Abstract
Triple negative breast cancer (TNBC) is characterized by highly aggressive phenotype, limited treatment options and a poor prognosis. In the present study, we examined the therapeutic effect of anti–claudin (CLDN)‐4 extracellular domain antibody, 4D3, on TNBC. When the expression of CLDN4 and CLDN1 in invasive ductal carcinoma (IDC) was examined in 114 IDC (78 cases from 2004 to 2009 in a single center and 36 cases of tissues array), CLDN1 had lower expression than CLDN4 and was correlated with histological grade. In contrast, expression of CLDN4 was correlated with histological grade, receptor subtype, and stage. CLDN4 expression in human IDC cell lines MCF‐7 (luminal subtype) and MDA‐468 (TNBC) was at the same level. In both cells, paclitaxel (PTX)‐induced growth suppression was enhanced by 4D3. Furthermore, 4D3 increased both intracellular PTX concentration (in both cells) and apoptosis. In the mouse model, 4D3 promoted the antitumor effect of PTX on subcutaneous tumors and reduced lung metastasis. The combination of PTX and 4D3 reduced M2 macrophages and mesenchymal stem cells in the tumor. 4D3 also reduced stemness of the tumors and increased the intratumoral pH. Moreover, concurrent treatment with 4D3, PTX and tamoxifen, or with PTX and tamoxifen in MDA‐468 also showed the same level of antitumor activity and survival as MCF‐7. Furthermore, in a bone metastasis model, combination of PTX and bisphosphonate with 4D3 promoted tumor growth in both cells. Thus, CLDN4 targeting of the antibody facilitated existing therapeutic effects.
Keywords: breast cancer, chemotherapy, claudin, microenvironment, tight junction
CLDN4 was overexpressed in breast cancer, including triple negative breast cancer (TNBC). The antibody against the CLDN4 extracellular domain altered the tumor microenvironment and promoted the effects of anticancer and bisphosphonates in both estrogen‐sensitive breast cancer and TNBC.

1. INTRODUCTION
Breast cancer is the third leading cause of cancer death in Japanese women.1 The most common histological type, invasive ductal carcinomas (IDC), frequently express hormone receptors (luminal subtype) and/or human epidermal growth factor receptor‐2 (HER2, Her2 subtype).2, 3 In contrast, approximately 15% of IDC express none of estrogen receptor (ER), progesterone receptor (PgR), or HER2, which is designated as triple‐negative breast cancer (TNBC).4 TNBC possess more malignant phenotypes than the usual IDC, with rapid growth and high frequency of recurrence and metastasis.5 The treatment of breast cancer commonly includes a combination of surgery, radiation, chemotherapy, hormone therapy and targeted therapy against HER2.6 However, TNBC lack molecular therapy targets and also currently lack effective molecular therapy, which, therefore, means there is an associated high mortality and poor prognosis.4, 7
Tight junctions are intercellular adhesion structures that control the para‐cellular traffic.8 In the epithelium, tight junctions act as a barrier or fence between the luminal space and the epithelium,8, 9 preventing the permeation of harmful substances and the leakage of physiologically active substances. Thus, tight junctions play a role in maintaining the microenvironment by inhibiting the invasion of anticancer drugs into tumor tissues and promoting intratumoral retention of growth factors in cancer.10, 11, 12
CLDN4 is a major component of the epithelial tight junction.13 CLDN form a family of 27 isoforms with very homologous structures.8, 9, 14 CLDN4 exhibits high expression in epithelial tissues, and the expression is also seen in epithelial malignant tumors.15, 16, 17, 18 We have studied the availability of CLDN4 targeting in cancer therapy by preparing antibodies specific to the extracellular domain of CLDN4.12 The anti–CLDN4 extracellular domain antibody (4D3) decreases the barrier function in cancer, promotes permeation of anticancer agents, and enhances its antitumor effect.10, 11, 12
Paclitaxel (PTX) is a first‐line therapeutic drug in the treatment of breast cancer.19 PTX exerts its antitumor activity by promoting the polymerization and stabilization of tubulin to form microtubules, stopping the cell cycle at the G2/M phase, which causes apoptosis.20 PTX is also applied as treatment for TNBC with immune checkpoint inhibitors.21, 22
In this study, we investigated the effect of 4D3 on IDC, especially TNBC, and assessed the efficacy in combination treatment with PTX and PD‐1 inhibitor.
2. MATERIALS AND METHODS
2.1. Surgical specimens
We reviewed the pathological diagnosis and clinical data of 78 patients diagnosed with IDC in the Department of Molecular Pathology, Nara Medical University from 2004 to 2009. There were 47 cases of luminal subtype, 23 cases of Her2 subtype and 8 cases of triple negative subtype (TNBC, 8 cases). As written informed consent was not obtained, any identifying information was removed from the samples prior to analysis, to ensure strict privacy protection (unlinkable anonymization). All procedures were performed in accordance with the Ethical Guidelines for Human Genome/Gene Research issued by the Japanese Government and were approved by the Ethics Committee of Nara Medical University (approval number 937).
2.2. Tissue array
A tissue array slide of TNBC was obtained from US Biomax (BR487a). Quadruplet slides were immunostained with ER, PgR, Her2, CLDN1 and CLDN4. IDC cases with triple negative expression (36 cases) were used for comparison between clinicopathological parameters and CLDN4 and CLDN1 expression.
2.3. Cell lines
MCF‐7 and MDA‐468 human IDC cell lines were purchased from Dainihon Pharmaceutical. Cells were cultured in DMEM supplemented with 10% charcoal‐stripped FBS (Sigma Chemical) at 37°C in 5% CO2. Cell growth was assessed using a tetrazolium (MTT) dye assay, as previously described.23 A pH 7.0 DMEM was prepared from regular DMEM (pH 7.4) adjusted by the addition of HCl.
2.4. Sphere assay
Breast cancer cells (5 × 104) were grown in stem cell medium (Sigma) in 6‐well bacteriological grade plates (Gibco) and incubated at 37°C in 5% CO2. After 5 hours, cells were treated with 4D3 for 24 hours.
2.5. Antibody and reagents
The anti–human CLDN4 extracellular domain antibody 4D3 was developed by immunizing rats with a plasmid vector encoding human CLDN4.12 The anti–human CLDN1 extracellular domain antibody 2C1 was also developed using the same method.24 PTX (Wako Pure Chemical) and estradiol (LKT Labs) were purchased.
2.6. Apoptosis
Apoptosis was assessed by staining the cells with the Hoechst 33 258 fluorescent dye (Wako). The number of apoptotic cells was determined by examining 1000 stained cells.23
2.7. Animals
BALB/c nude mice (4 weeks old, female) were purchased from SLC Japan. The mice were maintained according to the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, in accordance with the current regulations and standards of the Ministry of Health, Labor, and Welfare.
To establish a subcutaneous tumor model, cells (1 × 107) were inoculated subcutaneously into the scapular tissues of nude mice. Then, with five mice in each group, PTX (10 mg/kg body weight [BW])25 and/or 4D3 (1 mg/kg BW, diluted with saline) were injected into the peritoneal cavity simultaneously on days 1, 3 and 7. Tumor size was monitored weekly. According to the institutional humane endpoint for animal experiments, moribund mice were euthanized. For anti–estrogen therapy, tamoxifen citrate (TAM, 500 mg per mouse in peanut oil, daily, s.c., LKT Labs) was administrated.26
2.8. Lung metastasis model
The IDC cell suspension (1 × 106 cells/50 μL of PBS) was injected into the caudal vein. PTX (10 mg/kg BW)25 and 4D3 (1 mg/kg BW) were also administrated (i.p.) on days 1, 3 and 7.
2.9. Bone metastasis model
Under inhalation anesthesia with 3% isoflurane (WAKO), percutaneous intraosseal injection was performed by drilling a 26‐gauge needle into the tibia proximal to the tuberositas tibia, then the IDC cell suspension (5 × 105 cells/20 μL of PBS) was inserted.27 Mice were treated with bisphosphonate (BP, zoledronic acid, WAKO, 100 μg/kg in 100 μL PBS),28 PTX (10 mg/kg BW)25 and 4D3 (1 mg/kg BW) administrated (i.p.) on days 1, 3 and 7.
2.10. In vivo imaging of tumor
The IDC cell was labeled with VivoTrack 680 (PerkinElmer). A mouse was examined using a Clairvivo OPT in vivo imager (Shimazu) under anesthesia.12 Fluorescence intensity was calculated with software in the imager.
2.11. Intratumoral pH
Tumors were penetrated using a 18G needle, through which a fine needle probe of pH meter (Chemical Instruments) was inserted into tumors under anesthesia. pH was monitored for 5 minutes to calculate the mean value in one site. The measurement was performed at five sites for each tumor. Representative pH was a mean of values in the five sites.
2.12. Immunohistochemistry
Consecutive 4‐μm sections were immunohistochemically stained using 0.2 µg/mL of 4D3 or 2C1 with a previously described immunoperoxidase technique.29 Secondary antibodies (Medical and Biological Laboratories) were used at a concentration of 0.2 µg/mL. Tissue sections were color‐developed with diamine benzidine hydrochloride and counterstained with Meyer’s hematoxylin (Sigma). We counted immunopositive cells at the cytoplasmic membrane. Staining strength was scored from 0 to 3 (a score of 1 was used to describe the expression level in normal mammary duct epithelium). The staining index was calculated as the staining strength score multiplied by the staining area (%).10, 11, 12 For a negative control, non–immunized rat IgG (Santa‐Cruz Biotechnology) was used as the primary antibody.
2.13. Immunoblot analysis
Whole‐cell lysates were prepared as previously described.30 Lysates (20 μg) were subjected to immunoblot analysis using SDS‐PAGE (12.5%), followed by electrotransfer onto nitrocellulose filters. The filters were incubated with primary antibodies, followed by peroxidase‐conjugated IgG antibodies (Medical and Biological Laboratories). Anti–tubulin antibody was used to assess the levels of protein loaded per lane (Oncogene Research Products). The immune complex was visualized using an enhanced chemiluminescence western‐blot detection system (Amersham). Antibodies for CLDN4 (4D3), CLDN1 (2C1) and ER (Proteintech Group) were used as primary antibodies.
2.14. RT‐PCR
To assess human CLDN4 mRNA expression, RT‐PCR was performed with 0.5 µg total RNA extracted using an RNeasy Kit (Qiagen). The primer sets were as follows: mouse Batf2 (M1 macrophage), forward 5ʹ‐AGC ACG AAT CCT TGG AGA AA AA‐3′ and reverse 5ʹ‐GTT CCT GGC AGC CAT TGT AT‐3′ (National Center for Biotechnology Information [NCBI] Reference Sequence: BC024521.1); mouse Fizz1 (M2 macrophage), forward 5ʹ‐CCC TTC TCA TCT GCA TCT CC‐3′ and reverse 5ʹ‐CAG TAG CAG TCA TCC CAG CA‐3′ (NCBI Reference Sequence: AF316397.2); mouse CD73 (mesenchymal stem cell), forward 5ʹ‐CCT CTC AAA TCC AGG GAC AA‐3′ and reverse 5ʹ‐TTT GGA AGG TGG ATT TCC TG‐3′ (NCBI Reference Sequence: L12059.1), which were synthesized by Sigma Genosys). PCR products were electrophoresed using a 2% agarose gel and stained with ethidium bromide. The β‐actin (ACTB) mRNA was also amplified for use as an internal control (GenBank Accession No. NM_001101).
2.15. ELISA
ELISA kits were used to measure the concentrations of PTX (anti–5‐PTX antibody‐derived ELISA) according to the manufacturers’ instructions.
2.16. Statistical analysis
Statistical significance was calculated using χ 2, and the Kruskal–Wallis tests with InStat software (GraphPad). Survival analysis was performed using the Kaplan‐Meier method along with the log‐rank test (SPSS Statistics; IBM Japan). Statistical significance was defined as a two‐sided P‐value of <0.05.
3. RESULTS
3.1. Expressions of CLDN4 and CLDN1 in human invasive ductal carcinoma
Expressions of CLDN4 and CLDN1 were examined by immunohistochemistry in IDC (Figure 1). In a solid‐tubular/luminal type IDC case, CLDN4 was located at the cytoplasmic membrane in cancer cells (Figure 1A). The CLDN4 expression level was higher than that of CLDN1. In a scirrhous/triple negative IDC case, CLDN4 expression was retained at the cytoplasmic membrane (Figure 1B). In contrast, CLDN1 expression was not detected.
Figure 1.

Expression of CLDN4 and CLDN1 in invasive ductal carcinomas. An immunohistochemical evaluation to identify CLDN4 (A, C) and CLDN1 (B, D) at the cytoplasmic membrane of cancer cells using anti–CLDN4 antibody, 4D3 and anti–CLDN1 antibody, 2C1, respectively. A, B, Solid‐tubular type, nuclear grade (NG) 2, pT1N0, stage 1, luminal subtype. C, D, Scirrhous type, NG3, pT2N1, stage 2 and triple negative subtype. Bar, 100 μm
We next compared the CLDN4 expression with clinicopathological parameters in the 114 cases, including 36 tissue array cases (Table 1). CLDN4 expression index was associated with histological differentiation, receptor subtype, nodal metastasis (pN) and pathological stage. In contrast, CLDN1 expression index was associated with histological differentiation, and nodal metastasis (pN), but not with receptor subtype. Notably, CLDN4 expression in TNBC was higher than in luminal or HER2 subtypes. As shown in Table 2, TNBC cases showed more advanced primary tumor (pT), nodal metastasis (pN) and pathological stage than those in cases of luminal type and HER2 type. In TNBC cases, CLDN4 expression was associated with histological differentiation, primary tumor (pT), nodal metastasis (pN) and pathological stage.
Table 1.
Expression of CLDN4 and CLDN1 in 78 invasive ductal carcinomas
| Parameters c | n | Expression index a | |||
|---|---|---|---|---|---|
| CLDN1 | P b | CLDN4 | P b | ||
| Total | 114 | 45 ± 7 | 212 ± 10 | ||
| Age | |||||
| ‐50 | 58 | 47 ± 8 | NS | 218 ± 12 | NS |
| 51‐ | 56 | 45 ± 7 | 211 ± 13 | ||
| Histology | |||||
| Papillo‐tubular | 22 | 64 ± 24 | <0.0001 | 152 ± 27 | <0.0001 |
| Solid tubular | 48 | 30 ± 8 | 229 ± 21 | ||
| Scirrhous | 44 | 50 ± 7 | 232 ± 26 | ||
| Subtype | |||||
| Luminal | 47 | 45 ± 6 | NS | 197 ± 23 | <0.0001 |
| HER2 | 23 | 40 ± 8 | 195 ± 25 | ||
| Triple negative | 44 | 44 ± 16 | 245 ± 24 | ||
| Histological grade | |||||
| G1 | 22 | 46 ± 9 | NS | 216 ± 18 | NS |
| G2 | 67 | 45 ± 8 | 215 ± 15 | ||
| G3 | 25 | 48 ± 15 | 222 ± 19 | ||
| Pathological stage | |||||
| 1 | 14 | 45 ± 10 | NS | 226 ± 19 | <0.0001 |
| 2a | 62 | 45 ± 8 | 234 ± 11 | ||
| 2b | 24 | 42 ± 10 | 184 ± 22 | ||
| 3‐4 | 14 | 42 ± 4 | 194 ± 23 | ||
| Primary tumor | |||||
| pT1 | 17 | 41 ± 12 | NS | 208 ± 17 | NS |
| pT2 | 81 | 46 ± 11 | 214 ± 12 | ||
| pT3‐4 | 16 | 39 ± 13 | 211 ± 28 | ||
| Nodal metastasis | |||||
| pN0 | 82 | 48 ± 15 | 0.0003 | 222 ± 20 | 0.0059 |
| pN1‐2 | 32 | 37 ± 12 | 208 ± 32 | ||
The staining index was calculated as the staining strength score (0 to 3) multiplied by the staining area (%).
P value was calculated using the Kruskal–Wallis test.
Clinicopathological parameters were classified according to AJCC.45 pT1, tumor ≤ 2 cm in greatest dimension; pT2, tumor ≤ 5 cm in greatest dimension; pT3, tumor > 5 cm; pT4, tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodule; pN0, no regional lymph node metastasis; pN1, metastases in 1‐3 axillary lymph nodes; and/or in internal mammary nodes with metastases detected by sentinel lymph node biopsy but not clinically detected; pN2, metastases in 4‐9 axillary lymph nodes; or in clinically detected internal mammary lymph nodes in the absence of axillary lymph node metastases; stage 1, pT1/pN0; stage 2a, pT1/pN1 or pT2/pN0; stage 2b, pT2/pN1 or pT3/pN0; stage 3, pT1‐2/pN2, pT3/pN1‐2, pT4/any pN or any pT/pN3.
Table 2.
Expression of CLDN4 in triple negative breast cancer (TNBC), luminal and HER2 subtypes
| Parameters d | TNBC | P b | Luminal | P b | HER2 | P b | P c | |||
|---|---|---|---|---|---|---|---|---|---|---|
| n | CLDN4 a | n | CLDN4 a | n | CLDN4 a | |||||
| Histology | ||||||||||
| Papillo‐tubular | 11 | 260 ± 8 | .0246 | 7 | 181 ± 24 | NS | 4 | 213 ± 20 | NS | NS |
| Solid tubular | 18 | 272 ± 10 | 21 | 199 ± 19 | 9 | 171 ± 29 | ||||
| Scirrhous | 15 | 269 ± 14 | 19 | 212 ± 15 | 10 | 229 ± 22 | ||||
| Histological grade | ||||||||||
| G1 | 6 | 240 ± 10 | NS | 11 | 232 ± 22 | NS | 5 | 262 ± 19 | NS | NS |
| G2 | 26 | 244 ± 15 | 27 | 201 ± 14 | 14 | 191 ± 22 | ||||
| G3 | 12 | 249 ± 12 | 9 | 166 ± 20 | 4 | 175 ± 19 | ||||
| Pathological stage | ||||||||||
| 1 | 2 | 235 ± 14 | .0071 | 8 | 196 ± 17 | .0087 | 4 | 230 ± 44 | .0489 | <.0001 |
| 2a | 13 | 243 ± 11 | 32 | 220 ± 12 | 16 | 207 ± 17 | ||||
| 2b | 18 | 242 ± 11 | 6 | 125 ± 32 | 1 | 80 | ||||
| 3‐4 | 11 | 255 ± 8 | 1 | 195 | 2 | 185 ± 85 | ||||
| Primary tumor | ||||||||||
| pT1 | 3 | 238 ± 7 | .0017 | 10 | 191 ± 10 | NS | 4 | 230 ± 44 | NS | .0028 |
| pT2 | 28 | 241 ± 12 | 35 | 209 ± 81 | 18 | 194 ± 17 | ||||
| pT3‐4 | 13 | 255 ± 10 | 2 | 130 ± 14 | 1 | 270 | ||||
| Nodal metastasis | ||||||||||
| pN0 | 21 | 236 ± 20 | .0012 | 40 | 213 ± 68 | .0092 | 21 | 214 ± 15 | .0221 | <.0001 |
| pN1‐2 | 23 | 254 ± 14 | 7 | 136 ± 75 | 2 | 90 ± 10 | ||||
The staining index was calculated as the staining strength score (0 to 3) multiplied by the staining area (%).
P value was calculated by Kruskal‐Wallis test on expression indexes.
P value was calculated by χ2 test on case numbers.
Clinicopathological parameters were classified according to AJCC.41 pT1, tumor ≤ 2 cm in greatest dimension; pT2, tumor ≤ 5 cm in greatest dimension; pT3, tumor > 5 cm; pT4, tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodule; pN0, no regional lymph node metastasis; pN1, metastases in 1‐3 axillary lymph nodes; and/or in internal mammary nodes with metastases detected by sentinel lymph node biopsy but not clinically detected; pN2, metastases in 4‐9 axillary lymph nodes; or in clinically detected internal mammary lymph nodes in the absence of axillary lymph node metastases; stage 1, pT1/pN0; stage 2a, pT1/pN1 or pT2/pN0; stage 2b, pT2/pN1 or pT3/pN0; stage 3, pT1‐2/pN2, pT3/pN1‐2, pT4/any pN or any pT/pN3.
3.2. Effect of 4D3 in human invasive ductal carcinoma cell lines
Human IDC cell lines, MDA‐468 (triple negative subtype) and MCF‐7 (luminal subtype), were examined with the expressions of CLDN4, CLDN1 and ER with or without E2 treatment (Figure 2A). MDA‐468 cells expressed CLDN4 at higher levels than MCF‐7 cells. In contrast, CLDN1 expression was detected at lower levels in the two cell lines than that of CLDN4. ER expression was detected in only MCF‐7. CLDN4 and CLDN1 expression were not altered by E2 treatment in MDA‐468 cells. In contrast, both expressions were decreased in E2‐treated MCF‐7 cells.
Figure 2.
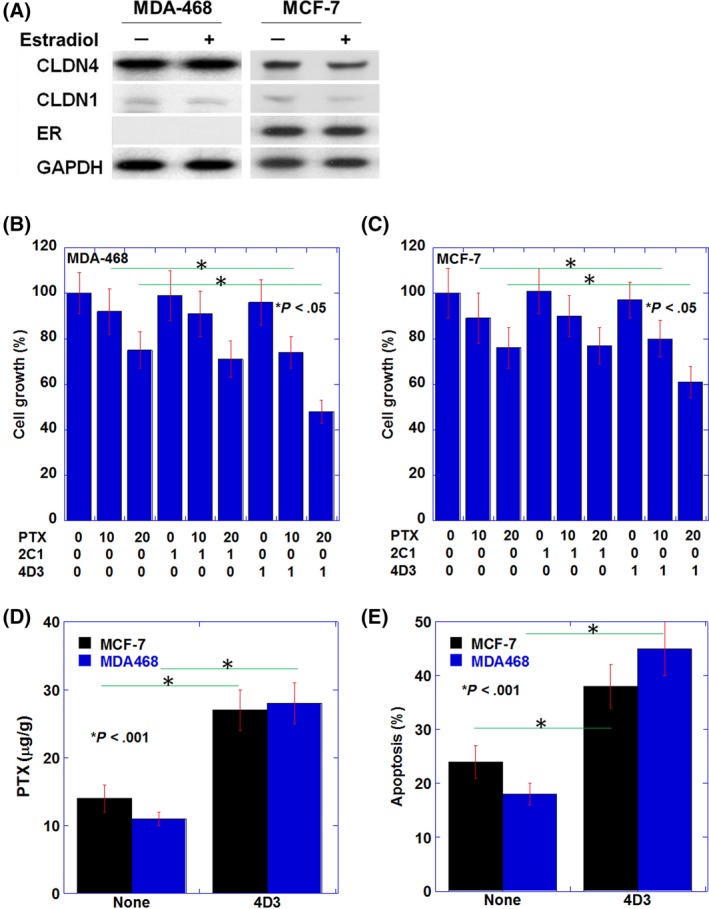
Effects induced by the 4D3 antibody on paclitaxel (PTX)‐induced antitumoral effect in human invasive ductal carcinoma cell lines. A, mRNA expressions of CLDN4, CLDN1 and estrogen receptor (ER) with or without estradiol (1 nM)43 in MDA‐468 and MCF‐7 cell lines. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was examined as loading control. B, C, Effect of 4D3 (1 μg/mL) on growth inhibition by PTX (10 or 20 nM)44 in comparison with that of 2C1 (1 μg/mL) in MDA‐468 cells (B) and MCF‐7 cells (C). D, The intracellular PTX concentration was measured by ELISA in cells treated with PTX (20 nM)44 exposed to 4D3 (1 μg/mL) or none. (E) Induction of apoptosis by PTX (20 nM)44 with or without exposure to 4D3 (1 μg/mL). Error bar, standard deviation (SD) from three independent examinations
We previously established 4D3 antibody for targeting CLDN4 in cancer cells.12 MDA‐468 and MCF‐7 cells were treated with 4D3 and compared with those treated with 2C124 with and without PTX treatment (Figure 2B and C). In both cell lines, 2C1 showed no growth inhibition alone, and no enhanced PTX effect. In contrast, 4D3 enhanced PTX‐induced growth inhibition at each PTX concentration in both cell lines.
Consistent with enhanced drug permeation into tumor tissues owing to impaired tight junction,12 intracellular PTX levels were found to increase in 4D3 treatment in the two cell lines (Figure 2D). PTX‐induced apoptosis was increased by 4D3 in the treatment involving both cell lines.
3.3. Effect of 4D3 on antitumoral effect of paclitaxel
We examined the antitumor effect of 4D3 on the two cell lines treated with PTX in nude mice (Figure 3). In a subcutaneous tumor model, the antitumor effect of PTX when treated in combination with 4D3 was 0.54 times and 0.65 times higher in MCF‐7 and MDA‐468 cells, respectively (Figure 3A). Similarly, in a lung metastasis model, the lung weight decreased by 0.63 times in MCF‐7 and 0.58 times and MDA‐468 cells, respectively (Figure 3B).
Figure 3.

The combined effects of paclitaxel (PTX) and 4D3 in vivo. A, Subcutaneous tumors of MDA‐468 and MCF‐7 cells in nude mice were treated with PTX (10 mg/kg BW)25 and/or 4D3 (1 mg/kg BW) on Days 1, 3 and 7. Tumor weights 4 weeks after inoculation are displayed. B, Lung metastasis resulted by caudal inoculation of MDA‐468 and MCF‐7 cells in nude mice that were treated with PTX (10 mg/kg BW)25 and/or 4D3 (1 mg/kg BW) on Days 1, 3 and 7. Lung weights, 4 weeks after inoculation, are displayed. C, mRNA expression of markers for mouse stromal cells in the subcutaneous tumors was examined by RT‐PCR. Batf2, M1 macrophage; Fizz, M2 macrophage; CD73, mesenchymal stem cell. D, mRNA expression of human stemness‐associated genes, CD133 and CD44, was examined by RT‐PCR. E, Intratumoral pH of the subcutaneous tumors was measured. F, Intracellular pH and extracellular pH were measured in spheres of MDA‐468 and MCF‐7 cells with or without 4D3 treatment. G, mRNA expression of human stemness‐associated genes was examined in invasive ductal carcinoma (IDC) cells cultured in media adjustment of the pH to 7.4 or 7.0. Error bar, standard deviation (SD) from five mice. β‐Actin (BACT) was examined as a loading control
In our previous report, 4D3 abrogated tumor microenvironment to reduce intratumoral accumulation of growth factors.12 In the MDA‐468 tumors, we examined the effects of 4D3 on tumor stromal cell population and cancer cell stemness (Figure 3C, D). PTX treatment increased mRNA expression of mouse M2 macrophage and MSC and decreased the expression of mouse M1 macrophage. In contrast, 4D3 alone decreased mRNA expression of mouse M2 macrophage and MSC and increased the expression of mouse M1 macrophage. Moreover, 4D3 abrogated the alteration of the stromal cell population induced by PTX with concurrent treatment. In tumor cells, mRNA expression of stem cell markers, CD133 and CD44, was increased by PTX treatment. In contrast, 4D3 alone decreased expressions of CD133 and CD44 and abrogated PTX‐induced expressions of CD133 and CD44 with concurrent treatment.
We next examined the tumor microenvironment by measuring the pH of the tumor tissue. With 4D3 treatment, tumor pH was elevated from weak acidic condition to neutral condition (Figure 3E). We then measured intracellular and extracellular pH in spheres of MDA‐468 and MCF‐7 cells with or without 4D3 treatment (Figure 3F). The results showed that intracellular pH was maintained with 4D3 treatment. In contrast, extracellular pH was acidic in cells without 4D3, whereas 4D3 treatment increased the extracellular pH to the level of the pH of the culture medium. To elucidate the effect of circumstantial pH on stemness, expressions of CD133 and CD44 were examined in cells cultured in media of pH 7.0 and pH 7.4 (Figure 3G). Expressions of CD133 and CD44 were higher in pH 7.0 than in pH 7.4 in both cell lines.
3.4. Effect of 4D3 on combination treatment of paclitaxel with tamoxifen
We examined the effect of 4D3 on combination treatment of PTX with TAM using a mouse subcutaneous tumor model (Figure 4A‐D). In MCF‐7, TAM or PTX alone showed an antitumor effect; however, the combination of both enhanced the effect (Figure 4A). Furthermore, the combined use of 4D3 enhanced the effects of TAM or PTX and further enhanced the combined effect of TAM and PTX. In contrast, in MDA‐468, TAM alone was not effective, and the combination of TAM and PTX was not different from PTX alone (Figure 4B). However, the combined use of 4D3 promoted the antitumor effect of PTX, and the same effect as seen in the case of TAM and PTX combination in MCF‐7 was obtained.
Figure 4.
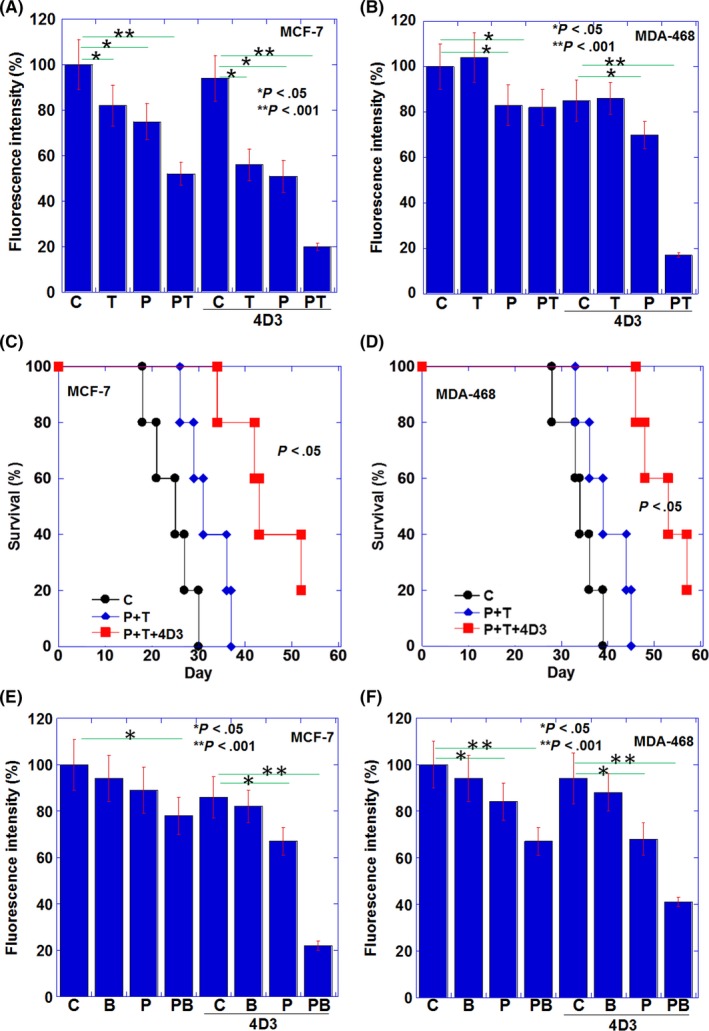
The combined effects of paclitaxel (PTX) and 4D3 with tamoxifen (TAM) or bisphosphonate (BP) in vivo. A, B, Lung tumors of MDA‐468 cells (A) and MCF‐7 cells (B) in nude mice were treated with PTX (10 mg/kg BW)25 and/or TAM (500 mg/mouse)26 and/or 4D3 (1 mg/kg BW) on Days 1, 3 and 7. Fluorescence intensities of tumors 4 weeks after inoculation are displayed. C, D, Survivals of mice examined in panels A and B. E, F, Intratibial tumors of MDA‐468 cells (E) and MCF‐7 cells (F) in nude mice were treated with PTX (10 mg/kg BW)25 and/or BP (zoledronic acid, 100 μg/kg BW)28 and/or 4D3 (1 mg/kg BW) on Days 1, 3 and 7. Fluorescence intensities of tumors 4 weeks after inoculation are displayed. Error bar, standard deviation (SD) from five mice
When examining mouse survival in a similar mouse model with inoculation of MCF‐7, the 50% survival periods were 26, 33 and 43 days in mice treated with none (control), PTX and TAM combination, and the three‐way combination of PTX, TAM and 4D3, respectively. In the case of MDA‐468, 50% survival periods were not significantly prolonged at 35 and 45 days in mice treated with nothing (control) or PTX and TAM combination, respectively. In contrast, a 50% survival period was significantly extended to 53 days in the three‐way combination. A significant prolongation of survival was found in the three‐way combination in both cell lines.
3.5. Effect of 4D3 on combination treatment of paclitaxel with bisphosphonate
Finally, the combined effect of BP (zoledronic acid) with PTX and antibody was examined using a mouse bone metastasis model (Figure 4E, F). In both MCF‐7 and MDA‐468 cell lines, there was no antitumor effect with BP alone, but an additional effect with PTX was observed. Furthermore, the combination of 4D3 enhanced the antitumor effect in both PTX and the combination of PTX and BP. The combination of PTX, BP and 4D3 reduced tumor growth to 21% and 41% in MCF‐7 and MDA‐468 tumors, respectively, compared to the control group.
4. DISCUSSION
In the present study, we examined the therapeutic effect of targeting CLDN4 to IDC, particularly TNBC. When the relationship between CLDN4 expression in IDC and clinicopathological parameters was examined, a correlation was found between stage and nodal metastasis. Furthermore, when correlations of CLDN4 expression with clinicopathological factors were compared between TNBC and non–TNBC (luminal and HER2 subtypes), CLDN4 expression in TNBC was higher than for non–TNBC and also correlated strongly with cancer progression. Increased expression of CLDN4 enhances maintenance of the tumor microenvironment and stemness,12, 31 while EMT reduces the expression.10 Thus, both correlation and inverse correlation have been reported for CLDN4 expression and prognosis.32, 33 The results of this study suggest that in TNBC, enhanced CLDN4 expression is correlated with pathological prognostic factors, and CLDN4 expression levels are expected to be correlated with poor prognosis.32 CLDN4 was considered to be more suitable as a molecular target for TNBC.
Interestingly, CLDN4 expression in non–TNBC was inversely correlated with stage and nodal metastasis, which is similar to what was reported in undifferentiated type gastric cancer.10 Contrary to the differentiated type gastric cancer, undifferentiated type gastric cancer shows increased, non–assembled (not integrated into tight junction) CLDN4, which is associated with stemness via an integrin signal.10 Because CLDN4 is known as an epithelial marker,8, 9, 14 downregulation of CLDN4 in non–TNBC might be associated with epithelial‐mesenchymal transition (EMT).10
In contrast to non–TNBC, CLDN4 expression was correlated with primary tumor expansion and nodal metastasis, which is similar to the situation in bladder cancer,12 colorectal cancer11 and differentiated type gastric cancer.10 In such tumors, CLDN4 overexpression might provide an isolated tumor microenvironment mediated through the tight junction, which retains epithelial growth factor and vascular endothelial growth factor in the tumor, and inhibits the permeation of anticancer drugs.10, 11, 12 In TNBC, we observed a novel barrier function, where the tight junction retained lactic acid in the tumor, which lowered intratumoral pH. Tissue pH was elevated by 4D3 treatment from acidic to neutral in the environment. Cancer cells release lactic acid into the cancer microenvironment through the Warburg effect, causing it to become acidic.34 Acidity in the environment affects the activity of immune cells.35, 36 We analyzed the alterations in immune cell properties in cancerous tissues with 4D3 treatment, which was examined by the expression of immune cell marker genes. As a result, M2 macrophages and MSC decreased and M1 macrophages increased with 4D3 treatment. M2 macrophages and MSC are known to promote the metastatic potential and resistance of cancer cells against anticancer drugs.37, 38, 39
4D3 treatment changed the pH in mouse tumor and extracellular pH in sphere culture from acidic to neutral. This indicates that it is difficult to maintain the microenvironment within the tumor due to tight junction damage caused by 4D3 treatment. It has been reported that the secretome of MSC changes depending on the extracellular pH.40 Our data suggest that MSCs bring stem cell‐promoting factors to cancer cells in an acidic intercellular environment. Since MSCs secrete factors such as CXCL12 in the ischemic acidic environment,41 similar factors might be involved in the acidic environment of cancer.
When cancer cells were co‐cultured with MSCs and pH was changed from an acidic environment to neutral environment and stemness decreased in our data, treatment with 4D3 alone showed no significant antitumor effect in in vitro but modest in mouse models. 4D3 also possesses antibody‐dependent cellular cytotoxicity activity.12 In the in vivo environment, a stronger antitumor effect than in the in vivo environment might be induced, including the immunostimulatory effect caused by the destruction of the cancer microenvironment.
Comparing the expression of the epithelial CLDN between CLDN4 and CLDN1, the expression level of CLDN4 was higher and correlation with clinicopathological factors was also observed. In contrast, the expression level was low in CLDN1 and less correlation with clinicopathological factors was found. From this, CLDN4 was considered to be a better molecular target than CLDN1. It was previously reported that CLDN4 is upregulated in bladder cancer, colon cancer, gastric cancer and pancreatic ductal cancer, and correlates with cancer progression.10, 11, 12, 42 CLDN4 is considered to be a molecular target with broad efficacy in epithelial malignancies.
We previously reported that 4D3 promoted the antitumor effect of CDDP, 5‐fluorouracil and FOLFIRINOX in bladder cancer, colon cancer, gastric cancer and pancreatic ductal cancer.10, 11, 12 Here, we examined the effects of 4D3 in breast cancer cells, and the use of 4D3 promoted the antitumor effect of PTX in both TNBC and luminal subtype. In the luminal subtype, the antitumor effect of TMX was promoted by 4D3 and a synergistic effect with PTX was observed. In contrast, in TNBC, a combination with 4D3 and PTX showed an effect comparable to the PTX and TAM combination in the luminal subtype. Furthermore, when the effect of the 4D3 on the effect of BP was examined in the bone metastasis model, the growth in bone metastasis was suppressed by the 4D3 combination in both TNBC and luminal subtype. Thus, 4D3 was found to promote its effect on any of the anticancer agents, anti–hormone agents, and BP.
Here, we examined the efficacy of 4D3 using a nude mouse model. However, because this 4D3 does not recognize mouse CLDN4, its toxicity to the host could not be evaluated. It is necessary, in the future, to evaluate the toxicity of 4D3 using an effective model
From the above findings, 4D3 enhanced the effect of PTX, TAM and BP in breast cancer in mouse models by impairing the barrier function of the tight junction. 4D3 is an effective molecular targeting therapeutic agent for IDC and is expected to be effective against TNBC. Application in future clinical trials is desired.
DISCLOSURE STATEMENT
The authors have no conflict of interest to declare.
ACKNOWLEDGMENTS
This work was supported by MEXT KAKENHI Grant Numbers 16H05164 (HK) and 17K19923 (HK), the Natural Science Foundation of Jiangsu Education Department Project (17KJB320010) (YL), and the National Natural Science Foundation of China (81702723) (YL). The authors thank Ms Tomomi Masutani for expert assistance with the preparation of this manuscript.
Luo Y, Kishi S, Sasaki T, et al. Targeting claudin‐4 enhances chemosensitivity in breast cancer. Cancer Sci. 2020;111:1840–1850. 10.1111/cas.14361
Yi Luo and Shingo Kishi contributed equally to this work.
REFERENCES
- 1. Wakao F, Nishimoto H, Kataonoda K, Tsukuma H, Mikami H, eds. Cancer Statistics in Japan, 2013, 2013 edn Tokyo, Japan: National Cancer Research Institute; 2013. [Google Scholar]
- 2. Meattini I, Bicchierai G, Saieva C, et al. Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: Single‐institution experience and review of published literature. Euro J Surg Oncol. 2017;43:642‐648. [DOI] [PubMed] [Google Scholar]
- 3. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19:312‐321. [DOI] [PubMed] [Google Scholar]
- 5. Andreopoulou E, Schweber SJ, Sparano JA, McDaid HM. Therapies for triple negative breast cancer. Exp Opin Pharmacotherap. 2015;16:983‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yagata H, Kajiura Y, Yamauchi H. Current strategy for triple‐negative breast cancer: appropriate combination of surgery, radiation, and chemotherapy. Breast Cancer. 2011;18:165‐173. [DOI] [PubMed] [Google Scholar]
- 7. Bagaria SP, Ray PS, Sim MS, et al. Personalizing Breast Cancer Staging by the Inclusion of ER, PR, and HER2. JAMA Surg. 2014;149:125‐129. [DOI] [PubMed] [Google Scholar]
- 8. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285‐293. [DOI] [PubMed] [Google Scholar]
- 9. Turksen K, Troy TC. Junctions gone bad: claudins and loss of the barrier in cancer. Biochim Biophys Acta. 2011;1816:73‐79. [DOI] [PubMed] [Google Scholar]
- 10. Nishiguchi Y, Fujiwara‐Tani R, Sasaki T, et al. Targeting claudin‐4 enhances CDDP‐chemosensitivity in gastric cancer. Oncotarget. 2019;10:2189‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujiwara‐Tani R, Sasaki T, Luo Y, et al. Anti–claudin‐4 extracellular domain antibody enhances the antitumoral effects of chemotherapeutic and antibody drugs in colorectal cancer. Oncotarget. 2018;9:37367‐37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuwada M, Chihara Y, Luo Y, et al. Pro‐chemotherapeutic effects of antibody against extracellular domain of claudin‐4 in bladder cancer. Cancer Lett. 2015;369:212‐221. [DOI] [PubMed] [Google Scholar]
- 13. Boivin FJ, Schmidt‐Ott KM. Transcriptional mechanisms coordinating tight junction assembly during epithelial differentiation. Ann N Y Acad Sci. 2017;1397:80‐99. [DOI] [PubMed] [Google Scholar]
- 14. Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hough CD, Sherman‐Baust CA, Pizer ES, et al. Large‐scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281‐6287. [PubMed] [Google Scholar]
- 16. Rangel LB, Agarwal R, D’Souza T, et al. Tight junction proteins claudin‐3 and claudin‐4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res. 2003;9:2567‐2575. [PubMed] [Google Scholar]
- 17. Michl P, Buchholz M, Rolke M, et al. Claudin‐4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678‐684. [DOI] [PubMed] [Google Scholar]
- 18. Sato N, Fukushima N, Maitra A, et al. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol. 2004;164:903‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gradishar WJ, Anderson BO. NCCN clinical practice guidelines in oncology ‐ breast cancer In: Kumar R, Shead DA, eds. NCCN.org; 2019. BINV1-25. [Google Scholar]
- 20. Maushagen R, Reers S, Pfannerstill AC, et al. Effects of paclitaxel on permanent head and neck squamous cell carcinoma cell lines and identification of anti–apoptotic caspase 9b. J Cancer Res Clin Oncol. 2016;142:1261‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cyprian FS, Akhtar S, Gatalica Z, Vranic S. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: A new clinical paradigm in the treatment of triple‐negative breast cancer. Bosnian J Basic Med Sci. 2019;19:227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Force J, Leal JHS, McArthur HL. Checkpoint blockade strategies in the treatment of breast cancer: Where we are and where we are heading. Curr Treat Option Oncol. 2019;20:35. [DOI] [PubMed] [Google Scholar]
- 23. Kuniyasu H, Yano S, Sasaki T, Sasahira T, Sone S, Ohmori H. Colon cancer cell‐derived high mobility group 1/amphoterin induces growth inhibition and apoptosis in macrophages. Am J Pathol. 2005;166:751‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukasawa M, Nagase S, Shirasago Y, et al. Monoclonal antibodies against extracellular domains of claudin‐1 block hepatitis C virus infection in a mouse model. J Virol. 2015;89:4866‐4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghoneum M, Badr El‐Din NK, Mahmoud AZ, Tolentino L, Pan D, Hassan TA. Dietary baker’s yeast sensitizes Ehrlich mammary adenocarcinoma to paclitaxel in mice bearing tumor. Oncol Rep. 2019;41:3155‐3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shibata T, Watari K, Izumi H, et al. Breast cancer resistance to antiestrogens is enhanced by increased ER degradation and ERBB2 expression. Cancer Res. 2017;77:545‐556. [DOI] [PubMed] [Google Scholar]
- 27. Uehara H, Takahashi T, Izumi K. Induction of retinol‐binding protein 4 and placenta‐specific 8 expression in human prostate cancer cells remaining in bone following osteolytic tumor growth inhibition by osteoprotegerin. Int J Oncol. 2013;43:365‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Du Y, Sun T, Xue H, Jin Z, Tian J. PD‐1 blockade in combination with zoledronic acid to enhance the antitumor efficacy in the breast cancer mouse model. BMC Cancer. 2018;18:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuniyasu H, Yasui W, Shinohara H, et al. Induction of angiogenesis by hyperplastic colonic mucosa adjacent to colon cancer. Am J Pathol. 2000;157:1523‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuniyasu H, Oue N, Wakikawa A, et al. Expression of receptors for advanced glycation end‐products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163‐170. [DOI] [PubMed] [Google Scholar]
- 31. Lin CH, Li HY, Liu YP, et al. High‐CLDN4 ESCC cells harbor stem‐like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma. Therap Advan Med Oncol. 2019;11:1758835919875324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katayama A, Handa T, Komatsu K, et al. Expression patterns of claudins in patients with triple‐negative breast cancer are associated with nodal metastasis and worse outcome. Pathol Int. 2017;67:404‐413. [DOI] [PubMed] [Google Scholar]
- 33. Duarte GM, Almeida NR, Tocchet F, et al. Claudin‐4 expression is associated with disease‐free survival in breast carcinoma‐in‐situ: Mean follow‐up of 8.2 years. Clin Breast Cancer. 2018;18:e1111‐e1116. [DOI] [PubMed] [Google Scholar]
- 34. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269‐270. [PubMed] [Google Scholar]
- 35. Sun L, Suo C, Li ST, Zhang H, Gao P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim Biophys Acta Rev Cancer. 2018;1870:51‐66. [DOI] [PubMed] [Google Scholar]
- 36. Noel G, Langouo Fontsa M, Willard‐Gallo K. The impact of tumor cell metabolism on T cell‐mediated immune responses and immuno‐metabolic biomarkers in cancer. Semin Cancer Biol. 2018;52:66‐74. [DOI] [PubMed] [Google Scholar]
- 37. Choi J, Gyamfi J, Jang H, Koo JS. The role of tumor‐associated macrophage in breast cancer biology. Histol Histopathol. 2018;33:133‐145. [DOI] [PubMed] [Google Scholar]
- 38. Nahas GR, Patel SA, Bliss SA, Rameshwar P. Can breast cancer stem cells evade the immune system? Curr Med Chem. 2012;19:6036‐6049. [DOI] [PubMed] [Google Scholar]
- 39. Melzer C, von der Ohe J, Hass R. Enhanced metastatic capacity of breast cancer cells after interaction and hybrid formation with mesenchymal stroma/stem cells (MSC). Cell Commun Signal. 2018;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell‐derived secretome. Cells. 2019;8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shiota Y, Nagai A, Sheikh AM, et al. Transplantation of a bone marrow mesenchymal stem cell line increases neuronal progenitor cell migration in a cerebral ischemia animal model. Sci Rep. 2018;8:14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kojima T, Kyuno D, Sawada N. Targeting claudin‐4 in human pancreatic cancer. Expert Opin Ther Targets. 2012;16:881‐887. [DOI] [PubMed] [Google Scholar]
- 43. Gschwantler‐Kaulich D, Weingartshofer S, Grunt TW, et al. Estradiol impairs the antiproliferative and proapoptotic effect of Zoledronic acid in hormone sensitive breast cancer cells in vitro. PLoS One. 2017;12:e0185566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer. 1993;68:1104‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. [Cited 2017.]. [Google Scholar]


