Abstract
Immunotherapy has become a hotspot in cancer therapy in recent years. Several immune checkpoints inhibitors have been used to treat lung cancer. CD137 is a kind of costimulatory molecule that mediates T cell activation, which regulates the activity of immune cells in a variety of physiological and pathological processes. Targeting CD137 or its ligand (CD137L) has been studied, aiming to enhance anticancer immune responses. Accumulating studies show that anti‐CD137 mAbs alone or combined with other drugs have bright antitumor prospects. In the following, we reviewed the biology of CD137, the antitumor effects of anti‐CD137 Ab monotherapy and the combined therapy in lung cancer.
Keywords: anti‐CD137 monoclonal antibody, CD137, CD137L, immune therapy, lung cancer
Several studies in cells and animal models of lung cancer underscore the essential role of CD137 in cancer therapy. Anti‐CD137 Ab is a potent cancer immunotherapy drug, which can regulate the immune system by acting on a variety of cells in tumor microenvironment. The therapeutic effect of anti‐CD137 mAbs monotherapy in lung cancer is not satisfactory, especially in poorly immunogenic tumors. Combination therapies of anti‐CD137 mAbs with other reagents have shown great potentials of antitumor activities. The clinical potential and the side‐effects of anti‐CD137 mAbs in lung cancer should be determined by more clinical trials. In conclusion, anti‐CD137 mAbs is an attractive candidate for the immunotherapy of lung cancer.
1. INTRODUCTION
Lung cancer is one of the most common malignant tumors; despite the application of new treatment strategies, the mortality rate is still very high.1 In recent years, the effects of immunotherapy have received considerable attention in the field of lung cancer treatment. T cell activation is a pivotal process to combat cancers, in which both coinhibition and costimulation signaling play an essential role. Currently, the application of immune checkpoint inhibitors has achieved great success in clinical practice.2, 3 In contrast, there is little research about the agonistic Abs on costimulation pathways. CD137 signaling plays a significant role in multiple cells and regulates the activity of many immune cells. It can strongly activate CD8+ T cells, induce cytokine release, and increase CTL activity.4 Accumulating evidence has shown that anti‐CD137 mAbs could be used in cancer therapy alone or combined with other Abs or reagents.5 Recently, several related clinical trials have been carried out. In the following, we review the biological characteristics of CD137 and the recent progress of anti‐CD137 mAbs in the fight against lung cancer.
2. EXPRESSION OF CD137
CD137 is a kind of surface glycoprotein, which was originally discovered in the late 1980s.6 It has been found that CD137 is expressed in a variety of cells, for example, T cells, B cells, natural killer (NK) cells, dendritic (DCs), eosinophils, and mast cells.7, 8, 9 A variety of tumor cells also express CD137, such as human leukemia cells and various lung tumor cell lines, such as H446, H1299, SPC‐A‐1, and H460.10, 11, 12 CD137 is also widely distributed in tissues; it has been found in vascular smooth muscles, tumor vessel walls, and liver tissue of hepatocellular carcinoma.13, 14 A study reported that CD137 is focally localized in the follicular structure of tonsil and lymph node.15 CD137 is an important target for enhancing the antitumor effect of immunotherapeutic Abs. Therefore, a comprehensive understanding of its distribution is essential for the discovery of potential therapeutic effects and adverse reactions.
3. BIOLOGICAL EFFECTS OF CD137 SIGNALING
Compared with CD4+ T cells, CD8+ T cells express higher levels of CD137, so CD137 mainly costimulates CD8+ T cells.16 Studies have shown that signaling through CD137 is induced by the ligand of CD137, CD137L, or by agonistic mAbs against CD137. CD137 ligand is a kind of transmembrane protein expressed on the cell surface. CD137 and CD137L form a bidirectional signaling pathway, which allows bidirectional signal exchange between receptor and ligand cells, thereby regulating their activities at the same time.5 CD137L is found on all types of antigen‐presenting cells.17 It has been shown that the interaction of CD137 with CD137L on activated antigen‐presenting cells contributes to the survival and activation of T cells.18 Additionally, the CD137L signaling pathway can influence the activation, maturation, and differentiation of CD137L expressing cells.19 These effects result from the activation of several signaling pathways, such as p38 MAPK.5 Some studies showed that the interaction of CD137 and CD137L could play a pivotal role in maintaining CD8+ T cells and the generation of their memory responses.20 All of these activations will promote the immune system fighting against tumors. For instance, the CD137/CD137L pathway could generate costimulatory signals on B cells to activate and induce their proliferation.21 In monocytes, CD137L signaling can increase their survival and proliferation and stimulate their migration and extravasation.22, 23 In addition, it induces the release of various proinflammatory factors.24 The CD137/CD137L pathway also affects non‐T cells. All of these activities lead to the influx of inflammatory monocytes into tissues and form an inflammatory environment, which is detrimental to tumors. The interaction of CD137 and CD137L is shown in Figure 1.
Figure 1.
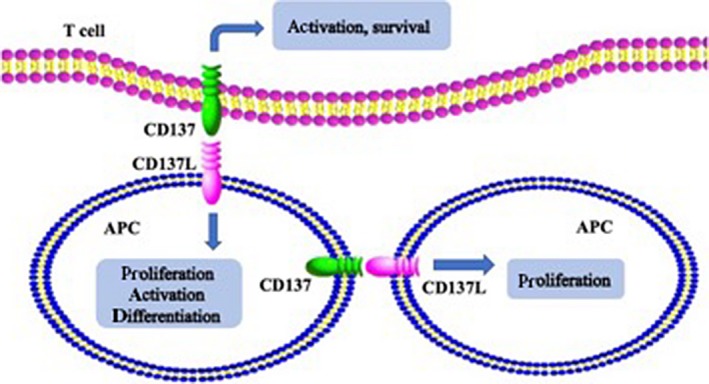
Role of CD137 and CD137 ligand (CD137L) signaling pathway. The interaction of CD137 with CD137L on activated antigen presenting cells (APC) contributes to the survival and activation of T cells. The CD137L signaling pathway can influence the activation, proliferation, and differentiation of CD137L expressing cells
The study undertaken by Melero et al25 first reported the potent antitumor property of anti‐CD137 mAbs, and this effect was also determined in a melanoma model of CD137 knockout mice.26 It was shown that, compared with wild type mice, CD137 knockout mice had more lung metastasis and shorter survival time. The mechanisms of anti‐CD137 mAbs in tumor regression depended on its effects on several immune cells in the tumor microenvironment. Anti‐CD137 mAbs could boost the activation of CD8+ T cells, promote their proliferation, and at the same time, enhance their cytolytic effect on tumor cells, and then killed tumor cells.27 The role of anti‐CD137 mAbs in the activity of CD4+ T cells remained controversial. A study reported that when CD137 costimulated CD4+ T cells, at first their activation was increased and then their apoptosis was promoted.20 When CD4+ T cells are activated, they can release useful cytokines, which are indispensable for the activation and maturation of CD8+ T cells. A study showed that the anti‐CD137 mAbs could enhance the antitumor response of CTLs.28 However, anti‐CD137 therapy was ineffective against the established or metastasized tumors with poor immunogenicity, such as TC‐1 lung cancer.29, 30 The underlying mechanism of the failure of anti‐CD137 mAbs is the immunological ignorance of CTLs. Anti‐CD137 treatment can also promote the proliferation and γ‐interferon (IFN‐γ) production of activated NK cells, modulating the activity of CD8+ T cells.20 Monoclonal Abs exert their antitumor effect through antibody dependent cell‐mediated cytotoxicity (ADCC). In this process, NK cells act on Ab‐targeted tumor cells and cause them to lysis. Dendritic cells also play a critical role in CD137‐mediated antitumor immunity.31 Depletion of DCs in vivo reduced the level of CTL stimulation, and then attenuated the effect of anti‐CD137 mAbs.32 Regulatory T cells (Tregs) can inhibit the cytotoxic effect of T cells, which is a mechanism of tumor immune tolerance. A study by Guo et al showed that, when anti‐CD137 mAbs were given, the infiltration of Tregs in the tumor was significantly reduced.33 Another study found that depleting Tregs in mice could enhance the antitumor effect of anti‐CD137 therapy.34 Therefore, attention should also be paid to the effect of Tregs on anti‐CD137 mAbs. The effects of anti‐CD137 mAbs on these cells are summarized in Figure 2. Interestingly, after treatment with anti‐CD137 mAbs, the number and the activities of memory T cells will increase, so this effect can prolong the clearance of tumor cells by the immune system.35 Moreover, it has been found that, when exposing tumor endothelial cells to anti‐CD137 mAbs, the expression of some adhesion molecules between cells was upregulated, which would promote T cells migrating to malignant tissue by stimulating the tumor endothelial cells.36 Therefore, the anti‐CD137 mAbs can act on multiple cells to play the antitumor role.
Figure 2.
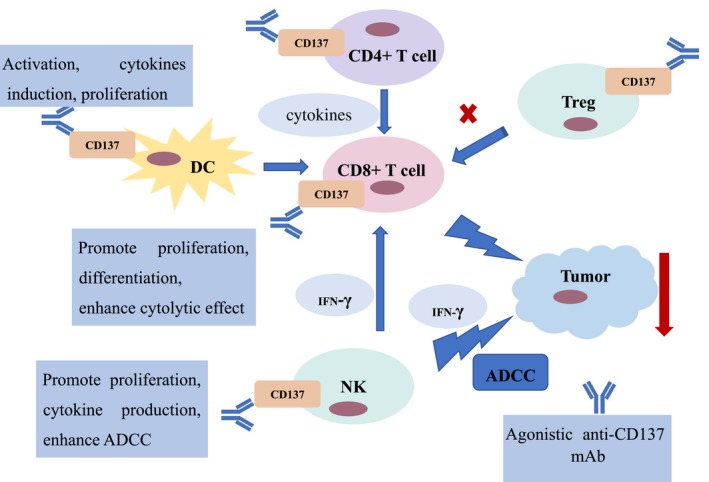
Immune regulation mechanisms of anti‐CD137 mAb. Agonistic anti‐CD137 mAb can promote the activation, proliferation, and differentiation of CD8+ T cells, and enhance their cytolytic effect on tumor cells. Anti‐CD137 mAb acts on CD4+ T cells, leading to the release of cytokines useful to the activation and maturation of CD8+ T cells. Anti‐CD137 mAb can enhance the antibody dependent cell‐mediated cytotoxicity (ADCC) of natural killer (NK) cells and promote their proliferation and γ‐interferon (IFN‐γ) production. In addition, anti‐CD137 mAb can promote the activation and proliferation of dendritic cells (DCs). In regulatory T cells (Tregs), anti‐CD137 mAb can depress their function to enhance the antitumor effect. He et al41 provides some information, but does not include the effect of CD4+ T cells on CD8+ T cells, the effect of NK cells on CD8+ T cells, nor the effect of IFN‐γ
4. AGONISTIC ANTI‐CD137 mAb MONOTHERAPY
The preclinical experiment showed that the anti‐CD137 mAbs can inhibit the growth of A549 lung adenocarcinoma cells.37 However, at present, there are few animal experiments to verify the effect of anti‐CD137 mAb monotherapy in lung cancer. A study undertaken by Shi et al investigated the antitumor effect of BMS‐469492, a kind of agonistic anti‐CD137 mAb. They used M109 mouse lung carcinoma cells in the study. The results showed that BMS‐469492 could not inhibit the growth of tumors in the mouse tumor model.38 Another mouse model of lung carcinoma has confirmed this finding. They showed that a poorly immunogenic tumor, TC‐1 lung carcinoma, was refractory to agonistic anti‐CD137 mAbs once established as solid tumors. They provided evidence that the immune neglect of specific CTLs might hinder the antitumor effect of anti‐CD137 mAbs.39 The effect of anti‐CD137 mAbs was reported in several other tumors, such as glioma, colon carcinoma, liver tumor, breast tumor, and some hematological tumors.5, 40 However, the effect of monotherapy was not obvious in some other poorly immunogenic tumors, such as B16/D5 melanoma.40 Thus, in order to break through the limitation of monotherapy and obtain more satisfactory therapeutic effects, researchers have focused on combination therapy.
5. ANTI‐CD137 mAb COMBINATION THERAPY
5.1. Combination with immune checkpoint inhibitors
Recently, immunotherapy has made a breakthrough in the treatment of various solid tumors.41, 42, 43 However, it was reported that the immunotherapy only benefits 30% of patients. As we all know, the essence of immunotherapy is to restore the normal antitumor immune responses of the body. The participation of T cells is indispensable in the process, so the activation of T cells is particularly important. Co stimulation and coinhibition signaling pathways both regulate the activation of T cells. Many papers have reported that anti‐CD137 mAbs had a synergistic effect with immunotherapeutic agents in several models of cancer.44, 45, 46 In lung cancer, a study built a kind of transplantable mouse non‐small‐cell lung cancer model to investigate the effect of combined immunotherapy. They found that the efficacy of single agents was lost after 17 days of treatment, whereas the combination treatment resulted in 3‐5 complete regressions; in the repetition experiment, 2 of 5 tumors experienced complete regression in the treatment of anti‐CD137 mAbs combined with anti‐programmed cell death‐1 (PD‐1)/PD‐1 ligand (PD‐L1) mAb. The synergistic effect was obtained in the tumor treated with combination therapy.47 A study established a TC‐1 lung carcinoma murine model to study the antitumor effect of combination of anti‐PD‐1/CD137 mAbs and cisplatin. The results showed that anti‐PD‐1 or anti‐CD137 mAb monotherapy could not annihilate tumors. Cisplatin monotherapy, or cisplatin combined with 1 of the 2 Ab treatments, can only suppress tumor growth slightly, but in the combination treatment of 2 Abs plus cisplatin, the tumor growth was significantly inhibited.48 Another study explored the effect of intratumoral injection of combinations of CD137/PD‐1/CTL‐associated protein‐4 (CTLA4)/CD19 mAbs in TC‐1 lung cancer. Compared with i.p. injection, intratumoral injection had a stronger antitumor effect. More than half of the tumor‐bearing mice achieved complete regression after intratumoral injection with CD137/PD‐1/CTLA4/CD19 mAbs, and the survival period was also prolonged.49 Thus, a number of clinical trials of anti‐CD137 mAbs have been developed because of this preclinical research.
5.2. Combination with vaccination
Cancer subunit vaccine based on tumor‐associated antigen is a promising tool for antitumor immunotherapy. Effective anticancer vaccines will achieve therapeutic effects by interfering with the innate, adaptive, and regulatory immune responses. As is known, vaccines are specific and can generate immunological memory, which is important to control tumor recurrence. Nevertheless, the antitumor efficacy of subunit vaccine is limited by low immunogenicity and immune evasion,50, 51 so potent adjuvants are used to overcome these limitations. Because of the pleiotropic and robust effects on immune response, costimulatory members of the tumor necrosis factor ligand family could be used as an appropriate adjuvant for antitumor vaccines.8 Sharma et al reported a new kind of soluble CD137L molecule, SA‐CD137L, which was chimeric with core streptavidin, and examined its efficacy in the survivin‐expressing 3LL lung cancer mouse model. The results showed that vaccination with CD137L in combination with a survivin protein could activate DCs, enhance the uptake of antigen, and generate the activity of CD8+ T cells, thus eradicating the 3LL tumors.52 In conclusion, CD137L molecule could be a candidate for therapeutic cancer vaccines due to its potent immunomodulatory activity and low toxicity.
5.3. Combination with antitumor Abs
Gangliosides are cell surface glycosphingolipids that play a significant role in cell recognition, adhesion, and signal transduction.53 Studies have shown that therapeutic Abs against gangliosides could inhibit the growth and metastasis of tumors. It has been found that fucosylated monosialotetrahexosylganglioside (FucGM1) is highly expressed in a large percentage of small‐cell lung cancer.54, 55 Anti‐FucGM1 mAbs have been reported to suppress tumor growth in nude mice.56, 57 A study undertaken by Ponath et al investigated the antitumor efficacy of anti‐CD137 mAbs in combination with BMS‐986012, a kind of anti‐FucGM1 mAbs. They found that BMS‐986012 showed antitumor activity in a human small‐cell lung cancer tumor xenograft model by enhancing ADCC effector function. The antitumor efficacy of BMS‐986012 was enhanced by anti‐CD137 mAbs.58 Several researchers have reported that anti‐CD137 mAbs could synergize with other antitumor Abs, such as rituximab, trastuzumab, and cetuximab.59, 60, 61
5.4. Combination with other treatments
As mentioned above, the agonistic CD137 mAb, BMS‐469492, cannot inhibit tumor growth significantly when used alone in the M109 tumor model. However, when BMS‐469492 was given to animals that had been primed with irradiated M109 cells 3 weeks prior to tumor inoculation, a significant antitumor effect was observed.38 It has been reported that intratumoral injection of Semliki Forest virus encoding interleukin‐12 combined with systemic treatment with agonist anti‐CD137 mAbs showed powerful synergistic antitumor effects in a TC‐1 lung carcinoma model.62
5.5. Current status of related clinical trials
Two kinds of humanized mAbs of CD137 have been studied in clinical trials, utomilumab (PF‐05082566) and urelumab (BMS‐663513). The characteristics of these two drugs are shown in Table 1. In clinical trials, utomilumab showed low efficacy and urelumab showed fatal hepatotoxicity; the mechanisms remain unclear, so their clinical development was hampered.63 A study showed that both the efficacy and the toxicity of anti‐CD137 mAbs are determined by their isotype and intrinsic agonistic strength. In addition, FcγRIIB‐mediated CD8+ T cell activation in the liver could cause anti‐CD137 mAb‐related hepatotoxicity.64 ADG106 and LVGN6051 are 2 new drugs that are just under clinical research, their characteristics need further study. So far, there are few clinical trials of anti‐CD137 mAbs in lung cancer. The relevant clinical trials are displayed in Table 2. Therefore, in‐depth exploration of the antitumor mechanisms and adverse reactions of anti‐CD137 mAbs is warranted to achieve optimal antitumor therapeutic potential.
Table 1.
Characteristics of anti‐CD137 Abs in recent clinical trials
| Drug | Brand name | Binding site | Property | Defect |
|---|---|---|---|---|
| Utomilumab | PF‐05082566 | CRDs III and IV | Humanized IgG2 mAb | Low efficacy |
| Urelumab | BMS‐663513 | CRD I | Human IgG4 mAb | Fatal hepatotoxicity |
Abbreviation: CRD, Carbohydrate recognition domain.
Table 2.
Clinical studies of anti‐CD137 mAbs in solid tumors in recent years
| NCT number | Intervention | Start year | Status | Phase | Condition |
|---|---|---|---|---|---|
| NCT00309023 | BMS‐663513 | 2005 | Terminated | I/II | Metastatic or locally advanced solid tumor |
| NCT00351325 | BMS‐663513 | 2007 | Terminated | I | Advanced solid malignancies |
| NCT00461110 | BMS‐663513 | 2008 | Terminated | I | Non‐small‐cell lung cancer |
| NCT01471210 | BMS‐663513 | 2012 | Completed | I |
Advanced and/or metastatic solid tumors Relapsed/refractory B‐cell non‐Hodgkin lymphoma |
| NCT02253992 | BMS‐663513 | 2014 | Completed | I/ II |
Advanced solid tumors Advanced B‐cell non‐Hodgkin lymphoma |
| NCT02179918 | PF‐05082566 | 2014 | Completed | Ib | Advanced solid tumors |
| NCT03707093 | ADG106 | 2018 | Recruiting | I |
Solid tumors Non‐Hodgkin lymphoma |
| NCT04130542 | LVGN6051 | 2019 | Recruiting | I | Cancer |
Some of the information is shown in He et al,41 but it does not include the clinical studies in 2018 and 2019.
Abbreviation: NCT, clinicaltrials.gov identifier.
6. CONCLUSION
A variety of in vivo and in vitro studies of lung cancer underscore the essential role of CD137 in cancer therapy. Anti‐CD137 Ab is a potent cancer immunotherapy drug, which can regulate the immune system by acting on various cells in the tumor microenvironment. The therapeutic effect of anti‐CD137 mAb monotherapy in lung cancer is not satisfactory, especially in poorly immunogenic tumors. Anti‐CD137 mAbs combined with other reagents have strong antitumor potential. The clinical potential and the side‐effects of anti‐CD137 mAbs in lung cancer should be determined by further clinical trials. In conclusion, anti‐CD137 mAb is an attractive candidate for the immunotherapy of lung cancer.
DISCLOSURE
There were no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported in part by a grant from the National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036), and the Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), National Key Research & Development Project (2016YFC0902300), Funding was also provided by the major disease clinical skills enhancement program of 3‐year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor” Outstanding Young Talents Program (fkyq1901), key disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), and a grant from the Shanghai Science and Technology Commission (16JC1405900).
Ye L, Jia K, Wang L, et al. CD137, an attractive candidate for the immunotherapy of lung cancer. Cancer Sci. 2020;111:1461–1467. 10.1111/cas.14354
Contributor Information
Yayi He, Email: 2250601@qq.com.
Caicun Zhou, Email: caicunzhoudr@126.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. He Y, Yu H, Rozeboom L, et al. LAG‐3 protein expression in non‐small cell lung cancer and its relationship with PD‐1/PD‐L1 and tumor‐infiltrating lymphocytes. J Thorac Oncol. 2017;12:814‐823. [DOI] [PubMed] [Google Scholar]
- 3. He Y, Bunn PA, Zhou C, et al. KIR 2D (L1, L3, L4, S4) and KIR 3DL1 protein expression in non‐small cell lung cancer. Oncotarget. 2016;13(7):82104‐82111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vinay DS, Kwon BS. 4–1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 2014;47:122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chun DT, Bac ND, Nguyen KH, et al. Update on Anti‐CD137 Antibodies in Immunotherapies for Cancer. Int J Mol Sci. 2019;20(8):1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwon BS, Weissman SM. cDNA sequences of two inducible T‐cell genes. Proc Natl Acad Sci U S A. 1989;86:1963‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinay DS, Kwon BS. Immunotherapy of cancer with 4–1BB. Mol Cancer Ther. 2012;11:1062‐1070. [DOI] [PubMed] [Google Scholar]
- 8. Croft M. The role of TNF superfamily members in T‐cell function and diseases. Nat Rev Immunol. 2009;9:271‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollok K, Kim Y, Zhou Z, et al. Inducible T cell antigen 4–1BB. Analysis of expression and function. J Immunol. 1993;150:771‐781. [PubMed] [Google Scholar]
- 10. Schwarz H, Blanco F, Valbracht J, et al. ILA, a member of the human NGF/TNF receptor family regulates T lymphocyte proliferation and survival. Blood. 1996;87:2839‐2845. [PubMed] [Google Scholar]
- 11. Palma C, Binaschi M, Bigioni M, et al. CD137 and CD137 ligand constitutively expressed on human T and B leukemia cells signal proliferation and survival. Int J Cancer. 2004;108:390‐398. [DOI] [PubMed] [Google Scholar]
- 12. Zhang GB, Dong QM, Hou JQ, et al. Characterization and application of three novel monoclonal antibodies against human 4–1BB: distinct epitopes of human 4–1BB on lung tumor cells and immune cells. Tissue Antigens. 2007;70:470‐479. [DOI] [PubMed] [Google Scholar]
- 13. Broll K, Richter G, Pauly S, et al. CD137 expression in tumor vessel walls. High correlation with malignant tumors. Am J Clin Pathol. 2001;115:543‐549. [DOI] [PubMed] [Google Scholar]
- 14. Wan YL, Zheng SS, Zhao ZC, et al. Expression of co‐stimulator 4–1BB molecule in hepatocellular carcinoma and adjacent non‐tumor liver tissue, and its possible role in tumor immunity. World J Gasteroenterol. 2004;10:195‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alfaro C, Echeveste JI, Rodriguez‐Ruiz ME, et al. Functional expression of CD137 (4–1BB) on T helper follicular cells. Oncoimmunology. 2015;4:e1054597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi C, Mittler RS, Vella AT. Cutting edge: 4–1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037‐5040. [PubMed] [Google Scholar]
- 17. Vinay DS, Kwon BS. Therapeutic potential of anti‐CD137 (4–1BB) monoclonal antibodies. Expert Opin Ther Targets. 2016;20:361‐373. [DOI] [PubMed] [Google Scholar]
- 18. Wang C, Lin GH, McPherson AJ, et al. Immune regulation by 4–1BB and 4–1BBL: complexities and challenges. Immunol Rev. 2009;229:192‐215. [DOI] [PubMed] [Google Scholar]
- 19. Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011;89:21‐29. [DOI] [PubMed] [Google Scholar]
- 20. Sun Y, Chen JH, Fu Y. Immunotherapy with agonistic anti‐CD137: two sides of a coin. Cell Mol Immunol. 2004;1:31‐36. [PubMed] [Google Scholar]
- 21. Pollok Karen E, Kim YJ, Hurtado J, et al. 4–1BB T‐cell antigen binds to mature B cells and macrophages, and costimulates anti‐µ‐primed splenic B cells. Eur J Immunol. 1994;24:367‐374. [DOI] [PubMed] [Google Scholar]
- 22. Langstein J, Schwarz H. Identification of CD137 as a potent monocyte survival factor. J Leukoc Biol. 1999;65:829‐833. [DOI] [PubMed] [Google Scholar]
- 23. Drenkard D, Becke FM, Langstein J, et al. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB J. 2007;21:456‐463. [DOI] [PubMed] [Google Scholar]
- 24. Langstein J, Michel J, Fritsche J, et al. CD137 (ILA/4‐1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol. 1998;160:2488‐2494. [PubMed] [Google Scholar]
- 25. Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4–1BB T‐cell activation molecule eradicate established tumors. Nat Med. 1997;3:682‐685. [DOI] [PubMed] [Google Scholar]
- 26. Ju SA, Lee SC, Kwon TH, et al. Immunity to melanoma mediated by 4–1BB is associated with enhanced activity of tumour‐infiltrating lymphocytes. Immunol Cell Biol. 2005;83:344‐351. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez‐Chacon JA, Li Y, Wu RC, et al. Costimulation through the CD137/4‐1BB pathway protects human melanoma tumor‐infiltrating lymphocytes from activation‐induced cell death and enhances antitumor effector function. J Immunother. 2011;34:236‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sin JI, Kim H, Ahn E, et al. Combined stimulation of TLR9 and 41BB augments Trp2 peptide vaccine‐mediated melanoma rejection by increasing Ag‐specific CTL activity and infiltration into tumor sites. Cancer Lett. 2013;330:190‐199. [DOI] [PubMed] [Google Scholar]
- 29. Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JA, Averbook BJ, Chambers K, et al. Divergent effects of 4–1BB antibodies on antitumor immunity and on tumor‐reactive T‐cell generation. Cancer Res. 2001;61:2031‐2037. [PubMed] [Google Scholar]
- 31. Miller RE, Jones J, Le T, et al. 4–1BB‐specific monoclonal antibody promotes the generation of tumor‐specific immune responses by direct activation of CD8 T cells in a CD40‐dependent manner. J Immunol. 2002;169:1792‐1800. [DOI] [PubMed] [Google Scholar]
- 32. Murillo O, Dubrot J, Palazon A, et al. In vivo depletion of DC impairs the anti‐tumor effect of agonistic anti‐CD137 mAb. Eur J Immunol. 2009;39:2424‐2436. [DOI] [PubMed] [Google Scholar]
- 33. Guo Z, Cheng D, Xia Z, et al. Combined TIM‐3 blockade and CD137 activation affords the long‐term protection in a murine model of ovarian cancer. J Transl Med. 2013;11:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Houot R, Goldstein MJ, Kohrt HE, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431‐3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Narazaki H, Zhu Y, Luo L, et al. CD137 agonist antibody prevents cancer recurrence: Contribution of CD137 on both hematopoietic and nonhematopoietic cells. Blood. 2010;115:1941‐1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palazón A, Teijeira A, Martínez‐Forero I, et al. Agonist anti‐CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71:801‐811. [DOI] [PubMed] [Google Scholar]
- 37. Zhu BQ, Ju SW, Shu YQ. CD137 enhances cytotoxicity of CD3(+)CD56(+) cells and their capacities to induce CD4(+) Th1 responses. Biomed Pharmacother. 2009;63:509‐516. [DOI] [PubMed] [Google Scholar]
- 38. Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4–1BB antibody (BMS‐469492) treatment. Anticancer Res. 2006;26:3445‐3453. [PubMed] [Google Scholar]
- 39. Wilcox RA, Files DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yonezawa A, Dutt S, Chester C, et al. Boosting cancer immunotherapy with anti‐CD137 antibody therapy. Clin Cancer Res. 2015;21:3113‐3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He Y, Rozeboom L, Rivard CJ, et al. PD‐1, PD‐L1 protein expression in non‐small cell lung cancer and their relationship with tumor‐infiltrating lymphocytes. Med Sci Monit. 2017;23:1208‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He Y, Liu S, Mattei J, et al. The combination of anti‐Kir monoclonal antibodies with anti‐PD‐1/PD‐l1 monoclonal antibodies could be a critical breakthrough in overcoming tumor immune escape in NSCLC. Drug Des Devel Ther. 2018;12:981‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He Y, Cao J, Zhao C, et al. TiM‐3, a promising target for cancer immunotherapy. Onco Targets Ther. 2018;11:7005‐7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti‐CTLA‐4, anti‐CD137, and anti‐PD1. J Immunotoxicol. 2012;9:241‐247. [DOI] [PubMed] [Google Scholar]
- 45. Ascierto PA, Kalos M, Schaer DA, et al. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19:1009‐1020. [DOI] [PubMed] [Google Scholar]
- 46. Morales‐Kastresana A, Sanmamed MF, Rodriguez I, et al. Combined immunostimulatory monoclonal antibodies extend survival in an aggressive transgenic hepatocellular carcinoma mouse model. Clin Cancer Res. 2013;19:6151‐6162. [DOI] [PubMed] [Google Scholar]
- 47. Azpilikueta A, Agorreta J, Labiano S, et al. Successful immunotherapy against a transplantable mouse squamous lung carcinoma with anti–PD‐1 and anti‐CD137 monoclonal antibodies. J Thorac Oncol. 2016;11:524‐536. [DOI] [PubMed] [Google Scholar]
- 48. Wei H, Zhao L, Li W, et al. Combinatorial PD‐1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin. PLoS ONE. 2013;8:e84927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dai M, Yip YY, Hellstrom I, et al. Curing mice with large tumors by locally delivering combinations of immunomodulatory antibodies. Clin Cancer Res. 2015;21:1127‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elpek KG, Lacelle C, Singh NP, et al. CD4+CD25+ T regulatory cells dominate multiple immune evasion mechanisms in early, but not late phases of tumor development in a B cell lymphoma model. J Immunol. 2007;178:6840‐6848. [DOI] [PubMed] [Google Scholar]
- 51. Schabowsky RH, Madireddi S, Sharma R, et al. Targeting CD4+CD25+FoxP3+ regulatory T‐cells for the augmentation of cancer immunotherapy. Curr Opin Investig Drugs. 2007;8:1002‐1008. [PubMed] [Google Scholar]
- 52. Sharma RK, Elpek KG, Yolcu ES, et al. Costimulation as a platform for the development of vaccines: a peptide‐based vaccine containing a novel form of 4–1BBL eradicates established tumors. Cancer Res. 2009;69:4319‐4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sonnino S, Mauri L, Chigorno V, et al. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1R‐13R. [DOI] [PubMed] [Google Scholar]
- 54. Brezicka FT, Olling S, Nilsson O, et al. Immunohistological detection of fucosyl‐GM1 ganglioside in human lung cancer and normal tissues with monoclonal antibodies. Cancer Res. 1989;49:1300‐1305. [PubMed] [Google Scholar]
- 55. Brezicka FT, Olling S, Bergman B, et al. Immunohistochemical detection of two small cell lung carcinoma‐ associated antigens defined by MAbs F12 and 123C3 in bronchoscopy biopsy tissues. APMIS. 1991;99:797‐802. [DOI] [PubMed] [Google Scholar]
- 56. Brezicka FT, Holmgren J, Kalies I, et al. Tumor‐cell killing by MAbs against fucosyl GM1, a ganglioside antigen associated with small‐cell lung carcinoma. Int J Cancer. 1991;49:911‐918. [DOI] [PubMed] [Google Scholar]
- 57. Brezicka F, Einbeigi Z, Bergman B. Functional assessment in vitro of human‐complement‐dependent antibody‐induced cytotoxicity of neoplastic cells. Cancer Immunol Immunother. 2000;49:235‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ponath P, Menezes D, Pan C, et al. A novel, fully human anti–fucosyl‐GM1 antibody demonstrates potent in vitro and in vivo antitumor activity in preclinical models of small cell lung cancer. Clin Cancer Res. 2018;24:5178‐5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kohrt HE, Colevas AD, Houot R, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124:2668‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Kohrt HE, Houot R, Goldstein MJ, et al. CD137 stimulation enhances the antilymphoma activity of anti‐CD20 antibodies. Blood. 2011;117:2423‐2432. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61. Kohrt HE, Houot R, Weiskopf K, et al. Stimulation of natural killer cells with a CD137‐specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62. Quetglas JI, Dubrot J, Bezunartea J, et al. Immunotherapeutic synergy between Anti‐CD137 mAb and intratumoral administration of a Cytopathic Semliki forest virus encoding IL‐12. Mol Ther. 2012;20:1664‐1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Squibb B‐M.A study of BMS‐663513 in combination with chemoradiation in Subjects with Non Small Cell Lung Carcinoma (NSCLC). http://clinicaltrials.gov/ct2/show/NCT00461110. Accessed July 20, 2013.
- 64. Qi X, Li F, Wu Y, et al. Optimization of 4–1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat Commun. 2019;10:2141. [DOI] [PMC free article] [PubMed] [Google Scholar]


