Abstract
Background. FOLFOXIRI plus Bevacizumab is one of the most frequently used first-line treatments for patients with BRAF-mutant colorectal cancer (CRC), while second-line treatment requires extensive further research. In this pooled analysis, we evaluate the impact of anti-angiogenics in patients with pre-treated BRAF-mutant CRC. Methods. We monitored patients in randomized, controlled studies who had advanced CRC and were undergoing second-line chemotherapy in addition to utilizing Bevacizumab, Ramucirumab or Aflibercept treatments. These data were pooled together with the data and results of BRAF-mutant patients enrolled in two phase III trials (TRIBE and TRIBE-2 study), who had been treated with second-line treatment both with or without Bevacizumab. Overall survival (OS), in relation to BRAF mutational status, was the primary focus. Results. Pooled analysis included 129 patients. Anti-angiogenics were found to have a significant advantage over the placebo in terms of OS (HR 0.50, 95%CI 0.29–0.85) (p = 0.01). Conclusions. Our pooled analysis confirms the efficacy of anti-angiogenics in pre-treated BRAF-mutant CRC, establishing the combination of chemotherapy plus Bevacizumab or Ramucirumab or Aflibercept as a valid treatment option.
Keywords: colorectal cancer, BRAF mutation, anti-angiogenics, chemotherapy, MSI-H
1. Background
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths worldwide, with approximately half of patients developing metastases during the course of the disease [1,2]. However, owing to the considerable progress made in the treatment of metastatic disease in recent decades, median survival in modern clinical trials is around 30 months [3,4]. When defining the treatment goal in relation to metastatic disease, both patient-related (e.g., age, performance status, comorbidities) and tumor-related factors (e.g., burden of disease, number of sites involved, respectability, molecular profile, primary tumor sidedness) along with patients’ wishes should be considered. A notable driver of CRC is the mutation of the BRAF gene, which concerns 8–10% of colon cancers and does not overlap with RAS mutations. Roughly 96% of all BRAF mutations are a T1799A transversion in exon 15, which results in a valine amino acid substitution: V600E [5]. BRAFV600E-mutant CRCs (referred to in the text as BRAF-mutant) share the following peculiar clinico-pathological features: they are often right-sided, microsatellite-high (MSI-H), more frequent in women, have a mucinous histology and are associated with a poor prognosis [6]. However, a small fraction of BRAF mutations affect codons 594 and 596, representing a distinctly smaller population from a clinical point of view when compared to a BRAFV600-mutant. These are found more often in males, are usually left-sided, associated with RAS mutations, have a better prognosis and are only rarely characterized by a peritoneal relapse [7,8]. In an effort to overcome the aggressiveness of BRAF-mutant CRCs, some authors have proposed a more intensive regimen of chemotherapy based on the combination of 5-Fluorouracil, Leucovorin, Irinotecan, Oxaliplatin (FOLFOXIRI) and Bevacizumab [9]. The phase III TRIBE trial used randomly selected patients with metastatic CRC to receive a first-line treatment with FOLFOXIRI plus Bevacizumab versus FOLFIRI plus Bevacizumab. The trial reached its primary endpoint, which was progression-free survival (PFS), and showed significantly improved OS with a median OS of 29.8 months (95%CI 26.0–34.3) in the FOLFOXIRI plus Bevacizumab group compared with 25.8 months (95%CI 22.5–29.1) in the FOLFIRI plus Bevacizumab group (hazard ratio [HR] 0.80, 95%CI 0.65–0.98; p = 0.03). However, in the absence of a significant interaction between BRAF mutational status and the treatment arm, improved median OS was reported with the triplet subgroup that used Bevacizumab (19 vs. 10.7 months; HR: 0.84, 95%CI 0.24–1.2), albeit with a poor prognosis [9]. Although these results may be skewed by the small sample size (28 patients) in a non-preplanned analysis, FOLFOXIRI plus Bevacizumab has become a preferred option for BRAF-mutant CRC patients. Likewise, second-line treatment in BRAF-mutant CRC undoubtedly requires further research. In the overall population, the addition of anti-angiogenics Aflibercept and Ramucirumab during standard chemotherapy yielded survival improvements in their respective pivotal trials [10,11]. Furthermore, treatment with Bevacizumab beyond first-line progression demonstrated a higher survival rate in both a large observational cohort study and randomized clinical trials [12,13,14]. These data led to a major step forward in the management of pre-treated metastatic CRC patients. Subgroup analyses of VELOUR and RAISE trials suggest a potential benefit of the addition of anti-angiogenics in patients with BRAF-mutant CRC, although results were restricted by the small sample size [15,16]. Conversely, no data have been published about the impact of Bevacizumab on BRAF mutation in second-line treatment. Therefore, we performed a pooled analysis aiming at evaluating the impact of anti-angiogenics in patients with pre-treated BRAF-mutant CRC.
2. Material and Methods
2.1. Study Design and Inclusion Criteria
In this pooled analysis, we used randomized, controlled studies and considered patients with BRAF-mutant CRC treated with second-line chemotherapy plus either antiangiogenic drugs (Ramicirumab or Aflibercept) or placebo. The resulting data were then pooled with the data and outcomes of BRAF-mutant patients enrolled in two phase III trials (TRIBE and TRIBE-2 study). These had been treated in second-line with chemotherapy plus Bevacizumab or chemotherapy alone. A primary analysis was planned to compare OS. Ethics approval and consent to participate do not apply to this research.
2.2. Search Strategy
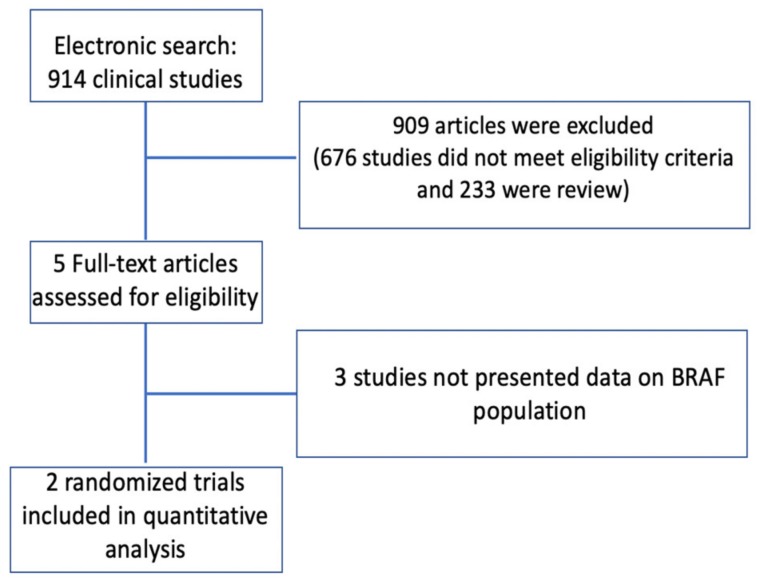
Figure 1 demonstrates the search strategy used in the meta-analysis. A bibliographic research of the PubMed, Cochrane Library, and Embase databases was conducted. Keywords used included colorectal cancer, either Aflibercept or Ramucirumab, Bevacizumab, chemotherapy, second-line, and BRAF. Articles published in English dating to March 2020 were retrieved. Relevant reviews and meta-analyses were also examined for the potential use of data. The annual meetings of the American Society of Clinical Oncology (ASCO and the ASCO gastrointestinal [ASCO GI] Cancers Symposium) and the European Society of Clinical Oncology (ESMO and ESMO GI) were comprehensively reviewed to detect unpublished data if pertinent.
Figure 1.
Summary of the evidence search and selection process (Flow diagram).
2.3. Data Extraction and Management
The titles and abstracts of each of the selected studies were screened independently by two authors (A.C.G. and E.T.). The abstracts of potentially eligible trials were then read independently by the same authors who decided whether the study in question would be selected. The authors then analyzed the full text of each selected paper in order to decide the trials to be included in the pooled analysis. When there were discrepancies in trial search or selection, they discussed with a third researcher (F.G.) to reach a final consensus. The internal validity of the trial was assessed by evaluating the methods used for randomization, blindness, allocation sequence, allocation concealment and the report of missing data. All selected trials published as full-text articles in a peer-reviewed journal were analyzed and classified using the Jadad score when possible [17]. Qualitative and quantitative analyses of the selected articles were independently performed by the same two authors (A.C.G. and E.T.). When discrepancies occurred, they consulted a third researcher (F.G.) to reach a final consensus. OS were the variables under analysis.
2.4. Statistical Analysis
Meta-analysis was performed in accordance with the PRISMA statement recommendations [18]. Data were entered into a computer database for transfer and statistical analysis in Review Manager 5.2. Heterogeneity among the trials was assessed with a descriptive aim using the I2 test. I2 values above 50% were deemed to suggest high heterogeneity, values of 25–50% were deemed to show modest heterogeneity, and values below 25% were deemed to represent low heterogeneity. A level of 5% was assumed to be statistically significant. Differences between categorical outcome parameters were quantified using the Odds Ratio (OR) and corresponding 95%CI. Summary statistics for dichotomous outcome data were assessed using the Mantel–Haenszel method. Summary statistics for generic inverse variance data were calculated using the inverse variance method. Pooled analysis of the OR was performed using a random-effect model, assuming an error of 5% as an index of statistical significance.
3. Results
3.1. Study Selection and Characteristics
The combined search yielded 914 potentially relevant articles, 912 of which were excluded because they were either reviews, non-randomized controlled trials or had no data relating to the BRAF-mutant population. Two trials (RAISE and VELOUR study), with a total of 77 patients with BRAF-mutant CRC, were included (Figure 1).
In the RAISE trial, the authors assessed the efficacy of Ramucirumab plus FOLFIRI versus FOLFIRI alone in patients with disease progression after first-line chemotherapy. In the VELOUR trial, the authors studied the efficacy of Aflibercept plus FOLFIRI versus FOLFIRI alone in the same setting. The results of the VELOUR and RAISE trials along with their effect on patients are summarized in Table 1. Of the 1226 patients enrolled in the RAISE and VELOUR trials, 77 patients (6.3%) were BRAF-mutant. Both studies were deemed to be of high quality. The modified Jadad score revealed that the quality of the two individual studies was sufficient for further analysis (Table 2).
Table 1.
Patient characteristics and results of VELOUR and RAISE studies.
| Velour | Raise Study | |
|---|---|---|
| Experimental arm | Aflibercept plus FOLFIRI | Ramucirumab plus FOLFIRI |
| Prior Bevacizumab | ||
| Yes | 30.4% | 100% |
| mOS | ||
| Arm with antiangiogenic | 13.5 months | 13.3 months |
| Arm without antiangiogfenic | 12.06 months | 11.7 months |
| mPFS | ||
| Arm with antiangiogenic | 6.9 months | 5.7 months |
| Arm without antiangiogenic | 4.67 months | 4.5 months |
Table 2.
Quality assessment of included studies using the modified Jadad score.
| Raise Trial | Velour Trial | |
|---|---|---|
| + | + | Random sequence generation (selection bias) |
| + | + | Allocation concealment (selection bias) |
| + | + | Blinding of participants and personnel (performance bias) |
| + | + | Incomplete outcome data (attrition bias) |
| + | + | Selective reporting (reporting bias) |
Of the 1187 patients enrolled in the TRIBE and TRIBE-2 trials, 52 patients (4.3%) were BRAF-mutant and received a second-line treatment. Of these, 46 (88.5%) were treated with an antiangiogenic drug and six (11.5%) with chemotherapy alone. Table 3 shows the characteristics of these patients.
Table 3.
Characteristics of BRAF-mutant patients in TRIBE and TRIBE-2 study.
| No. (%) | |
|---|---|
| First-Line Treatment | |
| FOLFOXIRI + Bevacizumab | 24 (46.1) |
| FOLFIRI + Bevacizumab | 5 (9.6) |
| FOLFOX + Bevacizumab | 23 (44.2) |
| Study | |
| TRIBE | 12 (23.1) |
| TRIBE-2 | 40 (76.9) |
| Primary tumor location | |
| Right | 37 (71.1) |
| Left | 11 (21.1) |
| Rectum | 4 (7.8) |
| Stage at diagnosis | |
| I–III | 10 (19.2) |
| IV | 42 (80.8) |
| Second line Therapy | |
| Chemotherapy plus antiangiogenic | 46 (88.5) |
| Chemotherapy | 6 (11.5) |
3.2. Publication Bias and Among-Trial Heterogeneity
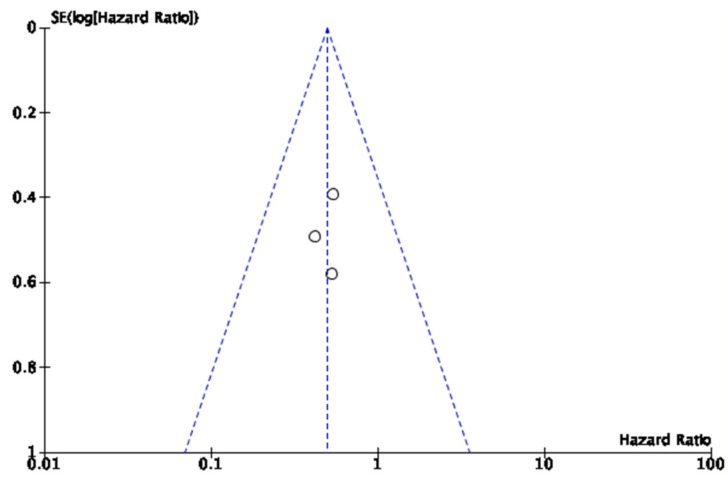
A total of 129 patients were included in the final pooled analysis. Figure 2 shows a funnel plot of the data. The funnel plot does not reveal significant publication bias.
Figure 2.
Funnel plots of publication bias.
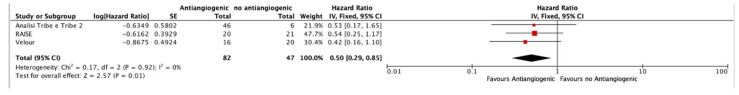
No heterogeneity was found between the studies in the analysis (I2TEST 0%). There was a significant advantage in favor of antiangiogenics versus chemotherapy alone in terms of OS in patients with BRAF mutation (HR 0.50, CI95% 0.29–0.85) (p = 0.01) (Figure 3).
Figure 3.
Forest plot of antiangiogenics versus no antiangiogenics in terms of OS in patients with BRAF mutation.
4. Discussion
BRAF mutation represents a well-recognized negative prognostic factor in patients with CRC. The worst prognoses of BRAF-mutant CRC have been largely discovered in both early-stage [19,20,21] and advanced-stage disease [22,23]. Many strategies have been developed to overcome the intrinsic resistance of BRAF-mutant CRCs, especially for those with BRAFV600-mutant tumors. One of these strategies is the intensification of first-line treatment by using a three-drugs regimen (FOLFOXIRI) in combination with Bevacizumab. The efficacy of this combination stems from an exploratory analysis of a phase II trial [24], subsequently echoed by the results of a subgroup analysis of the phase III TRIBE trial [9]. Preclinical evidence has shown that the MAPK pathway can increase expression of VEGF [25,26], thus suggesting that RAS and BRAF mutations can potentially influence the response to anti-angiogenics. Furthermore, post-hoc analyses of AVF2107g [27] and AGITG MAX trial [28] seem to suggest a numerical, although not statistically significant, survival advantage in BRAF-mutant CRC treated with Bevacizumab. However, besides the limited evidence resulting from retrospective analysis of small subgroups, one of the criticisms regarding the TRIBE trial concerns the added value of Bevacizumab in combination with FOLFOXIRI. In this respect, a propensity score-adjusted analysis of two randomized trials by Cremolini and colleagues demonstrated a survival advantage with the combination of FOLFOXIRI plus Bevacizumab versus FOLFOXIRI alone [29]. Despite the absence of randomized comparisons, this demonstrates the most successful attempt to answer this crucial question. Proving more difficult to treat, however, are pre-treated patients. While the introduction of BRAF inhibitors such as Vemurafenib, Dabrafenib and Encorafenib has recently revolutionized the treatment landscape of metastatic melanoma [30,31,32], the results in the treatment of CRC were largely unsatisfactory [33]. This can be attributed to the more complex molecular landscape of CRC compared to melanoma. Indeed, the inhibition of BRAF leads to a paradoxical restoration of MAPK signaling through a number of adaptive feedback mechanisms [34]. Therefore, many strategies have been developed to avoid the reactivation of the MAPK pathway and overcome the intrinsic resistance to BRAF inhibitors. One of these strategies is the simultaneous inhibition of a large number of components of the pathway. Recently, the results of the BEACON CRC phase III trial have been published. A total of 665 patients with pre-treated BRAF-mutant CRC were randomly selected to receive a triple combination of Encorafenib (a BRAF-inhibitor), Binimetinib (a MEK-inhibitor) and Cetuximab (an anti-EGFR), versus a double combination of Encorafenib plus Cetuximab versus an investigators’ choice (Irinotecan or FOLFIRI plus Cetuximab). Median OS was nine months for the triplet compared to 5.4 months for standard therapy (HR 0.52; p < 0.0001). The confirmed response rate from the blinded central review for the triplet therapy was 26% compared to 2% (p < 0.0001) for standard therapy [35]. Our pooled analysis seems to confirm the efficacy of anti-angiogenics in the peculiar subgroup of pre-treated patients with BRAF-mutant CRC. One possible explanation for the efficacy of anti-angiogenics could lie in the enrichment of this population with MSI-H tumors. In fact, in a pooled analysis of four phase III studies involving 250 BRAF-mutant CRCs, among deficient-MMR (dMMR) tumors, roughly one third of them had a BRAF mutation, while one fifth of BRAF-mutant also had a dMMR [36]. In this respect, in a subgroup analysis of CALGB/SWOG 80405, patients with MSI-H tumors showed longer OS in the Bevacizumab arm than in the Cetuximab arm (HR 0.13; interaction p < 0.001 for interaction between microsatellite status and the two arms) [37]. Although the unprecedented results of the BEACON CRC trial will hopefully change the treatment paradigm in BRAF-mutant CRCs, there are still patients who do not benefit from this chemotherapy-free therapy and who may potentially benefit from the combination of chemotherapy with an anti-angiogenic. Future research focusing on the biomarkers-driven selection of patients who may benefit from this triplet combination is eagerly awaited. Some limitations of this study are the limited number of trials included and the small number of patients with BRAF-mutant CRC enrolled in each trial. Furthermore, since the patients enrolled in each trial had different characteristics, we cannot exclude a clinical heterogeneity in our pooled analysis.
5. Conclusions
As of today, to the best of our knowledge, only post-hoc analyses of randomized trials have been published regarding the efficacy of anti-angiogenics in pre-treated BRAF-mutant CRC. Acknowledging the limitations of our pooled analysis, no definitive conclusions can be drawn and further evaluation is needed. However, recognizing that a randomized clinical trial would likely be anachronistic and unfeasible, our pooled analysis provides the best evidence available in favor of the addition of an anti-angiogenic to chemotherapy in the second-line treatment of BRAF-mutant CRC.
Author Contributions
E.T., F.G., A.C.-G., conceived the work, played an important role in interpreting the results, drafted and revised the manuscript; F.G., A.C.-G., A.S., M.G.V., I.G., M.S., M.T.E., F.C., C.S., S.C., E.T., D.R., A.B., G.M., C.C., A.F. acquired data, played an important role in interpreting the results and revised the manuscript. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO Consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 3.Venook A.P., Niedzwiecki D., Lenz H.J., Innocenti F., Fruth B., Meyerhardt J.A., Schrag D., Greene C., O’Neil B.H., Atkins J.N., et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemann V., von Weikersthal L.F., Decker T., Kiani A., Vehling-Kaiser U., Al-Batran S.E., Heintges T., Lerchenmüller C., Kahl C., Seipelt G., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran B., Kopetz S., Tie J., Gibbs P., Jiang Z.Q., Lieu C.H., Agarwal A., Maru D.M., Sieber O., Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones J.C., Renfro L.A., Al-Shamsi H.O., Schrock A.B., Rankin A., Zhang B.Y. Non-V600BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J. Clin. Oncol. 2017;35:2624–2630. doi: 10.1200/JCO.2016.71.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Shen J., Huang C., Cao M., Shen L. Clinocopathological significance of BRAFV600E mutation in colorectal cancer: An updated meta-analysis. J. Cancer. 2019;10:2332–2341. doi: 10.7150/jca.30789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cremolini C., Loupakis F., Antoniotti C., Lupi C., Sensi E., Lonardi S., Mezi S., Tomasello G., Ronzoni M., Zaniboni A., et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E., Tabernero J., Lakomy R., Prenen H., Prausová J., Macarulla T., Ruff P., van Hazel G.A., Moiseyenko V., Ferry D., et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 11.Tabernero J., Yoshino T., Cohn A.L., Obermannova R., Bodoky G., Garcia-Carbonero R., Ciuleanu T.E., Portnoy D.C., Van Cutsem E., Grothey A., et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 12.Grothey A., Sugrue M.M., Purdie D.M., Dong W., Sargent D., Hedrick E., Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE) J. Clin. Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 13.Bennouna J., Sastre J., Arnold D., Österlund P., Greil R., Van Cutsem E., von Moos R., Viéitez J.M., Bouché O., Borg C., et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 14.Masi G., Salvatore L., Boni L., Loupakis F., Cremolini C., Fornaro L., Schirripa M., Cupini S., Barbara C., Safina V., et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: Final results of the randomized BEBYP trial. Ann. Oncol. 2015;26:724–730. doi: 10.1093/annonc/mdv012. [DOI] [PubMed] [Google Scholar]
- 15.Wirapati P., Pomella V., Vandenbosch B., Kerr P., Maiello E., Jeffery G.M., Curca R.-O.D., Karthaus M., Bridgewater J.A., Mihailov A.C., et al. Velour trial biomarkers update: Impact of RAS, BRAF, and sidedness on aflibercept activity. J. Clin. Oncol. 2017;35:3538. doi: 10.1200/JCO.2017.35.15_suppl.3538. [DOI] [Google Scholar]
- 16.Yoshino T., Portnoy D.C., Obermannová R., Bodoky G., Prausová J., Garcia-Carbonero R., Ciuleanu T., García-Alfonso P., Cohn A.L., Van Cutsem E., et al. Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study. Ann. Oncol. 2019;30:124–131. doi: 10.1093/annonc/mdy461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials. 1996;17:1–2. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Fariña-Sarasqueta A., van Lijnschoten G., Moerland E., Creemers G.J., Lemmens V.E., Rutten H.J., van den Brule A.J. The BRAF V600E mutation is an independent prognostic factor for survival in stage ii and stage iii colon cancer patients. Ann. Oncol. 2010;21:2396–2402. doi: 10.1093/annonc/mdq258. [DOI] [PubMed] [Google Scholar]
- 20.Roth A.D., Tejpar S., Delorenzi M., Yan P., Fiocca R., Klingbiel D., Dietrich D., Biesmans B., Bodoky G., Barone C., et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00. Trial. J. Clin. Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 21.Ogino S., Shima K., Meyerhardt J.A., McCleary N.J., Ng K., Hollis D., Saltz L.B., Mayer R.J., Schaefer P., Whittom R., et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: Results from intergroup trial CALGB 89803. Clin. Cancer Res. 2012;18:890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegewisch-Becker S., Nöpel-Dünnebacke S., Hinke A., Graeven U., Reinacher-Schick A., Hertel J., Lerchenmüller C.A., Killing B., Depenbusch R., Al-Batran S.E., et al. Impact of primary tumour location and RAS/BRAF mutational status in metastatic colorectal cancer treated with first-line regimens containing oxaliplatin and bevacizumab: Prognostic factors from the AIO KRK0207 first-line and maintenance therapy trial. Eur. J. Cancer. 2018;101:105–113. doi: 10.1016/j.ejca.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Richman S.D., Seymour M.T., Chambers P., Elliott F., Daly C.L., Meade A.M., Taylor G., Barrett J.H. Quirke P.KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from Oxaliplatin or Irinotecan: Results from the MRC FOCUS trial. J. Clin. Oncol. 2009;27:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 24.Loupakis F., Cremolini C., Salvatore L., Masi G., Sensi E., Schirripa M., Michelucci A., Pfanner E., Brunetti I., Lupi C., et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur. J. Cancer. 2014;50:57–63. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Rak J., Mitsuhashi Y., Sheehan C., Tamir A., Viloria-Petit A., Filmus J., Mansour S.J., Ahn N.G., Kerbel R.S. Oncogenes and tumor angiogenesis: Differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490–498. [PubMed] [Google Scholar]
- 26.Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R.S. Mutant ras oncogenes upregulate VEGF/VPF expression: Implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 27.Ince W.L., Jubb A.M., Holden S.N., Holmgren E.B., Tobin P., Sridhar M., Hurwitz H.I., Kabbinavar F., Novotny W.F., Hillan K.J., et al. Association of K-Ras, B-Raf, and p53 status with the treatment effect of bevacizumab. J. Natl. Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 28.Price T.J., Hardingham J.E., Lee C.K., Weickhardt A., Townsend A.R., Wrin J.W., Chua A., Shivasami A., Cummins M.M., Murone C., et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J. Clin. Oncol. 2011;29:2675–2682. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 29.Cremolini C., Loupakis F., Masi G., Lonardi S., Granetto C., Mancini M.L., Chiara S., Moretto R., Rossini D., Vitello S., et al. FOLFOXIRI or FOLFOXIRI plus bevacizumab as first-line treatment of metastatic colorectal cancer: A propensity score-adjusted analysis from two randomized clinical trials. Ann. Oncol. 2016;27:843–849. doi: 10.1093/annonc/mdw052. [DOI] [PubMed] [Google Scholar]
- 30.Flaherty K.T., Puzanov I., Kim K.B., Ribas A., McArthur G.A., Sosman J.A., O’Dwyer P.J., Lee R.J., Grippo J.F., Nolop K., et al. Inhibition of mutated, activated BRAF in Metastatic Melanoma. N. Engl. J. Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long G.V., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., Grob J.J., et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in Melanoma. N. Engl. J. Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 32.Dummer R., Ascierto P.A., Gogas H.J., Arance A., Mandala M., Liszkay G.L. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant Melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 33.Kopetz S., Desai J., Chan E., Hecht J.R., O’Dwyer P.J., Maru D., Morris V., Janku F., Dasari A., Chung W., et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J. Clin. Oncol. 2015;33:4032–4038. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corcoran R.B., Ebi H., Turke A.B., Coffee E.M., Nishino M., Cogdill A.P., Brown R.D., Della Pelle P., Dias-Santagata D., Hung K.E., et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to raf inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopetz S., Grothey A., Yaeger R., Van Cutsem E., Desai J., Yoshino T., Wasan H., Ciardiello F., Loupakis F., Hong Y.S., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N. Engl. J. Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 36.Venderbosch S., Nagtegaal I.D., Maughan T.S., Smith C.G., Cheadle J.P., Fisher D., Kaplan R., Quirke P., Seymour M.T., Richman S.D., et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Innocenti F., Ou F.S., Qu X., Zemla T.J., Niedzwiecki D., Tam R., Mahajan S., Goldberg R.M., Bertagnolli M.M., Blanke C.D., et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J. Clin. Oncol. 2019;37:1217–1227. doi: 10.1200/JCO.18.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]