Abstract
The long‐term efficacy of nivolumab in esophageal squamous cell carcinoma and its association with disease biomarkers are currently not well known. Therefore, we investigated the association in Japanese patients with treatment‐refractory advanced esophageal cancer who participated in an open‐label, single‐arm, multicenter phase II study. Patients received nivolumab 3 mg/kg i.v. every 2 weeks until disease progression or unacceptable toxicity, and were followed up for 2 years after the initial dosing of the last patient. Archival tissue samples were collected before treatment and analyzed for programmed death ligand‐1 (PD‐L1) and CD8+ status of tumors and tumor‐infiltrating lymphocytes (TILs) and human leukocyte antigen class 1. Efficacy end‐points included objective response rate (ORR), overall survival (OS), progression‐free survival (PFS), time to response, and duration of response. Of 65 enrolled patients (83% male), 64 were evaluable for efficacy and 41 (63%) for biomarkers. The ORR, median OS, and survival rate were 17.2%, 10.78 months, and 17.2%, respectively. Time to response was 1.45 months and duration of response was 11.17 months. The PD‐L1 positivity of tumor cells was possibly associated with better PFS (2.04 vs 1.41 months, cut‐off 1%) and OS (11.33 vs 6.24 months, cut‐off 1%). Median OS was prolonged in patients with a median number of TILs greater than 63.75% vs 63.75% or less (11.33 vs 7.85 months). Nivolumab showed continued long‐term efficacy, as seen by the stability of PFS and OS, in Japanese patients with esophageal squamous cell carcinoma. Further investigation of PD‐L1 tumor expression and TILs as potential biomarkers for predicting patients likely to benefit from nivolumab therapy is warranted.
Keywords: CD8+ tumor‐infiltrating lymphocyte, esophageal squamous cell carcinoma, long‐term survival, nivolumab, programmed death‐1
An exploratory biomarker analysis was undertaken in Japanese patients with treatment‐refractory advanced esophageal cancer who were receiving nivolumab during the extension of a multicenter phase II study. Nivolumab showed continued efficacy in Japanese patients with esophageal squamous cell carcinoma, and the biomarker analysis suggested that higher levels of tumor‐infiltrating lymphocytes, especially CD8+ cells, could be associated with longer overall survival.

Abbreviations
- CI
confidence interval
- CPS
Combined Positive Score
- DFS
disease‐free survival
- HLA
human leukocyte antigen
- MSI
microsatellite instability
- NSCLC
non‐small‐cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PD‐1
programmed death‐1
- PD‐L1
programmed death ligand‐1
- PFS
progression‐free survival
- PS
performance status
- TIL
tumor‐infiltrating lymphocyte
- TMB
tumor mutation burden
1. INTRODUCTION
Immune checkpoint blockade has radically changed the treatment of certain cancers. 1 , 2 , 3 The PD‐1 pathway is critical to the regulation of host defenses aimed at eradicating tumors and has been implicated in immune system evasion by tumors. 4 , 5 Since the development of immunotherapy for cancer treatment, efforts have been directed towards the identification of biomarkers that can be used to predict response to therapy. Programmed death ligand‐1, CD8+ TILs, and HLA class 1 are common biomarkers that have been linked to outcomes with oncological immunotherapies. 6 In other tumor types, expression levels of PD‐L1 have been linked to OS, DFS, treatment efficacy, and treatment outcomes. 7 , 8 , 9 CD8+ TILs have been associated with outcomes, 10 , 11 and HLA class 1 expression has been linked to treatment efficacy, relapse‐free survival, and OS. 12 , 13
Nivolumab is a genetically engineered, fully human IgG4 mAb targeted at human PD‐1. 14 A multicenter, open‐label, uncontrolled, phase II study evaluated the efficacy and safety of nivolumab in 65 Japanese patients with advanced esophageal cancer refractory or intolerant to standard chemotherapy. 15 After a median follow‐up of 10.8 months, central assessment of clinical response revealed an ORR of 17%, with disease control in 42% of patients. 15 Centrally assessed PFS was 1.5 months, and 25% of patients had stable disease. According to investigator assessment, tumor burden and the size of target lesions decreased in 45% of patients. 15 These results suggested that nivolumab prolonged long‐term survival in these patients.
The long‐term efficacy of nivolumab in the treatment of esophageal cancer refractory or intolerant to standard chemotherapy in Japanese patients was further assessed for 2 years after the initial dosing of the last patient. This paper presents an update of the efficacy results obtained 2 years after the initial dosing of the last patient, and the results of a subgroup analysis investigating associations between the activity of nivolumab and the biomarkers PD‐L1, CD8+ TILs, and HLA class 1.
2. MATERIALS AND METHODS
2.1. Study design and patients
Details of the study design and patients enrolled in the study have been published previously. 15 Briefly, eligible patients were: (i) 20 years of age or older and had esophageal cancer, with the major lesion (either unresected or resected) located in the cervical or thoracic esophagus and pathologically proven to be of squamous cell carcinoma, adenosquamous cell carcinoma, or adenocarcinoma histology; (ii) refractory or intolerant to fluoropyrimidine‐, platinum‐, and taxane‐based chemotherapy; and (iii) not eligible for radical resection. Patients had an ECOG PS of 0‐1, a life expectancy of at least 90 days, and adequate organ function. Patients with apparent tumor invasion to adjacent organs, symptomatic brain metastases, or multiple primary cancers were excluded. Also excluded were patients with a current or past history of chronic or recurrent autoimmune disease, interstitial lung disease or pulmonary fibrosis, or diverticulitis or symptomatic gastrointestinal ulcerative disease. 15
All patients provided written informed consent, and the study protocol was reviewed by the institutional review board of each study site prior to study initiation. 15 Additional patient consent was required for participation in the biomarker analysis. The study was carried out in compliance with the ethical principles that have their origins in the Declaration of Helsinki.
2.2. Treatment
Patients received nivolumab 3 mg/kg as a 60‐minute i.v. infusion every 2 weeks in 6 weekly cycles (ie, 3 doses of nivolumab per cycle), until disease progression or unacceptable toxicity. 15
2.3. Efficacy end‐points
Efficacy outcomes for this study have been previously defined. 15 Briefly, the primary end‐point was centrally assessed ORR, defined as the proportion of patients with a best objective response of complete or partial response. Secondary end‐points included OS, ORR (assessed by investigator), PFS, change in tumor burden, time to response, time to disease progression, and duration of response.
2.4. Biomarker analysis
Patients who provided written consent were scheduled for tumor tissue collection during the screening phase and 28 days after the end of the treatment phase following completion of efficacy evaluations. Analyses were undertaken centrally, PD‐L1 by MOSAIC Laboratories, and CD8+ and HLA class 1 by LSI Medience.
Tumor expression of PD‐L1, CD8+, and HLA class 1 in human tissues was assessed in formalin‐fixed paraffin‐embedded tumor sections using immunohistochemistry. A single pathologist assessed PD‐L1 tumor expression. The PD‐L1 staining was carried out using the PD‐L1 IHC 28‐8 pharmDX (Dako/Agilent Technologies, Santa Clara, CA) according to the manufacturer’s instructions with the Dako Autostainer Link‐48 system (Dako/Agilent Technologies, Santa Clara, CA.). The percentage of positive cells was calculated, and cut‐off values of 1%, 5%, and 10% of stained cells were used to define PD‐L1 positivity after due consideration.
The CD8+, HLA class 1, and negative control staining were undertaken using 3 slides per sample. Positive control tissue was established for each and used to confirm a satisfactory staining process. Monoclonal mouse anti‐human Ab (clone, C8/144B; isotype, IgG1 kappa; Agilent Technologies) was used for staining CD8+ cells. Staining of HLA class 1 using anti‐HLA class I primary Ab (HLA‐A, B, and C, mouse IgG1; Medical & Biological Laboratories) and negative control was undertaken with a pretreatment system and automatic staining system of the Autostainer Link 48 (Dako/Agilent Technologies). The number of CD8+ T cells was counted, as well as the number of TILs. For HLA class 1 expression, the number of infiltrated lymphocytes in tumor cells was identified using H&E staining (40× objective magnification) and the positive rate of expression (%) and cellular localization were determined. Positive rate was calculated using the following formula: positive rate (%) = mean number of positive cells / mean total number of tumor cells × 100. The method used to analyze data for CD8+ expression was as follows: patients were stratified according to the median number of TILs (63.75% or less vs greater than 63.75%) and each of these groups was further dichotomized according to CD8+ expression (less than 50% vs 50% or more).
2.5. Statistical analysis
The full analysis set was defined as all patients who received at least 1 dose of nivolumab; the biomarker analysis set included all patients from the full analysis set who gave their consent to participate in the biomarker analyses. The primary and secondary end‐points were assessed in patients who received at least 1 dose of nivolumab and had 1 or more central assessments of tumor response. 15 For the efficacy analysis, OS and PFS were analyzed using Kaplan‐Meier curves and estimated median values with 95% CIs. The data cut‐off used for the main paper was 17 May 2015 (and 17 November 2015 for OS) 15 ; the data cut‐off for this analysis was 17 November 2016, 2 years after the initial dosing of the last patient.
The biomarker analysis examined the relationships between PD‐L1 expression levels (1%, 5%, and 10%), CD8+ TILs, and HLA class 1 tumor expression levels and the efficacy of nivolumab (ORR, time to response, duration of response, PFS, and OS). Except for OS, all the efficacy variables used in these analyses were centrally assessed. The Kaplan‐Meier method was used to estimate median values, Cox proportional hazards model was used to calculate hazard ratios, and 95% CIs were calculated using the Wilson method.
Overall survival was defined as the time from the first dose of nivolumab to death from any cause. Progression‐free survival was defined as the time from the first dose of nivolumab to disease progression or death from any cause. Clinical response was assessed using computed tomography scan or other imaging every 6 weeks according to RECIST version 1.1.
3. RESULTS
3.1. Patient characteristics
The baseline characteristics for this patient cohort have been previously reported. 15 Briefly, the group was predominantly male (83%) with an ECOG PS of 1 (55.4%). All patients were diagnosed with recurrent and metastatic esophageal cancer and 68% of patients (n = 44) received 3 or more prior treatment regimens.
3.2. Biomarker population
Of the 65 patients originally enrolled in the study, 41 consented to participation in the exploratory biomarker substudy. One patient was excluded from the efficacy analysis due to having multiple primary cancers. In addition, 3 patients were unevaluable for PD‐L1 expression analysis and 2 patients were unevaluable for CD8. No differences in HLA class 1 expression were observed between patients; therefore, HLA class 1 expression could not be used for efficacy assessment.
The majority of the 41 patients in the biomarker substudy group were male (85.4%; median age, 64 years) with an ECOG PS of 1 (63.4%; Table 1). All patients (n = 41) had squamous cell carcinoma and had received prior treatment regimens, 63.4% of patients (n = 26) received prior radiation therapy (Table 1).
Table 1.
Baseline characteristics of Japanese esophageal cancer patients treated with nivolumab who underwent biomarker analysis
| Characteristic | (N = 41) |
|---|---|
| Median age (range), years | 64.0 (50‐80) |
| Gender, n (%) | |
| Male | 35 (85.4) |
| Female | 6 (14.6) |
| ECOG performance status, n (%) | |
| 0 | 15 (36.6) |
| 1 | 26 (63.4) |
| Histological type a , n (%) | |
| Squamous cell carcinoma | 41 (100.0) |
| Prior treatment, n (%) | |
| Surgery | 27 (65.9) |
| Radiation therapy | 26 (63.4) |
| Prior treatment regimens, n (%) | |
| ≤2 | 12 (29.3) |
| 3 | 17 (41.5) |
| ≥4 | 12 (29.3) |
Based on the results at diagnosis.
3.3. Efficacy results obtained 2 years after the initial dosing of the last patient
The efficacy results which were obtained 2 years after the initial dosing of the last patient showed an ORR of 17.2% (Table S1). The Kaplan‐Meier curves showed that 2‐year PFS and OS were achieved by 8.6% and 17.2% of patients, respectively. Median PFS was 1.51 months, and median OS was 10.78 months (Figure S1).
Investigator assessment of tumor burden showed that generally the tumor burden at 1 year was maintained at 2 years, which showed continuation of long‐term efficacy (durable response and stabilization of target lesion) in the treatment of esophageal squamous cell carcinoma (Figure 1). Centrally assessed time to response was a median of 1.45 months (minimum, 1.4; maximum, 3.0), and the centrally assessed duration of response was a median of 11.17 months (95% CI, 3.02; not estimable).
Figure 1.
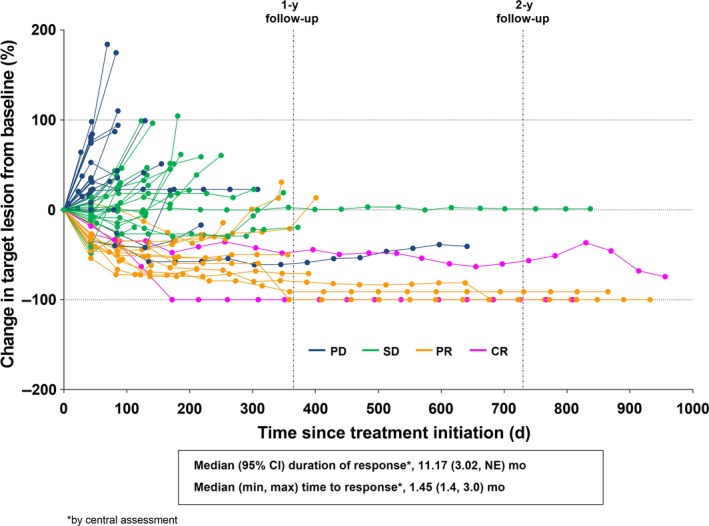
Change in investigator‐assessed tumor burden in Japanese esophageal cancer patients (n = 64) treated with nivolumab for up to 2 years after the initial dosing of the last patient. CI, confidence interval; max, maximum; min, minimum; NE, not evaluable
3.4. Correlation of PD‐L1 tumor expression and nivolumab efficacy
A numerically higher ORR and a trend towards longer PFS in PD‐L1‐positive patients was found using all expression cut‐off values compared with PD‐L1‐negative tumors (Table 2, Figure S2). A similar trend was seen with OS (Table 2, Figures 2 and ). In terms of best overall response, a higher proportion of PD‐L1‐positive patients had a CR or PR compared with PD‐L1‐negative patients. Time to response results for a small number of responding patients (n = 2‐5) were numerically shorter in negative patients compared with positive patients at all PD‐L1 cut‐off values (Table 2). Similarly, there was a numerically longer duration of response in negative patients vs positive patients, with the exception of the response in 10% cut‐off (Table 2), although subgroups of patients in these analyses were also small (n = 9‐28).
Table 2.
Efficacy of nivolumab according to programmed death ligand‐1 (PD‐L1) expression in the biomarker cohort of Japanese esophageal cancer patients a
| PD‐L1 cut‐off 1% | PD‐L1 cut‐off 5% | PD‐L1 cut‐off 10% | ||||
|---|---|---|---|---|---|---|
| Positive (n = 21) | Negative (n = 16) | Positive (n = 13) | Negative (n = 24) | Positive (n = 9) | Negative (n = 28) | |
| Progression‐free survival | ||||||
| Median, mo | 2.04 | 1.41 | 2.96 | 1.41 | 2.96 | 1.41 |
| HR (95% CI) | 0.67 (0.33, 1.37) | 0.50 (0.24, 1.05) | 0.49 (0.21, 1.15) | |||
| Overall survival | ||||||
| Median, mo | 11.33 | 6.24 | 12.12 | 7.36 | 9.53 | 8.95 |
| HR (95% CI) | 0.84 (0.41, 1.73) | 0.81 (0.39, 1.71) | 0.87 (0.37, 2.03) | |||
| Best overall response, n (%) | ||||||
| CR | 2 (9.5) | 0 (0.0) | 2 (15.4) | 0 (0.0) | 2 (22.2) | 0 (0.0) |
| PR | 3 (14.3) | 2 (12.5) | 3 (23.1) | 2 (8.3) | 1 (11.1) | 4 (14.3) |
| SD | 5 (23.8) | 0 (0.0) | 2 (15.4) | 3 (12.5) | 2 (22.2) | 3 (10.7) |
| PD | 10 (47.6) | 12 (75.0) | 5 (38.5) | 17 (70.8) | 3 (33.3) | 19 (67.9) |
| NE | 1 (4.8) | 2 (12.5) | 1 (7.7) | 2 (8.3) | 1 (11.1) | 2 (7.1) |
| Overall response rate, n (%) | ||||||
| ORR (CR + PR) | 5 (23.8) | 2 (12.5) | 5 (38.5) | 2 (8.3) | 3 (33.3) | 4 (14.3) |
| 95% CI | 10.6, 45.1 | 3.5, 36.0 | 17.7, 64.5 | 2.3, 25.8 | 12.1, 64.6 | 5.7, 31.5 |
| (n = 5) | (n = 2) | (n = 5) | (n = 2) | (n = 3) | (n = 4) | |
|---|---|---|---|---|---|---|
| Time to response | ||||||
| Median, mo | 2.76 | 1.43 | 2.76 | 1.43 | 2.79 | 1.43 |
| HR (95% CI) | 0.35 (0.05, 2.63) | 0.35 (0.05, 2.63) | 0.23 (0.02, 2.15) | |||
| Duration of response | ||||||
| Median, mo | 12.55 | NR b | 12.55 | NR b | NR b | 11.43 |
| HR (95% CI) | 1.40 (0.14, 13.66) | 1.40 (0.14, 13.66) | 0.39 (0.04, 3.81) | |||
Abbreviations: CI, confidence interval; CR, complete response; HR, hazard ratio; NE, not evaluable; NR, not reached; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Progression‐free survival, best overall response, time to response, and duration of response were centrally assessed.
Not estimable due to censoring.
Figure 2.
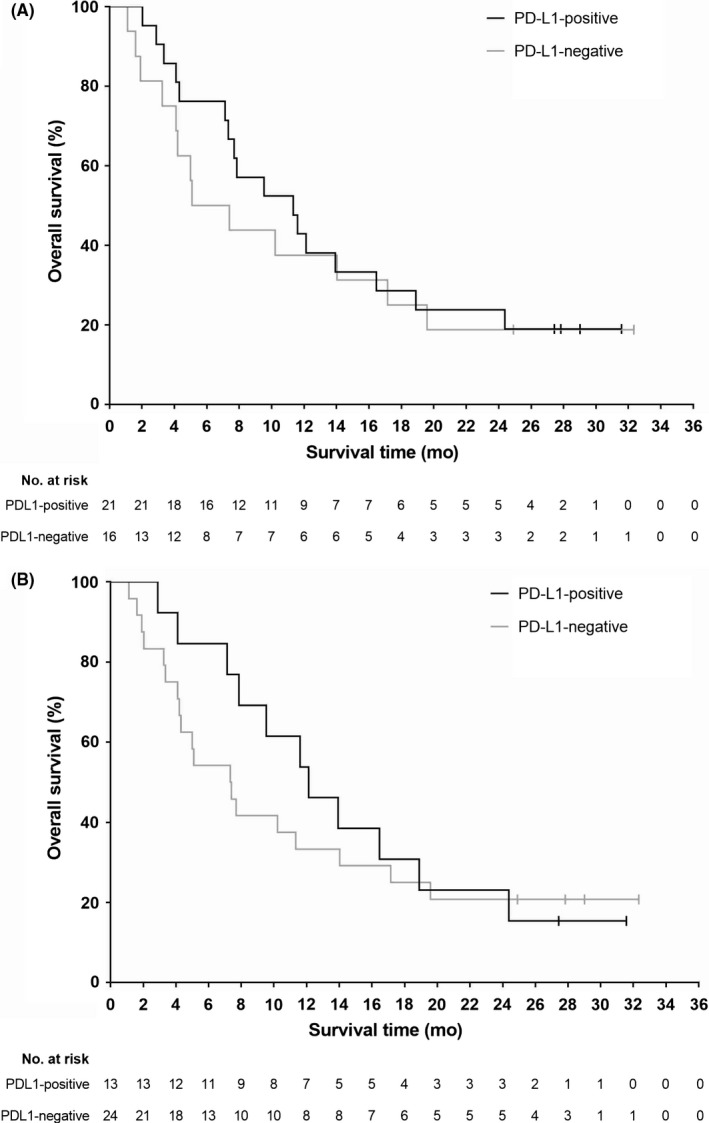
Kaplan‐Meier curves for overall survival in Japanese esophageal cancer patients treated with nivolumab and participating in the biomarker analysis, according to programmed death‐ligand 1 (PD‐L1) expression cut‐off values of (A) 1% and (B) 5%
3.5. Correlation of CD8+ TILs and nivolumab efficacy
The mean (SD) number of TILs was 68.04 (50.68) and the median (range) was 63.75 (6.5−208.0). A numerically higher ORR was shown in patients with more than 63.75% vs 63.75% or fewer TILs (Table 3). The median OS was numerically longer in patients with more than 63.75% vs 63.75% or fewer TILs (Table 3), but no differences in PFS or best overall response were detected (Table 3, Figure S4). Patients with more than 50% CD8+ TILs had a numerically prolonged OS vs those with 50% or fewer CD8+ TILs, irrespective of the number of TILs (Table 3).
Table 3.
Efficacy of nivolumab according to the presence of tumor‐infiltrating lymphocytes (TILs) and CD8 expression in the biomarker population of Japanese patients with esophageal cancer treated with nivolumab a
| Nivolumab (N = 38) | ||
|---|---|---|
|
>63.75% TILs (n = 19) |
≤63.75% TILs (n = 19) | |
| Progression‐free survival | ||
| Median, mo | 1.45 | 1.45 |
| HR (95% CI) | 1.01 (0.50, 2.02) | |
| Overall survival | ||
| Median, mo | 11.33 | 7.85 |
| HR (95% CI) | 0.94 (0.46, 1.93) | |
| Best overall response, n (%) | ||
| CR | 1 (5.3) | 1 (5.3) |
| PR | 3 (15.8) | 2 (10.5) |
| SD | 4 (21.1) | 1 (5.3) |
| PD | 10 (52.6) | 12 (63.2) |
| Unevaluable | 1 (5.3) | 3 (15.8) |
| (n = 4) | (n = 3) | |
|---|---|---|
| Time to response | ||
| Median, mo | 1.41 | 2.79 |
| HR (95% CI) | 6.29 (0.67, 58.70) | |
| Overall response rate, n (%) | ||
| ORR (CR + PR) | 4 (21.1) | 3 (15.8) |
| 95% CI | 8.5, 43.3 | 5.5, 37.6 |
| Duration of response | ||
| Median, mo | 11.43 | NR |
| HR (95% CI) | 2.54 (0.26, 24.61) | |
| >50% CD8+ (n = 11) | ≤50% CD8+ (n = 8) | >50% CD8+ (n = 7) | ≤50% CD8+ (n = 12) | |
|---|---|---|---|---|
| Progression‐free survival | ||||
| Median, mo | 1.45 | 2.18 | 1.41 | 1.45 |
| HR (95% CI) | 0.80 (0.30, 2.12) | 1.22 (0.43, 3.48) | ||
| Overall survival | ||||
| Median, mo | 12.12 | 8.43 | 11.60 | 7.77 |
| HR (95% CI) | 1.07 (0.38, 3.03) | 1.03 (0.37, 2.91) | ||
| Best overall response, n (%) | ||||
| CR | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (8.3) |
| PR | 1 (9.1) | 2 (25.0) | 1 (14.3) | 1 (8.3) |
| SD | 2 (18.2) | 2 (25.0) | 0 (0.0) | 1 (8.3) |
| PD | 6 (54.5) | 4 (50.0) | 5 (71.4) | 7 (58.3) |
| Unevaluable | 1 (9.1) | 0 (0.0) | 1 (14.3) | 2 (16.7) |
| Overall response rate, n (%) | ||||
| ORR (CR + PR) | 2 (18.2) | 2 (25.0) | 1 (14.3) | 2 (16.7) |
| 95% CI | 5.1, 47.7 | 7.1, 59.1 | 2.6, 51.3 | 4.7, 44.8 |
Abbreviations: CI, confidence interval; CR, complete response; HR, hazard ratio; NR, not reached; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Progression‐free survival, best overall response, time to response, and duration of response were centrally assessed.
4. DISCUSSION
These study results, obtained 2 years after the initial dosing of the last patient, showed that the use of nivolumab monotherapy for the treatment of Japanese patients with esophageal cancer was associated with continued clinical activity. Our results support the hypothesis that PD‐L1 tumor expression with a threshold of 1% and 5%, and the presence of higher levels of TILs, could be predictive of OS in Japanese patients with esophageal cancer and treated with nivolumab, although further research is warranted.
Long‐term use of nivolumab has been investigated in other tumor types, and the results of those studies are similar to the present study. In a phase II study of 76 Japanese patients with NSCLC receiving nivolumab for approximately 3 years, median OS was 17.1 months, median PFS was 2.8 months, and median time to response was 1.4 months; investigator‐assessed CR and PR occurred in 1.3% and 23.7% of patients. 16 Similarly, in the 3‐year follow‐up studies of nivolumab (3 mg/kg every 2 weeks) in patients with stage IIIB/IV squamous or nonsquamous NSCLC with disease recurrence/progression during or after prior platinum‐based chemotherapy (CheckMate 017 and CheckMate 057), the median OS and PFS and the ORR in the pooled population were 11.1 months, 2.6 months, and 19%, respectively. 17 Another study of patients with heavily pretreated advanced NSCLC receiving nivolumab for a median follow‐up time of 39 months reported a duration of response of 17 months. 18
Regarding the relationship between PD‐L1 expression and nivolumab efficacy, many studies have reported relationships between treatment benefit with nivolumab and PD‐L1 expression levels. 16 , 19 , 20 One study, using a 5% expression threshold, showed the potential of pretreatment PD‐L1 tumor expression as a predictive marker to indicate which patients would benefit from treatment. 19 A second phase I study, also using a 5% expression threshold, found a higher ORR in patients with PD‐L1‐positive melanoma, but also reported clinical responses to nivolumab in patients with PD‐L1‐negative melanoma. 20 The phase II study in Japanese patients with NSCLC discussed above showed that higher PD‐L1 expression levels led to a greater ORR than low expression levels at all cut‐offs tested. 16 In the present study, the PD‐L1 biomarker results provided some evidence that PD‐L1 positivity might be a marker of good response regardless of the expression cut‐off chosen, with a higher proportion of patients having a CR or PR and a lower proportion of patients having progressive disease following nivolumab treatment, although these results need further corroboration in a controlled clinical trial. Some patients with PD‐L1‐negative tumors did respond to nivolumab, albeit to a lesser extent than those with PD‐L1‐positive tumors. Studies investigating the relationship between response to treatment with other PD‐1/PD‐L1‐targeted agents have generally shown that the presence of PD‐L1 expression is related to response to treatment 21 , 22 , 23 , 24 ; this relationship needs to be explored further with nivolumab.
In the present study, irrespective of the number of TILs, patients with more than 50% CD8+ TILs had numerically longer median OS, compared with those with 50% or fewer CD8+ TILs; in other studies with various tumor types, CD8+ TILs have also been suggested as a good prognostic indicator. 25 , 26 , 27 , 28 , 29 , 30 , 31 Similar to the present study, patients with basal‐like breast cancer showed that those with CD8+ TILs survived 3.5 years longer than those who did not 31 in other studies of patients with breast cancer, the presence of TILs was prognostic for both DFS and OS 28 and event‐free survival. 29 Similarly, patients with rectal cancer showed a positive association between the number of CD8+ cells in tumor tissue and DFS and OS. 26 In the present study there was no relationship between CD8+ TILs and PFS. Although a numerically longer median OS was noted in PD‐L1‐positive vs PD‐L1‐negative patients (cut‐off 1% and 5%) among patients with TILs greater than 63.75%, the number of patients with available tumor tissue was small (n = 37), and therefore, we cannot definitively conclude that patients’ prognosis can be predicted by associating scores of TIL with PD‐L1 expression (Table S2). Further investigations are required to determine the relationship between CD8+ TILs and outcomes in a larger population of patients with esophageal cancer.
Predictive biomarkers of nivolumab efficacy other than PD‐L1 were recently reported. 32 , 33 Tumor mutation burden and MSI were shown to correlate with response to immune checkpoint inhibitors. Higher rates of TMB and MSI‐high status could induce the expression of immunogenic neoantigen, which leads to response to immune checkpoint inhibitor therapy. Although TMB and MSI status were unknown in our study, these biomarkers could influence nivolumab efficacy, rather than PD‐L1 expression and CD8+ infiltration. Further investigation of the biomarkers of nivolumab efficacy is warranted.
The main limitation of this analysis was the small size of the population examined, and the limited number of tumor specimens obtained. The majority of samples were obtained from surgical specimens, which were collected before the initiation of nivolumab; therefore, due to the changeable nature of biomarkers, the analysis of PD‐L1 and CD8+ might not reflect the status at nivolumab treatment. Recently, CPS, defined as PD‐L1 positivity in both tumor cells and TILs, has been used for the enrichment of patients who respond to immune therapy. In this study, analysis using CPS data was not planned. Although there could be a relationship between PD‐L1 and TILs and survival outcomes, firm conclusions could not be drawn due to the small number of patients and lack of statistical power. An ongoing phase III study of nivolumab in esophageal cancer is expected to confirm the present exploratory results (ClinicalTrials.gov identifier NCT02569242).
In conclusion, long‐term results with nivolumab were revealed to have continued efficacy in Japanese patients with esophageal squamous cell carcinoma. This was the first study to evaluate PD‐L1 tumor expression with a threshold of 1% and 5% as a positive biomarker for nivolumab therapy in this patient cohort and to show that higher levels of TILs, in particular CD8+ TILs, were associated with numerically longer OS. Although definite conclusions could not be drawn due to the limited number of tumor specimens analyzed, these results suggest that PD‐L1 tumor expression and TILs could be used to select patients most likely to benefit from nivolumab therapy. Further investigations into the relationship of nivolumab and these biomarkers will be undertaken as part of its ongoing phase III study.
CONFLICT OF INTERESTS
KK has received honoraria and research funds from Ono Pharmaceutical, honoraria from Bristol‐Myers Squibb and Eli Lilly, and research funds from Chugai Pharmaceutical, MSD, PFDeNA, and Shionogi. YD has received research funds from Ono Pharmaceutical. YH has received honoraria and research funds from Ono Pharmaceutical. TK has received research funds and remuneration from Shionogi, and research funds from Amgen Astellas BioPharma, Chugai Pharmaceutical, MSD, Oncolys BioPharma, Ono Pharmaceutical, and Parexel International. SH has received honoraria from Bristol‐Myers Squibb, Eli Lilly, Ono Pharmaceutical, and Taiho Pharmaceutical. HH has received honoraria and research funds from Eli Lilly and Ono Pharmaceutical, and research funds from AstraZeneca, BeiGene, Boehringer Ingelheim, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Incyte, LSK BioPharma, Merck Serono, MSD, Sumitomo Dainippon Pharma, and Taiho Pharmaceutical. TK has received research funds from Ono Pharmaceutical. SI has received honoraria from Eli Lilly and Taiho Pharmaceutical, and research funds from Bayer, Bristol‐Myers Squibb, Daiichi Sankyo, and Eli Lilly. KM has received honoraria and research funds from Sanofi, and honoraria from Bayer, Bristol‐Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Taiho Pharmaceutical, and Takeda Pharmaceutical, and research funds from Daiichi Sankyo, Mediscience Planning, Merck Serono, MSD, Parexel International, Pfizer, Shionogi, Solasia Pharma, and Sumitomo Dainippon Pharma. KM has received research funds from Ono Pharmaceutical. KY has received honoraria and research funds from Chugai Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical, Taiho Pharmaceutical, and Yakurt Honsha, honoraria from Bristol‐Myers Squibb, Eli Lilly, Merck Serono, and Takeda Pharmaceutical, and research funds from Boehringer Ingelheim, Eisai, Gilead Sciences, MSD, and Sumitomo Dainippon Pharma. AO has received research funds from Bristol‐Myers Squibb. Tomoko Ohtsu (wife of Atsushi Ohtsu) has received advisory fees from Celgene. YK has received advisory fees from Ageo Central General Hospital, HIRATA Clinic, and Medical Corporation Keiyoukai Keiai Clinic, honoraria and scholarships from Asahi Kasei, Chugai Pharmaceutical, Ono Pharmaceutical, and Taiho Pharmaceutical, honoraria from Ethicon, and scholarships from Daiichi Sankyo, Eisai, Medicon, Otsuka Pharmaceutical, Otsuka Pharmaceutical Factory, Pfizer, Shionogi, Takeda Pharmaceutical, and Tsumura. TU, UU, and HY have no potential conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and their families, and investigators and staff members participating in this study. Medical writing assistance for the preparation of the outline and first draft of this manuscript was provided by Sheridan Henness, PhD, of inScience Communications, Springer Healthcare. This assistance was funded by Ono Pharmaceuticals and Bristol‐Myers Squibb. This study was funded by Ono Pharmaceuticals and Bristol‐Myers Squibb.
Kato K, Doki Y, Ura T, et al. Long‐term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci. 2020;111:1676–1684. 10.1111/cas.14380
REFERENCES
- 1. Tsai HF, Hsu PN. Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J Biomed Sci. 2017;24:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emens LA, Silverstein SC, Khleif S, Marincola FM, Galon J. Toward integrative cancer immunotherapy: targeting the tumor microenvironment. J Transl Med. 2012;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J Gastrointest Oncol. 2015;6:561‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813‐824. [DOI] [PubMed] [Google Scholar]
- 6. Mehnert JM, Monjazeb AM, Beerthuijzen JMT, Collyar D, Rubinstein L, Harris LN. The challenge for development of valuable immuno‐oncology biomarkers. Clin Cancer Res. 2017;23:4970‐4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang K, Qu Y, Dai B, et al. PD‐L1 expression in Xp11.2 translocation renal cell carcinoma: Indicator of tumor aggressiveness. Sci Rep. 2017;7:2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu ZH, Zheng FF, Mao YL, et al. Effects of programmed death‐ligand 1 expression on OK‐432 immunotherapy following transurethral resection in non‐muscle invasive bladder cancer. Oncol Lett. 2017;13:4818‐4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Que Y, Xiao W, Guan YX, et al. PD‐L1 expression is associated with FOXP3+ regulatory T‐Cell infiltration of soft tissue sarcoma and poor patient prognosis. J Cancer. 2017;8:2018‐2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2‐mutation status with neoantigen load, number of tumor‐infiltrating lymphocytes and expression of PD‐1/PD‐L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587‐13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor‐infiltrating lymphocytes predict response to anthracycline‐based chemotherapy in estrogen receptor‐negative breast cancer. Breast Cancer Res. 2011;13:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carson WE 3rd, Unger JM, Sosman JA, et al. Adjuvant vaccine immunotherapy of resected, clinically node‐negative melanoma: long‐term outcome and impact of HLA class I antigen expression on overall survival. Cancer Immunol Res. 2014;2:981‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitamura H, Torigoe T, Honma I, et al. Effect of human leukocyte antigen class I expression of tumor cells on outcome of intravesical instillation of bacillus calmette‐guerin immunotherapy for bladder cancer. Clin Cancer Res. 2006;12:4641‐4644. [DOI] [PubMed] [Google Scholar]
- 14. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167‐3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous‐cell carcinoma: an open‐label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631‐639. [DOI] [PubMed] [Google Scholar]
- 16. Nishio M, Hida T, Atagi S, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non‐squamous non‐small cell lung cancer. ESMO Open. 2016;1:e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non‐small‐cell lung cancer (CheckMate 017 and CheckMate 057): 3‐year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29:959‐965. [DOI] [PubMed] [Google Scholar]
- 18. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long‐term safety of nivolumab (anti‐programmed death 1 antibody, BMS‐936558, ONO‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol. 2015;33:2004‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grosso J, Horak CE, Inzunza D, et al. Association of tumor PD‐L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti‐PD‐1; BMS‐936558; ONO‐4538). J Clin Oncol. 2013;31:abs 3016. [Google Scholar]
- 21. Daud AI, Hamid O, Ribas A, et al. Antitumor activity of the anti‐PD‐1 monoclonal antibody MK‐3475 in melanoma(MEL): Correlation of tumor PD‐L1 expression with outcome. Cancer Res. 2014;74:CT104. [Google Scholar]
- 22. Gandhi L, Balmanoukian A, Hui R, et al. MK‐3475 (anti‐PD‐1 monoclonal antibody) for non‐small cell lung cancer (NSCLC): antitumor activity and association with tumor PD‐L1 expression. Cancer Res. 2014;74:CT105. [Google Scholar]
- 23. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7–h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fridman WH, Pages F, Sautes‐Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298‐306. [DOI] [PubMed] [Google Scholar]
- 26. Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891‐1899. [DOI] [PubMed] [Google Scholar]
- 27. Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology. 2011;58:1107‐1116. [DOI] [PubMed] [Google Scholar]
- 28. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor‐infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node‐positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin‐based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860‐867. [DOI] [PubMed] [Google Scholar]
- 29. Salgado R, Denkert C, Campbell C, et al. Tumor‐infiltrating lymphocytes and associations with pathological complete response and event‐free survival in HER2‐positive early‐stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1:448‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perez EA, Ballman KV, Tenner KS, et al. Association of stromal tumor‐infiltrating lymphocytes with recurrence‐free survival in the N9831 adjuvant trial in patients with early‐stage HER2‐positive breast cancer. JAMA Oncol. 2016;2:56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal‐like breast cancer. Breast Cancer Res. 2012;14:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carbone DP, Reck M, Paz‐Ares L, et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med. 2017;376:2415‐2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


