellular carcinoma in a U.S. population. Cancer Epidemiol. 2014;38:523–527. doi: 10.1016/j.canep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGlynn K.A., Hagberg K., Chen J., Graubard B.I., London W.T., Jick S., Sahasrabuddhe V.V. Statin use and risk of primary liver cancer in the Clinical Practice Research Datalink. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohanty A., Tate J.P., Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology. 2016;150:430–440.e431. doi: 10.1053/j.gastro.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon T.G., Duberg A.S., Aleman S., Hagstrom H., Nguyen L.H., Khalili H., Chung R.T., Ludvigsson J.F. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann. Intern. Med. 2019;171:318–327. doi: 10.7326/M18-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran K.T., McMenamin U.C., Coleman H.G., Cardwell C.R., Murchie P., Iversen L., Lee A.J., Thrift A.P. Statin use and risk of liver cancer: Evidence from two population-based studies. Int. J. Cancer. 2020;146:1250–1260. doi: 10.1002/ijc.32426. [DOI] [PubMed] [Google Scholar]
- 26.Tsan Y.T., Lee C.H., Ho W.C., Lin M.H., Wang J.D., Chen P.C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J. Clin. Oncol. 2013;31:1514–1521. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- 27.Tsan Y.T., Lee C.H., Wang J.D., Chen P.C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 2012;30:623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 28.Sato S., Ajiki W., Kobayashi T., Awata N. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J. Epidemiol. 2006;16:201–206. doi: 10.2188/jea.16.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marelli C., Gunnarsson C., Ross S., Haas S., Stroup D.F., Cload P., Clopton P., DeMaria A.N. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J. Am. Coll. Cardiol. 2011;58:530–537. doi: 10.1016/j.jacc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Friis S., Poulsen A.H., Johnsen S.P., McLaughlin J.K., Fryzek J.P., Dalton S.O., Sorensen H.T., Olsen J.H. Cancer risk among statin users: a population-based cohort study. Int. J. Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita Y., Sugihara M., Kaburagi J., Ozawa M., Iwashita M., Yoshida S., Saito H., Hattori Y. Pravastatin use and cancer risk: a meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol. Drug Saf. 2010;19:196–202. doi: 10.1002/pds.1870. [DOI] [PubMed] [Google Scholar]
- 32.Emberson J.R., Kearney P.M., Blackwell L., Newman C., Reith C., Bhala N., Holland L., Peto R., Keech A., Collins R., et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H.Y., Singh N. Antimicrobial and immunomodulatory attributes of statins: relevance in solid-organ transplant recipients. Clin. Infect Dis. 2009;48:745–755. doi: 10.1086/597039. [DOI] [PubMed] [Google Scholar]
- 34.Marrone G., Russo L., Rosado E., Hide D., Garcia-Cardena G., Garcia-Pagan J.C., Bosch J., Gracia-Sancho J. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J. Hepatol. 2013;58:98–103. doi: 10.1016/j.jhep.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Villani R., Navarese E.P., Cavallone F., Kubica J., Bellanti F., Facciorusso A., Vendemiale G., Serviddio G. Risk of statin-induced hypertransaminasemia: A systematic review and meta-analysis of randomized controlled trials. Mayo Clin. Proc. Innov. Qual. Outcomes. 2019;3:131–140. doi: 10.1016/j.mayocpiqo.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami Y., Saigo K., Takashima H., Minami M., Okanoue T., Brechot C., Paterlini-Brechot P. Large scaled analysis of hepatitis B virus (HBV) DNA integration in HBV related hepatocellular carcinomas. Gut. 2005;54:1162–1168. doi: 10.1136/gut.2004.054452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demierre M.F., Higgins P.D., Gruber S.B., Hawk E., Lippman S.M. Statins and cancer prevention. Nat. Rev. Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 38.Facciorusso A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: recent findings and new perspectives. Curr. Diabetes Rev. 2013;9:382–386. doi: 10.2174/15733998113099990068. [DOI] [PubMed] [Google Scholar]
- 39.Weis M., Heeschen C., Glassford A.J., Cooke J.P. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 40.Tatsuta M., Iishi H., Baba M., Iseki K., Yano H., Uehara H., Yamamoto R., Nakaizumi A. Suppression by pravastatin, an inhibitor of p21ras isoprenylation, of hepatocarcinogenesis induced by N-nitrosomorpholine in Sprague-Dawley rats. Br. J. Cancer. 1998;77:581–587. doi: 10.1038/bjc.1998.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamelin B.A., Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol. Sci. 1998;19:26–37. doi: 10.1016/S0165-6147(97)01147-4. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [(accessed on 14 February 2020)]; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.