Abstract
Bromodomain and extraterminal domain (BET) proteins have evolved as key multifunctional super-regulators that control gene expression. These proteins have been shown to upregulate transcriptional machinery leading to over expression of genes involved in cell proliferation and carcinogenesis. Based on favorable preclinical evidence of BET inhibitors in various cancer models; currently, 26 clinical trials are underway in various stages of study on various hematological and solid organ cancers. Unfortunately, preliminary evidence for these clinical studies does not support the application of BET inhibitors as monotherapy in cancer treatment. Furthermore, the combinatorial efficiency of BET inhibitors with other chemo-and immunotherapeutic agents remain elusive. In this review, we will provide a concise summary of the molecular basis and preliminary clinical outcomes of BET inhibitors in cancer therapy, with special focus on triple negative breast cancer.
Keywords: bromodomains, breast cancer, immunotherapy, cancer biology, drug discovery
1. Introduction
Although all cells in a given organism have the same genomic DNA sequence, characteristic epigenetics allow for the unique identity of individual cell/tissue types to maintain their ability to differentially express genes suitable for their biological function [1]. DNA methylation and covalent histone modifications are the two major hallmarks of epigenetic regulation [2]. Abnormal epigenetic regulatory changes occur in cancers [3]. Under basal conditions, the upstream promoter region on DNA induces only limited gene expression, while higher levels of gene expression observed in cancers require highly regulated promoter–enhancer interactions [4]. Generally, transcription factors are referred to as enhancers, which upon binding with the promoter region induce gene expression by activating transcription machinery consisting of RNA polymerase II. This induction of structural changes in the otherwise cognate promoter region results in cell and microenvironment-specific gene expression [5]. Along with transcription factors, epigenetic mechanisms such as covalent changes to promoter region and histone modifications also exert a key enhancer functionality. As a general rule of thumb, in cancers, there is a wider genomic hypomethylation with localized hypermethylation of tumor suppressor gene promoters [6]. Multiple otherwise normal histone modifications, such as acetylation, methylation, phosphorylation, sumoylation and ubiquitination, along with mutations leading to dysregulation of histones can occur on cancers [7]. The acetylation of functionally active free amino-group of lysine residues in histones by enzymes histone acetyl transferase (HAT) and histone deacetylase (HDAC) induces the formation of transcriptionally active euchromatin [8]. The dysregulation of HAT and HDAC enzymes have been demonstrated in multiple cancers [9]. However, HDAC-based inhibition for cancer therapy has not proved to be very efficient due to the lack of target-specificity. An increasing body of literature evidence suggests that super-enhancer factors [10], in addition to the various mechanisms of action, possess a unique ability to identify acetylated-lysines on histones and modulate the epigenetic enhancer’s function which could span across a long range of genomic DNA to exert stronger transcriptional activation ability through addition of more transcriptional machinery, when compared to regular enhancers (Figure 1). Bromodomain and extraterminal domain (BET) proteins interact with acetylated-lysine portions of histones with a super-enhancer epigenetic role in upregulating gene transcription and thus potentially playing a critical role in carcinogenesis [11]. In this review, we will provide a concise summary of the molecular basis and preliminary clinical outcomes of BET inhibitors in cancer therapy, with special focus on triple negative breast cancer.
Figure 1.
General structure and mechanism of action of BET inhibitors. (A) Generic domain structure of the BET protein family. Each BET protein (BRD2, 3, 4 and BRDT) contains two bromodomains (BrD1 and BrD2) and an extra-terminal (ET) domain. An additional carboxy-terminal motif (CTM) is present in BRD4 and BRDT—BET proteins. (B) Acetylation of lysine moieties on histones leads to conversion of inactive heterochromatin to active euchromatin. (C) BET proteins through their interaction of bromodomain (BRD) motifs with acetylated histones activates transcriptional machinery leading to gene expression and carcinogenesis.
2. Bromodomain and Extraterminal Domain
The evolutionarily conserved bromodomain (BrD) motifs specifically bind to acetylated lysines in histones [12]. The binding of BrDs to the acetyl group of lysine neutralizes the lysine’s positive charge which then causes the relaxation of heterochromatin in nucleosomes, thereby allowing access to the binding of transcription factors and gene expression [13]. BrDs have a distinctly conserved signature structure with four α-helices, named, Z, A, B and C, with a hydrophobic pocket formed by two flexible linker loop regions (Z/A and B/C) [14]. While the original BrD was identified in Drosophila, in the human proteome, to date, eight sub-families of BrDs with 61 motifs have been identified in 46 proteins [15]. The protein–protein interactions between the hydrophobic pocket of BrDs and acetylated lysines on histones are extremely weak, which makes these motifs enticing targets for development of small molecule drug inhibitors [16].
Bromodomain and extraterminal domain (BET) proteins act as epigenetic super-enhancer modulators with a unique tertiary protein structure generally consisting of two tandem BrDs (BrD1 and BrD2), an extraterminal domain (ET), and a C-terminal domain with the ability to recognize acetylated portions of proteins [11]. The BrD motifs on BET proteins function to facilitate the neutralization of acetylated-lysines and also the recruitment of transcription factors for target gene expression. Literature evidence suggests distinctive functionality for the two BrD motifs of BET proteins, possibly resulting from differential interaction with lysine-acetylated histones or with the transcription factors [17]. For example, in human BRD4, the first BrD motif adheres to the diacetylated H4K5ac/K8ac portion of the histones on the promoter/enhancer region of the target gene, and the second BrD motif enables the recruitment of transcription factors such as pTEFb complex [18]. However, this phenomenon does not seem to be universal for all BET proteins [19]. For BRD3, the first BrD motif is shown to bind with the hematopoietic transcription factor GATA1 [20,21], thereby suggesting differential functionality of the two tandemly arranged BrDs motifs among various BET proteins. Along with BrD motifs, the extra-terminal (ET) domain of BET proteins has shown to play a critical role in overall protein functionality. The ET domain of BRD3 was shown to mediate the identification and eventual interaction of histone and non-histone proteins with BET molecules [22].
In addition to acetylated histones, BET proteins have the ability to recognize acetylated transcription factors. The BET family of proteins primarily consists of four proteins, namely, BRD2, BRD3, BRD4, and testis-restricted BRDT [23]. Studies based on in vitro cell culture and plasmid based overexpression of BRD4, have demonstrated that the extraterminal domain of BRD4 is involved in recruiting the positive transcription elongation factor complex (P-TEFb) and initiating the RNA polymerase complex for gene expression [24,25,26]. In addition, a P-TEFb independent gene upregulation by BRD4 has also been reported [27,28]. The accumulating evidence from these diverse data suggest that BRD4 plays a critical role in the transcription initiation and elongation of several genes promoting cell proliferation and cancer progression [18]. BET proteins are also directly involved in the expression of oncogenes such as, c-Myc, which is directly correlated with carcinogenesis [29]. BRD2 was suggested to modulate cell cycle through the expression of cyclin D1 by transcriptional regulatory genes E2F1 and E2F2 [30,31]. Along with BET proteins, BrD motifs have been identified in other proteins such as histone methyltransferase (ASH1L) and the mixed-lineage leukemia (MLL) associated proteins [32]. Along with acetylated-histones, BET proteins are also known to bind with acetylated non-histone proteins such as transcription factors [33]. For instance, BRD4 is shown to bind with the bromodomain region of acetylated-TWIST, a transcription factor associated with embryonic mesodermal development [34,35]. Similarly, BRD4 also modulates the activity of NF-κB complex through its interaction with acetylated RelA [36]. BET proteins can also interact with non-bromodomain motifs of p53, CEBP, etc. [37,38]. Further, although BrD motifs exist in histone acetyl transferases (HATs)—such as p300/CBP-associated factor (PCAF) and cAMP response binding protein (CREBBP)—their exact functionality and molecular regulation of transcription machinery are yet unknown [39,40].
3. BET Inhibitors
Some of the early BET inhibitors JQ1 and I-BET762 were reported by the Dana-Faber Cancer Institute in collaboration with the Structural Genomics Consortium (SGC) and, GlaxoSmithKline (GSK), respectively [41,42]. JQ1 (thieno-triazolo-1,4-diazepine) was shown to compete with the BRD proteins to bind with the acetylated-lysine residues in various solid organ tumors and hematological malignancies [43,44]. JQ1 causes a significant deletion of interleukin 7 receptor gene (IL7R) leading to the downregulation of oncogenes MYC and E2F1 [45,46]. Further, JQ1 has been reported to induce G1-cell cycle arrest and apoptosis in solid tumors [47,48].
In tamoxifen-resistant breast cancer, JQ1 through inhibition of WHSC1, a histone H3K36 methyltransferase suppresses ERα signaling pathway causing an anti-tumor effect along with having a synergistic effect with an ER proteolytic fulvestrant [49]. Sengupta et al. reported that JQ1 blocks estrogen (E2)-induced transcriptional activation by inhibiting the transition of RNA polymerase II from initiation to elongation phase [50]. Similarly, a combination of JQ1 with mocetinostat, a HDAC inhibitor, caused the inhibition of the RAS/MAPkinase signaling pathway, leading to decreased cell proliferation in both ER+ and triple negative breast cancers (TNBC) [51]. Selectively in TNBC, drug treatment with JQ1 exerted the downregulation of the cell cycle transcription factors Forkhead box M1 (FOXM1) and Lim domain only 4 (LMO4), angiogenic factors vascular endothelial growth factor A (VEGF-A) and carbonic anhydrase 9 (CA9), thus reducing cell proliferation, angiogenesis and metastasis [52].
A BRD2/3/4 inhibitor, OTX015 (MK-8628) is currently under clinical trial for evaluation in dose-finding studies and safety in TNBC [53]. In vitro cell culture studies with OTX015 caused inhibition of cellular proliferation and cell cycle arrest in leukemia cell lines [54]. I-BET151 (GSK) was reported to inhibit constitutively active JAK2 in glioblastoma leading to G1 arrest of cell cycle [55]. PFI-1 is a BET inhibitor with dual inhibition of BRD2 and BRD4 has been reported to exert anti-proliferative effect on leukemic cell lines [56]. Treatment with PFI-1 induced inhibition of MYC expression through the downregulation of oncogene Aurora kinase B, leading to cancer cell apoptosis [56]. The BRD4 inhibitor, MS436, was shown to exert anti-inflammatory effect on macrophages through downregulation of NF-κB mediated IL-6 expression [57]. However, there are no reports on the role of MS346 in cancer. Along with these, several newly discovered BET/BrD inhibitors, such as FT-1101, CPI-0610, BAY 1238097, INCB054329 TEN-010, BAY-299, etc., are currently under various phases of cancer clinical trials [58].
Numerous studies have reported that broad chemical inhibition of both BrD motifs of BET molecules effectively block genome-wide transcription of multiple key cancer and immune regulatory genes. However, the use of selective inhibitors of single BrDs could have a distinctive functional advantage. Gacias et al., have reported that specific BrD motif inhibition using a selective chemical inhibitor such as olinone to preferentially block one of the two BrDs on BET proteins inhibits lineage differentiation in neural oligodendrocytes [59]. A similar strategy targeting a single BrD motif on BET proteins could be of great interest for its future anti-cancer impact. The distinctive and unique ligand-binding selectivity between the two BrD motifs has been attributed to a few amino acid residues that distinguish the first and second BrDs within each BET protein, while all of them share nearly similar residues at the acetylated-lysine binding pocket [59]. More efforts are needed in this direction to enhance the current understanding of the specific molecular functions of the individual BrD motifs of BET proteins to develop more specific drug-targets.
Several mechanisms which enhance the chemotherapeutic sensitivity following BET inhibitor application have been noticed (Figure 2). The mammalian target of the rapamycin (mTOR) pathway, PI3K/AKT/mTOR, has been shown to be an important chemotherapeutic drug target in TNBC. Everolimus, a selective inhibitor of mTOR pathway, was suggested to exert anti-tumor efficient against the basal-like subtype of TNBC cell lines. However, in clinical breast cancer studies, the use of this mTOR inhibitor as a single-agent has resulted in minimally efficiency [60]. Studies by Stuhlmiller et al. have demonstrated that JQ1 enhanced the everolimus sensitivity of TNBC cells, leading to reduced cell proliferation and enhanced apoptosis [61]. Similarly, studies by Vazquez et al. have demonstrated that a combination of OTX015 with everolimus has enhanced the drug sensitivity in TNBC pre-clinical models [53]. The overexpression of the MYC oncogene is frequently reported in TNBC [62]. Efforts to directly target MYC expression and functionality have not been successful. Furthermore, there is evidence to suggest that BRD4-induced up-regulation of c-MYC played a critical role in inducing resistance to everolimus in ER+ breast cancer cells [63]. BET inhibitors OTX015 and JQ1 have been reported to induce c-MYC down-regulation in several cancer types including TNBC [64,65].
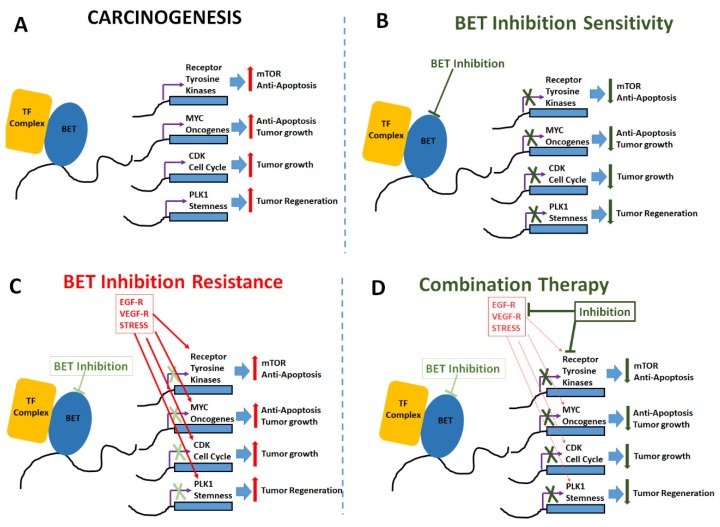
Figure 2.
Mechanisms of BET inhibitor activity, resistance and combinatorial application. (A) Carcinogenic changes mediated by upregulation of tumorigenic transcription factors, anti-apoptotic genes, oncogenes and cell cycle inducers following epigenetic enhancement by BET proteins. (B) BET inhibitors induce anti-tumor effect by enhancing apoptosis and reducing cell proliferation. (C) Cell adaptation mechanisms to overcome BET inhibition by upregulation of receptors for epidermal growth factor (EGF-R), vascular endothelial growth factor (VEGF-R) and other stress mediated factors such as HIF1α etc. (D) Combinatorial treatment with addition of drugs targeted at mTOR pathway and other oncogenic pathways along with BET inhibition to enhance anti-cancer impact.
There is increasing evidence that tumor-initiating stem cells (TISCs) play a critical role in tumor recurrence and treatment failure in several solid and hematological cancers [66]. Treatment failure to paclitaxel and cisplatin in TNBC patients is suggested to be associated with TISC generation [67,68]. BET proteins have been demonstrated to play a crucial role in the stem-functionality of TISCs. Studies by Vazquez et al. have demonstrated that OTX015 down-regulated the expression of stem cell functionality associated genes NANOG and OCT4. Similarly, Horne et al. have demonstrated the downregulation of NANOG expression following treatment with JQ1 on murine embryonic stem cells [67]. The binding of BRD4 with the promoter region of stemness associated gene WNT5A is shown to enhance the tumor cell regeneration and invasiveness of TISCs in in basal-like breast cancer [34]. The BET inhibitor JQ1 is also known to inhibit the stem cell associated with acute myeloid leukemia [69]. All these data strongly suggest a potential role of BET inhibitors in targeting TISCs in various cancers. BET inhibitors were also suggested to inhibit stem cell functionality through inhibition of JAK/STAT pathway [70,71]. In TISCs, the polo-like kinase (PLK1) induces the M-phase of the cell cycle [72,73]. Studies by Mao et al., have demonstrated that the BET inhibitor produced a cell cycle arrest at G1, while the volasertib, a PLK1 inhibitor, induced cells to arrest at M-phase [74,75]. Further BET inhibitors induced not only arrest at G1-cell cycle, but also reduced the levels of kinases such as PLK1 involved in mitosis [53]. Taken together, these data suggest that BET inhibition is an attractive targeted therapy against TISCs in TNBC patients.
Currently, there are very limited BET inhibitor based clinical trials in TNBC. Of the 25 clinical trials (listed in www.clinicaltrials.gov), only four trials included breast cancer (Table 1), while the remaining 21 trials (not provided in the table) were on hematological malignancies. Unfortunately, the preliminary results from these studies are not promising. The majority of these preclinical studies indicate resistance to BET inhibitors. The cancer resistance does not seem to be due to changes in the BET protein’s gene expression pattern such as copy-number changes or somatic mutations on gate-keeper genes induced by specific BET inhibitors [76]. For example, in TNBC preclinical proteomic studies, BET inhibitor resistance was suggested to be due to the downregulation of protein phosphates 2A (PP2A) leading to hyper-phosphorylation of BRD4 and the enhanced interaction and activity of MED1, a mediator of RNA polymerase II, leading to the upregulation of transcription machinery and cell proliferation [76,77]. Similarly, in other hematological cancers, BET inhibitor treatment was shown to induce delayed WNT/β-catenin signaling-mediated MYC oncogene expression [78,79]. Furthermore, pre-existing mutations in PIK3CA in breast cancers is associated with BET inhibitor resistance [80,81,82]. Furthermore, pre-existing LKB1 and KRAS mutations in lung cancer have been associated with BET inhibitor resistance [83,84].
Table 1.
Clinical trials with BET inhibitors on triple negative breast cancers. (# Identifier number on www.clinicaltrials.gov).
| Drug | Identifier # | Tumor Type | Clinical Phase | Status |
|---|---|---|---|---|
| MK-8628/OTX105 | NCT02259114 | NUT Midline Carcinoma | Phase IB | Completed [93] |
| Non-small Cell Lung Cancer | ||||
| Castrate-resistant Prostate Cancer | ||||
| Pancreatic Ductal Adenocarcinoma | ||||
| GSK525762 | NCT01587703 | All solid tumors, Midline | Phase 1 | Active, not recruiting |
| MK-8628 | NCT02698176 | NUT Midline Carcinoma | Phase 1 | Terminated |
| Non-small Cell Lung Cancer | ||||
| Castrate-resistant Prostate Cancer | ||||
| Pancreatic Ductal Adenocarcinoma | ||||
| INCB054329 | NCT02431260 | Solid Tumors and Hematologic Malignancy | Phase 1 Phase 2 |
Terminated |
Structure activity relationship studies targeted at the better optimization of BET inhibitors was met with some major road-blocks, predominantly arising from the lack of current understanding of bromodomain motifs [85]. For example, unlike pan-bromodomain inhibitors, selective bromodomain-1 (MS-436, Olinone, and BI-2536) and bromodoamain-2 specific (RVX-208 and RVX-297) compounds were developed [86,87,88,89]. However, these individual sub-motif specific compounds have led to unexpected outcomes. Olinone, a bromodomain-1 specific BET inhibitor caused terminal primary differentiation of oligodendrocytes, while pan-BET inhibitors induced inhibition of oligodendrocyte growth and activity [59]. Further, BET inhibition was noted as an important off-target effect. Drugs such as dinaciclib (CDK inhibitor), TG101209 (JAK2 inhibitor) and BI-2536 (PLK inhibitor) have shown a strong BET inhibitor potential [90,91,92]. These off-target effects pose an opportunity to use these compounds for their dual-effect versus a challenge to limit their use due to unintended side effects. More stringent dose-dependent clinical studies should be performed to evaluate the utility of this off-target effect.
4. Challenges
While original functional and genomic studies on BRD4 were performed on NUT midline carcinoma, because of the lower incidence of this disease (as compared to other cancers), diverse scientific opinions exist on a more generalized applicability in other malignancies [94]. In hematological malignancies, based on shRNA-based knock-down and other proteomic data, BRD4 is correlated with the rearrangement of the mixed lineage leukemia (MLL1) gene (renamed as lysine specific methyl transferase 2A, KMT2A) [95]. Based on these studies, several clinical trials were initiated to demonstrate the efficacy of BET inhibitors in hematological malignancies. In spite of the initiation of BET inhibitor-based clinical studies, the precise genetic signatures and gene pathway clusters modulated by BET inhibitors have been areas of debate. Studies with JQ1/iBET on various cell lines from solid organ tumors demonstrated divergent results with the induction of terminal cell differentiation in some cancer cell lines to apoptosis in other cell lines [96,97]. Large scale profiling studies demonstrated that BET inhibitors induced the suppression of some oncogenes such as MYC, BCL2, CDK6, etc., while they had no impact on house-keeping genes [15,98].
In preclinical animal models of cancer, BET inhibitors have shown some unique effects on normal tissues too. Nicodeme et al. have demonstrated that treatment of animals with iBET helped overcome septic shock due to their anti-inflammatory effect and ability to inhibit the expression of inflammatory cytokines leading to immunosuppression [41]. In cardiac studies, BET inhibitors were able to inhibit cardiomyocyte damage and overcome pressure-overload effect in hypertension and congestive heart failure models [99]. In addition to these effects, BET inhibitors have also been shown to induce temporary infertility in men with their ability to inhibit spermatogenesis in the testes [100,101]. In addition, BET inhibitors are correlated with memory impairment, autism-like disorder and worsened bacterial co-infections in HIV-mediated immunosuppressive disorders [102,103]. In clinical studies, several important side-effects have been noted in patients being treated with OTX015, TEN-010, and CPI-0610 based BET inhibitor therapy [17,104]. Patients treated with high-dose OTX015 (120–160 mg/day) had adverse side-effects, such as thrombocytopenia, gastrointestinal bleeding and severe fatigue (Table 2).
Table 2.
Toxicities reported from clinical trials with BET inhibitors.
| Therapeutic Agents | Malignancies | Toxicities |
|---|---|---|
| MK-8268/OTX-015 [88] | Relapsed/refractory leukemia | diarrhea, fatigue, anorexia. Toxicities also included dysgeusia, abdominal pain, decreased clotting factor VII |
| MK-8628/OTX015 [89] | Relapsed/refractory lymphoma or multiple myeloma | thrombocytopenia, neutropenia, hyponatremia; diarrhea, dysgeusia, fatigue, anemia |
| MK-8628/OTX-015 [90] | NUT midline carcinoma | thrombocytopenia, nausea, dysgeusia, hyperglycemia, fatigue |
| BAY1238097 [91] | Advanced solid tumors or NHL | headache, vomiting, low back pain, Recurrent headaches |
5. Future Role of Novel Drug Design and Combinatorial Therapy
To-date, application of BET inhibitors has resulted in limited success. The only clinical trials with OTX015 (MK-8628, NCT02259114) in TNBC were discontinued due to a lack of clinical efficacy, in spite of its efficiency in preclinical TNBC models [53]. Therefore, more research is needed for the discovery of drugs which would exert more a sustained and efficient inhibition of BET proteins. Compounds with bivalent efficiency to simultaneously block two bromodomain motifs such as AZD5153, biBET and MT1 have demonstrated favorable outcomes in preclinical cell culture-based studies [105,106]. The development of dual-action proteolysis targeting chimera-based compounds with a BET inhibitor compound merged with protein degrading E3 ubiquitin ligase proteasome complex allows for selective degradation of BET proteins [107,108]. Along these lines, the original BET inhibitor BETi-211 was modified to BETd-246 and BETd-260 to include E3 ubiquitin ligase activity and has shown more efficient outcomes in preclinical TNBC studies [109]. Similarly, dual-functional compounds such as ARV-825 and ARV-771 have shown significantly higher anti-cancer effects over BET inhibitors such as, JQ1 or OTX015 [110].
Preclinical studies in various cancer models have demonstrated an apparent combinatorial synergism of BET inhibitors with various previously anti-cancer chemo-and immunotherapeutic agents (Table 3). In breast cancer studies, a combination of BET inhibitors with PI3K inhibitors demonstrated significantly decreased expression of downstream PI3K signaling genes such as EGFR and IGF growth factors, leading to reduced cell proliferation, as compared to treatment with PI3K inhibitor alone [53,61,82]. Similarly, a combination of BET inhibitor with PARP inhibitor, olaparib, has demonstrated significant reduction in transcription of BRAC1 and RAD51 genes [111,112]. In hematological cancers, active clinical trials are underway to study the combinatorial benefit of combining BET inhibitors with BCL2 inhibitors [113]. Interestingly, some of the known anti-cancer kinases inhibitors which target PLK1 and JAK2 (TG-101348) have also shown BET inhibitor capability [114]. Further dose-dependent clinical studies are needed to better understand the multi-functional efficiency of these drugs. Studies by Hogg et al. have demonstrated that treatment with BET inhibitors decreased the expression of PD-L1 in tumor cells through the inhibition of BRD4 binding to CD274 locus on chromosome 9 [115]. These data suggest a potential role of a combinatorial therapy of immune-modulating agents such as anti-PD1, anti-CTLA4 and CAR-T cells with BET inhibitors [116]. In the context of preclinical breast and prostate cancer models, BET inhibitors have suggested a combinatorial benefit with hormone receptor-modulating agents fulvestrant and enzalutamide [117]. All these various combinations should undergo stringent clinical trials to prove clinical applicability.
Table 3.
Various combinations used with BET inhibitors on pre-clinical models.
| Combination Therapy | Pre-Clinical Models Tested |
|---|---|
| JQ1 and FLT3-TK1 [118] | Immunodeficient mice injected with OCIAML3 or MOLM13 cells |
| JQ1/dBET1 and Ponatinib [119] | Colon (HCT116, HT29), breast (MCF-7, SKBR3) and ovarian (A2780, SKOV3) cancer cells |
| I-BET151 and panobinostat [120] | Immunodeficient mice injected with patient-derived melanoma cells resistant to vemurafenib |
| JQ1 and panobinostat [121] | Syngeneic orthotopic murine tumors, SK-N-BE (2) neuroblastoma cells |
| JQ1 and romidepsin [48] | Murine tumor models of NT2/D1 and NCCIT embryonal carcinoma |
| JQ1 and rapamycin [122] | Immunodeficient mice injected with MNNG/HOS osteosarcoma cells |
| CPI203 and rapamycin [123] | Immunodeficient mice injected with BON-1 pancreatic neuroendocrine tumor cells |
| JQ1 and trametinib [124] | Immunodeficient mice injected with ES2 ovarian clear cell carcinoma cells |
| JQ1 and vemurafenib [125] | Immunodeficient mice injected with A375 melanoma cells |
| JQ1 and fulvestrant [49] | Immunodeficient mice injected with tamoxifen-resistant MCF7 breast cancer cells |
| I-BET151 and lapatinib [126] | Immunodeficient mice injected with Her2þ BT474 breast cancer cells |
| JQ1 and lenalidomide [127] | Immunodeficient mice injected with BC-3 lymphoma cells |
| JQ1 and unidentified PD-1 inhibitor [128] | KRASmt NSCLC murine tumor model |
| RVX2135 and ATR inhibitor AZ20 [129] | Syngeneic λ820 and λ2749 murine Myc-induced lymphoma xenografts |
6. Conclusions
In conclusion, despite promising evidence from preclinical models, the clinical application of BET inhibitors remains elusive. Similar to chemotherapeutic agents such as alkylating and cell-cycle disrupting agents, BET inhibitors target transcriptional machinery with a higher impact of rapidly dividing cancer cells over normal cells. Although the final results from several of the BET inhibitor-based clinical trials are eagerly awaited, there could be detrimental side-effects, possibly explaining their limited success in the initial evidence from clinical trials. However, in spite of these challenges, we think that BET inhibitors have a promising role in combinatorial therapy and the future development of novel dual-BRD-motifs targeting inhibitors. Further studies are needed to determine the specific biomarkers which would implicate the long-term success of BET inhibitors in the p therapeutic application of a cancer patient, paving the way for personalized medicine.
Author Contributions
D.K. and V.T. participated in manuscript drafting, and revision of this review article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH-5U54CA163066 (VT).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kim M., Costello J. DNA methylation: An epigenetic mark of cellular memory. Exp. Mol. Med. 2017;49:e322. doi: 10.1038/emm.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang K.C., Chang H.Y. Epigenomics: Technologies and Applications. Circ. Res. 2018;122:1191–1199. doi: 10.1161/CIRCRESAHA.118.310998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanwal R., Gupta S. Epigenetic modifications in cancer. Clin. Genet. 2012;81:303–311. doi: 10.1111/j.1399-0004.2011.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C., Yue D., Lei L., Wang H., Lu J., Zhou Y., Liu S., Ding T., Guo M., Xu L. Promoter-Operating Targeted Expression of Gene Therapy in Cancer: Current Stage and Prospect. Mol. Nucleic Acids. 2018;11:508–514. doi: 10.1016/j.omtn.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradner J.E., Hnisz D., Young R.A. Transcriptional Addiction in Cancer. Cell. 2017;168:629–643. doi: 10.1016/j.cell.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audia J.E., Campbell R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016;8:a019521. doi: 10.1101/cshperspect.a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Seto E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016;6:a026831. doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes C.E., English D.M., Cowley S.M. Acetylation & Co: An expanding repertoire of histone acylations regulates chromatin and transcription. Essays Biochem. 2019;63:97–107. doi: 10.1042/EBC20180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi Y. The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins. Int. J. Mol. Sci. 2016;17:1849. doi: 10.3390/ijms17111849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez R., Zhou M.M. The role of human bromodomains in chromatin biology and gene transcription. Curr. Opin. Drug Discov. Dev. 2009;12:659–665. [PMC free article] [PubMed] [Google Scholar]
- 13.Zaware N., Zhou M.M. Bromodomain biology and drug discovery. Nat. Struct. Mol. Biol. 2019;26:870–879. doi: 10.1038/s41594-019-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippakopoulos P., Picaud S., Mangos M., Keates T., Lambert J.P., Barsyte-Lovejoy D., Felletar I., Volkmer R., Muller S., Pawson T., et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J., Vakoc C.R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell. 2014;54:728–736. doi: 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller S., Filippakopoulos P., Knapp S. Bromodomains as therapeutic targets. Expert Rev. Mol. Med. 2011;13:e29. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Vakoc C.R. Targeting Cancer Cells with BET Bromodomain Inhibitors. Cold Spring Harb. Perspect. Med. 2017;7:a026674. doi: 10.1101/cshperspect.a026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donati B., Lorenzini E., Ciarrocchi A. BRD4 and Cancer: Going beyond transcriptional regulation. Mol. Cancer. 2018;17:164. doi: 10.1186/s12943-018-0915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeney J.T., Belkina A.C., Shirihai O.S., Corkey B.E., Denis G.V. BET Bromodomain Proteins Brd2, Brd3 and Brd4 Selectively Regulate Metabolic Pathways in the Pancreatic beta-Cell. PLoS ONE. 2016;11:e0151329. doi: 10.1371/journal.pone.0151329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamonica J.M., Deng W., Kadauke S., Campbell A.E., Gamsjaeger R., Wang H., Cheng Y., Billin A.N., Hardison R.C., Mackay J.P., et al. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc. Natl. Acad. Sci. USA. 2011;108:E159–E168. doi: 10.1073/pnas.1102140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamsjaeger R., Webb S.R., Lamonica J.M., Billin A., Blobel G.A., Mackay J.P. Structural basis and specificity of acetylated transcription factor GATA1 recognition by BET family bromodomain protein Brd3. Mol. Cell Biol. 2011;31:2632–2640. doi: 10.1128/MCB.05413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wai D.C.C., Szyszka T.N., Campbell A.E., Kwong C., Wilkinson-White L.E., Silva A.P.G., Low J.K.K., Kwan A.H., Gamsjaeger R., Chalmers J.D., et al. The BRD3 ET domain recognizes a short peptide motif through a mechanism that is conserved across chromatin remodelers and transcriptional regulators. J. Biol. Chem. 2018;293:7160–7175. doi: 10.1074/jbc.RA117.000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French C.A. Small-Molecule Targeting of BET Proteins in Cancer. Adv. Cancer Res. 2016;131:21–58. doi: 10.1016/bs.acr.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Itzen F., Greifenberg A.K., Bosken C.A., Geyer M. Brd4 activates P-TEFb for RNA polymerase II CTD phosphorylation. Nucleic Acids Res. 2014;42:7577–7590. doi: 10.1093/nar/gku449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J., Li Q., Lievens S., Tavernier J., You J. Abrogation of the Brd4-positive transcription elongation factor B complex by papillomavirus E2 protein contributes to viral oncogene repression. J. Virol. 2010;84:76–87. doi: 10.1128/JVI.01647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel M.C., Debrosse M., Smith M., Dey A., Huynh W., Sarai N., Heightman T.D., Tamura T., Ozato K. BRD4 coordinates recruitment of pause release factor P-TEFb and the pausing complex NELF/DSIF to regulate transcription elongation of interferon-stimulated genes. Mol. Cell Biol. 2013;33:2497–2507. doi: 10.1128/MCB.01180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F., Shao W., Fujinaga K., Peterlin B.M. Bromodomain-containing protein 4-independent transcriptional activation by autoimmune regulator (AIRE) and NF-kappaB. J. Biol. Chem. 2018;293:4993–5004. doi: 10.1074/jbc.RA117.001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R., Yik J.H., Lew Q.J., Chao S.H. Brd4 and HEXIM1: Multiple roles in P-TEFb regulation and cancer. Biomed. Res. Int. 2014;2014:232870. doi: 10.1155/2014/232870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togel L., Nightingale R., Chueh A.C., Jayachandran A., Tran H., Phesse T., Wu R., Sieber O.M., Arango D., Dhillon A.S., et al. Dual Targeting of Bromodomain and Extraterminal Domain Proteins, and WNT or MAPK Signaling, Inhibits c-MYC Expression and Proliferation of Colorectal Cancer Cells. Mol. Cancer. 2016;15:1217–1226. doi: 10.1158/1535-7163.MCT-15-0724. [DOI] [PubMed] [Google Scholar]
- 30.Sinha A., Faller D.V., Denis G.V. Bromodomain analysis of Brd2-dependent transcriptional activation of cyclin A. Biochem. J. 2005;387:257–269. doi: 10.1042/BJ20041793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeRoy G., Rickards B., Flint S.J. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohan M., Lin C., Guest E., Shilatifard A. Licensed to elongate: A molecular mechanism for MLL-based leukaemogenesis. Nat. Rev. Cancer. 2010;10:721–728. doi: 10.1038/nrc2915. [DOI] [PubMed] [Google Scholar]
- 33.Hajmirza A., Emadali A., Gauthier A., Casasnovas O., Gressin R., Callanan M.B. BET Family Protein BRD4: An Emerging Actor in NFkappaB Signaling in Inflammation and Cancer. Biomedicines. 2018;6:16. doi: 10.3390/biomedicines6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi J., Wang Y., Zeng L., Wu Y., Deng J., Zhang Q., Lin Y., Li J., Kang T., Tao M., et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J., Cao J., Zhou B.P. Twist-BRD4 complex: Potential drug target for basal-like breast cancer. Curr. Pharm. Des. 2015;21:1256–1261. doi: 10.2174/1381612821666141211153853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang B., Yang X.D., Zhou M.M., Ozato K., Chen L.F. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol. Cell. Biol. 2009;29:1375–1387. doi: 10.1128/MCB.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.E., Park Y.K., Park S., Jang Y., Waring N., Dey A., Ozato K., Lai B., Peng W., Ge K. Brd4 binds to active enhancers to control cell identity gene induction in adipogenesis and myogenesis. Nat. Commun. 2017;8:2217. doi: 10.1038/s41467-017-02403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roe J.S., Mercan F., Rivera K., Pappin D.J., Vakoc C.R. BET Bromodomain Inhibition Suppresses the Function of Hematopoietic Transcription Factors in Acute Myeloid Leukemia. Mol. Cell. 2015;58:1028–1039. doi: 10.1016/j.molcel.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wapenaar H., Dekker F.J. Histone acetyltransferases: Challenges in targeting bi-substrate enzymes. Clin. Epigenetics. 2016;8:59. doi: 10.1186/s13148-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan H.M., La Thangue N.B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 2001;114:2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 41.Nicodeme E., Jeffrey K.L., Schaefer U., Beinke S., Dewell S., Chung C.W., Chandwani R., Marazzi I., Wilson P., Coste H., et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mirguet O., Gosmini R., Toum J., Clement C.A., Barnathan M., Brusq J.M., Mordaunt J.E., Grimes R.M., Crowe M., Pineau O., et al. Discovery of epigenetic regulator I-BET762: Lead optimization to afford a clinical candidate inhibitor of the BET bromodomains. J. Med. Chem. 2013;56:7501–7515. doi: 10.1021/jm401088k. [DOI] [PubMed] [Google Scholar]
- 43.Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I., et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.French C.A., Ramirez C.L., Kolmakova J., Hickman T.T., Cameron M.J., Thyne M.E., Kutok J.L., Toretsky J.A., Tadavarthy A.K., Kees U.R., et al. BRD-NUT oncoproteins: A family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 45.Ott C.J., Kopp N., Bird L., Paranal R.M., Qi J., Bowman T., Rodig S.J., Kung A.L., Bradner J.E., Weinstock D.M. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120:2843–2852. doi: 10.1182/blood-2012-02-413021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mio C., Gerratana L., Bolis M., Caponnetto F., Zanello A., Barbina M., Di Loreto C., Garattini E., Damante G., Puglisi F. BET proteins regulate homologous recombination-mediated DNA repair: BRCAness and implications for cancer therapy. Int. J. Cancer. 2019;144:755–766. doi: 10.1002/ijc.31898. [DOI] [PubMed] [Google Scholar]
- 47.Wen N., Guo B., Zheng H., Xu L., Liang H., Wang Q., Wang D., Chen X., Zhang S., Li Y., et al. Bromodomain inhibitor jq1 induces cell cycle arrest and apoptosis of glioma stem cells through the VEGF/PI3K/AKT signaling pathway. Int. J. Oncol. 2019;55:879–895. doi: 10.3892/ijo.2019.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jostes S., Nettersheim D., Fellermeyer M., Schneider S., Hafezi F., Honecker F., Schumacher V., Geyer M., Kristiansen G., Schorle H. The bromodomain inhibitor JQ1 triggers growth arrest and apoptosis in testicular germ cell tumours in vitro and in vivo. J. Cell. Mol. Med. 2017;21:1300–1314. doi: 10.1111/jcmm.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng Q., Zhang Z., Shea M.J., Creighton C.J., Coarfa C., Hilsenbeck S.G., Lanz R., He B., Wang L., Fu X., et al. An epigenomic approach to therapy for tamoxifen-resistant breast cancer. Cell Res. 2014;24:809–819. doi: 10.1038/cr.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengupta S., Biarnes M.C., Clarke R., Jordan V.C. Inhibition of BET proteins impairs estrogen-mediated growth and transcription in breast cancers by pausing RNA polymerase advancement. Breast Cancer Res. Treat. 2015;150:265–278. doi: 10.1007/s10549-015-3319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borbely G., Haldosen L.A., Dahlman-Wright K., Zhao C. Induction of USP17 by combining BET and HDAC inhibitors in breast cancer cells. Oncotarget. 2015;6:33623–33635. doi: 10.18632/oncotarget.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.da Motta L.L., Ledaki I., Purshouse K., Haider S., De Bastiani M.A., Baban D., Morotti M., Steers G., Wigfield S., Bridges E., et al. The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer. Oncogene. 2017;36:122–132. doi: 10.1038/onc.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vazquez R., Riveiro M.E., Astorgues-Xerri L., Odore E., Rezai K., Erba E., Panini N., Rinaldi A., Kwee I., Beltrame L., et al. The bromodomain inhibitor OTX015 (MK-8628) exerts anti-tumor activity in triple-negative breast cancer models as single agent and in combination with everolimus. Oncotarget. 2017;8:7598–7613. doi: 10.18632/oncotarget.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coude M.M., Braun T., Berrou J., Dupont M., Bertrand S., Masse A., Raffoux E., Itzykson R., Delord M., Riveiro M.E., et al. BET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cells. Oncotarget. 2015;6:17698–17712. doi: 10.18632/oncotarget.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pastori C., Daniel M., Penas C., Volmar C.H., Johnstone A.L., Brothers S.P., Graham R.M., Allen B., Sarkaria J.N., Komotar R.J., et al. BET bromodomain proteins are required for glioblastoma cell proliferation. Epigenetics. 2014;9:611–620. doi: 10.4161/epi.27906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picaud S., Da Costa D., Thanasopoulou A., Filippakopoulos P., Fish P.V., Philpott M., Fedorov O., Brennan P., Bunnage M.E., Owen D.R., et al. PFI-1, a highly selective protein interaction inhibitor, targeting BET Bromodomains. Cancer Res. 2013;73:3336–3346. doi: 10.1158/0008-5472.CAN-12-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang G., Plotnikov A.N., Rusinova E., Shen T., Morohashi K., Joshua J., Zeng L., Mujtaba S., Ohlmeyer M., Zhou M.M. Structure-guided design of potent diazobenzene inhibitors for the BET bromodomains. J. Med. Chem. 2013;56:9251–9264. doi: 10.1021/jm401334s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Salvia M., Esteller M. Bromodomain inhibitors and cancer therapy: From structures to applications. Epigenetics. 2017;12:323–339. doi: 10.1080/15592294.2016.1265710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gacias M., Gerona-Navarro G., Plotnikov A.N., Zhang G., Zeng L., Kaur J., Moy G., Rusinova E., Rodriguez Y., Matikainen B., et al. Selective chemical modulation of gene transcription favors oligodendrocyte lineage progression. Chem. Biol. 2014;21:841–854. doi: 10.1016/j.chembiol.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pascual J., Turner N.C. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann. Oncol. 2019;30:1051–1060. doi: 10.1093/annonc/mdz133. [DOI] [PubMed] [Google Scholar]
- 61.Stuhlmiller T.J., Miller S.M., Zawistowski J.S., Nakamura K., Beltran A.S., Duncan J.S., Angus S.P., Collins K.A., Granger D.A., Reuther R.A., et al. Inhibition of Lapatinib-Induced Kinome Reprogramming in ERBB2-Positive Breast Cancer by Targeting BET Family Bromodomains. Cell Rep. 2015;11:390–404. doi: 10.1016/j.celrep.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klauber-DeMore N., Schulte B.A., Wang G.Y. Targeting MYC for triple-negative breast cancer treatment. Oncoscience. 2018;5:120–121. doi: 10.18632/oncoscience.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bihani T., Ezell S.A., Ladd B., Grosskurth S.E., Mazzola A.M., Pietras M., Reimer C., Zinda M., Fawell S., D’Cruz C.M. Resistance to everolimus driven by epigenetic regulation of MYC in ER+ breast cancers. Oncotarget. 2015;6:2407–2420. doi: 10.18632/oncotarget.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mertz J.A., Conery A.R., Bryant B.M., Sandy P., Balasubramanian S., Mele D.A., Bergeron L., Sims R.J., 3rd Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. USA. 2011;108:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delmore J.E., Issa G.C., Lemieux M.E., Rahl P.B., Shi J., Jacobs H.M., Kastritis E., Gilpatrick T., Paranal R.M., Qi J., et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khandekar D., Amara S., Tiriveedhi V. Immunogenicity of Tumor Initiating Stem Cells: Potential Applications in Novel Anticancer Therapy. Front. Oncol. 2019;9:315. doi: 10.3389/fonc.2019.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horne G.A., Stewart H.J., Dickson J., Knapp S., Ramsahoye B., Chevassut T. Nanog requires BRD4 to maintain murine embryonic stem cell pluripotency and is suppressed by bromodomain inhibitor JQ1 together with Lefty1. Stem Cells Dev. 2015;24:879–891. doi: 10.1089/scd.2014.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhola N.E., Balko J.M., Dugger T.C., Kuba M.G., Sanchez V., Sanders M., Stanford J., Cook R.S., Arteaga C.L. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013;123:1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fong C.Y., Gilan O., Lam E.Y., Rubin A.F., Ftouni S., Tyler D., Stanley K., Sinha D., Yeh P., Morison J., et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525:538–542. doi: 10.1038/nature14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kroon P., Berry P.A., Stower M.J., Rodrigues G., Mann V.M., Simms M., Bhasin D., Chettiar S., Li C., Li P.K., et al. JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res. 2013;73:5288–5298. doi: 10.1158/0008-5472.CAN-13-0874. [DOI] [PubMed] [Google Scholar]
- 71.Chan C.H., Fang C., Yarilina A., Prinjha R.K., Qiao Y., Ivashkiv L.B. BET bromodomain inhibition suppresses transcriptional responses to cytokine-Jak-STAT signaling in a gene-specific manner in human monocytes. Eur. J. Immunol. 2015;45:287–297. doi: 10.1002/eji.201444862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu N., Hu G., Wang H., Li Z., Guo Z. PLK1 inhibitor facilitates the suppressing effect of temozolomide on human brain glioma stem cells. J. Cell. Mol. Med. 2018;22:5300–5310. doi: 10.1111/jcmm.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia Z., Nie Y., Yue F., Kong Y., Gu L., Gavin T.P., Liu X., Kuang S. A requirement of Polo-like kinase 1 in murine embryonic myogenesis and adult muscle regeneration. eLife. 2019;8:e47097. doi: 10.7554/eLife.47097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao F., Li J., Luo Q., Wang R., Kong Y., Carlock C., Liu Z., Elzey B.D., Liu X. Plk1 Inhibition Enhances the Efficacy of BET Epigenetic Reader Blockade in Castration-Resistant Prostate Cancer. Mol. Cancer. 2018;17:1554–1565. doi: 10.1158/1535-7163.MCT-17-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nieto-Jimenez C., Alcaraz-Sanabria A., Perez-Pena J., Corrales-Sanchez V., Serrano-Heras G., Galan-Moya E.M., Serrano-Oviedo L., Montero J.C., Burgos M., Llopis J., et al. Targeting basal-like breast tumors with bromodomain and extraterminal domain (BET) and polo-like kinase inhibitors. Oncotarget. 2017;8:19478–19490. doi: 10.18632/oncotarget.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shu S., Lin C.Y., He H.H., Witwicki R.M., Tabassum D.P., Roberts J.M., Janiszewska M., Huh S.J., Liang Y., Ryan J., et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529:413–417. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiang C.M. Phospho-BRD4: Transcription plasticity and drug targeting. Drug Discov. Today Technol. 2016;19:17–22. doi: 10.1016/j.ddtec.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abedin S.M., Boddy C.S., Munshi H.G. BET inhibitors in the treatment of hematologic malignancies: Current insights and future prospects. Oncol. Targets. 2016;9:5943–5953. doi: 10.2147/OTT.S100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhattacharya S., Piya S., Borthakur G. Bromodomain inhibitors: What does the future hold? Clin. Adv. Hematol. Oncol. 2018;16:504–515. [PubMed] [Google Scholar]
- 80.Ocana A., Nieto-Jimenez C., Pandiella A. BET inhibitors as novel therapeutic agents in breast cancer. Oncotarget. 2017;8:71285–71291. doi: 10.18632/oncotarget.19744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marcotte R., Sayad A., Brown K.R., Sanchez-Garcia F., Reimand J., Haider M., Virtanen C., Bradner J.E., Bader G.D., Mills G.B., et al. Functional Genomic Landscape of Human Breast Cancer Drivers, Vulnerabilities, and Resistance. Cell. 2016;164:293–309. doi: 10.1016/j.cell.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stratikopoulos E.E., Dendy M., Szabolcs M., Khaykin A.J., Lefebvre C., Zhou M.M., Parsons R. Kinase and BET Inhibitors Together Clamp Inhibition of PI3K Signaling and Overcome Resistance to Therapy. Cancer Cell. 2015;27:837–851. doi: 10.1016/j.ccell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimamura T., Chen Z., Soucheray M., Carretero J., Kikuchi E., Tchaicha J.H., Gao Y., Cheng K.A., Cohoon T.J., Qi J., et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin. Cancer Res. 2013;19:6183–6192. doi: 10.1158/1078-0432.CCR-12-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jauset T., Masso-Valles D., Martinez-Martin S., Beaulieu M.E., Foradada L., Fiorentino F.P., Yokota J., Haendler B., Siegel S., Whitfield J.R., et al. BET inhibition is an effective approach against KRAS-driven PDAC and NSCLC. Oncotarget. 2018;9:18734–18746. doi: 10.18632/oncotarget.24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alqahtani A., Choucair K., Ashraf M., Hammouda D.M., Alloghbi A., Khan T., Senzer N., Nemunaitis J. Bromodomain and extra-terminal motif inhibitors: A review of preclinical and clinical advances in cancer therapy. Future Sci. OA. 2019;5:FSO372. doi: 10.4155/fsoa-2018-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steegmaier M., Hoffmann M., Baum A., Lenart P., Petronczki M., Krssak M., Gurtler U., Garin-Chesa P., Lieb S., Quant J., et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 87.Picaud S., Wells C., Felletar I., Brotherton D., Martin S., Savitsky P., Diez-Dacal B., Philpott M., Bountra C., Lingard H., et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc. Natl. Acad. Sci. USA. 2013;110:19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLure K.G., Gesner E.M., Tsujikawa L., Kharenko O.A., Attwell S., Campeau E., Wasiak S., Stein A., White A., Fontano E., et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS ONE. 2013;8:e83190. doi: 10.1371/journal.pone.0083190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kharenko O.A., Gesner E.M., Patel R.G., Norek K., White A., Fontano E., Suto R.K., Young P.R., McLure K.G., Hansen H.C. RVX-297—A novel BD2 selective inhibitor of BET bromodomains. Biochem. Biophys. Res. Commun. 2016;477:62–67. doi: 10.1016/j.bbrc.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 90.Martin M.P., Olesen S.H., Georg G.I., Schonbrunn E. Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. ACS Chem. Biol. 2013;8:2360–2365. doi: 10.1021/cb4003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ember S.W., Zhu J.Y., Olesen S.H., Martin M.P., Becker A., Berndt N., Georg G.I., Schonbrunn E. Acetyl-lysine binding site of bromodomain-containing protein 4 (BRD4) interacts with diverse kinase inhibitors. ACS Chem. Biol. 2014;9:1160–1171. doi: 10.1021/cb500072z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ciceri P., Muller S., O’Mahony A., Fedorov O., Filippakopoulos P., Hunt J.P., Lasater E.A., Pallares G., Picaud S., Wells C., et al. Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. Nat. Chem. Biol. 2014;10:305–312. doi: 10.1038/nchembio.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lewin J., Soria J.C., Stathis A., Delord J.P., Peters S., Awada A., Aftimos P.G., Bekradda M., Rezai K., Zeng Z., et al. Phase Ib Trial with Birabresib, a Small-Molecule Inhibitor of Bromodomain and Extraterminal Proteins, in Patients with Selected Advanced Solid Tumors. J. Clin. Oncol. 2018;36:3007–3014. doi: 10.1200/JCO.2018.78.2292. [DOI] [PubMed] [Google Scholar]
- 94.Wang R., You J. Mechanistic analysis of the role of bromodomain-containing protein 4 (BRD4) in BRD4-NUT oncoprotein-induced transcriptional activation. J. Biol. Chem. 2015;290:2744–2758. doi: 10.1074/jbc.M114.600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winters A.C., Bernt K.M. MLL-Rearranged Leukemias-An Update on Science and Clinical Approaches. Front. Pediatr. 2017;5:4. doi: 10.3389/fped.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li N., Yang L., Qi X.K., Lin Y.X., Xie X., He G.P., Feng Q.S., Liu L.R., Xie X., Zeng Y.X., et al. BET bromodomain inhibitor JQ1 preferentially suppresses EBV-positive nasopharyngeal carcinoma cells partially through repressing c-Myc. Cell Death Dis. 2018;9:761. doi: 10.1038/s41419-018-0789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wadhwa E., Nicolaides T. Bromodomain Inhibitor Review: Bromodomain and Extra-terminal Family Protein Inhibitors as a Potential New Therapy in Central Nervous System Tumors. Cureus. 2016;8:e620. doi: 10.7759/cureus.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rathert P., Roth M., Neumann T., Muerdter F., Roe J.S., Muhar M., Deswal S., Cerny-Reiterer S., Peter B., Jude J., et al. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature. 2015;525:543–547. doi: 10.1038/nature14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anand P., Brown J.D., Lin C.Y., Qi J., Zhang R., Artero P.C., Alaiti M.A., Bullard J., Alazem K., Margulies K.B., et al. BET bromodomains mediate transcriptional pause release in heart failure. Cell. 2013;154:569–582. doi: 10.1016/j.cell.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matzuk M.M., McKeown M.R., Filippakopoulos P., Li Q., Ma L., Agno J.E., Lemieux M.E., Picaud S., Yu R.N., Qi J., et al. Small-molecule inhibition of BRDT for male contraception. Cell. 2012;150:673–684. doi: 10.1016/j.cell.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berkovits B.D., Wolgemuth D.J. The role of the double bromodomain-containing BET genes during mammalian spermatogenesis. Curr. Top. Dev. Biol. 2013;102:293–326. doi: 10.1016/B978-0-12-416024-8.00011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sullivan J.M., Badimon A., Schaefer U., Ayata P., Gray J., Chung C.W., von Schimmelmann M., Zhang F., Garton N., Smithers N., et al. Autism-like syndrome is induced by pharmacological suppression of BET proteins in young mice. J. Exp. Med. 2015;212:1771–1781. doi: 10.1084/jem.20151271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banerjee C., Archin N., Michaels D., Belkina A.C., Denis G.V., Bradner J., Sebastiani P., Margolis D.M., Montano M. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J. Leukoc. Biol. 2012;92:1147–1154. doi: 10.1189/jlb.0312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amorim S., Stathis A., Gleeson M., Iyengar S., Magarotto V., Leleu X., Morschhauser F., Karlin L., Broussais F., Rezai K., et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: A dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3:e196–e204. doi: 10.1016/S2352-3026(16)00021-1. [DOI] [PubMed] [Google Scholar]
- 105.Rhyasen G.W., Hattersley M.M., Yao Y., Dulak A., Wang W., Petteruti P., Dale I.L., Boiko S., Cheung T., Zhang J., et al. AZD5153: A Novel Bivalent BET Bromodomain Inhibitor Highly Active against Hematologic Malignancies. Mol. Cancer. 2016;15:2563–2574. doi: 10.1158/1535-7163.MCT-16-0141. [DOI] [PubMed] [Google Scholar]
- 106.Tanaka M., Roberts J.M., Seo H.S., Souza A., Paulk J., Scott T.G., DeAngelo S.L., Dhe-Paganon S., Bradner J.E. Design and characterization of bivalent BET inhibitors. Nat. Chem. Biol. 2016;12:1089–1096. doi: 10.1038/nchembio.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu J., Qian Y., Altieri M., Dong H., Wang J., Raina K., Hines J., Winkler J.D., Crew A.P., Coleman K., et al. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem. Biol. 2015;22:755–763. doi: 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakamoto K.M., Kim K.B., Kumagai A., Mercurio F., Crews C.M., Deshaies R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bai L., Zhou B., Yang C.Y., Ji J., McEachern D., Przybranowski S., Jiang H., Hu J., Xu F., Zhao Y., et al. Targeted Degradation of BET Proteins in Triple-Negative Breast Cancer. Cancer Res. 2017;77:2476–2487. doi: 10.1158/0008-5472.CAN-16-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raina K., Lu J., Qian Y., Altieri M., Gordon D., Rossi A.M., Wang J., Chen X., Dong H., Siu K., et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA. 2016;113:7124–7129. doi: 10.1073/pnas.1521738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karakashev S., Zhu H., Yokoyama Y., Zhao B., Fatkhutdinov N., Kossenkov A.V., Wilson A.J., Simpkins F., Speicher D., Khabele D., et al. BET Bromodomain Inhibition Synergizes with PARP Inhibitor in Epithelial Ovarian Cancer. Cell Rep. 2017;21:3398–3405. doi: 10.1016/j.celrep.2017.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang L., Zhang Y., Shan W., Hu Z., Yuan J., Pi J., Wang Y., Fan L., Tang Z., Li C., et al. Repression of BET activity sensitizes homologous recombination-proficient cancers to PARP inhibition. Sci. Transl. Med. 2017;9:eaal1645. doi: 10.1126/scitranslmed.aal1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bui M.H., Lin X., Albert D.H., Li L., Lam L.T., Faivre E.J., Warder S.E., Huang X., Wilcox D., Donawho C.K., et al. Preclinical Characterization of BET Family Bromodomain Inhibitor ABBV-075 Suggests Combination Therapeutic Strategies. Cancer Res. 2017;77:2976–2989. doi: 10.1158/0008-5472.CAN-16-1793. [DOI] [PubMed] [Google Scholar]
- 114.Ember S.W., Lambert Q.T., Berndt N., Gunawan S., Ayaz M., Tauro M., Zhu J.Y., Cranfill P.J., Greninger P., Lynch C.C., et al. Potent Dual BET Bromodomain-Kinase Inhibitors as Value-Added Multitargeted Chemical Probes and Cancer Therapeutics. Mol. Cancer. 2017;16:1054–1067. doi: 10.1158/1535-7163.MCT-16-0568-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hogg S.J., Vervoort S.J., Deswal S., Ott C.J., Li J., Cluse L.A., Beavis P.A., Darcy P.K., Martin B.P., Spencer A., et al. BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1. Cell Rep. 2017;18:2162–2174. doi: 10.1016/j.celrep.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lai X., Stiff A., Duggan M., Wesolowski R., Carson W.E., 3rd, Friedman A. Modeling combination therapy for breast cancer with BET and immune checkpoint inhibitors. Proc. Natl. Acad. Sci. USA. 2018;115:5534–5539. doi: 10.1073/pnas.1721559115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sammons S., Kornblum N.S., Blackwell K.L. Fulvestrant-Based Combination Therapy for Second-Line Treatment of Hormone Receptor-Positive Advanced Breast Cancer. Target Oncol. 2019;14:1–12. doi: 10.1007/s11523-018-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fiskus W., Sharma S., Qi J., Shah B., Devaraj S.G., Leveque C., Portier B.P., Iyer S., Bradner J.E., Bhalla K.N. BET protein antagonist JQ1 is synergistically lethal with FLT3 tyrosine kinase inhibitor (TKI) and overcomes resistance to FLT3-TKI in AML cells expressing FLT-ITD. Mol. Cancer. 2014;13:2315–2327. doi: 10.1158/1535-7163.MCT-14-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bauer K., Berger D., Zielinski C.C., Valent P., Grunt T.W. Hitting two oncogenic machineries in cancer cells: Cooperative effects of the multi-kinase inhibitor ponatinib and the BET bromodomain blockers JQ1 or dBET1 on human carcinoma cells. Oncotarget. 2018;9:26491–26506. doi: 10.18632/oncotarget.25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heinemann A., Cullinane C., De Paoli-Iseppi R., Wilmott J.S., Gunatilake D., Madore J., Strbenac D., Yang J.Y., Gowrishankar K., Tiffen J.C., et al. Combining BET and HDAC inhibitors synergistically induces apoptosis of melanoma and suppresses AKT and YAP signaling. Oncotarget. 2015;6:21507–21521. doi: 10.18632/oncotarget.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shahbazi J., Liu P.Y., Atmadibrata B., Bradner J.E., Marshall G.M., Lock R.B., Liu T. The Bromodomain Inhibitor JQ1 and the Histone Deacetylase Inhibitor Panobinostat Synergistically Reduce N-Myc Expression and Induce Anticancer Effects. Clin. Cancer Res. 2016;22:2534–2544. doi: 10.1158/1078-0432.CCR-15-1666. [DOI] [PubMed] [Google Scholar]
- 122.Lee D.H., Qi J., Bradner J.E., Said J.W., Doan N.B., Forscher C., Yang H., Koeffler H.P. Synergistic effect of JQ1 and rapamycin for treatment of human osteosarcoma. Int. J. Cancer. 2015;136:2055–2064. doi: 10.1002/ijc.29269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wong C., Laddha S.V., Tang L., Vosburgh E., Levine A.J., Normant E., Sandy P., Harris C.R., Chan C.S., Xu E.Y. The bromodomain and extra-terminal inhibitor CPI203 enhances the antiproliferative effects of rapamycin on human neuroendocrine tumors. Cell Death Dis. 2014;5:e1450. doi: 10.1038/cddis.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jing Y., Zhang Z., Ma P., An S., Shen Y., Zhu L., Zhuang G. Concomitant BET and MAPK blockade for effective treatment of ovarian cancer. Oncotarget. 2016;7:2545–2554. doi: 10.18632/oncotarget.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paoluzzi L., Hanniford D., Sokolova E., Osman I., Darvishian F., Wang J., Bradner J.E., Hernando E. BET and BRAF inhibitors act synergistically against BRAF-mutant melanoma. Cancer Med. 2016;5:1183–1193. doi: 10.1002/cam4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matkar S., Sharma P., Gao S., Gurung B., Katona B.W., Liao J., Muhammad A.B., Kong X.C., Wang L., Jin G., et al. An Epigenetic Pathway Regulates Sensitivity of Breast Cancer Cells to HER2 Inhibition via FOXO/c-Myc Axis. Cancer Cell. 2015;28:472–485. doi: 10.1016/j.ccell.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gopalakrishnan R., Matta H., Tolani B., Triche T., Jr., Chaudhary P.M. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene. 2016;35:1797–1810. doi: 10.1038/onc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adeegbe D.O., Liu S., Hattersley M.M., Bowden M., Zhou C.W., Li S., Vlahos R., Grondine M., Dolgalev I., Ivanova E.V., et al. BET Bromodomain Inhibition Cooperates with PD-1 Blockade to Facilitate Antitumor Response in Kras-Mutant Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2018;6:1234–1245. doi: 10.1158/2326-6066.CIR-18-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muralidharan S.V., Bhadury J., Nilsson L.M., Green L.C., McLure K.G., Nilsson J.A. BET bromodomain inhibitors synergize with ATR inhibitors to induce DNA damage, apoptosis, senescence-associated secretory pathway and ER stress in Myc-induced lymphoma cells. Oncogene. 2016;35:4689–4697. doi: 10.1038/onc.2015.521. [DOI] [PubMed] [Google Scholar]