Abstract
Photobiomodulation (PBM) might be an effective treatment for Parkinson’s disease (PD) in human patients. PBM of the brain uses red or near infrared light delivered from a laser or an LED at relatively low power densities, onto the head (or other body parts) to stimulate the brain and prevent degeneration of neurons. PD is a progressive neurodegenerative disease involving the loss of dopamine-producing neurons in the substantia nigra deep within the brain. PD is a movement disorder that also shows various other symptoms affecting the brain and other organs. Treatment involves dopamine replacement therapy or electrical deep brain stimulation. The present systematic review covers reports describing the use of PBM to treat laboratory animal models of PD, in an attempt to draw conclusions about the best choice of parameters and irradiation techniques. There have already been clinical trials of PBM reported in patients, and more are expected in the coming years. PBM is particularly attractive as it is a non-pharmacological treatment, without any major adverse effects (and very few minor ones).
Keywords: Parkinson’s disease, animal models, photobiomodulation, low-level laser therapy, transcranial, abscopal, parameters
1. Introduction
Parkinson’s disease (PD) is a multifactorial and multisystem disease, characterized by the loss of the dopamine producing neuronal cells of the substantia nigra pars compacta (SNc) in the brain [1,2]. The lack of dopamine primarily affects the motor function, but there are many other signs and symptoms that affect mood, cognition, digestive system, sense of smell, etc. The motor symptoms include bradykinesia, muscular rigidity, tremor at rest, and postural instability. The dopamine producing neurons die off, and one somewhat controversial theory to explain this is the accumulation of Lewy bodies containing aggregated α-synuclein inside the cells. The causes of PD are not completely understood. Only about 15% of PD patients are likely to have a genetic cause, among which mutations in leucine-rich repeat kinase 2 (LRRK2), GBA1 (glucocerebrosidase), and SNCA (α-synuclein) are the most common [3]. The environmental causes are complex, but recent evidence has implicated mitochondrial dysfunction [4] and changes in the gut microbiome [5]. Over 1 million individuals in the US suffer from PD and the annual financial burden is estimated to be $52 billion [6]. The accepted treatment is replacement of the lost dopamine using Levodopa, which helps the motor symptoms but does not modify the course of the disease [7]. Monoamine oxidase-B inhibitors and dopamine agonists might be used later in the course of the disease. Deep brain stimulation (DBS) using an electrode implanted into the subthalamic nucleus and other brain regions has also shown promising results [8].
Photobiomodulation (PBM) involves the use of low-powered red and near-infrared (NIR) light from a laser or light-emitting diode (LED) to stimulate, heal, and regenerate damaged or dying tissues [9]. PBM was previously known as low-level laser (light) therapy (LLLT) [10]. PBM was discovered by Endre Mester soon after the first ruby laser was discovered by Ted Maiman in 1960 [11]. For many years, it was thought that a coherent laser beam was necessary for effective PBM [12], but now it is appreciated that in many situations, LEDs might be a better choice [13]. The mechanism of action primarily involves absorption of the light through the mitochondria, leading to an increased membrane potential, electron transport, oxygen consumption, and ATP synthesis [9]. Since the brain is heavily dependent on mitochondrial activity, it is not surprising that PBM has been extensively tested to treat various brain disorders [14]. Many signaling pathways are activated by PBM, including those mediated by reactive oxygen species (ROS), leading to the up-regulation of anti-oxidant defenses [15]. Anti-apoptotic and pro-survival signaling is also activated [16]. Moreover the ability to switch mitochondrial respiration from glycolysis towards oxidative phosphorylation has two other important effects. First, stem cells are mobilized from their hypoxic niche and can migrate towards sites of injury where they can repair the damage [17]. Second, the mitochondrial alteration can switch the macrophage and microglial phenotype from the pro-inflammatory M1 state, to the anti-inflammatory and phagocytic M2 state [18]. In the brain, neurotrophic factors (such as brain-derived neurotrophic factor [BDNF]) are up-regulated [19], adult hippocampal neurogenesis is stimulated [20], and synaptogenesis and neuroplasticity is encouraged [19].
These latter effects can be thought of as “helping the brain to repair itself”, and suggest that PBM can be useful for many traumatic brain disorders, such as stroke [21] and traumatic brain injury [22], as well as neurodegenerative brain disorders like Alzheimer’s disease [23] and PD [24]. One question that is often asked about PBM for the brain, is how important is it to apply the light to the head and for the photons to actually penetrate into the brain tissue, or else how important is it for the light to be absorbed by the circulating blood or bone marrow? The latter pathways might explain the systemic or abscopal effects of PBM, which have been reported by many authors [25]. The recent discovery of respiratory-competent cell free mitochondria that are circulating in the blood of normal individuals [26] might offer an explanation for how the beneficial effects of light that is incident on the body can be transmitted to distant organs including the brain. Calculation or measurement of the fraction of photons that are incident on the scalp and which penetrate the cortex, and especially into deeper brain structures (such as the SNc), is not particularly encouraging [27], suggesting that for PD, the abscopal effect, or the application of light to the abdomen, to affect the gut microbiome (“photobiomics” [28]), might be important.
The goal of the present paper was to undertake a systematic review of published studies, which have examined the use of PBM therapy to treat PD in animal models, to see if any conclusions about the parameters and methods can be drawn.
2. Materials and Methods
2.1. Search Strategy
The primary search was conducted from 1990 to November 2019. Bibliographic databases (i.e., MEDLINE through PubMed, SCOPUS, Web of Science, EMBASE and Cochrane Library) were searched electronically for studies on the neuroprotective effects of PBM on animal models of PD, through the keywords “photobiomodulation”, “low-level light therapy”, “low-level laser therapy”, “near-infrared light”, “red light”, “Parkinson’s disease”, and “Parkinsonism”. Two independent investigators screened the title, abstract and the full text of the articles and judged the searched materials against the inclusion and exclusion criteria. The search was limited to the original studies performed in animals and to publications written in English. Therefore, ex vivo, in vitro or clinical original articles, as well as review articles were not included.
2.2. Inclusion and Exclusion Criteria
We included all in vivo studies reporting the effects of PBM, as opposed to vehicles, on the behavioral and molecular outcomes in PD models. Studies conducting PBM via transcranial, intracranial, systemic irradiation (remotely or laser acupuncture irradiations) as well as whole-body irradiation approaches in PD models were included. Studies performed on ex vivo or in vitro (primary cultures or cell line), as well as clinical trials, were excluded. Additionally, studies conducted on intact (healthy) animals were excluded from our review. Moreover, non-English language publications and studies involving NIR spectroscopy and conference papers were excluded.
2.3. Data Extraction
The author, publication year, animals and species, number of animals in each experimental group, gender and age, type of PD model, light source/wavelength, output power, irradiance (power density), irradiation time, fluence (energy density) or energy (dose), total fluence or dose, irradiation approach/site, number of treatment sessions, and outcome(s) were extracted. However, the time of outcome evaluation was not extracted from the studies.
3. Results
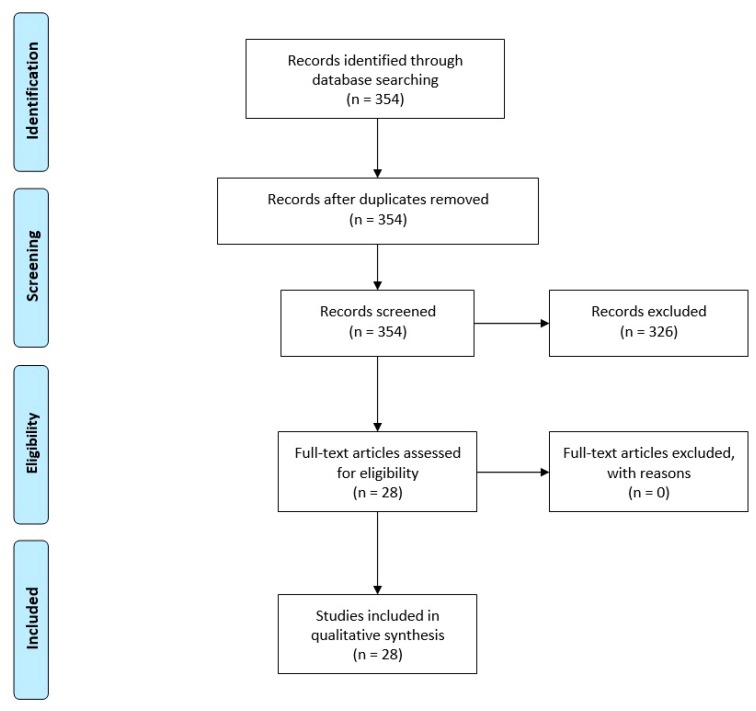
The initial systematic search of the mentioned databases identified 354 articles, of which 28 studies met the inclusion criteria (Figure 1). Twenty-two articles reported experiments in rodents, five articles reported studies in primates (macaque monkey, Macaca fascicularis), and one study was conducted in a Pink1 mutant PD model. Of the twenty-two studies on rodents, sixteen studies assessed the effects of PBM in mice, of which thirteen were on the albino BALB/c strain and three were on the C57BL/6 strain. Additionally, six rodent studies were performed on rats, of which five were on the Sprague–Dawley strain and one was on the albino Wistar strain. It should be noted that in one study, more than one experiment was conducted using three different animal species of BALB/c mice, Wistar rats, and macaque monkeys; and also in one study, two different types of irradiation methods, transcranial or remote-tissue were performed; in these cases, each experiment was regarded as a separate study and was included in the systematic review.
Figure 1.
Systematic review flow chart for the inclusion of eligible studies.
Animal models of PD were induced using injections of methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice or primates. Other models used 6-hydroxydopamine (6OHDA) in rats, and rotenone in Drosophila Pink1 mutants. In the context of molecular and biochemical assessments, the possible neuroprotective effects of PBM were evaluated in various brain regions, including the SNc, subthalamic nucleus (STN), striatum, zona incerta (ZI), zona incerta-hypothalamus (ZI-Hyp), caudate putamen (CPu) and periaqueductal grey matter (PaG).
In fifteen studies, laser or LED light was delivered to the head of the animal in a transcranial approach. On the other hand, nine studies used an intracranial irradiation approach via implantation of an optical fiber connected to a light source into the region of interest inside the brain. In addition, four studies performed systemic PBM using remote-tissue irradiation (abscopal effect) or laser acupuncture methods. Whole-body PBM was carried out in one study of Pink1 Drosophila mutant PD model. Eighteen studies applied LED-based devices, while eleven studies used lasers as light sources. Twenty six studies performed PBM with red/far-red wavelengths (627 nm [one study], 630 nm [one study], 670 nm [twenty one studies], and 675 nm [two studies]), whereas, four studies used NIR light (808 nm) and only in one study blue light (405 nm) was delivered via an acupuncture point. The operation mode of light sources in all studies was a continuous wave (CW). Other physical treatment parameters, such as output power, irradiance, irradiation time, fluence, total delivered dose, numbers and duration of treatment sessions are summarized in Table 1.
Table 1.
Summary of Studies on the Effects of Photobiomodulation Therapy in Animal Models of Parkinson’s Disease.
| Study/Year | Animal/Species (n) | Gender/Age | PD Model | Light Source | Output Power | Irradiance | Irradiation Time per Session | Fluence or Dose per Session | Total Fluence or Dose | Irradiation Approach/Sites | Number of Treatment Sessions | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shaw et al., (2010) [29] | Mouse Albino BALB/c (n Saline = 20) (n Saline + PBM = 20) (n MPTP = 20) (n MPTP + PBM = 20) |
Male 8 weeks old |
MPTP Mild: 50 mg/kg per mouse Strong: 100 mg/kg per mouse |
LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | 14.4 J/cm2 (at scalp) | Transcranial Holding probe at 1 cm from the head |
4 simultaneous irradiations over 30 h | Increased TH+ terminals in the caudate-putamen complex; no effect on the overall volume of the SNc and ZI-Hyp; increased TH+ cells in the SNc and ZI-Hyp regions; no effect on the morphology of TH+ cells in both the SNc and ZI-Hyp; increased number of TH+ cells in the SNc (in both 50 and 100 mg/kg MPTP doses); no effect on the number of TH+ cells in the ZI-Hyp (in 50 and 100 mg/kg MPTP doses) |
| Peoples et al., (2012) [30] | Mouse Albino BALB/c (n Saline = 20) (n Saline + PBM = 20) (n MPTP = 20) (n MPTP+PBM = 20) |
Male 8 weeks old |
MPTP: 200 mg/kg per mouse | LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Simultaneous group: 36 J/cm2 (at scalp) Post-treatment group: 36 J/cm2 (at scalp) | TranscranialHolding probe at 1–2 cm from the head | Simultaneous group: 10 irradiations over 5 weeks Post-treatment group: 10 irradiations over 3 weeks |
For both simultaneous and post-treatment series: increased TH+ cell number in the SNc, but not in the PaG and ZI-Hyp regions |
| Shaw et al., (2012) [31] | Mouse Albino BALB/c (n Saline = 24) (n Saline + PBM = 24) (n MPTP = 24) (n MPTP + PBM = 24) |
Male 8 weeks old |
MPTP Acute: 100 mg/kg per mouse Chronic: 200 mg/kg per mouse |
LED, 670 nm |
NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Acute regimen: 14.4 J/cm2 (at scalp) Chronic regimen: 36 J/cm2 (at scalp) |
TranscranialHolding probe at 1–2 cm from the head | Acute regimen: 4 simultaneous irradiations over 30 h Chronic regimen: 10 simultaneous irradiations over 5 weeks |
For acute regimen: decreased Fos+ cell number in the STN and ZI regions in group with six-day survival period For chronic regimen: decreased Fos+ cell number in the STN and ZI regions |
| Peoples et al., (2012) [32] | Mouse Albino BALB/c (n Saline = 21) (n Saline + PBM = 19) (n MPTP = 22) (n MPTP + PBM = 18) |
Male 8 weeks old |
MPTP Acute: 100 mg/kg per mouse Chronic: 200 mg/kg per mouse |
LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Simultaneous acute group: 14.4 J/cm2 (at scalp) Simultaneous chronic group: 36 J/cm2 (at scalp) Post-treatment acute group: 14.4 J/cm2 (at scalp) Post-treatment chronicgroup: 36 J/cm2 (at scalp) |
TranscranialJust above the mouse head and in full view of the eyes | Simultaneous group: 4 irradiations over 30 h (acute regimen) or 10 irradiations over 5 weeks (chronic regimen) Post-treatment group: 4 irradiations over 2 days (acute regimen) or 10 irradiations over 3 weeks (chronic regimen) | For all group and regimens: no effect on the retinal areas For all groups except simultaneous group with acute regimen: increased TH+ cell number in the retina |
| Moro et al., (2013) [33] | Mouse Albino BALB/c: (n Saline = 10) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP + PBM = 10) Black C57BL/6: (n Saline = 10) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP + PBM = 10) |
Male 8–10 weeks old |
MPTP: 50 mg/kg per mouse | LED, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | 14.4 J/cm2 (at scalp) | TranscranialHolding probe at 1–2 cm from the head | 4 simultaneous irradiations over 30 h | For Albino BALB/c mice: increased TH+ cell number in the SNc; improved locomotor activities via increase of velocity and high mobility, and decrease of immobility For C57BL/6 mice: no effect on the TH+ cell number in the SNc; no effect on the locomotor activities |
| Purushothuman et al., (2013) [34] | Mouse K3 transgenic model (n WT = 5) (n K3 = 5) (n K3 + PBM = 5) |
NR 5 months old |
K369I tau transgenic model | LED 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 4 J/cm2 (at scalp) | 80 J/cm2 (at scalp) | TranscranialHolding probe at 1–2 cm from the head | 20 irradiations over 4 weeks | Decreased markers of oxidative stress, over expression of hyperphosphorylated tau, and increased TH+ cell number in the SNc |
| Vos et al., (2013) [35] | Drosophila Pink1 null mutants | NA | Rotenone (250 μM) | Laser, 808 nm | NR | 25 mW/cm2 | 100 s | 2.5 J/cm2 | 2.5 J/cm2 | Whole-body | One session (single dose) | Improved CCO-dependent oxygen consumption and ATP production; rescued major systemic and mitochondrial defects |
| Wattanathorn and Sutalangka, (2014) [36] | Rat Albino Wistar (n Control = 12) (n 6OHDA = 12) (n 6OHDA + Sham PBM = 12) (n 6OHDA + Sham PBM = 12) |
Male 8 weeks old |
6OHDA (6 μg per rat) | Laser, 405 nm | 100 mW | NR | 10 min | NR | NR | Laser acupuncture at HT7 acupoint |
Once daily for 14 days | Improved spatial memory in Morris water maze test; attenuated the decreased neuron density in CA3 and dentate gyrus, but not CA1 and CA2 regions; decreased activity of monoamine oxidase-B and acetylcholinesterase in the hippocampus; mitigated the decreased GSH-Px activity and the elevation of MDA level |
| Johnstone et al., (2014) [25] | Mouse Albino BALB/c: 50 mg/kg MPTP: (n MPTP = 36) (n MPTP + Transcranial PBM = 12) (n MPTP + Remote PBM = 11) 75 mg/kg MPTP: (n MPTP = 8) (n MPTP + Transcranial PBM = 8) (n MPTP + Remote PBM = 8) 100 mg/kg MPTP: (n MPTP = 9) (n MPTP + Transcranial PBM = 19) (n MPTP + Remote PBM = 9) |
Male 8 weeks old |
MPTP: 50 mg/kg per mouse 75 mg/kg per mouse 100 mg/kg per mouse |
LED, 670 nm | NR | 50 mW/cm2 (at scalp) | 90 s | 4 J/cm2 (at scalp) | 50 mg/kg MPTP: 8 J/cm2 (at scalp) 75 mg/kg MPTP: 12 J/cm2 (at scalp) 100 mg/kg MPTP: 16 J/cm2 (at scalp) |
Transcranial irradiation to the head Remote irradiation to the dorsum |
50 mg/kg MPTP: 2 irradiations over 2 days 75 mg/kg MPTP: 3 irradiations over 3 days 100 mg/kg MPTP: 4 irradiations over 4 days |
In 50 but not 75 or 100 mg/kg MPTP doses: increased TH+ cell number in the SNc with both transcranial and remote irradiations |
| Moro et al., (2014) [25] | Mouse Albino BALB/c (n Saline = 5) (n Saline + Pulse PBM = 5) (n Saline + Continuous PBM = 5) (n MPTP = 5) (n MPTP + Pulse PBM = 5) (n MPTP + Continuous PBM = 5) |
Male NR |
MPTP: 50 mg/kg per mouse | LEDs, 670 nm | 0.16 mW | Pulse irradiation 1.5 mW/cm2 Continuous irradiation 14.5 mW/cm2 |
Pulse irradiation: 90 s Continuous irradiation: 6 days continuously | Pulse irradiation: 0.13 J/cm2 Continuous irradiation: 7516.8 J/cm2 |
Pulse irradiation: 0.54 J/cm2 Continuous irradiation: 7516.8 J/cm2 |
Intracranial, implanted in the lateral ventricles |
Pulse irradiation: 4 simultaneous irradiations over 30 h Continuous irradiation: 6 days continuously |
For pulse irradiation group: significantly increased TH+ cell number in the SNc For continuous irradiation group: Non-significantly increased TH+ cell number in the SNc |
| Reinhart et al., (2015) [37] | Mouse Albino BALB/c (n Saline = 11) (n Saline + PBM = 11) (n MPTP = 11) (n MPTP + PBM = 11) |
Male 8–10 weeks old |
MPTP: 50 mg/kg per mouse | LEDs, 810 nm | 0.16 mW | NR | 90 s | 14.4 mJ (at scalp) | 57.6 mJ (at scalp) | Transcranial | 4 simultaneous irradiations over 30 h | Improved locomotor activity at different time points including at immediately after first MPTP injection, at after sond PBM, at after fourth PBM, and 6 days after the last MPTP injection; increased TH+ cell number in the SNc |
| Darlot et al., (2015) [38] | Macaque monkey Macaca fascicularis (n Control = 5) (n MPTP (1.5 mg/kg) = 6) (n MPTP (2.1 mg/kg) = 5) (n MPTP (1.5 mg/kg) + PBM = 5) (n MPTP (2.1 mg/kg) + PBM = 4) |
Male 4–5 years old |
MPTP: 1.5 mg/kg per monkey 2.1 mg/kg per monkey |
Laser, 670 nm | 10 mW | NR | MPTP (1.5 mg/kg) continuous irradiation (5 s ON/60 s OFF) for 5 days MPTP (2.1 mg/kg) continuous irradiation (5 s ON/60 s OFF) for 7 days |
NA | MPTP (1.5 mg/kg): 25 J MPTP (2.1 mg/kg): 35 J |
Intracranial, Implanted 1 to 2 mm to the left side of the midline in the midbrain |
MPTP (1.5 mg/kg): continuous irradiation for 5 days MPTP (2.1 mg/kg): continuous irradiation for 7 days |
For both irradiation groups: Improved clinical scores and behavioral activities as indicated by locomotive traces and distance moved and velocity as well as increased nigral dopaminergic cells For PBM (25 J) group: increased striatal TH+ terminals |
| Oueslati et al., (2015) [39] | AAV-Based Rat Genetic Model Sprague-Dawley (n α-syn = 9) (n α-syn + PBM (2.5 mW/cm2) = 7) (n α-syn + PBM (5 mW/cm2) = 7) |
Female NR |
α-syn-induced toxicity: 2 μL of viral suspension per rat | Laser, 808 nm | NR | PBM (2.5 mW/cm2): 20.4 mW/cm2 (at scalp) or 2.5 mW/cm2 (at midbrain) PBM (5 mW/cm2): 40.8 mW/cm2 (at scalp) or 5 mW/cm2 (at midbrain) |
100 s | PBM (2.5 mW/cm2): 4.08 J/cm2 (at scalp) or 0.50 J/cm2 (at midbrain) PBM (5 mW/cm2): 8.16 J/cm2 (at scalp) or 1 J/cm2 (at midbrain) |
PBM (2.5 mW/cm2): 114.24 J/cm2 (at scalp) or 14 J/cm2 (at midbrain) PBM (5 mW/cm2): 228.48 J/cm2 (at scalp) or 28 J/cm2 (at midbrain) |
Transcranial 2 irradiation spots of about 1 cm2 bilaterally on the head |
All groups: once a day for 4 weeks | For both irradiation groups: decreased motor deficits (akinesia) as indicated by improvement of the use of the contralateral forepaw For PBM (5 mW/cm2) group: decreased nigral and striatal dopaminergic fiber loss |
| Moro et al., (2016) [40] | Macaque monkey Macaca fascicularis (n Control = 3) (n MPTP) = 5) (n MPTP + PBM = 7) |
Male 4–5 years old |
MPTP: 1.8–2.1 mg/kg per monkey | Laser, 670 nm | 10 mW | NR | Continuous irradiation (5 s ON/60 s OFF) for 25 days | NA | 125 J | Intracranial, implanted in region 1–2 mm to the left hand side of the midline in the mid-brain |
Continuous irradiation for 25 days | Improved clinical scores as indicated by locomotive traces; increased TH+ cell number in the SNc; no effect on the striatal TH+ terminal density |
| Salgado et al., (2016) [41] | Rat Albino Wistar (n 6OHDA = 20) (n 6OHDA + LED PBM=20) (n 6OHDA + Laser PBM = 20) |
NR NR |
6OHDA bilateral microinjections of 15 μg per rat | LEDs, 627 nm Laser, 630 nm |
LEDs: 70 mW Laser: 45 mW |
LEDs: 70 mW/cm2 (at scalp) Laser: 45 mW/cm2 (at scalp) |
LEDs: 57 s Laser: 88 s |
LEDs: 4 J/cm2 (at scalp) Laser: 4 J/cm2 (at scalp) |
LEDs: 28 J/cm2 (at scalp) Laser: 28 J/cm2 (at scalp) |
Transcranial | All groups: once a day for 7 days | For laser and LEDs sources: increased locomotive traces in open field test; decreased TNF-α levels For LEDs source: increased IFN-γ levels For laser source: increased IL-2 levels no effect on the IL-4, IL-6 and IL-10 levels |
| Reinhart et al., (2016) [42] | Rat Wistar (n Saline = 8) (n 6OHDA = 15) (n 6OHDA + Pulse PBM = 16) (n 6OHDA + Continuous PBM (0.16 mW) = 13) (n 6OHDA + Continuous PBM (333 nW) = 9) |
Male 8 weeks old |
6OHDA 7.5 μg/μL per rat |
LEDs, 670 nm | Pulse irradiation: 0.16 mW Continuous irradiation (0.16 mW): 0.16 mW Continuous irradiation (333 nW): 333 nW |
NR | Pulse irradiation: 90 s Continuous irradiation (0.16 mW): continuous irradiation for 23 days Continuous irradiation (333 nW): continuous irradiation for 23 days |
NA | Pulse irradiation: 634 mJ Continuous irradiation (0.16 mW): 304 J Continuous irradiation (333 nW): 634 mJ |
Intracranial, implanted in region near the SNc, incorporating the red nucleus and ventral tegmental area, toward the midline |
Pulse irradiation: twice a day for 23 days Continuous irradiation (0.16 mW): continuous irradiation for 23 days Continuous irradiation (333 nW): continuous irradiation for 23 days |
For pulse irradiation group: decreased rotational behavior at 21 days post-surgery; increased TH+ cell number in the SNc For continuous irradiation (0.16 mW) group: decreased rotational behavior at 14 and 21 days post-surgery; no effect on the TH+ cell number in the SNc For continuous irradiation (333 nW) group: no effect on the rotational behavior; no effect on the TH+ cell number in the SNc |
| Reinhart et al., (2016) [42] | Mouse Albino BALB/c (n Saline = 9) (n MPTP = 9) (n MPTP + Pre-PBM = 9) (n MPTP + Simultaneous PBM = 9) (n MPTP + Post-PBM = 9) (n MPTP + Pre- & Simultaneous PBM = 9) (n MPTP + Post- & Simultaneous PBM = 9) (n MPTP + Pre- & Post- & Simultaneous PBM = 9) |
Male 8–10 weeks old |
MPTP: 50 mg/kg per mouse | LEDs, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 3.6 J/cm2 (at scalp) | Pre-PBM: 14.4 J/cm2 Simultaneous-PBM: 14.4 J/cm2 Post-PBM: 14.4 J/cm2 Pre- & Simultaneous PBM: 28.4 J/cm2 Post- & Simultaneous PBM: 28.4 J/cm2 Pre- & Post- & Simultaneous PBM: 43.2 J/cm2 |
Transcranial | Pre-PBM: twice a day for 2 days Simultaneous-PBM: twice a day for 2 days Post-PBM: twice a day for 2 days Pre- & Simultaneous PBM: twice a day for 4 days Post- & Simultaneous PBM: twice a day for 4 days Pre- & Post- & Simultaneous PBM: twice a day for 6 days |
In all irradiation groups: increased locomotor activity in open field test by a similar magnitude and increased TH+ cell number in the SNc |
| El Massri et al., (2016) [43] | Macaque monkey Macaca fascicularis (n Control = 5) (n MPTP) = 11) (n MPTP + PBM = 6) |
Male 4–5 years old |
MPTP: 1.5–2.1 mg/k per monkey | Laser, 670 nm | 10 mW | NR | Continuous irradiation (5 s ON/60 s OFF) for 5 or 7 days | NA | 25 or 35 J | Intracranial, Implanted in 1 to 2 mm to the left side of the midline in the midbrain |
Continuous irradiation for 5 or 7 days | Decreased number of GFAP+ astrocytes and astrocyte cell body size in the SNc and striatum; decreased microglia cell body size in the SNc and striatum |
| El Massri et al., (2016) [44] | Mouse Albino BALB/c: 2 days group (n Saline = 7) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP+PBM = 10) 7 days group: (n Saline = 7) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP+PBM=10) 14 days group: (n Saline = 7) (n Saline + PBM = 10) (n MPTP = 10) (n MPTP + PBM (2 J/cm2) = 10) (n MPTP + PBM (4 J/cm2) = 10) |
Male 8–10 weeks old |
MPTP: 50 or 100 mg/kg per mouse | LEDs, 670 nm | NR | 40 mW/cm2 (at scalp) | 90 s | 4 J/cm2 (at scalp) or 0.5 J/cm2 (at brain) | 2 days group: 8 J/cm2 (at scalp) or 1 J/cm2 (at brain) 7 days group: 8 J/cm2 (at scalp) or 1 J/cm2 (at brain) 14 days group (2 J/cm2): 16 J/cm2 (at scalp) or 2 J/cm2 (at brain) 14 days group (4 J/cm2): 32 J/cm2 (at scalp) or 4 J/cm2 (at brain) |
Transcranial Holding probe at 1 cm from the head |
2 days group: once a day for 2 days 7 days group: once a day for 2 days 14 days group (2 J/cm2): once a day for 4 days 14 days group (4 J/cm2): once a day for 8 days |
In 7 days irradiation group: increased TH+ cell number in the SNc In 14 days (4 J/cm2 ) irradiation group: increased TH+ cell number in the SNc; decreased number of GFAP+ cells in the CPu |
| El Massri et al., (2017) [45] | Mouse Albino BALB/c (n Saline = 5) (n Saline + PBM = 3) (n MPTP = 5) (n MPTP + PBM = 4) Rat Wistar (n Saline = 5) (n 6OHDA = 5) (n 6OHDA + PBM = 4) Macaque monkey Macaca fascicularis (n Saline = 3) (n Saline + PBM = 5) (n MPTP = 5) (n MPTP + PBM = 3) |
Mouse: ~8 weeks old Rat: ~8 weeks old Monkey: 4–5 years old |
Mouse: MPTP (50 mg/kg per mouse) Rat: 6OHDA (7.5 μg/μL) Monkey: MPTP (1.5 mg/kg per monkey) |
Laser, 670 nm | Mouse: 0.16 mW Rat: 0.16 mW Monkey: 10 mW |
NR | NR | NR | NR | Intracranial, Mouse: implanted in lateral ventricle Rat and Monkey: implanted in midline region of the midbrain |
Mouse: Continuous irradiation for 30 h Rat: Continuous irradiation for 23 days Monkey: Continuous irradiation for 6 days |
Mouse: no effect Rat: no effect Monkey: increased TH+ cell number and terminal density in the striatum; increased GDNF expression in the striatum |
| Reinhart et al., (2017) [46] | Mouse Albino BALB/c (n Saline = 8) (n MPTP = 8) (n MPTP + 670 nm PBM = 8) (n MPTP + 810 nm PBM = 8) (n MPTP + Sequentially 670 & 810 nm PBM (15 mW) = 8) (n MPTP + Sequentially 670 & 810 nm PBM (30 mW) = 8) (n MPTP + Concurrently 670 & 810 nm PBM (15 mW) = 8) (n MPTP + Concurrently 670 & 810 nm PBM (30 mW) = 8) |
Male 8–10 weeks old |
MPTP: 50 mg/kg per mouse | LED, 670 or 810 nm | 15 or 30 mW | NR | 45 or 90 s | 2.7 J (at scalp) | 670 nm PBM: 11 J 810 nm PBM: 11 J Sequentially 670 & 810 nm PBM (15 mW): 11 J Sequentially 670 & 810 nm PBM (30 mW): 22 J Concurrently 670 & 810 nm PBM (15 mW): 11 J Concurrently 670 & 810 nm PBM (30 mW): 22 J |
Transcranial | All groups: twice a day for 2 days | In all irradiation groups: increased locomotor activity in open field test and increased TH+ cell number in the SNc Note: combination treatment groups exhibited a greater overall beneficial outcome |
| El Massri et al., (2018) [47] | Macaque monkey Macaca fascicularis (n Control = 3) (n Control + PBM = 3) (n MPTP = 3) (n MPTP + PBM = 3) |
Male 4–5 years old |
MPTP: 1.5 mg/kg per monkey | Laser, 670 nm | 10 mW | NR | Continuous irradiation (5 s ON/60 s OFF) for 5 days | NA | 25 J | Intracranial, Implanted in 1 to 2 mm to the left side of the midline in the midbrain |
Continuous simultaneous irradiation for 5 days | No effect on the number and somal sizes of encephalopsin +cells in the striatum |
| Kim et al., (2018) [48] | Mouse C57BL/6: (n Saline = 10) (n MPTP = 10) (n MPTP + PBM = 10) |
Male 10 weeks old |
MPTP: 50 mg/kg per mouse | LED, 670 nm | NR | 50 mW/cm2 (at skin) | 180 s | 9 J/cm2 (at skin) | 18 J/cm2 (at skin) | Remotely; irradiation to the dorsum | Twice (24 h apart) | Increased TH+ cell number in the SNc; no effect on the density of TH+ terminations in the dorsal CPu |
| O’Brien and Austin (2019) [49] | Rat Sprague–Dawley (n Vehicle = various) (n Lipopolysaccharide = various) (n Lipopolysaccharide + PBM = various) |
Male NR |
Lipopolysaccharide 10 μg per rat 20 μg per rat |
LED, 675 nm | 500 mW | 40 mW/cm2 (at scalp) | 88 s | 3.6 J/cm2 (at scalp) | 46.8 J/cm2 (at scalp) | Transcranial Holding probe at 1 cm from the head |
Thirteen (once 2 h following the completion of the lipopolysaccharide injection + twice daily for 6 days) | With 10 µg lipopolysaccharide: increased TH+ cell number in the SNc; no effect on the IBA1+ cell densities in the SNc With 20 µg lipopolysaccharide: no significant effect on the motor behavior in the cylinder, rotarod and adjusted stepping tests |
| Miguel et al., (2019) [50] | Mouse C57BL/6: (n Saline = 8) (n MPTP = 6) (n MPTP + PBM = 6) |
Male 12 weeks old |
MPTP: 80 mg/kg per mouse | LED, 675 nm |
NR | 50 mW/cm2 (at scalp) | 180 s | 9 J/cm2 (at scalp) | 63 J/cm2 (at scalp) | Transcranial | Once a day for 7 days | Decreased vascular leakage in the SNc and CPu |
| Ganeshan et al., (2019) [51] | Mouse Albino BALB/c (n Saline = 10) (n MPTP = 10) (n MPTP + PBM (2 days) = 10) (n MPTP + PBM (5 days) = 10) (n MPTP + PBM (10 days) = 10) |
Male 10 weeks old |
MPTP: 50 mg/kg per mouse | LED, 670 nm | NR | 50 mW/cm2 (at skin) | 90 s | 4.5 J/cm2 (at skin) | PBM (2 days): 9 J/cm2 (at skin) PBM (5 days): 22.5 J/cm2 (at skin) PBM (10 days): 45 J/cm2 (at skin) |
Remotely; irradiation to the dorsum and hind limbs | Once a day for 2, 5 or 10 days | In PBM (2 days) group: decreased Fos+ cell number in the CPu In PBM (5 days) group: decreased Fos+ cell number in the CPu In PBM (10 days) group: increased TH+ cell number in the SNc; decreased Fos+ cell number in the CPu; upregulated cell signaling and migration (including CXCR4+ stem cell and adipocytokine signaling), oxidative stress response pathways and modulated blood-brain barrier |
4. Discussion
The evidence that has been presented in this systematic review does suggest that PBM (and in particular transcranial PBM) is an effective method to treat animal models of PD. The discovery of the toxic effects of MPTP, which is an impurity found in recreational drugs consumed by individuals in San Francisco in 1982, for the first time allowed the creation of laboratory animal models of PD [52]. Besides MPTP, other compounds have been used to produce PD-like models [53], including 6-hydroxydopamine (6-OHDA) paraquat, rotenone, and Maneb (a polymeric Mn complex of ethylene bis (dithiocarbamate). The mechanism of action of these compounds usually involves metabolism into intermediates that can undergo redox cycling and thereby damage the mitochondria, and in particular Complex 1. There have also been genetic models of PD involving mutations to genes such as α-synuclein, Parkin (an ubiquitin E3 ligase), PINK1 (PTEN-induced putative kinase 1), and LRRK2 (leucine-rich repeat kinase 2). Although the animal models of PD do not completely mimic the human disease, they have been useful for studying the pathophysiology of PD, and for testing the effectiveness of novel treatments, including DBS and PBM. It is expected that further animal studies will use PBM in genetically engineered models of PD rather than toxin-induced models, because these are now considered to be more representative of the human disease.
Although most of animal studies have used red light (670 nm, 675 nm or 630 nm), this does not necessarily mean that red wavelengths are better than NIR wavelengths (810 nm). This preponderance might simply reflect the wider use of red LEDs in ophthalmology and wound healing. The power density levels employed were generally between 20–50 mW/cm2, but occasionally lower or higher values were employed. Moderate illumination times (minutes) generally provided fluences in the range of 10–60 J/cm2 on the scalp. The intracranial fibers that were implanted into the brain delivered fairly low powers (up to 14 mW), but when the illumination was continued for several days, the total energy density delivered could be quite large. It should be noted that the regions of the brain where optical fibers are implanted are different from the regions where electrodes are implanted in the DBS procedure. In DBS, electrodes are usually implanted into the globus pallidus internus to improve the motor function [54] or into the subthalamic nucleus [55] or the caudal zona incerta to improve tremor [56]. The optical fibers in PD animal models have been implanted into the mid-brain, with the goal of delivering the light as close as possible to the SNc, to preserve the dopamine producing neurons.
Pulsing is an interesting parameter for brain PBM therapy, as it has been found that pulsing the light at certain frequencies is more effective than CW light [57]. The two most popular frequencies are 10 Hz (the so-called alpha rhythm) and 40 Hz (the so-called gamma rhythm). The idea is that these frequencies can resonate with intrinsic brain rhythms, and therefore, can improve brain function to a greater extent than CW light [57]. The repetition regimens that have been used for treating the animal models of PD range from a few times per day to every few days, for periods that could be as long as 4 weeks. As PD in humans is a chronic degenerative disease, it is expected that PBM therapy would need to be continued for the foreseeable future.
The encouraging results that have been obtained in the animal studies reviewed above have led to the initiation of clinical studies of PBM therapy for PD patients. Hamilton and colleagues described the construction of “light buckets” lined with LEDs (670, 810 and 850 nm) to treat patients with PD [58] (Figure 2). These devices delivered a power density of 10 mW/cm2 to the entire head, and in addition an intranasal device with a power of 4 mW/cm2 was employed. Patients were treated twice a day (1800 J per session) for 30 days. The initial symptoms of tremor, akinesia, gait, difficulty in swallowing and speech, poor facial animation, and reduced fine motor skills, loss of the sense of smell, and impaired social confidence were all improved in ~75% of the subjects, while ~25% remained the same and none got worse. The improvements were still maintained over an extended period (up to 24 months). Santos et al. conducted a randomized controlled trial in Parkinson’s patients using a CW 670 nm LED array (WARP 10) over 10 cm2, on 6 sites on both temples at 60 mW/cm2, delivering 6 J/cm2 and a total energy of 2160 J [59]. A total of 18 sessions were given over 9 weeks leading to clinical improvements.
Figure 2.
Photograph of the “light bucket” described by Hamilton et al. [24].
Additional clinical trials are in progress that, in addition to applying light to the head also apply light to the abdomen, with the goal of improving the gut microbiome. The results are eagerly awaited.
Acknowledgments
The authors express their sincere gratitude to Fariba Pashazadeh M.Sc., for assisting with the literature research.
Abbreviations
| 6OHDA | 6-hydroxydopamine |
| α-syn | alpha synuclein |
| CPu | caudate putamen |
| CXCR4 | chemokine receptor 4 |
| CW | continuous wave |
| DMS | delayed match-to-sample |
| GDNF | glial cell line-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| GFAP+ | glial fibrillary acidic protein positive |
| GSH-Px | glutathione peroxidase |
| IBA1 | ionized calcium-binding adapter molecule 1 |
| IFN-γ | interferon-gamma |
| IL2 | Interleukin 2 |
| LED | light-emitting diode |
| MDA | malondialdehyde |
| MPTP | 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine |
| NA | not available |
| NR | not reported |
| PaG | periaqueductal grey matter |
| PBM | photobiomodulation |
| PD | Parkinson’s disease |
| PVT | psychomotor vigilance task |
| SNc | substantia nigra pars compacta |
| STN | subthalamic nucleus |
| TH | tyrosine hydroxylase |
| TNF-α | tumor necrosis factor-alpha |
| WT | wild type |
| ZI | zona incerta |
| ZI-Hyp | zona incerta-hypothalamus |
Author Contributions
F.S. Writing—Original Draft, Data Curation, Formal analysis; M.R.H.: Conceptualization, Supervision, Writing—Original Draft, Writing—Review & Editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
MRH was funded by US NIH Grants R01AI050875 and R21AI121700.
Conflicts of Interest
F.S. is on the Scientific Advisory Board and is a consultant of Niraxx Light Therapeutics, Inc., Irvine, CA and a consultant of ProNeuroLIGHT LLC. Phoenix, AZ. M.R.H. is on the following Scientific Advisory Boards: Transdermal Cap, Inc.; Cleveland, OH; BeWell Global, Inc.; Wan Chai, Hong Kong; Hologenix, Inc. Santa Monica, CA; LumiThera, Inc., Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon, Inc., Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV, Inc., Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL;UltraluxUV, Inc., Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc., Boston, MA; M.R.H. has been a consultant for Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V.; Johnson & Johnson, Inc., Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; M.R.H. is a stockholder in Global Photon, Inc., Bee Cave, TX; Mitonix, Newark, DE.
References
- 1.Elsworth J.D. Parkinson’s Disease Treatment: Past, Present, and Future. J. Neural Transm. 2020 doi: 10.1007/s00702-020-02167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 3.Toffoli M., Vieira S.R.L., Schapira A.H.V. Genetic causes of PD: A pathway to disease modification. Neuropharmacology. 2020;170:108022. doi: 10.1016/j.neuropharm.2020.108022. [DOI] [PubMed] [Google Scholar]
- 4.Zaia A., Maponi P., Zannotti M., Casoli T. Biocomplexity and Fractality in the Search of Biomarkers of Aging and Pathology: Mitochondrial DNA Profiling of Parkinson’s Disease. Int. J. Mol. Sci. 2020;21:1758. doi: 10.3390/ijms21051758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullich C., Keshavarzian A., Garssen J., Kraneveld A., Perez-Pardo P. Gut Vibes in Parkinson’s Disease: The Microbiota-Gut-Brain Axis. Mov. Disord. Clin. Pr. 2019;6:639–651. doi: 10.1002/mdc3.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkinson’s Disease Economic Burden On Patients, Families And The Federal Government Is $52 Billion, Doubling Previous Estimates. [(accessed on 16 March 2020)]; Available online: https://www.prnewswire.com/news-releases/parkinsons-disease-economic-burden-on-patients-families-and-the-federal-government-is-52-billion-doubling-previous-estimates-300867192.html.
- 7.De Bie R.M.A., Clarke C.E., Espay A.J., Fox S.H., Lang A.E. Initiation of pharmacological therapy in Parkinson’s disease: When, why, and how. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30036-3. [DOI] [PubMed] [Google Scholar]
- 8.Merola A., Romagnolo A., Krishna V., Pallavaram S., Carcieri S., Goetz S., Mandybur G., Duker A.P., Dalm B., Rolston J.D., et al. Current Directions in Deep Brain Stimulation for Parkinson’s Disease-Directing Current to Maximize Clinical Benefit. Neurol. Ther. 2020 doi: 10.1007/s40120-020-00181-9. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung H., Dai T., Sharma S.K., Huang Y.Y., Carroll J.D., Hamblin M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2011;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anders J.J., Lanzafame R.J., Arany P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015;33:183–184. doi: 10.1089/pho.2015.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mester A., Mester A. The History of Photobiomodulation: Endre Mester (1903–1984) Photomed. Laser Surg. 2017;35:393–394. doi: 10.1089/pho.2017.4332. [DOI] [PubMed] [Google Scholar]
- 12.Moskvin S.V. Only lasers can be used for low level laser therapy. Biomedicine. 2017;7:22. doi: 10.1051/bmdcn/2017070422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiskanen V., Hamblin M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018;17:1003–1017. doi: 10.1039/C8PP00176F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamblin M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Freitas L.F., Hamblin M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016;22:7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang H.L., Whelan H.T., Eells J.T., Meng H., Buchmann E., Lerch-Gaggl A., Wong-Riley M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamse H., Hamblin M.R. Photomedicine and Stem Cells. Morgan & Claypool Publishers; San Rafael, CA, USA: 2017. [Google Scholar]
- 18.De Sousa K., Rodrigues M., de Santos D., Mesquita-Ferrari R.A., Nunes F.D., da Silva D.d.T., Bussadori S.K., Fernandes K.P.S. Differential expression of inflammatory and anti-inflammatory mediators by M1 and M2 macrophages after photobiomodulation with red or infrared lasers. Lasers Med Sci. 2019;35:337–343. doi: 10.1007/s10103-019-02817-1. [DOI] [PubMed] [Google Scholar]
- 19.Xuan W., Agrawal T., Huang L., Gupta G.K., Hamblin M.R. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics. 2015;8:502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan W., Vatansever F., Huang L., Hamblin M.R. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt. 2014;19:108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamblin M.R. Photobiomodulation for traumatic brain injury and stroke. J. Neurosci. Res. 2017;96:731–743. doi: 10.1002/jnr.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thunshelle C., Hamblin M.R. Transcranial Low-Level Laser (Light) Therapy for Brain Injury. Photomed. Laser Surg. 2016;34:587–598. doi: 10.1089/pho.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamblin M.R. Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned? Photonics. 2019;6:77. doi: 10.3390/photonics6030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton C.L., el Khoury H., Hamilton D., Nicklason F., Mitrofanis J. “Buckets”: Early Observations on the Use of Red and Infrared Light Helmets in Parkinson’s Disease Patients. Photobiomodul. Photomed. Laser Surg. 2019;37:615–622. doi: 10.1089/photob.2019.4663. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone D., el Massri N., Moro C., Spana S., Wang X., Torres N., Chabrol C., de Jaeger X., Reinhart F., Purushothuman S. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism–an abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Dache Z.A.A., Otandault A., Tanos R., Pastor B., Meddeb R., Sanchez C., Arena G., Lasorsa L., Bennett A., Grange T., et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020;34:3616–3630. doi: 10.1096/fj.201901917RR. [DOI] [PubMed] [Google Scholar]
- 27.Salehpour F., Cassano P., Rouhi N., Hamblin M.R., de Taboada L., Farajdokht F., Mahmoudi J. Penetration Profiles of Visible and Near-Infrared Lasers and Light-Emitting Diode Light Through the Head Tissues in Animal and Human Species: A Review of Literature. Photobiomodul. Photomed. Laser Surg. 2019;37:581–595. doi: 10.1089/photob.2019.4676. [DOI] [PubMed] [Google Scholar]
- 28.Liebert A., Bicknell B., Johnstone D.M., Gordon L.C., Kiat H., Hamblin M.R. “Photobiomics”: Can Light, Including Photobiomodulation, Alter the Microbiome? Photobiomodul. Photomed. Laser Surg. 2019;37:681–693. doi: 10.1089/photob.2019.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw V.E., Spana S., Ashkan K., Benabid A.L., Stone J., Baker G.E., Mitrofanis J. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 2010;518:25–40. doi: 10.1002/cne.22207. [DOI] [PubMed] [Google Scholar]
- 30.Peoples C., Shaw V.E., Stone J., Jeffery G., Baker G.E., Mitrofanis J. Survival of dopaminergic amacrine cells after near-infrared light treatment in MPTP-treated mice. ISRN Neurol. 2012;2012:850150. doi: 10.5402/2012/850150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw V.E., Peoples C., Spana S., Ashkan K., Benabid A.-L., Stone J., Baker G.E., Mitrofanis J. Patterns of cell activity in the subthalamic region associated with the neuroprotective action of near-infrared light treatment in MPTP-treated mice. Park. Dis. 2012;2012:296875. doi: 10.1155/2012/296875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peoples C., Spana S., Ashkan K., Benabid A.-L., Stone J., Baker G.E., Mitrofanis J. Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson’s disease. Park. Relat. Disord. 2012;18:469–476. doi: 10.1016/j.parkreldis.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Moro C., Torres N., el Massri N., Ratel D., Johnstone D.M., Stone J., Mitrofanis J., Benabid A.-L. Photobiomodulation preserves behaviour and midbrain dopaminergic cells from MPTP toxicity: Evidence from two mouse strains. BMC Neurosci. 2013;14:40. doi: 10.1186/1471-2202-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purushothuman S., Nandasena C., Johnstone D.M., Stone J., Mitrofanis J. The impact of near-infrared light on dopaminergic cell survival in a transgenic mouse model of parkinsonism. Brain Res. 2013;1535:61–70. doi: 10.1016/j.brainres.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 35.Vos M., Lovisa B., Geens A., Morais V.A., Wagnières G., van den Bergh H., Ginggen A., de Strooper B., Tardy Y., Verstreken P. Near-infrared 808 nm light boosts complex IV-dependent respiration and rescues a Parkinson-related pink1 model. PLoS ONE. 2013;8:e78562. doi: 10.1371/journal.pone.0078562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wattanathorn J., Sutalangka C. Laser acupuncture at HT7 acupoint improves cognitive deficit, neuronal loss, oxidative stress, and functions of cholinergic and dopaminergic systems in animal model of parkinson’s disease. Evidence-Based Complement. Altern. Med. 2014;2014:937601. doi: 10.1155/2014/937601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinhart F., El Massri N., Darlot F., Torres N., Johnstone D.M., Chabrol C., Costecalde T., Stone J., Mitrofanis J., Benabid A.-L. 810 nm near-infrared light offers neuroprotection and improves locomotor activity in MPTP-treated mice. Neurosci. Res. 2015;92:86–90. doi: 10.1016/j.neures.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Darlot F., Moro C., El Massri N., Chabrol C., Johnstone D.M., Reinhart F., Agay D., Torres N., Bekha D., Auboiroux V. Near-infrared light is neuroprotective in a monkey model of P arkinson disease. Ann. Neurol. 2016;79:59–75. doi: 10.1002/ana.24542. [DOI] [PubMed] [Google Scholar]
- 39.Oueslati A., Lovisa B., Perrin J., Wagnières G., van den Bergh H., Tardy Y., Lashuel H.A. Photobiomodulation suppresses alpha-synuclein-induced toxicity in an AAV-based rat genetic model of Parkinson’s disease. PLoS ONE. 2015;10:e0140880. doi: 10.1371/journal.pone.0140880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moro C., el Massri N., Darlot F., Torres N., Chabrol C., Agay D., Auboiroux V., Johnstone D.M., Stone J., Mitrofanis J. Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson’s disease. Brain Res. 2016;1648:19–26. doi: 10.1016/j.brainres.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Salgado A.S., Ribeiro L.G., Oliveira T.B., Rolão M.P., Gomes J.C., Carraro E., Perreira M.C., Suckow P.T., Kerppers I.I. Effects of Light Emitting Diode and Low-intensity Light on the immunological process in a model of Parkinson’s disease. Med Res. Arch. 2017;4 doi: 10.18103/mra.v4i8.652. Issue 8, December, 2016. [DOI] [Google Scholar]
- 42.Reinhart F., el Massri N., Chabrol C., Cretallaz C., Johnstone D.M., Torres N., Darlot F., Costecalde T., Stone J., Mitrofanis J. Intracranial application of near-infrared light in a hemi-parkinsonian rat model: The impact on behavior and cell survival. J. Neurosurg. 2016;124:1829–1841. doi: 10.3171/2015.5.JNS15735. [DOI] [PubMed] [Google Scholar]
- 43.Reinhart F., El Massri N., Johnstone D.M., Stone J., Mitrofanis J., Benabid A.-L., Moro C. Near-infrared light (670 nm) reduces MPTP-induced parkinsonism within a broad therapeutic time window. Exp. Brain Res. 2016;234:1787–1794. doi: 10.1007/s00221-016-4578-8. [DOI] [PubMed] [Google Scholar]
- 44.El Massri N., Johnstone D.M., Peoples C.L., Moro C., Reinhart F., Torres N., Stone J., Benabid A.-L., Mitrofanis J. The effect of different doses of near infrared light on dopaminergic cell survival and gliosis in MPTP-treated mice. Int. J. Neurosci. 2015;126:76–87. doi: 10.3109/00207454.2014.994063. [DOI] [PubMed] [Google Scholar]
- 45.El Massri N., Lemgruber A.P., Rowe I.J., Moro C., Torres N., Reinhart F., Chabrol C., Benabid A.-L., Mitrofanis J. Photobiomodulation-induced changes in a monkey model of Parkinson’s disease: Changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp. Brain Res. 2017;235:1861–1874. doi: 10.1007/s00221-017-4937-0. [DOI] [PubMed] [Google Scholar]
- 46.Reinhart F., El Massri N., Torres N., Chabrol C., Molet J., Johnstone D.M., Stone J., Benabid A.-L., Mitrofanis J., Moro C. The behavioural and neuroprotective outcomes when 670 nm and 810 nm near infrared light are applied together in MPTP-treated mice. Neurosci. Res. 2017;117:42–47. doi: 10.1016/j.neures.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 47.El Massri N., Cullen K.M., Stefani S., Moro C., Torres N., Benabid A.-L., Mitrofanis J. Evidence for encephalopsin immunoreactivity in interneurones and striosomes of the monkey striatum. Exp. Brain Res. 2018;236:955–961. doi: 10.1007/s00221-018-5191-9. [DOI] [PubMed] [Google Scholar]
- 48.Kim B., Mitrofanis J., Stone J., Johnstone D.M. Remote tissue conditioning is neuroprotective against MPTP insult in mice. IBRO Rep. 2018;4:14–17. doi: 10.1016/j.ibror.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien J.A., Austin P.J. Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague–Dawley Rat. Biomolecules. 2019;9:381. doi: 10.3390/biom9080381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miguel M.S., Martin K.L., Stone J., Johnstone D.M. Photobiomodulation Mitigates Cerebrovascular Leakage Induced by the Parkinsonian Neurotoxin MPTP. Biomolecules. 2019;9:564. doi: 10.3390/biom9100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganeshan V., Skladnev N.V., Kim J.Y., Mitrofanis J., Stone J., Johnstone D.M. Pre-conditioning with remote photobiomodulation modulates the brain transcriptome and protects against MPTP insult in mice. Neuroscience. 2019;400:85–97. doi: 10.1016/j.neuroscience.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 52.Langston J.W., Palfreman J. The Case of the Frozen Addicts: How the Solution of a Medical Mystery Revolutionized the Understanding of Parkinson’s Disease. IOS Press; Amsterdam, The Netherlands: 2014. [Google Scholar]
- 53.Gubellini P., Kachidian P. Animal models of Parkinson’s disease: An updated overview. Rev. Neurol. 2015;171:750–761. doi: 10.1016/j.neurol.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Bezchlibnyk Y.B., Sharma V.D., Naik K.B., Isbaine F., Gale J.T., Cheng J., Triche S.D., Miocinovic S., Buetefisch C.M., Willie J.T., et al. Clinical outcomes of globus pallidus deep brain stimulation for Parkinson disease: A comparison of intraoperative MRI- and MER-guided lead placement. J. Neurosurg. 2020 doi: 10.3171/2019.12.JNS192010. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Jorge A., Dastolfo-Hromack C., Lipski W.J., Kratter I.H., Smith L.J., Gartner-Schmidt J.L., Richardson R.M. Anterior Sensorimotor Subthalamic Nucleus Stimulation Is Associated With Improved Voice Function. Neurosurgery. 2020;2020:nyaa024. doi: 10.1093/neuros/nyaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandstrom L., Blomstedt P., Karlsson F., Hartelius L. The Effects of Deep Brain Stimulation on Speech Intelligibility in Persons With Essential Tremor. J. Speech, Lang. Hear. Res. 2020;63:456–471. doi: 10.1044/2019_JSLHR-19-00014. [DOI] [PubMed] [Google Scholar]
- 57.Ando T., Xuan W., Xu T., Dai T., Sharma S.K., Kharkwal G.B., Huang Y.Y., Wu Q., Whalen M.J., Sato S., et al. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS ONE. 2011;6:e26212. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton C., Hamilton D., Nicklason F., el Massri N., Mitrofanis J. Exploring the use of transcranial photobiomodulation in Parkinson’s disease patients. Neural Regen. Res. 2018;13:1738–1740. doi: 10.4103/1673-5374.238613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santos L., Olmo-Aguado S.D., Valenzuela P.L., Winge K., Iglesias-Soler E., Arguelles-Luis J., Alvarez-Valle S., Parcero-Iglesias G.J., Fernandez-Martinez A., Lucia A. Photobiomodulation in Parkinson’s disease: A randomized controlled trial. Brain Stimul. 2019;12:810–812. doi: 10.1016/j.brs.2019.02.009. [DOI] [PubMed] [Google Scholar]