Abstract
Tumor angiogenesis is an important therapeutic target in colorectal cancer (CRC). We aimed to identify novel genes associated with angiogenesis in CRC. Using RNA sequencing analysis in normal and tumor endothelial cells (TECs) isolated from primary CRC tissues, we detected frequent upregulation of adipocyte enhancer‐binding protein 1 (AEBP1) in TECs. Immunohistochemical analysis revealed that AEBP1 is upregulated in TECs and stromal cells in CRC tissues. Quantitative RT‐PCR analysis showed that there is little or no AEBP1 expression in CRC cell lines, but that AEBP1 is well expressed in vascular endothelial cells. Levels of AEBP1 expression in Human umbilical vein endothelial cells (HUVECs) were upregulated by tumor conditioned medium derived from CRC cells or by direct coculture with CRC cells. Knockdown of AEBP1 suppressed proliferation, migration, and in vitro tube formation by HUVECs. In xenograft experiments, AEBP1 knockdown suppressed tumorigenesis and microvessel formation. Depletion of AEBP1 in HUVECs downregulated a series of genes associated with angiogenesis or endothelial function, including aquaporin 1 (AQP1) and periostin (POSTN), suggesting that AEBP1 might promote angiogenesis through regulation of those genes. These results suggest that upregulation of AEBP1 contributes to tumor angiogenesis in CRC, which makes AEBP1 a potentially useful therapeutic target.
Keywords: angiogenesis, aquaporin 1, cancer stroma, periostin, tumor endothelium
We identified that adipocyte enhancer‐binding protein 1 (AEBP1) is upregulated in tumor endothelial cells in primary colorectal cancers. Upregulation of AEBP1 in vascular endothelial cells promotes cell proliferation, migration, and angiogenesis. We also found that AEBP1 might mediate tumor angiogenesis through regulating expression of angiogenesis‐related genes, including aquaporin 1 (AQP1) and periostin (POSTN).
1. INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide and accounts for approximately 10% of all newly diagnosed malignant diseases. 1 Early stage CRCs are usually curable with endoscopic resection or surgery, whereas more advanced cases have poorer prognoses. A number of therapeutic agents, including chemotherapeutic drugs and mAbs, have been developed for the treatment of advanced CRCs, but the prognoses of patients with metastatic disease are still unsatisfactory. 2 , 3
Active angiogenesis is a hallmark of cancer and is deeply involved in the progression and metastasis of CRC. For that reason, antiangiogenic therapy is an important strategy used in the treatment of advanced disease. 4 For example, bevacizumab, a mAb that binds to vascular endothelial growth factor (VEGF), is a standard component of the treatment for patients with metastatic CRC. 5 However, current antiangiogenic therapies have yielded only moderate benefit in CRC.
Although they have not yet been fully characterized, it seems clear that tumor endothelial cells (TECs) play an essential role in tumor angiogenesis. 6 Notably, the molecular features of TECs distinctly differ from those of normal endothelial cells (NECs). This makes a fundamental understanding of the differences between TECs and NECs indispensable for development of specific and effective antiangiogenic therapies. Several studies have analyzed the gene expression patterns in TECs and their normal counterparts. For instance, using serial analysis of gene expression with endothelial cells isolated from CRC tissues, St. Croix et al 7 identified a series of tumor endothelial markers (TEMs). A subsequent study showed that one of those markers, TEM8 (also known as anthrax toxin receptor 1, ANTXR1), is a potential therapeutic target in cancer. 8 Another study used subtractive hybridization to compare gene expression profiles in endothelial cells isolated from CRC and normal tissues, and identified 17 genes specifically overexpressed in TECs. 9 More recently, Naschberger et al 10 isolated TECs from CRCs with different clinical outcomes. They undertook microarray analyses to screen for differentially expressed genes between TECs from CRCs with better or worse prognoses and found that SPARC‐like protein 1 (SPARCL1) regulates vessel homeostasis and contributes to a favorable prognosis of CRC. These studies proved that transcriptome analysis in endothelial cells isolated from clinical specimens is a potentially useful strategy for unraveling the mechanism of tumor angiogenesis.
In the present study, we aimed to identify novel TEC markers and potential therapeutic targets in CRC. To that end, we isolated TECs and corresponding NECs from surgically resected CRC tissues, and carried out RNA sequencing (RNA‐seq) analysis. Our findings revealed that upregulation of adipocyte enhancer‐binding protein 1 (AEBP1) might promote tumor angiogenesis in CRCs.
2. MATERIALS AND METHODS
2.1. Tissue samples and cell lines
Primary CRC tissues and adjacent normal colonic tissues were collected from Japanese patients (n = 14) who underwent surgical resection at Sapporo Medical University Hospital. Informed consent was obtained from all patients before collection of the specimens. Approval of this study was obtained from the Intuitional Review Board of Sapporo Medical University. Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza and cultured in Endothelial Cell Basal Medium‐2 (EBM‐2) supplemented with an Endothelial Cell Growth Medium‐2 (EGM‐2) SingleQuots kit (Lonza) at 37°C under 5% CO2. Where indicated, HUVECs were treated for 24 h with 10 ng/mL transforming growth factor‐β (TGF‐β; PeproTech). Human dermal microvascular endothelial cells (HMVECs) were purchased from Lonza and cultured in EBM‐2 supplemented with an EGM‐2 MV SingleQuots kit (Lonza) at 37°C under 5% CO2. The CRC cell lines were obtained and cultured as described previously. 11
2.2. Isolation of endothelial cells
Endothelial cells were isolated from CRC and normal colonic tissue as described previously. 7 , 9 In brief, we isolated endothelial cells from surgically resected tissues using Ab‐coated magnetic beads. Tissues were minced with surgical blades and digested with collagenase type 1 (Worthington Biochemical) and DNase I (Worthington Biochemical) for 2 h at 37°C. The solution derived from the tissue was filtered through mesh and added to ACK buffer (Thermo Fisher Scientific) to remove red blood cells. The resultant cell suspensions were then subjected to selection of epithelial cell populations using Dynabeads Epithelial Enrich (Thermo Fisher Scientific), after which endothelial cells were selected using Dynabeads Pan mouse IgG (Thermo Fisher Scientific) coated with anti‐human CD146 Ab (clone SHM‐57; BioLegend).
2.3. Immunohistochemistry
Immunohistochemical staining was carried out as described previously. 11 A mouse anti‐human CD146 mAb (clone SHM‐57; BioLegend), a rabbit anti‐CD31 polyclonal Ab (ab28364; Abcam), and a mouse anti‐AEBP1 mAb (ab54820; Abcam) were used.
2.4. Reverse transcription‐PCR
Single‐stranded cDNA was prepared using a PrimeScript RT Reagent Kit with gDNA Eraser Perfect Real Time (Takara Bio). Reverse transcription‐PCR was carried out as described previously. 12 Quantitative RT‐PCR (qRT‐PCR) was carried out using TaqMan Gene Expression Assays or SYBR Select Master Mix (Thermo Fisher Scientific) with a 7500 Fast Real‐Time PCR System (Thermo Fisher Scientific). GAPDH or β‐actin (ACTB) was used as endogenous control. TaqMan assay IDs and primer sequences are listed in Tables S1 and S2.
2.5. Western blot analysis
Total cell lysate extraction and western blot analysis were undertaken as described previously. 12 A mouse anti‐β‐actin mAb (1:3000 dilution, clone AC‐15; Sigma‐Aldrich), a rabbit anti‐AEBP1 mAb (1:3000 dilution, ab168355; Abcam) or a mouse anti‐V5 mAb (1:5000 dilution, R960‐25; Thermo Fisher Scientific) were used.
2.6. Preparation of tumor conditioned medium
To prepare tumor conditioned medium (TCM), CRC cells were cultured in a 10‐cm dish in growth medium containing 10% FBS. After replacing this medium with serum‐free medium, the cells were incubated for an additional 48 h. The TCM was then collected and filtered through a 0.2‐μm filter.
2.7. Small interfering RNA and expression vectors
For RNA interference‐induced knockdown of AEBP1, 1 × 106 cells were transfected with 100 pmol Silencer Select Pre‐designed siRNA (AEBP1 siRNA1, s1145; AEBP1 siRNA2, s1146; Thermo Fisher Scientific) or a Silencer Select Negative Control No. 1 siRNA (Thermo Fisher Scientific) using a HUVEC Nucleofector Kit (Lonza). An ORF Expression Gateway Shuttle Clone containing full‐length AEBP1 variant 1 was obtained from GeneCopoeia. Full‐length AEBP1 variant 2 cDNA was amplified by PCR using cDNA derived from HUVECs. Primer sequences are listed in Table S2. The AEBP1 variants were cloned into pLenti6/V5‐DEST, and lentiviral expression vectors (Lenti‐V5‐AEBP1v1 and Lenti‐V5‐AEBP1v2) were then constructed using a ViraPower Lentiviral Expression System (Thermo Fisher Scientific).
2.8. Cell viability assays
Cells were transfected with siRNAs or infected with lentiviral vectors and seeded into 96‐well plates (5 × 103 cells per well). Cell viability assays were carried out using CCK‐8 (Dojindo) according to the manufacturer’s instructions.
2.9. Wound healing assays
Cells transfected with siRNAs were incubated for 48 hours, after which they were seeded into 6‐well plates (2 × 105 cells per well). Linear wounds were drawn in the monolayer using a pipette tip, and the cells were incubated for an additional 24 hours. Photomicrographs of the wounds and invading cells were then taken to evaluate wound closure. The experiments were carried out in triplicate under each condition.
2.10. In vitro tube formation assay
Cells transfected with siRNAs were incubated for 48 hours, or those infected with lentiviral vectors were incubated for 10‐14 days, after which they were seeded onto Matrigel (Corning) in 24‐well plates (2.5 × 104 cells per well). After incubation for 3, 6, 12, or 24 hours, 5 randomly selected fields in each well were photographed. Branch numbers and tube lengths were measured using ImageJ analysis software (NIH).
2.11. Statistical analysis
Quantitative variables were analyzed using Student’s t tests or ANOVA with post hoc Tukey’s tests. Survival was analyzed using the log‐rank test for 2‐group comparisons. Values of P less than .05 (2‐sided) were considered statistically significant. Statistical analyses were carried out using JMP version 12 (SAS Institute) or GraphPad Prism version 5 (GraphPad Software).
2.12. RNA sequencing, microarray, and xenograft studies
Methods of RNA‐seq, microarray, and xenograft studies are described in Methods S1.
3. RESULTS
3.1. AEBP1 is upregulated in tumor endothelial cells of CRC
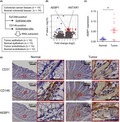
To identify novel tumor endothelium‐associated genes in CRC, we isolated endothelial and epithelial cells from a series of 14 CRC tissue samples and corresponding normal colorectal tissues (Table S3). We first isolated epithelial cells, using EpCAM as an epithelium marker, and subsequently isolated endothelial cells, using CD146 as an endothelium marker (Figure 1A). This enabled us to obtain total RNA from endothelial cells (EpCAM−, CD146+) derived from 14 CRC tissue and 12 normal tissue samples, and from epithelial cells (EpCAM+) derived from 14 CRC tissue and 13 normal tissue samples.
Figure 1.
Detection of adipocyte enhancer‐binding protein 1 (AEBP1) upregulation in tumor endothelial cells (TECs). A, Workflow to isolate endothelial and epithelial cells from primary colorectal cancer (CRC) and corresponding normal colorectal tissues. B, Summary of RNA sequencing analysis to identify genes differentially expressed between normal endothelial cells and TECs. Genes upregulated in TECs are indicated in red. C, Relative expression of AEBP1 in endothelial cells (EPCAM−, CD146+) isolated from normal and CRC tissues. Expression levels are normalized to GAPDH expression. Error bars depict SEM. **P < .01. D, Immunohistochemical staining of CD31, CD146, and AEBP1 in representative primary CRC and adjacent normal tissues. Magnified views of the respective boxed areas are shown to the right. ANTXR1, anthrax toxin receptor 1
We undertook RNA‐seq analysis using 3 samples each of tumor endothelium (patients 19, 21, and 24) and normal endothelium (patients 19, 20, and 22) from which we were able to collect sufficient amounts of good quality RNA. RNA sequencing analysis confirmed enrichment of endothelial markers (PECAM1, MCAM, and CD34) in the EpCAM−/CD146+ cells as compared to EpCAM+ cells (Figure S1). This analysis revealed 18 genes significantly upregulated in TECs compared to NECs (Figure 1B, Table 1). The list of genes included a previously identified tumor endothelial marker, TEM8 (ANTXR1), which confirmed that our endothelial cell isolation was successful. We validated the RNA‐seq results by undertaking TaqMan assays with all the samples, and most of the listed genes showed higher expression in TECs than in NECs or epithelial cells (Figures S2 and S3). Among these genes, we noted significant upregulation of AEBP1 in TECs (Figure 1C). Immunohistochemical analysis showed that AEBP1 was abundantly expressed in the vascular endothelium and stroma of primary CRC tissues (Figure 1D). Moreover, analysis using the RNA‐seq data obtained from primary CRC tissues in The Cancer Genome Atlas (TCGA) dataset suggested that higher expression of AEBP1 is associated with poorer overall survival (Figure S4).
Table 1.
Genes upregulated in tumor endothelial cells
| Gene ID | Gene symbol | Fold change (log2) | P value |
|---|---|---|---|
| 7472 | WNT2 | 8.077 | .037 |
| 3381 | IBSP | 7.967 | .027 |
| 2328 | FMO3 | 3.582 | .047 |
| 493869 | GPX8 | 3.372 | .027 |
| 85360 | SYDE1 | 3.277 | .027 |
| 100289470 | LOC100289470 | 3.142 | .042 |
| 201191 | SAMD14 | 3.130 | .031 |
| 6909 | TBX2 | 3.088 | .037 |
| 100506462 | SRD5A3‐AS1 | 2.959 | .031 |
| 283298 | OLFML1 | 2.800 | .027 |
| 79990 | PLEKHH3 | 2.796 | .044 |
| 84168 | ANTXR1 | 2.640 | .031 |
| 114757 | CYGB | 2.594 | .037 |
| 219793 | TBATA | 2.549 | .027 |
| 51393 | TRPV2 | 2.544 | .034 |
| 165 | AEBP1 | 2.496 | .049 |
| 158572 | USP27X‐AS1 | 1.867 | .027 |
| 3575 | IL7R | 0.555 | .037 |
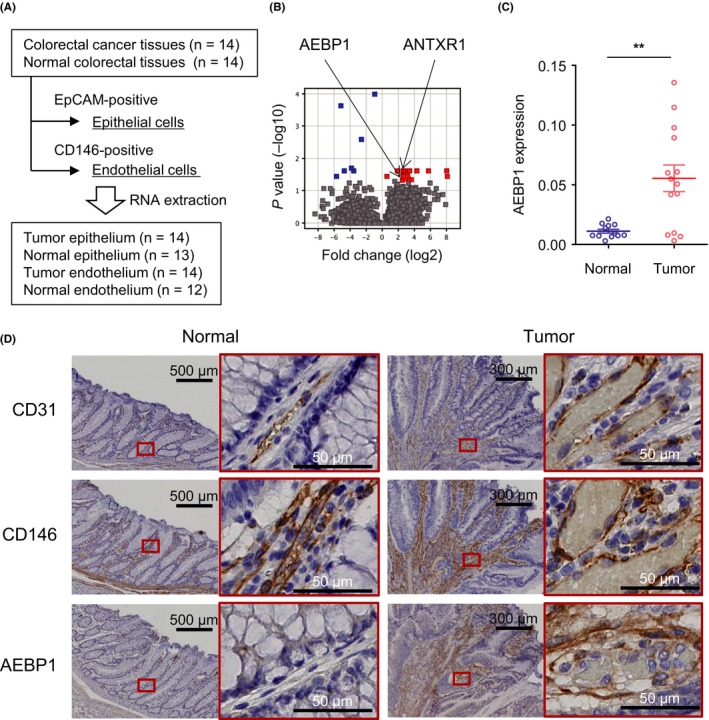
Earlier studies showed that AEBP1 is expressed in adipocytes, fibroblasts, and vascular smooth muscle cells, but its expression in vascular endothelial cells has not been well documented. 13 , 14 , 15 Two transcriptional variants of AEBP1 have been identified in the human genome, and the TaqMan assay detects both variants. 16 We therefore designed a RT‐PCR primer pair that would amplify both variants but would yield different sized PCR products (Figure 2A). We found that endothelial cells mainly express AEBP1 variant 1, whereas CRC cells express variant 2 (Figure 2B). Moreover, endothelial cells express AEBP1 at significantly higher levels than do CRC cells (Figure 2B). We also designed qRT‐PCR primer pairs to specifically detect the respective variants and observed similar results (Figure S5). Western blot analysis revealed expression of the 2 AEBP1 variants in HUVECs (Figure 2C). Bands at approximately 170 kDa and 150 kDa and those at 100 kDa and 80 kDa are considered to be variants 1 and 2, respectively. The larger bands (170 kDa and 100 kDa) likely represent glycosylated forms, as described previously. 15 , 17 Fluorescent immunostaining showed AEBP1 to be present in both the nucleus and cytoplasm of HUVECs (Figure S6).
Figure 2.
Expression of adipocyte enhancer‐binding protein 1 (AEBP1) in endothelial cells. A, Structures of genes encoding the indicated AEBP1 variants. Locations of the RT‐PCR primers used in (B) are indicated by arrows below. B, RT‐PCR of AEBP1 variants in endothelial cells and colorectal cancer (CRC) cell lines. C, Western blot analysis of AEBP1 in human umbilical vein endothelial cells (HUVECs). D, Quantitative RT‐PCR of the indicated AEBP1 variants in HUVECs treated with control medium or tumor conditioned medium (TCM) derived from DLD1 cells with or without supplemented FBS. Results are normalized to ACTB expression. Shown are means of 3 replications. E, Quantitative RT‐PCR analysis of the indicated AEBP1 variants in HUVECs treated with PBS (Ctrl), transforming growth factor (TGF)‐β1 or TGF‐β3. Shown are means of 3 replications. Error bars depict SEMs. **P < .01
Notably, we found that treatment with TCM derived from CRC cells upregulated both AEBP1 variants in HUVECs (Figures 2D and S7). Induction of AEBP1 was also observed when HUVECs were directly cocultured with CRC cells (Figure S8). An earlier study showed that TGF‐β induces AEBP1 expression in preadipocytes. 18 Analysis using a dataset from TCGA showed significant positive correlations between expression levels of TGFB1 or TGFB3 and those of AEBP1 in primary CRC (Figure S9). We therefore treated HUVECs with TGF‐β1 or TGF‐β3 and confirmed that they upregulate AEBP1 expression (Figure 2E).
3.2. AEBP1 promotes proliferation and in vitro tube formation by HUVECs
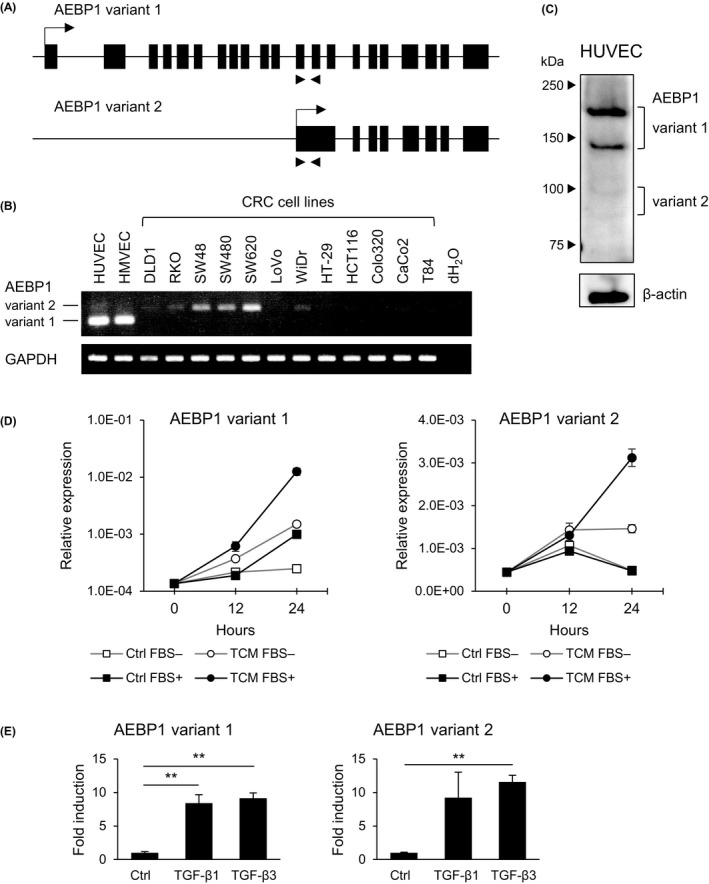
To clarify the function of AEBP1 in endothelial cells, we undertook a series of knockdown experiments using HUVECs. We first confirmed successful depletion of AEBP1 mRNA and protein in HUVECs transiently transfected with siRNAs targeting the 2 AEBP1 variants (Figures 3A,B and S10). Cell viability assays showed that knocking down AEBP1 suppressed HUVEC proliferation (Figure 3C); cell cycle analysis revealed that depleting AEBP1 induced G1 arrest (Figures 3D and S11). In addition, wound healing assays showed that AEBP1 knockdown might suppress HUVEC migration, although the results could be significantly affected by the reduced cell proliferation (Figure 3E). We also found that AEBP1 knockdown attenuated in vitro tube formation by HUVECs (Figure 3F). To assess the functional difference between the 2 AEBP1 variants, we endeavored to design siRNAs specifically targeting the respective variants. We found that a siRNA targeting variant 1 had suppressive effects on HUVEC proliferation and in vitro tube formation similar to the siRNA targeting both variants (data not shown). In contrast, specific knockdown of variant 2 was difficult due to its very low expression level in HUVECs and limited locations that could be used to design variant 2‐specific siRNAs.
Figure 3.
Depletion of adipocyte enhancer‐binding protein 1 (AEBP1) in endothelial cells attenuates proliferation and in vitro tube formation. A, Quantitative RT‐PCR of AEBP1 in human umbilical vein endothelial cells (HUVECs) transfected with a control siRNA (siCtrl) or siRNAs targeting AEBP1. Total RNA was extracted at the indicated time points after transfection, and AEBP1 expression was analyzed in TaqMan assays. Shown are means of 3 replications. B, Western blot analysis of AEBP1 in HUVECs transfected with the indicated siRNAs. Cell lysates were extracted 72 h after transfection. C, Proliferation of HUVECs transfected with the indicated siRNAs. Shown are means of 8 replications. D, Cell cycle analysis of HUVECs transfected with the indicated siRNAs. Shown are means of 3 replications. E, Wound healing assays using HUVECs transfected with the indicated siRNAs. The wound was made 48 h after transfection, and photographs were taken at the indicated times. Representative results are shown on the left, and summarized results of 3 replications are shown on the right. F, In vitro tube formation by HUVECs transfected with the indicated siRNAs. Representative results are shown on the left, and summarized results of 3 replications are shown on the right. Error bars depict SEMs. *P < .05, **P < .01
We next constructed lentiviral expression vectors encoding the respective AEBP1 variants and confirmed ectopic expression of the mRNAs and proteins in HUVECs (Figure 4A,B). We observed that both protein variants were present in the nucleus and cytoplasm (Figure 4C), and that their ectopic expression upregulated proliferation and in vitro tube formation by HUVECs (Figure 4D, E).
Figure 4.
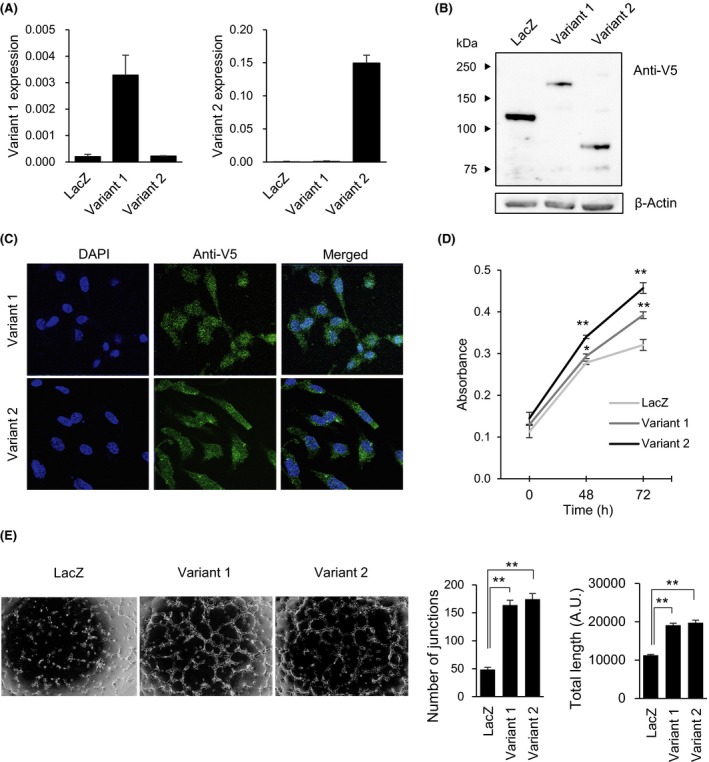
Ectopic expression of adipocyte enhancer‐binding protein 1 (AEBP1) in endothelial cells promotes proliferation and in vitro tube formation. A, Quantitative RT‐PCR analysis of AEBP1 variants 1 (left) and 2 (right) in human umbilical vein endothelial cells (HUVECs) infected with the indicated lentiviral vectors. Expression levels are normalized to ACTB expression. B, Western blot analysis of AEBP1 in HUVECs infected with the indicated vectors. Ectopic expression of AEBP1 or LacZ was detected using an anti‐V5 Ab. C, Fluorescent immunostaining of AEBP1 variants in HUVECs transfected with the indicated vectors. (D) Proliferation of HUVECs infected with the indicated vectors. (E) In vitro tube formation by HUVECs transfected with indicated vectors. Representative results are shown on the left, and summarized results of 3 replications are on the right. Error bars depict SEMs. *P < .05, **P < .01
3.3. AEBP1 promotes in vivo tumor angiogenesis
We used a xenograft model to evaluate the contribution of AEBP1 to in vivo tumor angiogenesis. We first confirmed that cotransplantation of DLD1 cells and HUVECs into nude mice significantly enhanced xenograft formation as compared to transplantation of DLD1 cells alone (Figure S12). We then cotransplanted DLD1 cells plus HUVECs transfected with a siRNA targeting AEBP1 or a control siRNA into nude mice. We found that AEBP1 knockdown in HUVECs diminished microvessel formation in xenograft tumors, although it did not affect tumor volumes (Figure 5A). To clarify whether AEBP1 in mouse endothelial cells plays a role in determining the microenvironment of xenograft tumors, we transplanted DLD1 cells into nude mice and then treated the tumors with siRNAs targeting mouse Aebp1. We found that treatment with the siRNAs suppressed tumor growth and microvessel formation (Figure 5B‐D). Conversely, cotransplantation of DLD1 cells and HUVECs ectopically expressing AEBP1 variant 2 promoted tumor growth (Figure 5E,F). In addition, we observed that ectopic expression of both AEBP1 variants in HUVECs promoted microvessel formation (Figure 5G). These results suggest that upregulation of AEBP1 in endothelial cells promotes tumor angiogenesis.
Figure 5.
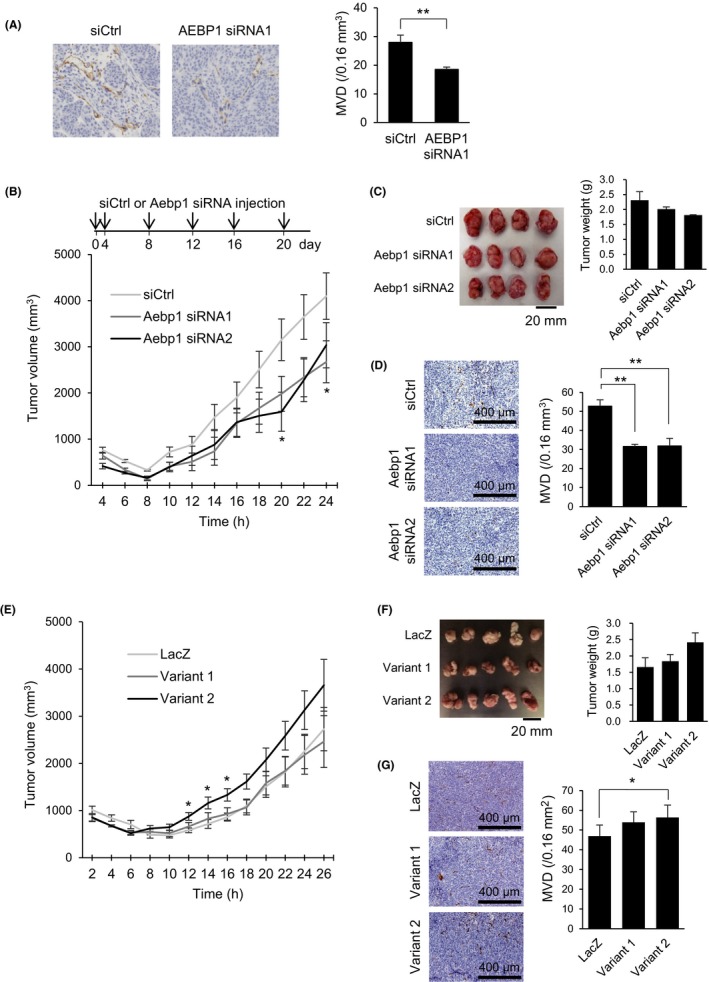
Adipocyte enhancer‐binding protein 1 (AEBP1) promotes tumor angiogenesis in CRC xenograft models. A, Immunohistochemical detection of CD31 in xenograft tumors derived from DLD1 cells plus human umbilical vein endothelial cells (HUVECs) transfected with the indicated siRNAs. Representative views are shown on the left, and summaries of microvessel densities (MVDs) are on the right. B, Growth of xenograft tumors treated with siRNAs targeting mouse Aebp1. Intratumoral injection of siRNAs was undertaken at indicated time points. Shown are means of 4 replications. C, Photographs and weights of resected tumors treated with the indicated siRNAs. D, Representative views of CD31 staining (left) and summaries of microvessel densities (right) in tumors treated with the indicated siRNAs. E, Growth of xenograft tumors derived from DLD1 cells plus HUVECs ectopically expressing LacZ or the indicated AEBP1 variants. Shown are means of 5 replications. F, Pictures and weights of resected tumors expressing the indicated gene. G, Representative views of CD31 staining (left) and summaries of microvessel densities (right) in tumors expressing the indicated gene. Error bars depict SEMs. *P < .05, **P < .01
3.4. AEBP1 regulates expression of angiogenesis‐related genes in endothelial cells
To clarify the mechanism by which AEBP1 regulates angiogenesis, we sought to identify target genes downstream of AEBP1 in endothelial cells. Gene expression microarray analysis of HUVECs in which AEBP1 was knocked down revealed that depleting AEBP1 in these cells downregulated (2‐fold or more) a number of genes reportedly associated with angiogenesis or endothelial functions, including AQP1 (aquaporin 1), POSTN (periostin), SELE (selectin E), SEMA3G (semaphorin 3G), and GJA4 (gap junction protein, alpha‐4) (Figure 6A). We validated the microarray results using qRT‐PCR (Figure 6B). We also found that ectopic expression of AEBP1 upregulated expression of AQP1, SELE, and GJA4 in HUVECs (Figure 6C). These data suggest that AEBP1 might promote tumor angiogenesis by targeting multiple angiogenesis‐related genes.
Figure 6.
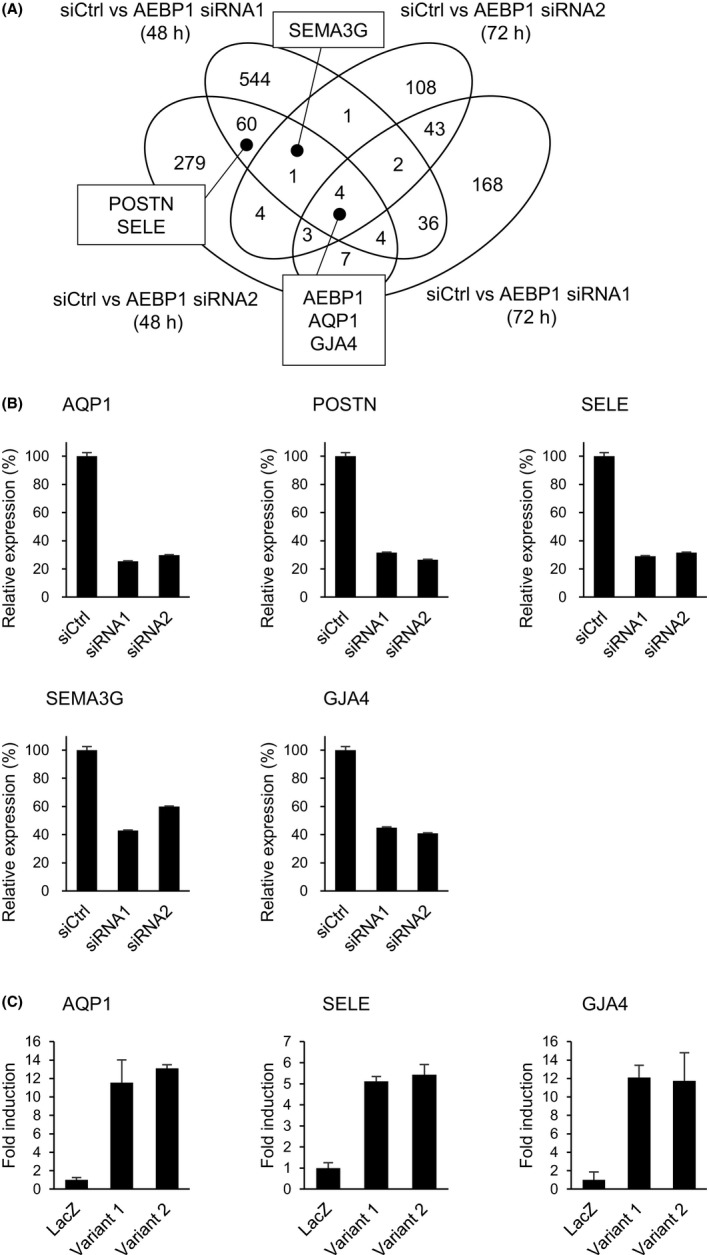
Identification of downstream adipocyte enhancer‐binding protein 1 (AEBP1) targets in endothelial cells. A, Venn diagram showing genes whose expression was downregulated by AEBP1 knockdown in human umbilical vein endothelial cells (HUVECs). B, Quantitative RT‐PCR validating the microarray results for the indicated genes. Expression levels are normalized to ACTB expression. Shown are means of 3 replications. C, Relative expression of the indicated genes in HUVECs ectopically expressing LacZ or the indicated AEBP1 variant. Expression levels are normalized to ACTB expression. Shown are means of 3 replications. Error bars depict SEMs. AQP, aquaporin; GJA4, gap junction protein, alpha‐4; POSTN, periostin; SELE, selectin E; SEMA3G, semaphorin 3G; siCtrl, control siRNA
4. DISCUSSION
In this study, we found that elevated expression of AEBP1 promotes tumor angiogenesis in CRC. Adipocyte enhancer‐binding protein 1 was first identified as a transcriptional repressor with carboxypeptidase activity in mouse preadipocytes. 13 A human homologue of AEBP1 was found to be uniquely expressed in osteoblasts and adipose tissue, suggesting its involvement in determining bone morphology. 19 Later, an N‐terminally extended isoform of AEBP1 was identified as aortic carboxypeptidase‐like protein (ACLP), which is upregulated during vascular smooth muscle cell differentiation. 14
Adipocyte enhancer‐binding protein 1 (encoded by AEBP1 variant 2) is abundantly expressed in preadipocytes and macrophages, where it acts as a proinflammatory mediator and induces nuclear factor‐κB (NF‐κB) signaling. 20 , 21 It is also expressed in the stromal component of mammary glands, and its overexpression in mammary macrophages promotes mammary epithelial hyperplasia. 22 Aortic carboxypeptidase‐like protein (encoded by AEBP1 variant 1) is characterized by a signal peptide at its N‐terminus and a discoidin‐like domain. 14 Aortic carboxypeptidase‐like protein is a secreted protein that associates with the ECM. In mice, ACLP is abundantly detected in the ECM of collagen‐rich tissues, including the vasculature, dermis, and connective tissue, and ACLP‐null mice show abdominal wall disruption and deficient wound healing. 23 Aortic carboxypeptidase‐like protein is also highly expressed in human fibrotic lung tissue and fibroblasts, and it enhances myofibroblast differentiation. 15 , 24
Adipocyte enhancer‐binding protein 1 has been implicated in various human malignancies. For instance, AEBP1 expression is upregulated in glioblastoma cells, where it promotes proliferation and survival through regulation of various target genes involved in cell growth, differentiation, and apoptosis. 25 Another study reported that AEBP1 is upregulated in melanoma cells with acquired resistance to BRAF inhibition, and that AEBP1 could be a therapeutic target in BRAF inhibitor‐resistant melanoma. 26 Recently, AEBP1 was found to be overexpressed in gastric cancer and CRC cells, where it promotes proliferation, migration, invasion, and metastasis by activating NF‐κB signaling. 27 , 28 These results suggest that AEBP1 might exert oncogenic effects in multiple types of malignancy.
But although evidence suggests AEBP1 is expressed in stromal tissues, its role in cancer stroma remains unclear. In an earlier study, Bhati et al 29 used laser capture microdissection to isolate vascular cells from breast tumors and normal breast tissue, and analyzed the respective gene expression profiles using a cDNA microarray. They identified a series of genes upregulated in tumor vascular cells, including AEBP1, although its role in breast cancer angiogenesis remains to be clarified. In the present study, we found that AEBP1 is upregulated in TECs and in the stroma of primary CRC tissues. Notably, vascular endothelial cells expressed both AEBP1 variants, although variant 1 was the dominantly expressed isoform. Knocking down AEBP1 suppressed proliferation and in vitro tube formation by HUVECs. In addition, our xenograft experiments suggested that, when CRC cells are cotransplanted with HUVECs, depletion of AEBP1 in the HUVECs attenuates microvessel formation. We also observed that tumor growth and microvessel formation are suppressed when tumors are treated with siRNAs targeting mouse AEBP1. The experimental results obtained with HUVECs ectopically expressing AEBP1 are largely consistent with the knockdown experiments, however, there are some discrepancies. For instance, AEBP1 knockdown in HUVECs had no effect on xenograft tumor volumes, whereas ectopic expression of variant 2 in HUVECs promoted tumor growth. These results could be due to the limited expression level of endogenous variant 2 in HUVECs.
We also found that AEBP1 might exert its function by altering expression of downstream target genes. Aquaporin 1 is a water channel protein widely expressed in epithelial and endothelial cells, and is involved in water transport and cell migration. 30 Expression of AQP1 is elevated in various tumor types and is associated with cell migration, angiogenesis, and metastasis. 31 In CRC tissues, AQP1 expression is elevated early during tumorigenesis and is maintained through the late stages of cancer progression. 32 Aquaporin 1 is highly expressed in proliferating tumor microvessels, 33 and a cancer model in AQP1‐null mice showed impaired tumor angiogenesis. 34 A recent study also showed that pharmacological blockade of AQP1 suppressed in vitro tube formation by HUVECs. 35
Also known as osteoblast‐specific factor 2, POSTN is a secreted ECM protein originally identified in osteoblasts. 36 Periostin belongs to the superfamily of TGF‐β‐inducible proteins and promotes integrin‐dependent cell adhesion and motility. It is normally expressed in collagen‐rich fibrous connective tissues, including periosteum, as well as in fibroblasts of normal tissues. By activating integrin‐mediated signaling pathways, POSTN promotes cell survival, angiogenesis, and resistance to hypoxia‐induced cell death, and its expression is elevated in esophageal, gastric, colorectal, and pancreatic cancers. 36 In CRC, POSTN is expressed by stromal cells, and elevation of its expression is associated with metastasis and a poor prognosis. 37 , 38 , 39 , 40 Periostin also reportedly promotes tumor angiogenesis. In breast cancer, POSTN mediates angiogenesis by upregulating VEGF receptor (FLK1/KDR) through an integrin αvβ3‐FAK‐mediated signaling pathway. 41 In addition, POSTN promotes proliferation, migration, and in vitro tube formation by HUVECs, and that it promotes tumor angiogenesis through Erk/VEGF signaling in pancreatic cancer. 42
E‐selectin is a major adhesion receptor on endothelial cells, and its expression is induced by inflammatory cytokines, including interleukin‐1β and tumor necrosis factor‐α. 43 Multiple lines of evidence suggest that E‐selectin‐mediated adhesion of CRC cells to endothelial cells is an important determinant of metastasis. E‐selectin is expressed on activated endothelial cells and is upregulated during metastatic colonization of the liver. 44 Anti‐E‐selectin Abs or antisense oligonucleotides that suppress E‐selectin expression have been shown to attenuate experimental liver metastasis. 45 These reports suggest that AEBP1 might promote CRC metastasis by inducing E‐selectin expression in endothelial cells, although further study will be needed to evaluate this possibility.
As a class 3 semaphorin, SEMA3G is primarily expressed by endothelial cells that acts to control endothelial and smooth muscle function. 46 Recently, SEMA3G was shown to promote peroxisome proliferator‐activated receptor‐γ‐driven migration of HUVECs. 47 GJA4, also known as connexin 37 (Cx37), is a transmembrane protein that forms gap junctions and mediates cell‐cell communication. Among the 21 connexin isoforms, HUVECs express Cx37, Cx40, and Cx43, and knocking down that expression suppresses angiogenic activity. 48
There are several limitations in our study. Although we found that AEBP1 could induce angiogenesis‐related genes in endothelial cells, it remains unclear whether AEBP1 acts directly within the nucleus to regulate transcription of target genes. To clarify the molecular mechanism by which AEBP1 promotes tumor angiogenesis, identification of factors that associate with AEBP1 in endothelial cells will be necessary. Second, it remains unclear whether or not the 2 AEBP1 variants play similar roles in endothelial cells. We found that ectopic expression of variant 2 exerted similar or even stronger effects than variant 1 in HUVECs, but our results should be carefully interpreted, given the low expression levels of endogenous variant 2. In addition, the effect of glycosylation on AEBP1 function remains to be clarified. Finally, we found that AEBP1 is abundantly expressed in the stroma of CRC tissues, but its role in cell types other than endothelial cells remains to be determined. Further study will be needed to clarify the function of AEBP1 in cancer stromal cells, including cancer‐associated fibroblasts.
In summary, we found that expression of AEBP1 is frequently elevated in TECs within CRC tissues, and that upregulation of AEBP1 might contribute to tumor angiogenesis. Endothelial expression of AEBP1 is induced by CRC cells, and AEBP1 could promote angiogenesis by inducing angiogenesis‐related genes, including AQP1. These results suggest that AEBP1 signaling could be a useful therapeutic target in CRC.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Fig S1‐S12
Table S1
Table S2
Table S3
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms Mutsumi Toyota and Tomo Hatahira for technical assistance, and Dr William F. Goldman for editing the manuscript. This study was supported in part by a Grant‐in‐Aid for Scientific Research (C) from the Japan Society for Promotion of Science (JSPS KAKENHI 15K08973, 18K07949, E. Yamamoto), Grant‐in‐Aid for Young Investigators from Japan Society for Promotion of Science (JSPS KAKENHI 19K18736, A. Yorozu), the Japan Agency for Medical Research and Development (AMED, JP18lm0203001), MEXT KAKENHI (No. 221S0002), The NOVARTIS Foundation for the Promotion of Science (H. Suzuki), and the Yasuda Memorial Medical Foundation (H. Suzuki).
Yorozu A, Yamamoto E, Niinuma T, et al. Upregulation of adipocyte enhancer‐binding protein 1 in endothelial cells promotes tumor angiogenesis in colorectal cancer. Cancer Sci. 2020;111:1631–1644. 10.1111/cas.14360
REFERENCES
- 1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cersosimo RJ. Management of advanced colorectal cancer, Part 2. Am J Health Syst Pharm. 2013;70:491‐506. [DOI] [PubMed] [Google Scholar]
- 3. Brenner H, Bouvier AM, Foschi R, et al. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 1980s;131:1649‐1658. [DOI] [PubMed] [Google Scholar]
- 4. Winder T, Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology. 2010;138:2163‐2176. [DOI] [PubMed] [Google Scholar]
- 5. Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2:a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197‐1202. [DOI] [PubMed] [Google Scholar]
- 8. Byrd TT, Fousek K, Pignata A, et al. TEM8/ANTXR1‐specific CAR T cells as a targeted therapy for triple‐negative breast cancer. Cancer Res. 2018;78:489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Beijnum JR, Dings RP, van der Linden E, et al. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339‐2348. [DOI] [PubMed] [Google Scholar]
- 10. Naschberger E, Liebl A, Schellerer VS, et al. Matricellular protein SPARCL1 regulates tumor microenvironment‐dependent endothelial cell heterogeneity in colorectal carcinoma. J Clin Invest. 2016;126:4187‐4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aoki H, Yamamoto E, Takasawa A, et al. Epigenetic silencing of SMOC1 in traditional serrated adenoma and colorectal cancer. Oncotarget. 2018;9:4707‐4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niinuma T, Kitajima H, Kai M, et al. UHRF1 depletion and HDAC inhibition reactivate epigenetically silenced genes in colorectal cancer cells. Clin Epigenetics. 2019;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He GP, Muise A, Li AW, Ro HS. A eukaryotic transcriptional repressor with carboxypeptidase activity. Nature. 1995;378:92‐96. [DOI] [PubMed] [Google Scholar]
- 14. Layne MD, Endege WO, Jain MK, et al. Aortic carboxypeptidase‐like protein, a novel protein with discoidin and carboxypeptidase‐like domains, is up‐regulated during vascular smooth muscle cell differentiation. J Biol Chem. 1998;273:15654‐15660. [DOI] [PubMed] [Google Scholar]
- 15. Schissel SL, Dunsmore SE, Liu X, Shine RW, Perrella MA, Layne MD. Aortic carboxypeptidase‐like protein is expressed in fibrotic human lung and its absence protects against bleomycin‐induced lung fibrosis. Am J Pathol. 2009;174:818‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ro HS, Kim SW, Wu D, Webber C, Nicholson TE. Gene structure and expression of the mouse adipocyte enhancer‐binding protein. Gene. 2001;280:123‐133. [DOI] [PubMed] [Google Scholar]
- 17. Shijo M, Honda H, Suzuki SO, et al. Association of adipocyte enhancer‐binding protein 1 with Alzheimer's disease pathology in human hippocampi. Brain Pathol. 2018;28:58‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gagnon A, Landry A, Proulx J, Layne MD, Sorisky A. Aortic carboxypeptidase‐like protein is regulated by transforming growth factor beta in 3T3‐L1 preadipocytes. Exp Cell Res. 2005;308:265‐272. [DOI] [PubMed] [Google Scholar]
- 19. Ohno I, Hashimoto J, Shimizu K, et al. A cDNA cloning of human AEBP1 from primary cultured osteoblasts and its expression in a differentiating osteoblastic cell line. Biochem Biophys Res Commun. 1996;228:411‐414. [DOI] [PubMed] [Google Scholar]
- 20. Majdalawieh A, Zhang L, Fuki IV, Rader DJ, Ro HS. Adipocyte enhancer‐binding protein 1 is a potential novel atherogenic factor involved in macrophage cholesterol homeostasis and inflammation. Proc Natl Acad Sci U S A. 2006;103:2346‐2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Majdalawieh A, Zhang L, Ro HS. Adipocyte enhancer‐binding protein‐1 promotes macrophage inflammatory responsiveness by up‐regulating NF‐kappaB via IkappaBalpha negative regulation. Mol Biol Cell. 2007;18:930‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holloway RW, Bogachev O, Bharadwaj AG, et al. Stromal adipocyte enhancer‐binding protein (AEBP1) promotes mammary epithelial cell hyperplasia via proinflammatory and hedgehog signaling. J Biol Chem. 2012;287:39171‐39181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Layne MD, Yet SF, Maemura K, et al. Impaired abdominal wall development and deficient wound healing in mice lacking aortic carboxypeptidase‐like protein. Mol Cell Biol. 2001;21:5256‐5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tumelty KE, Smith BD, Nugent MA, Layne MD. Aortic carboxypeptidase‐like protein (ACLP) enhances lung myofibroblast differentiation through transforming growth factor beta receptor‐dependent and ‐independent pathways. J Biol Chem. 2014;289:2526‐2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ladha J, Sinha S, Bhat V, Donakonda S, Rao SM. Identification of genomic targets of transcription factor AEBP1 and its role in survival of glioma cells. Mol Cancer Res. 2012;10:1039‐1051. [DOI] [PubMed] [Google Scholar]
- 26. Hu W, Jin L, Jiang CC, et al. AEBP1 upregulation confers acquired resistance to BRAF (V600E) inhibition in melanoma. Cell Death Dis. 2013;4:e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu JY, Jiang L, Liu JJ, et al. AEBP1 promotes epithelial‐mesenchymal transition of gastric cancer cells by activating the NF‐kappaB pathway and predicts poor outcome of the patients. Sci Rep. 2018;8:11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xing Y, Zhang Z, Chi F, et al. AEBP1, a prognostic indicator, promotes colon adenocarcinoma cell growth and metastasis through the NF‐kappaB pathway. Mol Carcinog. 2019;58:1795‐1808. [DOI] [PubMed] [Google Scholar]
- 29. Bhati R, Patterson C, Livasy CA, et al. Molecular characterization of human breast tumor vascular cells. Am J Pathol. 2008;172:1381‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ribatti D, Ranieri G, Annese T, Nico B. Aquaporins in cancer. Biochim Biophys Acta. 1840;2014:1550‐1553. [DOI] [PubMed] [Google Scholar]
- 32. Moon C, Soria JC, Jang SJ, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699‐6703. [DOI] [PubMed] [Google Scholar]
- 33. Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saadoun S, Papadopoulos MC, Hara‐Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin‐1 gene disruption. Nature. 2005;434:786‐792. [DOI] [PubMed] [Google Scholar]
- 35. Dorward HS, Du A, Bruhn MA, et al. Pharmacological blockade of aquaporin‐1 water channel by AqB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J Exp Clin Cancer Res. 2016;35:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morra L, Moch H. Periostin expression and epithelial‐mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bao S, Ouyang G, Bai X, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329‐339. [DOI] [PubMed] [Google Scholar]
- 38. Li Z, Zhang X, Yang Y, et al. Periostin expression and its prognostic value for colorectal cancer. Int J Mol Sci. 2015;16:12108‐12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu X, Chang W, Yuan J, et al. Periostin expression in intra‐tumoral stromal cells is prognostic and predictive for colorectal carcinoma via creating a cancer‐supportive niche. Oncotarget. 2016;7:798‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oh HJ, Bae JM, Wen XY, Cho NY, Kim JH, Kang GH. Overexpression of POSTN in tumor stroma is a poor prognostic indicator of colorectal cancer. J Pathol Transl Med. 2017;51:306‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shao R, Bao S, Bai X, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up‐regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992‐4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Li F, Gao F, et al. Periostin promotes tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling. Oncotarget. 2016;7:40148‐40159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169‐177. [DOI] [PubMed] [Google Scholar]
- 44. Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. Rapid induction of cytokine and E‐selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59:1356‐1361. [PubMed] [Google Scholar]
- 45. Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, Brodt P. Inhibition of hepatic endothelial E‐selectin expression by C‐raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 2002;62:5393‐5398. [PubMed] [Google Scholar]
- 46. Kutschera S, Weber H, Weick A, et al. Differential endothelial transcriptomics identifies semaphorin 3G as a vascular class 3 semaphorin. Arterioscler Thromb Vac Biol. 2011;31:151‐159. [DOI] [PubMed] [Google Scholar]
- 47. Liu W, Li J, Liu M, Zhang H, Wang N. PPAR‐gamma promotes endothelial cell migration by inducing the expression of Sema3g. J Cell Biochem. 2015;116:514‐523. [DOI] [PubMed] [Google Scholar]
- 48. Gartner C, Ziegelhoffer B, Kostelka M, Stepan H, Mohr FW, Dhein S. Knock‐down of endothelial connexins impairs angiogenesis. Pharmacol Res. 2012;65:347‐357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S12
Table S1
Table S2
Table S3
Supplementary Material


