Abstract
Epstein‐Barr virus (EBV) BamHI A rightward transcripts (BART) encoded microRNAs (EBV‐miR‐BARTs) are abnormally highly expressed in nasopharyngeal carcinoma (NPC). This study aims to investigate the diagnostic and prognostic performance of miR‐BART7‐3p and miR‐BART13‐3p. Plasma levels of EBV DNA, miR‐BART7‐3p, and miR‐BART13‐3p were examined by quantitative PCR in 483 treatment‐naïve NPC patients and 243 controls without NPC. The prognostic performance was examined by comparing plasma levels with rates of distant metastasis during follow‐up. The area under the receiver operating characteristic curve for diagnosing NPC was 0.926 for EBV DNA, 0.964 for plasma miR‐BART7‐3p, 0.973 for miR‐BART13‐3p, and 0.997 for all three indices. Among 465 NPC patients without distant metastasis, the above‐median miR‐BART7‐3p and EBV DNA were independent risk for shorter distant metastasis‐free survival (DMFS) (hazard ratio [HR] = 2.94, 95% confidence interval [CI], 1.44‐5.97, P = .003; HR = 2.27, 95% CI, 1.26‐4.10, P = .006) in multivariate Cox regression. Epstein‐Barr virus DNA, miR‐BART7‐3p, and miR‐BART13‐3p after radiotherapy were detectable in 28.6%, 17.6%, and 54.7% of patients, respectively. In multivariate Cox regression, detectable miR‐BART7‐3p and EBV DNA were independent risks for shorter DMFS (HR = 4.13, 95% CI, 1.89‐9.01, P < .001; HR = 2.14, 95% CI, 1.04‐4.42, P = .039). The 4‐year DMFS rate was 92.0% in subjects (n = 156) with neither detectable miR‐BART7‐3p nor EBV DNA, 80.0% in subjects (n = 65) with either detectable miR‐BART7‐3p or EBV DNA, and 52.9% in subjects (n = 24) with both detectable miR‐BART7‐3p and EBV DNA after radiotherapy (P < .001). Circulating levels of miR‐BART7‐3p and miR‐BART13‐3p show excellent diagnostic performance for NPC. The combination of plasma levels of miR‐BART7‐3p and EBV DNA at diagnosis and after radiotherapy could help stratify patients by risk of poor DMFS.
Keywords: biomarker, Epstein‐Barr virus, metastasis, microRNA, nasopharyngeal carcinoma
This is so far the most extensive retrospective study to report the diagnostic and prognostic value of circulating levels of the Epstein‐Barr virus BamHI A rightward transcripts encoded microRNAs (EBV miR‐BARTs) in nasopharyngeal carcinoma. Compared with circulating EBV DNA, this study found that circulating EBV miR‐BARTs is not inferior to EBV DNA in diagnosis and prognosis. Furthermore, the combination of circulating levels of EBV DNA and miR‐BARTs at diagnosis can improve the potential for diagnostic and prognostic evaluation.

Abbreviations
- AUC
area under the receiver operating characteristic curve
- BART
BamHI A rightward transcripts
- CI
confidence interval
- DMFS
distant metastasis‐free survival
- EBV
Epstein‐Barr virus
- HNSCC
head and neck squamous cell carcinoma
- HR
hazard ratio
- LRRFS
locoregional recurrence‐free survival
- miR
microRNA
- NPC
nasopharyngeal carcinoma
- NPV
negative predictive value
- OS
overall survival
1. INTRODUCTION
Nasopharyngeal carcinoma is common in Southeast Asia, especially in southern China. 1 , 2 , 3 As early NPC is almost asymptomatic, 80% of patients present with locally advanced disease or distant metastasis at diagnosis. 4 With the application of intensity‐modulated radio‐ and chemotherapy, treatment outcomes have dramatically improved. The 5‐year OS of stage I NPC is as high as 95%, and the survival of stage IV cancer is over 60%. 5 , 6 , 7 , 8 Nevertheless, 20%‐30% of patients suffer distant metastasis after radical chemoradiotherapy. 1 , 4 , 9 The survival rate and quality of life of these patients are difficult to satisfy for patients with treatment failure, which is the bottleneck to improve the OS rate. Therefore, finding stable and reliable biomarkers for diagnosis, predicting prognosis, and monitoring treatment response are essential directions, which will help guide individualized treatment of NPC and further improve overall outcome.
The pathogenesis of NPC is closely associated with EBV infection; over 95% of tumors are EBV‐positive in high‐incidence areas. 10 A current hypothesis proposes that EBV plays a crucial role in transforming nasopharyngeal epithelial cells into invasive cancer. 11 Circulating cancer‐derived EBV DNA has been established as a biomarker for NPC, with sensitivities ranging from 53% to 96%. 12 One study suggested that plasma EBV DNA is useful for screening for early asymptomatic NPC, with 97.1% sensitivity and 98.6% specificity. 13 The EBV DNA level is also a strong predictor of NPC poor outcomes, especially high risk of distant metastasis. 14 , 15 , 16 , 17 , 18 However, current methods for assaying plasma levels of EBV DNA show high variability. 19 , 20 , 21 , 22 , 23 The NRG‐HN001 trial reported relatively poor interlaboratory concordance for a PCR‐based assay. 19 At present, there is no standard assay for quantification of EBV DNA for clinical or analytical purposes. Establishing standard assay quantification of EBV DNA and searching other stable biomarkers for NPC are necessary.
Epstein‐Barr virus infection is typically latent in NPC. Only a few proteins are expressed in EBV‐associated NPC, such as LMP1, LMP2, and Epstein‐Barr nuclear antigen, and they are expressed at low levels. 13 MicroRNAs derived from the EBV gene BART are highly expressed in NPC tissues. 24 These so‐called miR‐BARTs play essential roles in the development, invasion, metastasis, and immune escape of NPC, based on preclinical studies. 19 , 25 , 26 , 27 , 28 A previous study from our laboratory found high plasma levels of miR‐BARTs in NPC patients. 29 However, the diagnostic and prognostic performance of plasma miR‐BART7‐3p and miR‐BART13‐3p were not well confirmed.
In the current study, we examined the potential diagnostic performance of plasma levels of miR‐BART7‐3p and miR‐BART13‐3p in NPC by comparing levels in patients with levels in control individuals without NPC. We also examined prognostic performance by comparing plasma levels between patients with radiotherapy.
2. MATERIALS AND METHODS
2.1. Study subjects and sample processing
This study was approved by the Hospital Review Board of Fujian Cancer Hospital, Fujian, China (2015‐010‐02). All participants provided written consent for their blood to be sampled and analyzed. The study was undertaken according to the Reporting Recommendations for Tumor Marker Prognostic Studies guidelines. 30 From July 2012 to March 2015, 1394 subjects at our hospital were diagnosed with NPC based on histology of biopsies, of whom 483 patients (18 with distant metastasis at diagnosis) were included in the study because plasma samples at diagnosis were available. Among the 465 NPC patients without distant metastasis at diagnosis, plasma samples within 3 days after completion of radiotherapy were available for 245. Nasopharyngeal carcinoma was reclassified according to the 8th edition of the AJCC/UICC staging system. 8 From July 2012 to March 2015, 243 non‐NPC controls were included in this study, included 207 healthy adults, 12 patients with chronic nasopharyngitis, and 24 patients with histologically confirmed HNSCC excepting NPC.
2.2. Diagnostic and prognostic performance of biomarkers
The diagnostic performance of miR‐BART7‐3p, miR‐BART13‐3p, and EBV DNA was examined by comparing plasma levels between NPC patients and non‐NPC controls. The prognostic performance of miR‐BART7‐3p, miR‐BART13‐3p, and EBV DNA was evaluated based on DMFS among the 465 NPC patients without distant metastasis at diagnosis, and among the 245 NPC patients for whom plasma samples were available immediately after radiotherapy. The workflow of data collection and analysis is shown in Figure 1.
Figure 1.
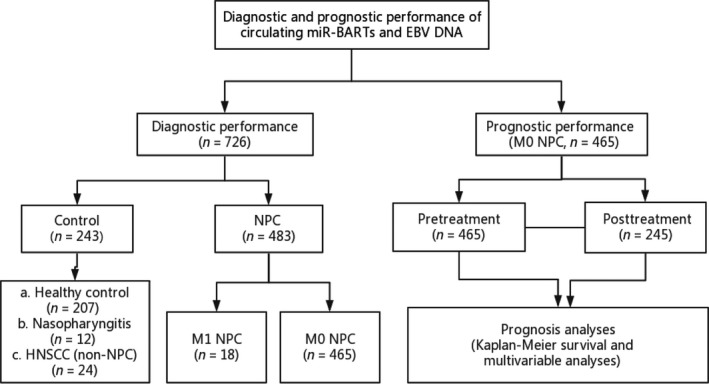
Workflow of data generation and analysis. EBV, Epstein‐Barr virus; HNSCC, head and neck squamous cell carcinoma; mir‐BART, BamHI A rightward transcripts encoded microRNA; NPC, nasopharyngeal carcinoma
2.3. Patient treatment and follow‐up
All patients received intensity‐modulated radiotherapy according to our institutional protocols. 5 In general, stage I disease was treated by radiotherapy alone, whereas stage II and IV tumors were treated by a combination of chemo‐ and radiotherapy. The main chemotherapy strategies are induction chemotherapy and concurrent chemotherapy. The most commonly used chemotherapy regimen for induction and adjuvant chemotherapy was platinum (cisplatin 80 mg/m2, or nedaplatin 80 mg/m2 i.v. in 3 daily doses), plus paclitaxel (135 mg/m2 i.v. on day 1) or gemcitabine (1000 mg/m2 i.v. on days 1 and 8). The concurrent chemotherapy regimen was cisplatin (80mg/m2 i.v. in 3 daily doses) or nedaplatin (80mg/m2 i.v. in 3 daily doses). After treatment completion, follow‐up was typically undertaken at 3‐monthly intervals for 2 years, and at 6‐monthly intervals thereafter.
2.4. RNA extraction
Venous blood samples were processed within 6 hours after collection in EDTA‐containing tubes. Plasma was collected after centrifugation at 1500 g for 10 minutes, then stored at −80°C until use. (RNAiso Plus, Takara, Japan) was used to purify total RNA from plasma samples. In brief, 480 μL plasma was mixed thoroughly with 1000 μL TRIzol reagent and 5 μL Caenorhabditis elegans miRNA 39‐3p (5 μmol/μL), then incubated for 10 minutes at room temperature, and finally mixed with 200 μL chloroform. RNA was then purified according to the manufacturer’s protocol, 31 except that centrifugation was extended to 15 minutes following acid‐phenol/chloroform extraction. RNA was eluted in 20.4 μL RNAse‐free water and stored at −80°C until further processing.
2.5. Reverse transcription and quantification of miRNA
Reverse transcription of miRNA was carried out using the TaqMan MicroRNA Reverse Transcription Kit (catalog no. 4366597; Thermo Fisher Scientific) and miRNA‐specific stem‐loop primers. The program for reverse transcription involved 16°C for 30 minutes, 42°C for 30 minutes, 85°C for 5 minutes, then a hold at 4°C. Quantitative PCR was carried out using TaqMan Universal Master Mix II, no UNG (catalog no. 4440048; Thermo Fisher Scientific), and carried out in duplicate on the ViiA 7 Real‐Time PCR System (Applied Biosystems) with the following conditions: 95°C for 10 minutes, 45 cycles of 15 seconds at 95°C, and 1 minute at 60°C. To estimate the copy number of a particular miRNA in plasma samples, a standard curve was established by quantitative PCR using serially diluted synthetic miRNA mimics. Five microliters of miRNA mimics (3 × 109 copies/μL) was added into the reaction system for reverse transcription, and then the cDNA of miRNA mimics was 10‐fold diluted from 1 × 109 to 1 × 102 copies/μL. Data were collected and analyzed using the ViiA 7 DX Software (Applied Biosystems). Serially diluted mimics were run along with the tested samples to generate the standard curve. Specific information on miRNA‐specific stem‐loop primers, Taqman Probes, PCR primers, and the reaction system of reverse transcription and PCR are described in Table S1. Multiple negative water blanks were included in every analysis.
2.6. Assay of EBV DNA plasma levels
Plasma EBV DNA concentrations were measured by quantitative PCR as previously described. 32 In brief, plasma samples (450 μL) were subjected to DNA extraction using a magnetic bead kit (catalog no. EA20160201; PerkinElmer) using an automatic nucleic acid extraction workstation (Pre‐NAT; PerkinElmer). DNA was eluted from the extraction column in 60 μL nuclear‐free water (catalog no 1902060; Invitrogen). Circulating EBV DNA concentrations were measured using a real‐time quantitative PCR system (catalog no. DA‐D065; Da An Gene) that amplified a DNA segment in the BamHI‐W fragment region of the EBV genome. Data were collected using an ABI Prism 7500 Sequence Detector and analyzed using the 7500 Software (version 2.0.6; Applied Biosystems). The sequences of the forward primers, reverse primers, and probe were listed in Table S1. Results were expressed as copies of EBV genomes per milliliter of plasma. Multiple negative water blanks were included in every analysis.
2.7. Statistical analyses
Statistical analyses were undertaken using GraphPad Prism version 8.0.2 (GraphPad Software), SPSS version 18.0 (SPSS) and R version 3.6.1 (R Foundation for Statistical Computing). Differences in miRNA and DNA levels between NPC and non‐NPC controls were assessed for significance using the Mann‐Whitney U test. Differences in levels at diagnosis and after radiotherapy were assessed using the Wilcoxon test. The DMFS, OS, and LRRFS were analyzed using Kaplan‐Meier survival analyses, and differences between groups were assessed using the log‐rank test. Multivariate analyses using Cox proportional hazard modeling were undertaken to estimate the risk of distant metastasis, death, or locoregional recurrence. Potential confounders included sex, age, clinical stage, and number of chemotherapy cycles. P < .05 (2‐sided) was considered statistically significant.
3. RESULTS
3.1. Plasma EBV‐miR BART7‐3p and BART13‐3p in NPC patients vs non‐NPC controls
The analysis included 483 NPC patients with a median age of 48 years (range, 18‐83 years) and 243 control subjects (Table 1). Epstein‐Barr virus DNA was detected in 93.8% (453/483) of NPC patients, 7.2% (8/207) of healthy subjects, 16.7% (2/12) of subjects with chronic nasopharyngitis, and 12.5% (3/24) of HNSCC patients (Figure 2A). MicroRNA‐BART7‐3p and miR‐BART13‐3p were detected in 96.1% (464/483) and 97.9% (473/483) of NPC cases, but in only 3.9% (8/207) and 3.9% (8/207) of healthy controls (Figure 2B,C). Neither miR‐BART7‐3p nor miR‐BART13‐3p was detected in patients with chronic nasopharyngitis or HNSCC.
Table 1.
Demographic and clinical characteristics of nasopharyngeal carcinoma (NPC) patients and non‐NPC controls
| Characteristic | Controls | NPC (n = 483) | ||
|---|---|---|---|---|
| Healthy (n = 207) | Nasopharyngitis (n = 12) | HNSCC (n = 24) | ||
| Age (y) | ||||
| Median | 45 | 49 | 55 | 48 |
| Range | 18‐75 | 19‐69 | 26‐72 | 18‐89 |
| Sex (n, %) | ||||
| Female | 68 (32.9) | 4 (33.3) | 12 (50.0) | 144 (29.8) |
| Male | 139 (67.1) | 8 (66.7) | 12 (50.0) | 339 (70.2) |
| Clinical stage (n, %) | ||||
| I | 12 (2.50) | |||
| II | 126 (26.1) | |||
| III | 182 (37.7) | |||
| IVA | 145 (30.0) | |||
| IVB | 18 (3.70) | |||
| miR‐BART7‐3p (copies/mL) | ||||
| Median (range) | 0 (0‐271) | 0 (0‐0) | 0 (0‐0) | 3860 (0‐635 720) |
| miR‐BART13‐3p (copies/mL) | ||||
| Median (range) | 0 (0‐1265) | 0 (0‐0) | 0 (0‐0) | 10 220 (0‐1 031 760) |
| EBV DNA (copies/mL) | ||||
| Median (range) | 0 (0‐216) | 0 (0‐84) | 0 (0‐112) | 5430 (0‐6 610 000) |
Abbreviations: EBV, Epstein‐Barr virus; HNSCC, head and neck squamous cell carcinoma; miR‐BART, BamHI A rightward transcripts (BART) encoded microRNA.
Figure 2.
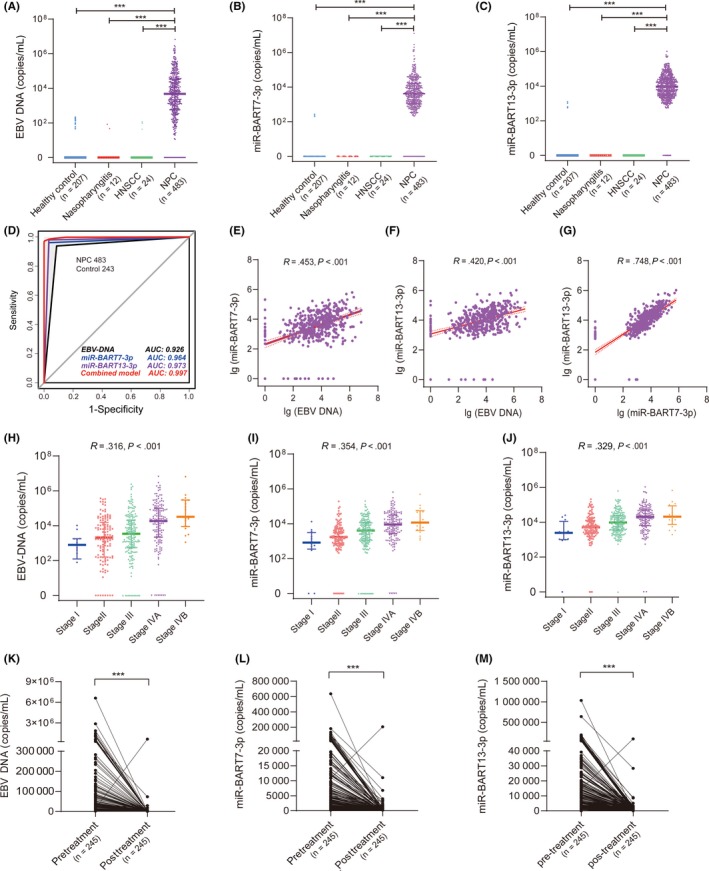
Diagnostic performance of plasma levels of Epstein‐Barr virus (EBV) DNA, BamHI A rightward transcripts encoded microRNA (miR‐BART)7‐3p, and miR‐BART13‐3p. A‐C, Plasma EBV DNA, miR‐BART7‐3p, and miR‐BART13‐3p levels in healthy controls and subjects with nasopharyngitis, head and neck squamous cell carcinoma (HNSCC), and nasopharyngeal carcinoma (NPC). D, Receiver operating characteristic curves of EBV DNA, miR‐BART7‐3p, miR‐BART13‐3p, and combination of the 3 nucleic acid species. All 3 nucleic acid molecules can be detected as 1 point, and undetectable as 0 points. Total score = 3.188 × EBV DNA + 4.165 × miR‐BART7‐3p + 5.445 × miR‐BART13‐3p − 5.531. A score greater than 0 is positive, and a score less than 0 is negative. E‐G, Correlation analysis of the expression of EBV DNA, miR‐BART7‐3p, and miR‐BART13‐3p. Expression of the 3 nucleic acid species was transformed by log10. H‐J, Correlation of levels of EBV DNA, miR‐BART7‐3p, and miR‐BART7‐3p with clinical stage of NPC patients. A correlation coefficient (r) and the corresponding P value for this correlation were estimated by Spearman’s correlation. K‐M, Difference in plasma EBV DNA, miR‐BART7‐3p, and miR‐BART7‐3p between pretreatment and posttreatment samples
When compared with positive diagnosis by histology, miR‐BART7‐3p showed 96.1% sensitivity, 96.7% specificity and an AUC of 0.964 using the detection level as cut‐off value; the corresponding values for miR‐BART13‐3p were 97.9%, 96.7%, and 0.973 (Figure 1D and Table 2). Similarly, the sensitivity, specificity, and AUC of EBV DNA were 93.7%, 91.4%, and 0.926, slightly inferior to those of miR‐BART7‐3p and miR‐BART13‐3p (Table 2). When the plasma levels of all 3 nucleic acid species were considered together, the sensitivity, specificity, and AUC were 97.3%, 99.6%, and 0.997. In a subgroup analysis that included only the 138 patients with stage I‐II NPC, the AUC was 0.921 for EBV DNA, 0.965 for miR‐BART7‐3p, and 0.980 for miR‐BART13‐3p (Table 2). When plasma levels of the 3 nucleic acid species were considered together, the AUC was 0.994, higher than any single biomarker (Figure S1).
Table 2.
Diagnostic performance of plasma biomarkers for detection of nasopharyngeal carcinoma (NPC) (cut‐off = 0 copies/mL)
| Comparison | TP (n) | FN (n) | TN (n) | FP (n) | SE (%) | SP (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|---|---|---|
| EBV DNA | |||||||||
| Early stage vs control | 128 | 10 | 222 | 21 | 92.8 | 91.4 | 85.9 | 95.7 | 0.921 |
| NPC vs control | 453 | 30 | 222 | 21 | 93.7 | 91.4 | 95.6 | 88.1 | 0.926 |
| miR‐BART7‐3p | |||||||||
| Early stage vs control | 133 | 5 | 235 | 8 | 96.4 | 96.7 | 94.3 | 97.9 | 0.965 |
| All NPC vs control | 464 | 19 | 235 | 8 | 96.1 | 96.7 | 98.3 | 92.5 | 0.964 |
| miR‐BART13‐3p | |||||||||
| Early stage vs control | 137 | 1 | 235 | 8 | 99.3 | 96.7 | 94.5 | 99.6 | 0.980 |
| All NPC vs control | 473 | 10 | 235 | 8 | 97.9 | 96.7 | 98.3 | 95.9 | 0.974 |
| All 3 nucleic acid species combined | |||||||||
| Early stage vs control | 137 | 1 | 242 | 1 | 99.3 | 99.6 | 99.3 | 99.6 | 0.994 |
| All NPC vs control | 471 | 13 | 242 | 1 | 97.5 | 99.6 | 99.7 | 94.9 | 0.997 |
Abbreviations: AUC, area under the receiver operating characteristic curve; EBV, Epstein‐Barr virus; FN, false negative; FP, false positive; miR‐BART, BamHI A rightward transcripts (BART) encoded microRNA; NPV, negative predictive value; PPV, positive predictive value; SE, sensitivity; SP, specificity; TN, true negative; TP, true positive.
The EBV DNA levels correlated moderately with levels of both miR‐BART7‐3p (r = .453, P < .001) and miR‐BART13‐3p (r = .420, P < .001) (Figure 2E,F). Levels of miR‐BART7‐3p correlated positively with those of miR‐BART13‐3p (r = .748, P < .001) (Figure 2G).
3.2. Association between plasma miR‐BARTs and NPC burden
Patients with advanced NPC stage had higher plasma levels of miR‐BART7‐3p (r = .354, P < .001), miR‐BART13‐3p (r = .329, P < .001), and EBV DNA (r = .316, P < .001) (Figure 2H‐J). There was a trend towards higher plasma levels of miR‐BART7‐3p, miR‐BART13‐3p, and EBV DNA with advanced N‐ and T‐classification (Figure S2). In a majority of the 245 NPC study subjects with plasma samples after radiotherapy, miR‐BART7‐3p and miR‐BART13‐3p levels were undetectable or significantly reduced in comparison with pretreatment levels (Figure 2L,M). Similar results were observed for EBV DNA (Figure 2K).
3.3. Prognostic performance of plasma levels of miR‐BARTs
The prognostic performance of circulating miR‐BARTs was first examined in the 465 NPC patients without distant metastasis at initial diagnosis. Clinicopathological features of these subjects are listed in Table 3. The median follow‐up time was 55 (range, 2‐83) months. Median circulating levels at diagnosis were 4770 (range, 0‐6 610 000) for EBV DNA, 3669 (0‐635 730) for miR‐BART7‐3p, and 9441 (0‐1 031 760) copies/mL for miR‐BART13‐3p. The median expression value is used as the cut‐off value for high and low expression of miR‐BARTs and EBV DNA. The 4‐year DMFS rate was 81.3% in patients with high EBV DNA expression vs 92.6% in those with low expression (P < .001; Figure 3A), 80.9% in subjects with high miR‐BART7‐3p expression vs 93.2% with low expression (P < .001; Figure 3B), and 83.1% in subjects with high miR‐BART13‐3p expression vs 90.8% with low expression (P = .005; Figure 3C). In the multivariate analysis, short DMFS was independently associated with high levels of miR‐BART7‐3p (HR 2.94; 95% CI, 1.44‐5.98; P = .003) and EBV DNA (HR 2.27; 95% CI, 1.26‐4.10; P = .006), but not high levels of miR‐BART13‐3p (HR 0.67; 95% CI, 0.35‐1.28; P = .227) (Table 4).
Table 3.
Demographic and clinical characteristics of nasopharyngeal carcinoma patients before and after treatment
| Covariate | Pretreatment (n = 465) (n, %) | Posttreatment (n = 245) (n, %) |
|---|---|---|
| Age (y) | ||
| ≤45 | 189 (40.6) | 109 (44.5) |
| >45 | 276 (59.4) | 136 (55.5) |
| Sex | ||
| Male | 324 (69.7) | 172 (70.2) |
| Female | 141 (30.3) | 73 (29.8) |
| Pathology | ||
| Keratinizing squamous cell | 2 (0.4) | 2 (0.8) |
| Nonkeratinizing, differentiated | 45 (9.7) | 22 (9.0) |
| Nonkeratinizing, undifferentiated | 418 (89.9) | 221 (90.2) |
| T category | ||
| T1 | 120 (25.8) | 70 (28.6) |
| T2 | 86 (18.5) | 46 (18.8) |
| T3 | 158 (40.0) | 83 (33.9) |
| T4 | 91 (19.7) | 46 (18.8) |
| N category | ||
| N0 | 37 (8.00) | 22 (9.00) |
| N1 | 226 (48.6) | 117 (47.8) |
| N2 | 138 (29.7) | 73 (29.8) |
| N3 | 64 (13.7) | 33 (13.5) |
| Clinical stage | ||
| I | 12 (2.6) | 7 (2.9) |
| II | 126 (27.1) | 68 (27.8) |
| III | 182 (39.1) | 95 (38.8) |
| IVa | 145 (31.2) | 75 (30.6) |
| Chemotherapy (cycles) | ||
| ≤3 | 155 (33.3) | 70 (28.6) |
| >3 | 310 (66.7) | 175 (71.4) |
| Follow‐up time (mo) | ||
| Median (range) | 55 (2‐83) | 55 (2‐82) |
Figure 3.
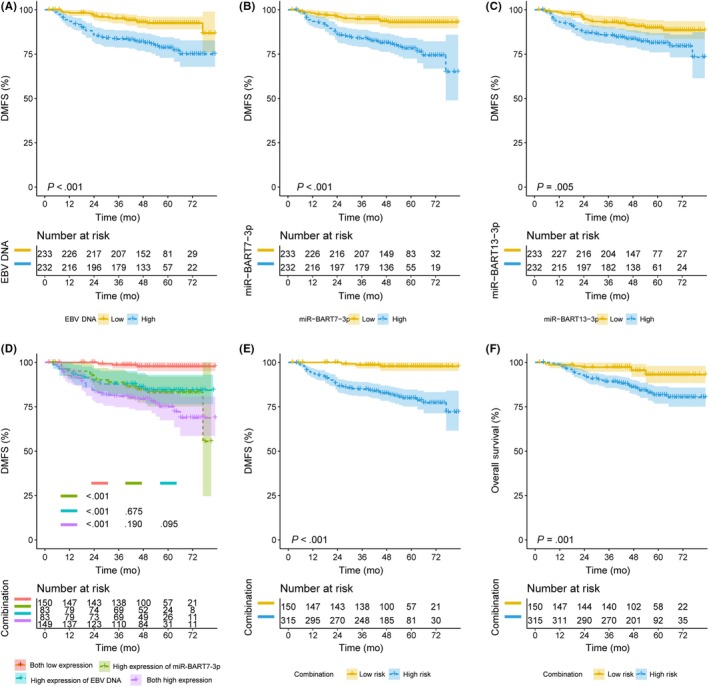
Kaplan‐Meier curves for distant metastasis‐free survival (DMFS) and overall survival (OS) in nasopharyngeal carcinoma patients. A, DMFS of patients with low or high levels of pretreatment Epstein‐Barr virus (EBV) DNA. B, DMFS of patients with low or high levels of pretreatment BamHI A rightward transcripts encoded microRNA (miR‐BART)7‐3p. C, DMFS of patients with low or high levels of pretreatment miR‐BART13‐3p. D, DMFS of 4 patient subgroups based on the combination of EBV DNA and miR‐BART7‐3p. Color coding is explained at the bottom of the panel. E, DMFS of patient groups at low or high risk of poor DMFS based on the combination of EBV DNA and miR‐BART7‐3p. F, OS of patient groups at low or high risk of poor OS based on the combination of EBV DNA and miR‐BART7‐3p
Table 4.
Univariate and multivariate analysis of distant metastasis‐free survival (DMFS) of in patients with nasopharyngeal carcinoma according to pretreatment levels of BamHI A rightward transcripts encoded microRNA (miR‐BART)7‐3p, miR‐BART13‐3p, and Epstein‐Barr virus (EBV) DNA
| Covariate | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| 5‐year DMFS (%) | P value | HR (95% CI) | P value | |
| miR‐BART7‐3p | ||||
| Low | 93.2 | <.001 | 1.00 | .003 |
| High | 80.8 | 2.94 (1.44‐5.97) | ||
| miR‐BART13‐3p | ||||
| Low | 90.8 | .005 | 1.00 | .227 |
| High | 83.1 | 0.67 (0.35‐1.28) | ||
| EBV DNA | ||||
| Low | 92.6 | <.001 | 1.00 | .006 |
| High | 81.3 | 2.27 (1.26‐4.10) | ||
| Age (y) | ||||
| ≤45 | 87.7 | .151 | 1.00 | .204 |
| >45 | 82.1 | 1.41 (0.83‐2.40) | ||
| Sex | ||||
| Male | 81.9 | .004 | 1.00 | .009 |
| Female | 92.1 | 0.40 (0.20‐0.80) | ||
| Chemotherapy cycles | ||||
| ≤3 | 87.1 | .302 | 1.00 | .620 |
| >3 | 83.1 | 1.16 (0.65‐2.05) | ||
| Clinical stage | ||||
| I‐II | 95.1 | <.001 | 1.00 | .018 |
| III‐IV | 81.0 | 2.83 (1.19‐6.72) | ||
Abbreviations: CI, confidence interval; HR, hazard ratio.
3.4. Combining miR‐BART7‐3p and EBV DNA levels improves prognostic performance
The 4‐year DMFS rate of subjects with both high levels of EBV DNA and miR‐BART7‐3p, subjects with high EBV DNA expression but low miR‐BART7‐3p expression, and subjects with high miR‐BART7‐3p expression but low EBV DNA expression were similar (78.7%, 83.3%, and 84.8%, respectively; all P > .05 for comparison between any 2 groups) (Figure 3D). However, the 4‐year DMFS of subjects with both low EBV DNA and miR‐BART7‐3p expression (n = 150) was significantly better than other 3 groups (P < .001) (Figure 3D). Based on these findings, we classified as “high risk” those subjects with high levels of EBV DNA and/or miR‐BART7‐3p. This high‐risk group showed a 4‐year DMFS rate of 82.3% (Figure 3E) and 4‐year OS rate of 85.8% (Figure 3F). The 4‐year LRRFS did not differ between the high‐ and low‐risk groups (92.1% vs 91.6%, P = .329; Figure S3). In multivariate analysis, the abovementioned “high‐risk” status was independently associated with short DMFS (HR 8.74; 95% CI, 2.73‐27.98; P < .001) and short OS (HR 2.45; 95% CI, 1.16‐5.19; P = .019), but not RFS (HR 1.24; 95% CI, 0.61‐2.50, P = .550) (Table 5).
Table 5.
Multivariate analysis based on the pretreatment levels of both BamHI A rightward transcripts encoded microRNA (miR‐BART)7‐3p and Epstein‐Barr virus DNA in nasopharyngeal carcinoma patients
| Covariate | DMFS | OS | LRRFS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Combination | ||||||
| Low risk | 1.00 | <.001 | 1.00 | .019 | 1.00 | .550 |
| High risk | 8.74 (2.73‐27.98) | 2.45 (1.16‐5.19) | 1.24 (0.92‐4.51) | |||
| Age (y) | ||||||
| ≤45 | 1.00 | .239 | 1.00 | .447 | 1.00 | .512 |
| >45 | 1.38 (0.81‐2.35) | 1.24 (0.71‐2.15) | 1.25 (0.64‐2.42) | |||
| Sex | ||||||
| Male | 1.00 | .014 | 1.00 | .135 | 1.00 | .572 |
| Female | 0.43 (0.22‐0.85) | 0.62 (0.33‐1.16) | 1.21 (0.63‐2.31) | |||
| Chemotherapy cycles | ||||||
| ≤3 | 1.00 | .337 | 1.00 | .493 | 1.00 | .058 |
| >3 | 1.32 (0.75‐2.33) | 0.82 (0.47‐1.43) | 0.54 (0.28‐1.02) | |||
| Clinical stage | ||||||
| I‐II | 1.00 | .010 | 1.00 | .001 | 1.00 | .078 |
| III‐IV | 3.06 (1.31‐7.16) | 7.05 (2.19‐22.71) | 2.04 (0.92‐4.51) | |||
Bold values indicate significance.
Abbreviations: CI, confidence interval; DMFS, distant metastasis‐free survival; HR, hazard ratio; LRRFS, locoregional recurrence‐free survival; OS, overall survival.
3.5. Plasma miR‐BART7‐3p levels at the end of radiotherapy can predict outcomes
This analysis included 245 NPC patients (Table 3) followed up for a median of 55 months. At the end of radiotherapy, EBV DNA was detectable in 28.6% (70/245) patients, miR‐BART7‐3p in 17.6% (43/245) patients, and miR‐BART13‐3p in 54.7% (134/245) patients. The 4‐year DMFS rate was 73.0% in subjects with detectable EBV DNA vs 89.7% in those without (P < .001), 61.4% in subjects with detectable miR‐BART7‐3p vs 90.0% (P < .001), and 82.7% in subjects with detectable miR‐BART13‐3p vs 89.5% (P = .035) (Figure 4A‐C). In multivariate analysis, independent risk factors for short DMFS included detectable levels of miR‐BART7‐3p (HR 4.13; 95% CI, 1.89‐9.01; P < .001) and EBV DNA (HR 2.14; 95% CI, 1.04‐4.42; P = .039) at the end of radiotherapy, but not of BART13‐3p (HR 1.19; 95% CI, 0.52‐2.72; P = .672) (Table 6).
Figure 4.
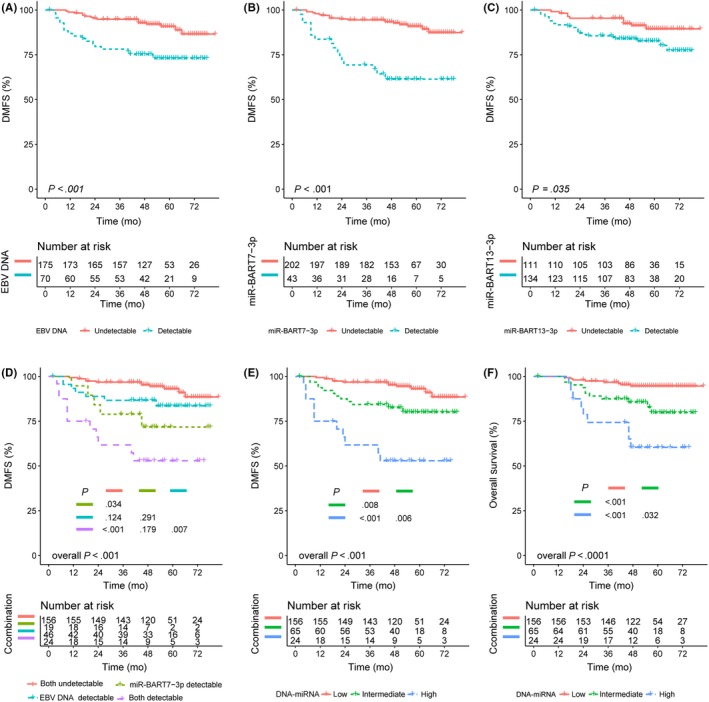
Kaplan‐Meier curves of the 3 biomarkers at the end of radiotherapy for distant metastasis‐free survival (DMFS) and overall survival (OS) in nasopharyngeal carcinoma patients. A, DMFS of patients with detectable or undetectable Epstein‐Barr virus (EBV) DNA at the end of radiotherapy. B, DMFS of patients with detectable or undetectable BamHI A rightward transcripts encoded microRNA (miR‐BART7)‐3p at end of radiotherapy. C, DMFS of patients with detectable or undetectable miR‐BART13‐3p at end of radiotherapy. D, DMFS of patients in 4 subgroups based on the combination of EBV DNA and miR‐BART7‐3p at end of radiotherapy. Color coding is explained at the bottom of the panel. E, DMFS of patients at different risk of poor DMFS based on the combination of EBV DNA and miR‐BART7‐3p at end of radiotherapy. Low, intermediate, and high risk was defined as undetectable EBV DNA and miR‐BART7‐3p, detectable EBV DNA or miR‐BART7‐3p, and detectable EBV DNA and miR‐BART7‐3p, respectively. F, OS of patients at different risk of poor OS based on the combination of EBV DNA and miR‐BART7‐3p at end of radiotherapy
Table 6.
Multivariate analysis of distant metastasis‐free survival (DMFS) according to posttreatment levels of BamHI A rightward transcripts encoded microRNA (miR‐BART)7‐3p, miR‐BART13‐3p, and Epstein‐Barr virus (EBV) DNA in patients with nasopharyngeal carcinoma
| Covariate | DMFS | |
|---|---|---|
| HR (95% CI) | P value | |
| EBV DNA | ||
| Undetectable | 1.00 | .039 |
| Detectable | 2.14 (1.04‐4.42) | |
| miR‐BART7‐3p | ||
| Undetectable | 1.00 | <.001 |
| Detectable | 4.13 (1.89‐9.01) | |
| miR‐BART13‐3p | ||
| Undetectable | 1.00 | .672 |
| Detectable | 1.19 (0.52‐2.72) | |
| Chemotherapy cycles | ||
| ≤3 | 1.00 | .762 |
| >3 | 0.89 (0.42‐1.90) | |
| Age (y) | ||
| ≤45 | 1.00 | .059 |
| >45 | 2.10 (0.97‐4.54) | |
| Gender | ||
| Male | 1.00 | .011 |
| Female | 0.25 (0.09‐0.73) | |
| Clinical stage | ||
| I‐II | 1.00 | .060 |
| III‐IV | 2.78 (0.96‐8.09) | |
Bold values indicate significance.
Abbreviations: CI, confidence interval; DMFS, distant metastasis‐free survival; HR, hazard ratio.
The 4‐year DMFS rates were similar between those with only EBV DNA detectable (83.6%) and those with only miR‐BART7‐3p detectable (71.8%, P = .293) (Figure 4D). Based on these findings, we divided the patients into a low‐risk group (neither EBV DNA nor miR‐BART7‐3p detectable), an intermediate‐risk group (either detectable), and a high‐risk group (both detectable). The groups showed 4‐year DMFS rates of 92.0%, 80.0%, and 52.9%, respectively (P < .001) (Figure 4E), and respective 4‐year OS rates of 94.6%, 79.8%, and 60.4% (P < .001) (Figure 4F).
3.6. Prognostic value of the combination of risk groups at pretreatment and end of radiotherapy
Considering that the pretreatment risk groups and the end‐radiotherapy risk groups both can screen high‐risk patients with distant metastasis, we further analyzed the value of the combination of the 2 kinds of risk groups. Survival analysis found that, among patients with pretreatment low risk, patients in the low‐risk, intermediate‐risk, and high‐risk groups at the end of radiotherapy had similar 4‐year DMFS (98.0% vs 100% vs 100%, respectively, P = .879) and OS (98.0% vs 87.5% vs 100%, respectively, P = .464) (Figure 5A,B). In the pretreatment high‐risk group, the DMFS rate of patients in the low‐risk, intermediate‐risk, and high‐risk groups at the end of radiotherapy was decreased to 91.9%, 75.0%, and 45.9% (P < .001), respectively (Figure 5C). The groups showed respective 4‐year OS rates of 92.4%, 82.7%, and 59.4% (P = .001) (Figure 5D).
Figure 5.
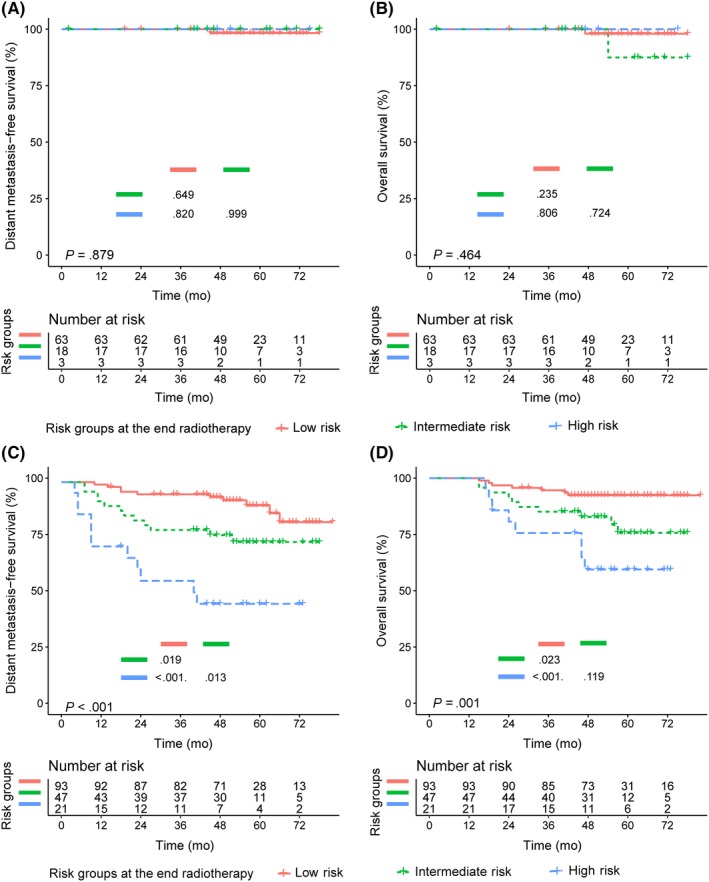
Kaplan‐Meier curves of the combination of risk groups at pretreatment and end of radiotherapy for distant metastasis‐free survival (DMFS) and overall survival (OS) in nasopharyngeal carcinoma patients. A, DMFS of pretreatment low‐risk group. B, OS of pretreatment low‐risk group. C, DMFS of pretreatment high‐risk group. D, OS of pretreatment high‐risk group
4. DISCUSSION
This retrospective study found that plasma levels of miR‐BART7‐3p and miR‐BART13‐3p could be useful as diagnostic and prognostic biomarkers for NPC, and their biomarker performance appears to be at least as good as that of the well‐established EBV DNA biomarker. Furthermore, the combination of miR‐BART7‐3p, miR‐BART13‐3p, and EBV DNA levels at diagnosis could show better diagnostic performance than any of the biomarkers on their own. The combination of miR‐BART7‐3p and EBV DNA at diagnosis and at the end of radiotherapy could perform better than any of the biomarkers on their own for predicting risk of distant metastasis in NPC.
Our previous study found that EBV‐positive NPC cells release miR‐BARTs into culture supernatants, and high expression of miR‐BARTs can be detected in the plasma of NPC patients, especially miR‐BART7‐3p and miR‐BART13‐3p. 29 Our present study validates these findings in an expanded patient cohort, confirming that miR‐BART7‐3p and miR‐BART13‐3p can be used as diagnostic biomarkers for NPC. The AUC of miR‐BART7‐3p and miR‐BART13‐3p for the diagnosis of early NPC was as high as 0.90. Consistent with this, another study 33 identified levels of plasma miR‐BART7‐3p and miR‐BART13‐3p as potential biomarkers. The detection of miR‐BARTs is both a qualitative (detectable or undetectable) and quantitative determination, which is significantly different from human miRNAs that show relatively high or low expression.
In addition, the AUC of miR‐BART7‐3p (0.964) and miR‐BART13‐3p (0.973) in our study was higher than that of EBV DNA (0.926) and they also showed higher positive and negative predictive values. These results indicate that miR‐BART7‐3p and miR‐BART13‐3p are at least as effective as EBV DNA for the diagnosis of NPC. A study from Hong Kong established the value of plasma EBV DNA screening for NPC with sensitivity and specificity of 97.1% and 98.6%. 13 A metaanalysis of 15 studies found pooled sensitivity of 89.1% (95% CI, 87.0%‐90.9%) and specificity of 85.0% (95% CI, 83.0%‐86.9%). 20 The sensitivity and specificity of EBV DNA in our study were 93.8% and 91.4%, slightly lower than that reported by Chan et al, 13 but higher than that reported by Liu et al, 20 suggesting that the detection level of EBV DNA in our study was comparable to that in other studies. Although sequencing analysis of the EBV DNA could lead to even more accurate diagnosis, 34 this might still be too expensive for routine screening in many medical centers. We were able to inexpensively improve performance by combining plasma levels of miR‐BART7‐3p, miR‐BART13‐3p, and EBV DNA, yielding diagnostic sensitivity of 97.5% and specificity of 99.6%. The combination of DNA as a genomic biomarker and miRNAs as transcribed biomarkers could better capture EBV presence and activity in NPC. This possibility should be explored in larger population cohorts.
In the present work, pretreatment plasma levels of miR‐BART7‐3p were a prognostic marker for NPC, with high expression indicating higher risk of distant metastasis. Similarly, detectable posttreatment miR‐BART7‐3p levels were a poor predictor of DMFS and OS, as confirmed in multivariate analysis. 14 , 15 , 16 , 17 , 18 Overall, 28.6% (70/245) of patients still had detectable EBV DNA at posttreatment, which were with higher risk of metastasis. Several studies have established the prognostic value of EBV DNA levels pre‐ and posttreatment in NPC. A multicenter trial found that, in 27.4% of patients, EBV DNA was detectable at 6‐8 weeks after radiotherapy, and detectable levels were associated with 3.14‐fold greater risk of distant metastasis risk. 16 Another study 18 detected EBV DNA in 13.4% of NPC patients at 1 week after completion of radiotherapy, and detectable levels were associated with higher risk of distant metastasis. These 2 studies, 16 , 18 like ours, suggest that posttreatment EBV DNA could be a useful prognostic factor for NPC.
We found that the combination of pretreatment plasma levels of miR‐BART7‐3p and EBV DNA could reliably classify patients as being at low or high risk of distant metastasis. This might help identify individuals in whom more aggressive treatment and close follow‐up could inhibit distant metastasis and improve outcomes, which might also help reduce overtreatment of those at low risk of distant metastasis. Similarly, the combination of circulating levels of miR‐BART7‐3p and EBV DNA at the end of radiotherapy could improve patient stratification by risk of distant metastasis. It will be necessary to undertake clinical trials to explore the treatment value of adjuvant chemotherapy, maintenance chemotherapy, and immunotherapy for patients at intermediate or high risk of distant metastasis after treatment.
Although miR‐BART13‐3p and miR‐BART7‐3p were detectable in the plasma of more than 90% of NPC patients, the prognostic value of miR‐BART13‐3p was less than that of miR‐BART7‐3p. Two studies, in vitro and in vivo, have suggested that miR‐BART13‐3p can promote metastasis of NPC cells by downregulating NKIRAS2 26 and ABI2 35 expression. A previous study showed that miR‐BART7‐3p can promote tumor cell metastasis in vitro and in vivo by targeting PTEN. 25 It seems that biological behavior might not explain why miR‐BART13‐3p is not an independent prognostic factor for NPC. There is a strong correlation between the expression of MiR‐BART13‐3p and miR‐BART7‐3p (r = .748). Therefore, the 2 miR‐BARTs might be mutually exclusive in multivariate analysis. Of course, it is also possible that the sample size of our study is relatively small, highlighting the need for further study of this potential biomarker.
Our study presents several limitations. First, this is a retrospective, single‐center analysis and therefore we could not avoid potential selection biases. Multicenter and prospective studies should be undertaken to validate the diagnostic and prognostic performance of miR‐BARTs in NPC. Second, the detection method of miR‐BARTs was quantitative PCR, which is susceptible to some variability in factors such as plasma volume, extraction method, and PCR reagents. Even though the diagnostic and prognostic value of EBV DNA has been confirmed in several studies, the detection level is still inconsistent in different centers. 23 Therefore, in order to obtain stable and consistent results, a standard detection system should be established and confirmed in multicenter studies.
This study confirmed that the diagnostic performance of circulating miR‐BART7‐3p and miR‐BART13‐3p was at least as good as that of circulating EBV DNA, and the combination of the 3 nucleic acid markers could further improve performance. We also found that circulating miR‐BART7‐3p levels before and after treatment can be used as prognostic markers for NPC, and the combination of EBV DNA and miR‐BART7‐3p might further improve performance.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant from the National Natural Science Foundation of China (Nos. U1405221, U1705282, and 81972717), Fujian Provincial Natural Science Foundation of China (Nos. 2019J01194, 2019Y0061, and 2019J05140), and Joint Funds for the Innovation of Science and Technology of Fujian province (Nos. 2018Y9109 and 2018Y9114), Fujian Provincial Health Technology Project (No. 2019‐ZQN‐14), Science and Technology Program of Fujian Province (No. 2018Y2003), and Startup Fund for Scientific Research, Fujian Medical University (Nos. 2017XQ1213 and 2018QH1222). This work was also supported by the Fujian Key Laboratory of Translational Cancer Medicine.
Lu T, Guo Q, Lin K, et al. Circulating Epstein‐Barr virus microRNAs BART7‐3p and BART13‐3p as novel biomarkers in nasopharyngeal carcinoma. Cancer Sci. 2020;111:1711–1723. 10.1111/cas.14381
Tianzhu Lu, Qiaojuan Guo, and Keyu Lin contributed equally to this study.
Contributor Information
Jingfeng Zong, Email: zongjingfeng@126.com.
Jianji Pan, Email: panjianji1956@fjmu.edu.cn.
REFERENCES
- 1. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356‐3364. [DOI] [PubMed] [Google Scholar]
- 2. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64‐80. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 4. Agulnik M, Epstein JB. Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol. 2008;44:617‐627. [DOI] [PubMed] [Google Scholar]
- 5. Lin S, Pan J, Han L, et al. Update report of nasopharyngeal carcinoma treated with reduced‐volume intensity‐modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014;110:385‐389. [DOI] [PubMed] [Google Scholar]
- 6. Liu YP, Lv X, Zou X, et al. Minimally invasive surgery alone compared with intensity‐modulated radiotherapy for primary stage I nasopharyngeal carcinoma. Cancer Commun. 2019;39:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang LL, Chen YP, Mao YP, et al. Validation of the 8th edition of the UICC/AJCC staging system for nasopharyngeal carcinoma From endemic areas in the intensity‐modulated radiotherapy era. J Natl Compr Canc Netw. 2017;15(7):913‐919. [DOI] [PubMed] [Google Scholar]
- 8. Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity‐modulated radiotherapy. Cancer. 2016;122(4):546‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Q, Lu T, Hui Huang S, et al. Depicting distant metastatic risk by refined subgroups derived from the 8th edition nasopharyngeal carcinoma TNM. Oral Oncol. 2019;91:113‐120. [DOI] [PubMed] [Google Scholar]
- 10. Pathmanathan R, Prasad U, Chandrika G, Sadler R, Flynn K, Raab‐Traub N. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein‐Barr virus‐infected neoplasia. Am J Pathol. 1995;146:1355‐1367. [PMC free article] [PubMed] [Google Scholar]
- 11. Young LS, Yap LF, Murray PG. Epstein‐Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16:789‐802. [DOI] [PubMed] [Google Scholar]
- 12. Fung SY, Lam JW, Chan KC. Clinical utility of circulating Epstein‐Barr virus DNA analysis for the management of nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5:18. [DOI] [PubMed] [Google Scholar]
- 13. Chan KCA, Woo JKS, King A, et al. Analysis of plasma epstein‐barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513‐522. [DOI] [PubMed] [Google Scholar]
- 14. Lv J, Chen Y, Zhou G, et al. Liquid biopsy tracking during sequential chemo‐radiotherapy identifies distinct prognostic phenotypes in nasopharyngeal carcinoma. Nat Commun. 2019;10:3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein‐Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461‐2470. [DOI] [PubMed] [Google Scholar]
- 16. Chan ATC, Hui EP, Ngan RKC, et al. Analysis of plasma epstein‐barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high‐risk patients for adjuvant chemotherapy: A randomized controlled trial. J Clin Oncol. 2018;Jco2018777847:3091–3100. [DOI] [PubMed] [Google Scholar]
- 17. Leung SF, Zee B, Ma BB, et al. Plasma Epstein‐Barr viral deoxyribonucleic acid quantitation complements tumor‐node‐metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006;24:5414‐5418. [DOI] [PubMed] [Google Scholar]
- 18. Wang WY, Lin TY, Twu CW, et al. Long‐term clinical outcome in nasopharyngeal carcinoma patients with post‐radiation persistently detectable plasma EBV DNA. Oncotarget. 2016;7:42608‐42616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le QT, Zhang Q, Cao H, et al. An international collaboration to harmonize the quantitative plasma Epstein‐Barr virus DNA assay for future biomarker‐guided trials in nasopharyngeal carcinoma. Clin Cancer Res. 2013;19:2208‐2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Fang Z, Liu L, Yang S, Zhang L. Detection of Epstein‐Barr virus DNA in serum or plasma for nasopharyngeal cancer: a meta‐analysis. Genet Test Mol Biomark. 2011;15:495‐502. [DOI] [PubMed] [Google Scholar]
- 21. Tan LP, Tan GW, Sivanesan VM, et al. Systematic comparison of plasma EBV DNA, anti‐EBV antibodies and miRNA levels for early detection and prognosis of nasopharyngeal carcinoma. Int J Cancer. 2020;146(8):2336‐2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanosyan A, Fayd'herbe de Maudave A, Bollore K, et al. The impact of targeting repetitive BamHI‐W sequences on the sensitivity and precision of EBV DNA quantification. PLoS ONE. 2017;12:e0183856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim KY, Le QT, Yom SS, et al. Current state of PCR‐based epstein‐barr virus DNA testing for nasopharyngeal cancer. J Natl Cancer Inst. 2017;109:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raab‐Traub N. Nasopharyngeal Carcinoma: An evolving role for the epstein‐barr virus. Curr Top Microbiol Immunol. 2015;390:339‐363. [DOI] [PubMed] [Google Scholar]
- 25. Cai LM, Lyu XM, Luo WR, et al. EBV‐miR‐BART7‐3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene. 2015;34:2156‐2166. [DOI] [PubMed] [Google Scholar]
- 26. Xu YJ, Zhou R, Zong JF, et al. Epstein‐Barr virus‐coded miR‐BART13 promotes nasopharyngeal carcinoma cell growth and metastasis via targeting of the NKIRAS2/NF‐kappaB pathway. Cancer Lett. 2019;447:33‐40. [DOI] [PubMed] [Google Scholar]
- 27. Lin C, Zong J, Lin W, et al. EBV‐miR‐BART8‐3p induces epithelial‐mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF‐kappaB and Erk1/2 pathways. J Exp Clin Cancer Res. 2018;37:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng X, Wang J, Wei L, et al. Epstein‐Barr Virus MicroRNA miR‐BART5‐3p Inhibits p53 Expression. J Virol. 2018;92:e01022‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang G, Zong J, Lin S, et al. Circulating Epstein‐Barr virus microRNAs miR‐BART7 and miR‐BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int J Cancer. 2015;136:E301‐312. [DOI] [PubMed] [Google Scholar]
- 30. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide‐ and proteoglycan‐rich sources. Biotechniques. 1995;19:942‐945. [PubMed] [Google Scholar]
- 32. Lo YM, Chan LY, Lo KW, et al. Quantitative analysis of cell‐free Epstein‐Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Can Res. 1999;59:1188‐1191. [PubMed] [Google Scholar]
- 33. Ramayanti O, Verkuijlen S, Novianti P, et al. Vesicle‐bound EBV‐BART13‐3p miRNA in circulation distinguishes nasopharyngeal from other head and neck cancer and asymptomatic EBV‐infections. Int J Cancer. 2019;144:2555‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lam WKJ, Jiang P, Chan KCA, et al. Sequencing‐based counting and size profiling of plasma Epstein‐Barr virus DNA enhance population screening of nasopharyngeal carcinoma. Proc Natl Acad Sci USA. 2018;115:E5115‐e5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang J, Qin Y, Yang C, et al. Downregulation of ABI2 expression by EBV‐miR‐BART13‐3p induces epithelial‐mesenchymal transition of nasopharyngeal carcinoma cells through upregulation of c‐JUN/SLUG signaling. Aging. 2020;12:340‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


