Abstract
This study provides the benchmark statistics on medically treated patients with non–small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) in Japan. Demographic background, treatment, and prognosis were obtained from patients with lung cancer pathologically diagnosed in 2012, who received nonsurgical treatment. Descriptive statistics and their associations with survival were analyzed. In total, 12 320 patients were registered from 314 institutions in Japan. The median age was 70 years, and 73% of the patients were male. The number (%) of stages I, II, III, and IV diseases were 468 (3.8%), 421 (3.4%), 3260 (26.5%), and 8171 (66.3%), respectively. NSCLC and SCLC accounted for 9872 (80.1%) and 2353 (19.1%) patients, respectively. Thoracic radiotherapy‐based therapy, chemotherapy, and palliative care alone were administered to 2572 (20.9%), 7790 (63.2%), and 1952 (15.8%) patients, respectively. Clinical TNM stage was one of the strongest prognostic factors with the 3‐year survival rates of 62.9%, 47.3%, 40.0%, 27.8%, 37.5%, 26.5%, and 18.2% for stages IA, IB, IIA, IIB, IIIA, IIIB, and IV, respectively. Among 6158 patients with NSCLC treated with chemotherapy, the 3‐year survival rate was 33.4% in patients receiving epidermal growth factor receptor‐tyrosine kinase inhibitors (EGFR‐TKIs) at some point in their clinical course, whereas it was 17.4% in patients who did not. The 3‐year survival rate of SCLC was only 15.9%. In conclusion, approximately two‐thirds of the patients were diagnosed as stage IV at the initial diagnosis. Use of EGFR‐TKIs significantly improved the survival of patients with NSCLC.
Keywords: epidermal growth factor receptor, non–small cell lung cancer, small cell lung cancer, TNM stage, tyrosine kinase inhibitors
This study provides the benchmark statistics on medically treated patients with lung cancer in Japan. Here, 3‐year survival rates of patients treated with concurrent chemoradiotherapy (n = 1689), sequential chemoradiotherapy (n = 221), thoracic radiotherapy alone (n = 662), chemotherapy alone (n = 7790) and palliative care alone (n = 1952) were 43.6%, 29.1%, 40.0%, 19.9%, and 0.3.4%, respectively.

1. INTRODUCTION
Lung cancer is the most common cancer worldwide with approximately 1.8 million new cases in 2012, and its incidence continues to increase as a result of population growth and aging.1, 2 Lung cancer is also the leading cause of mortality with 1.6 million patients dying of this disease in 2012,1, 2 because the survival of patients with lung cancer remains very poor. The age‐standardized 5‐year survival rate is in the range 10%‐20% for most countries.3 Furthermore, these basic statistics vary greatly with different regions. Thus, it is crucial to understand the demographics, tumor‐specific backgrounds, treatment, and prognoses by related factors in nationwide registration studies to improve prevention, diagnosis, and treatment approaches for lung cancer in each country.
The three major societies that deal with patients with lung cancer in Japan, the Japan Lung Cancer Society, the Japanese Association for Chest Surgery, and the Japanese Respiratory Society, established the Japanese Joint Committee of Lung Cancer Registry (JJCLCR) in 1994 to perform registry studies on clinicopathologic profiles of lung cancer. This committee has periodically performed retrospective studies every 5 years on patients who had been surgically resected, as well as a study of prospective enrollment and retrospective analyses on medically treated patients in 2002.4, 5, 6, 7 The current study, the sixth lung cancer registry project, is a nationwide prospective follow‐up study aimed at providing the benchmark statistics on nonsurgical patients pathologically diagnosed in 2012.
2. MATERIALS AND METHODS
The committee asked 846 teaching institutions in Japan to join this study, of which 314 (37.1%) participated. This registry was opened on January 1, 2012, and follow‐up survey was closed on April 30, 2016. The participating institutions took part in this registry by accessing a web site established by the JJCLCR, after receiving their identification number (ID), password, and a universal serial bus (USB) flash memory stick that contained software to be used for the registry.
Inclusion criteria of patients were as follows: (1) pathological or cytological diagnosis of any type of lung cancer at a participating institution; (2) diagnosis obtained from January 1 to December 31 in 2012; and (3) receiving nonsurgical treatment including supportive care alone. Patients who received palliative surgery for metastatic sites (eg brain metastasis) and those who received salvage surgery after chemoradiotherapy for locally advanced disease were eligible. Patients who developed recurrence after curative primary surgery were excluded. The registered patient information was as follows: (1) demographic background, (a) date of registry, (b) gender, (c) birth month and year, and (d) month and year of diagnosis; (2) general conditions at the diagnosis, (a) smoking status, (b) body weight loss, (c) presence of concomitant lung disease, (d) Eastern Cooperative Oncology Group performance status, (e) serum calcium, and (f) serum albumin; (3) tumor‐related factors, (a) clinical TNM factors and stage, (b) presence of superior vena cava syndrome, (c) metastatic sites, (d) histology, and (e) epidermal growth factor receptor (EGFR) mutation status; (4) therapeutic factors, (a) 1st‐line therapy, and (b) 2nd‐line or later line therapy, and; (5) prognostic information, (a) alive or dead, (b) final follow‐up month and year, and (c) cause of death. These items collected for this study were decided and given to every investigator before 2012 so as to obtain the data properly. The clinical TNM factors and stage were classified according to UICC‐TNM 7th edition (2009).8 The histology was classified according to World Health Organization Histological Typing of Lung and Pleural Tumours, 3rd edition (1999). “Large cell neuroendocrine carcinoma” was classified as variant, large cell carcinoma, non–small cell lung cancer (NSCLC).9 Descriptive statistics and their association with survival were analyzed. Survival period was defined as 0.5 plus the number of months between the month of pathological diagnosis and the month of death or the latest month of confirmed survival. Survival curves were estimated according to the Kaplan–Meier method for the clinical subset groups. Differences in survival were tested using the log‐rank method.
This registry study followed the ethical guideline for epidemiologic studies published jointly by the Japan Ministry of Science, Culture, and Education and the Japan Ministry of Health, Labor, and Welfare on June 17, 2002, and was revised on August 16, 2007. The protocol of this study was further amended to follow the ethical guideline for medical studies for humans issued on December 22, 2014. In addition, it was approved by the institutional review board of Osaka University Medical Hospital, where the registry office is located.
3. RESULTS
In total, 14 260 patients were registered from the 314 institutions in Japan. Of these, 1940 patients were excluded because of no data inputted (n = 244), ineligible (n = 1292), stage not confirmed (n = 188), or lost of follow‐up (n = 216). Thus, finally, 12 320 patients were subjects of this study.
Patient characteristics are summarized in Table 1. The median age for all patients was 70 years, and the average was 69.3 years. Seventy‐three percent of the patients were men. Two‐thirds of patients had M1 disease, but distant metastasis (M1b) was noted in less than half of patients. Stages III and IV accounted for the majority of patients, but there were also patients with stage I (3.8%) and stage II (3.5%) diseases. The percentages of small cell lung cancer (SCLC) and NSCLC were 19% and 80%, respectively. Adenocarcinoma accounted for 64% of NSCLC and about a half of all lung malignant tumors. As high as 10% of patients had a poor performance status at the initial diagnosis that give rise to difficulties in treatment; 7.5% and 3.2% of patients had a performance status of 3 and 4, respectively.
TABLE 1.
Patient characteristics
| Characteristics | No. of patients (%) |
|---|---|
| Gender | |
| Male | 8997 (73.0) |
| Female | 3323 (27.0) |
| Age | |
| Median (range) | 70 (22‐102) |
| Mean (standard deviation) | 69.3 (±10.2) |
| Smoking status | |
| Never smoker | 2489 (20.2) |
| Former smoker | 4808 (39.0) |
| Current smoker | 5023 (40.8) |
| TNM stage | |
| T‐factor | |
| TX | 353 (2.9) |
| T0 | 31 (0.3) |
| Tis | 16 (0.3) |
| T1a | 856 (6.9) |
| T1b | 1246 (10.1) |
| T2a | 2678 (21.7) |
| T2b | 1084 (8.8) |
| T3 | 2243 (18.2) |
| T4 | 3813 (30.9) |
| N‐factor | |
| NX | 165 (1.3) |
| N0 | 2056 (16.7) |
| N1 | 1274 (10.3) |
| N2 | 4058 (32.9) |
| N3 | 4767 (38.7) |
| M‐factor | |
| M0 | 4155 (33.7) |
| M1a | 2450 (19.9) |
| M1b | 5715 (46.4) |
| Stage | |
| IA | 261 (2.1) |
| IB | 207 (1.7) |
| IIA | 253 (2.1) |
| IIB | 168 (1.4) |
| IIIA | 1535 (12.5) |
| IIIB | 1725 (14.0) |
| IV | 8171 (66.3) |
| Histology | |
| Non–small cell lung cancer | 9872 (80.1) |
| Adenocarcinoma | 6276 (50.9) |
| Squamous cell carcinoma | 2602 (21.1) |
| Large cell | 165 (1.3) |
| Adenosquamous | 60 (0.5) |
| Not otherwise specified | 632 (5.1) |
| Others | 137 (1.1) |
| Small cell lung cancer | 2353 (19.1) |
| Others | 19 (0.2) |
| Unknown | 76 (0.6) |
| Performance status | |
| 0 | 3783 (30.7) |
| 1 | 5701 (46.3) |
| 2 | 1515 (12.3) |
| 3 | 921 (7.5) |
| 4 | 400 (3.2) |
In total, 2993 (24.3%) patients were treated with palliative care alone as the initial treatment. Of these, 1041 patients successfully received curative‐intent therapy at the second‐line therapy, but the remaining 1952 (15.8%) received palliative care alone through their clinical course. Curative‐intent radiation‐based therapy (concurrent chemoradiotherapy, sequential chemoradiotherapy and thoracic radiotherapy alone) was delivered to 20.9% of patients. Chemotherapy was administered to 63.2% of patients (Table 2).
TABLE 2.
Treatment
| Type of treatment | No. of patients (%) |
|---|---|
| 1st line treatment | |
| Concurrent chemoradiotherapy | 1598 (13.0) |
| Sequential chemoradiotherapy | 200 (1.6) |
| Thoracic radiotherapy | 782 (6.3) |
| Chemotherapy | 6736 (54.7) |
| Palliative care | 2993 (24.3) |
| Others | 11 (0.1) |
| Total | 12 320 (100.0) |
| Chief treatment through all lines | |
| Concurrent chemoradiotherapy | 1689 (13.7) |
| Sequential chemoradiotherapy | 221 (1.8) |
| Thoracic radiotherapy | 662 (5.4) |
| Chemotherapy | 7790 (63.2) |
| NSCLC use of EGFR‐TKIs yes | 2053 (26.4) a |
| NSCLC use of EGFR‐TKIs no | 4105 (52.6) a |
| SCLC use of EGFR‐TKIs no | 1613 (20.7) a |
| SCLC use of EGFR‐TKIs yes | 6 |
| Histology unknown | 13 |
| Palliative care alone | 1952 (15.8) |
| Others | 6 (0.1) |
| Total | 12 320 (100.0) |
Abbreviations: EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; NSCLC, non–small cell lung cancer; SCLC, small cell lung cancer.
The percentage among patients treated with chemotherapy alone.
Association between T‐factor and survival in patients with NSCLC was not clear compared with patients who had been surgically resected; 3‐year survival was 38.2%, 37.1%. 27.9%, 17.9%, 16.4%, and 19.5% for T1a, T1b, T2a, T2b, T3, and T4, respectively. The log‐rank test results were not significantly different between T1a and T1b (P = .359), between T2b and T3 (pa‐0.053), and between T2b and T4 (P = .959). In contrast, N‐factor was significantly associated with survival; 3‐year survival was 36.6%, 27.8%, 22.7%, and 17.7% for N0, N1, N2, and N3, respectively (Table S1). M‐factor also affected significantly on survival; 3‐year survival was 35.0%, 23.3%, and 15.7% for M0, M1a, and M1b, respectively (Table S1 and Figure 1). Clinical TNM stage was one of the strongest prognostic factors in this patient cohort. Three‐y survival was 62.9%, 47.3%, 40.0%, 27.8%, 37.5%, 26.5%, and 18.2% for stages IA, IB, IIA, IIB, IIIA, IIIB, and IV, respectively. Thus, there was no difference in survival between stage IIA and stage IIIA (P = .202), and between stage IIB and stage IIIB (P = .885; Table S1 and Figure 2). A crude survival analysis showed that histological subtypes and EGFR genotypes affected overall survival in patients with NSCLC. A 3‐year survival rate of non‐squamous NSCLC with EGFR mutation was 40%, while that in the other NSCLC subtypes was around 20% (Table S2 and Figure 3). A 3‐year survival rate of SCLC was 15.9%. Performance status was another strong prognostic factor that has been frequently demonstrated in several studies; 3‐year survival of patients with performance status (PS) 0, 1, 2, 3, and 4 were 0.34.3%, 20.1%, 10.2%, 5.6%, and 4.1%, respectively (Table S2).
FIGURE 1.
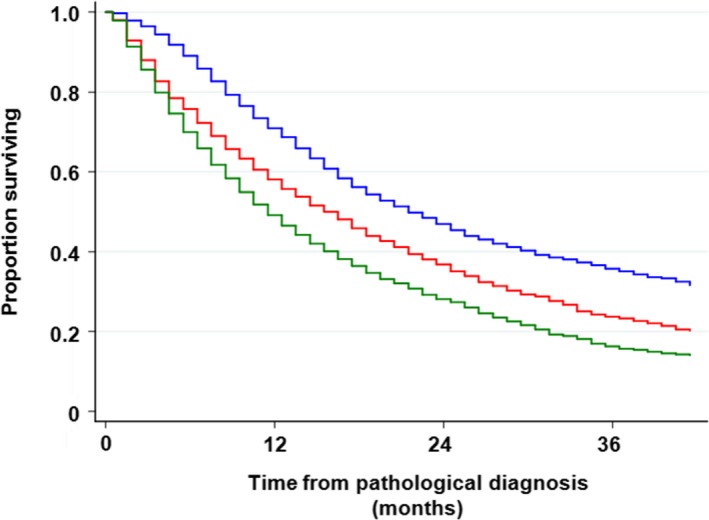
Overall survival rate with regard to clinical M stage. Patients with M1a disease had a significantly better survival rate than those with M1b disease. The 3‐y survival rate was 23.3% vs 15.7%, respectively (P < .001). Blue line, M0; red line, M1a; and dark green line, M1b
FIGURE 2.
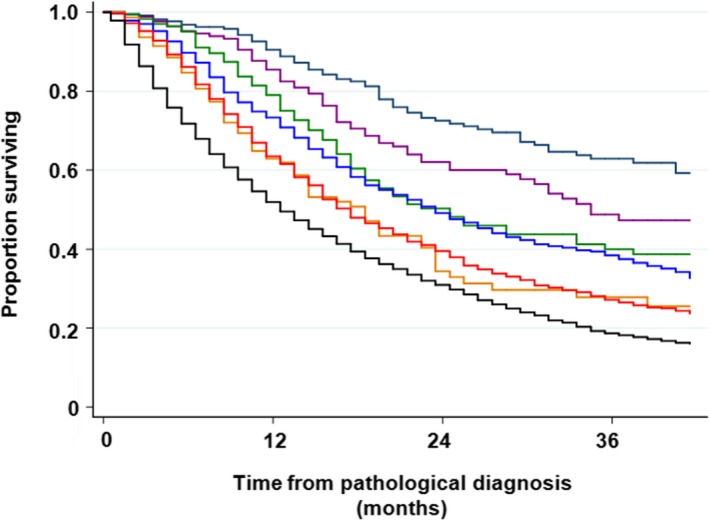
Overall survival with regard to clinical TNM stage in patients with non–small cell lung cancer. There was no significant difference in survival between stage IIA and stage IIIA (P = .202) and between stage IIB and stage IIIB (P = .885). Navy line, stage IA; purple line, stage IB; dark green line, stage IIA; dark orange line, stage IIB; blue line, stage IIIA; red line, stage IIIB; and black line, stage IV
FIGURE 3.
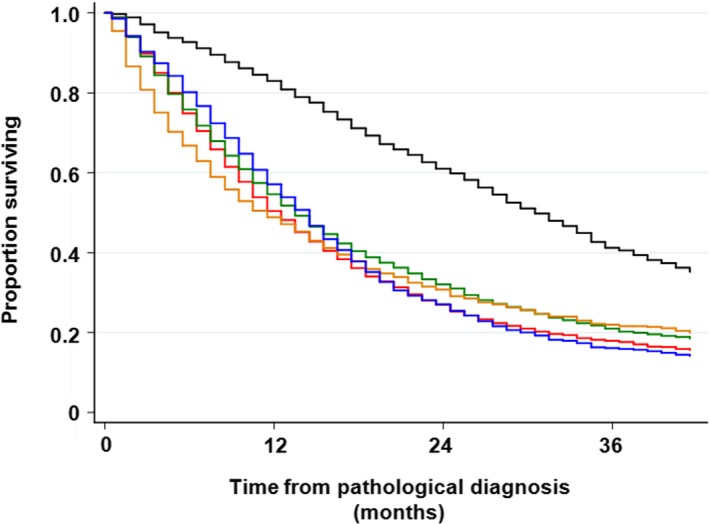
Overall survival with regard to histology. Red line, squamous cell carcinoma (Sq); black line, non‐Sq non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutation; dark green line, non‐Sq NSCLC without EGFR mutation; dark orange line, non‐Sq NSCLC of unknown EGFR status; blue line, small cell lung cancer
Chief treatment was also associated with survival; 3‐year survival of patients treated with concurrent chemoradiotherapy, sequential chemoradiotherapy, thoracic radiotherapy, chemotherapy, and supportive care alone was 43.6%, 29.1%, 40%, 19.9%, and 3.4%, respectively (Table S2 and Figure 4). The use of EGFR‐tyrosine kinase inhibitors (TKIs) affected survival significantly among 7790 patients who were treated with chemotherapy; a 3‐year survival rate was 33.4% in patients who received EGRT‐TKIs at some time in their clinical course, while it was 17.4% in patients with NSCLC who had never received EGRT‐TKIs, and only 7.4% among patients with SCLC (Table S2 and Figure 5).
FIGURE 4.
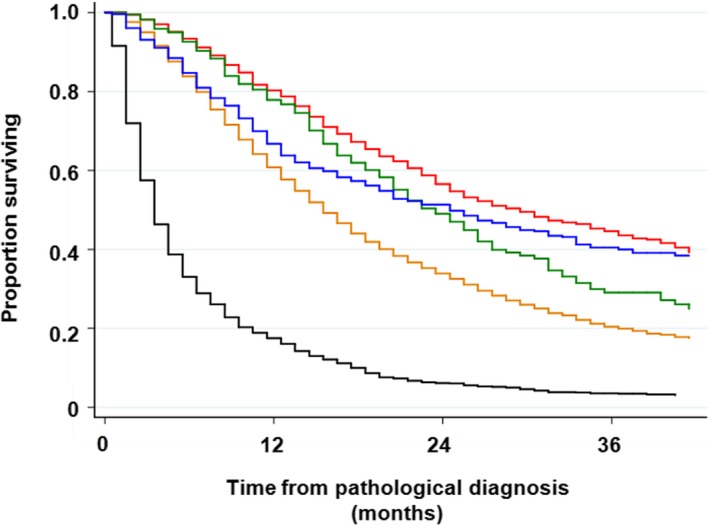
Overall survival with regard to chief treatment through all lines. Patients who received concurrent chemoradiotherapy had better survival than those who received sequential chemoradiotherapy (3‐y survival rate, 43.6% vs 29.1%, respectively; P = .002). There was no difference in survival between patients who received sequential chemoradiotherapy and thoracic radiotherapy alone (3‐y survival rate, 29.1% vs 40.0%, respectively; P = .675). Red line, concurrent chemoradiotherapy; dark green line, sequential chemoradiotherapy; blue line, radiotherapy alone; dark orange line, chemotherapy; black line, palliative care alone
FIGURE 5.
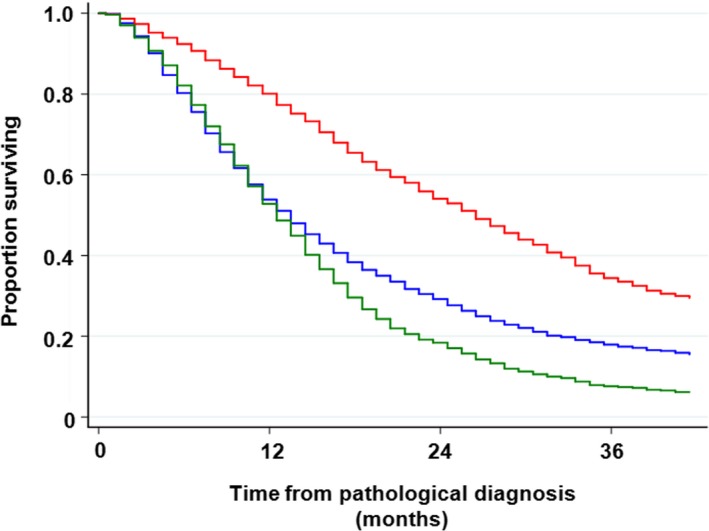
Overall survival in patients treated with chemotherapy. Red line, patients with non–small cell lung cancer (NSCLC) who received epidermal growth factor receptor‐tyrosine kinase inhibitor (EGFR‐TKI); blue line, patients with NSCLC who did not receive EGFR‐TKI; dark green line, patients with small cell lung cancer
4. DISCUSSION
This is the first nationwide registry study that was exclusively focused on medically treated patients with lung cancer. Although our previous registry study in 2002 also included medically treated patients,5 they accounted for only 39% of all patients analyzed, and therefore, the overall picture of these patients could not be fully obtained. This study clarified: (1) descriptive statistics, (2) treatment information, and (3) prognostic information of medically treated lung cancer patients in Japan.
One of the differences in demographics between medically and surgically treated patients was that the former patients were 3 years older in average than the latter patients (69 vs 66 years old).7 This is easily imagined because elderly patients generally exhibit more complications that could affect their general condition than younger patients, but the 3‐year difference was firstly disclosed in this study. There were more men in this study than patients who had been surgically treated (73% vs 63%).7 As smoking are more common in men than in women, male patients might have chronic pulmonary and heart diseases more frequently, and this might hamper them from undergoing surgical treatment.
T3 disease was associated with poorer survival than T4 disease (3‐year survival, 16.4% vs 19.5%). This may be attributable to difficulty in the exact diagnosis of tumor invasion to adjacent tissues without surgical evaluation. As a result, there might be a subpopulation with good prognosis among patients with clinical T4 disease. However, such patients would have a good prognosis if they underwent surgery. Thus, in patients with unresectable T4 disease, it is unlikely that the subpopulation included in clinical T4 disease had an effect to push up the survival rate.
This study disclosed that patients with NX, regional lymph node cannot be assessed, had an extremely poor survival (3‐year survival, 6.6% vs 17.7% for N3). The “NX” could be associated with several clinical situations, but mainly with deterioration in patients' physical conditions such that even a computed tomography (CT) scan could not be performed. Patients with NX accounted for only 1.1% (134/12 320) of the whole study population, and therefore, this was an exceptional case.
The survival curve of stage IIIA disease fitted closely with that of stage IIA and was better than that of stage IIB (3‐year survival, 37.5%, 40% vs 27.8%, respectively). Considering that patient survival rates are generally worse in clinical practice than in clinical trials, the survival of stage IIIA in this study “sounded too good” when compared with 3‐year survival rates of approximately 30%‐35% in clinical trials of unresectable stage III NSCLC treated with concurrent chemoradiotherapy.10, 11 This suggests that substantial numbers of pathological N0 and N1 diseases were mixed into clinical N2 disease in this study. A positron emission tomography (PET) scan was performed in only 62.1% of patients with stage IIIA disease in this study, and might lead to overdiagnosis of N2 disease. Besides, in patients with stage III NSCLC who underwent chemoradiotherapy, an effect of the treatment might be a more crucial factor for survival than the anatomical extent of local tumor burden described by the TNM staging system. Tumor sensitivity to an anticancer therapy is becoming more important for survival, especially in the case of immune checkpoint inhibitors.12
This study showed an overall picture of medically inoperable stage I‐II NSCLC in Japan, which accounted for 7.2% of all the medically treated NSCLC cases. The standard treatment of patients with stage I‐II NSCLC with a good general condition has been primary surgery with or without adjuvant chemotherapy. Patients with stage I‐II disease registered in this study had impaired organ functions that led them to be so‐called “medically inoperable” cases. Stereotactic radiotherapy has been established as the standard treatment of medically inoperable stage I NSCLC, with 3‐year overall survival rate of 46%‐60%.13, 14, 15 Thus, 3‐year survivals of 62.9% and 47.3% for stage IA and stage IB, respectively, in this study are comparable with those found in the medical literature. In contrast, treatment of medically inoperable stage II NSCLC has been controversial. Because stereotactic radiotherapy cannot be applied to N1 disease, conventional thoracic radiotherapy has been used to treat this condition, but its dose is obviously not enough to control the primary tumor and hilar lymph node metastasis. A retrospective analysis using the Surveillance, Epidemiology, and End Results (SEER) program showed that 3‐year lung cancer‐specific survival in 375 patients with inoperable stage II NSCLC treated with radiotherapy was 25% (95% confidence interval, 19%‐30%). This study did not report the overall survival because of the high prevalence of comorbidities leading to cancer‐unrelated deaths.16 Chemoradiotherapy can be one option for this disease,17 but patients who cannot undergo surgical resection are frequently not a candidate for chemoradiotherapy, either. Thus, patients with stage II disease were probably received suboptimal treatment for this disease. This could explain poor survival rates in patients with stage IIA and stage IIB diseases in this study in addition to concomitant severe diseases that are potentially fatal.
When we select the optimal treatment for a patient, PS of the patient is one of the most important factors that should be fully taken into account. However, we have had only limited information on the effect of PS on the patient population of lung cancer and their prognosis, because patients are intentionally selected by PS in clinical trials. This study showed that PS 3 and 4, the poor PS scores that are a serious obstacle to cancer treatment, was noted in as low as 11% of medically treated lung cancer patients in Japan. For these patients earlier diagnosis of lung cancer is obviously needed, but we have failed to find a feasible solution, because the barrier against early diagnosis of lung cancer includes social and psychological as well as medical problems.
This study showed for the first time that chemotherapy as the chief treatment accounted for as high as 63% of medically treated lung cancer patients, followed by thoracic radiation‐based therapy in 21% of the patients, and palliative care alone in 16% of the patients.
Significant numbers of patients with lung cancer, one‐quarter of patients in this study, were treated first with palliative care. This indicates that their lung cancer progressed very rapidly and affected general conditions of the patients significantly. Of 2993 patients receiving palliative care as the first‐line therapy, 1952 (65%) patients continued to receive palliative care alone throughout their clinical course, but the remaining 35% of the patients proceeded to chemotherapy or thoracic radiotherapy. This indicated that the purpose of palliative care may not be only to keep the quality of life of patients, but also to improve their PS so as to receive curative‐intent therapy. Thus, effective palliative therapy for the latter purpose is one of the unmet needs and an important research field.
In total, 6164 patients with NSCLC were treated with chemotherapy. Of these, 2059 (33.4%) received an EGFR‐TKI sometime in their clinical course. Thus, EGFR‐TKIs have a great effect on prognosis of advanced NSCLC population in Japan, because as high as one‐third of these patients were candidates for EGFR‐TKIs, and they were highly effective against EGFR‐mutated NSCLC. One point of note is that EGFR mutations may be associated with relatively long survival of patients regardless of the use of EGFR‐TKIs. However, because almost all such patients are currently treated with an EGFR‐TKI in clinical practice, it is impossible to demonstrate the effect of EGFR mutation on the prognosis of lung cancer.
The survival curve of thoracic radiotherapy alone crossed the curve for sequential chemoradiotherapy at about 18 months from diagnosis and the 3‐year survival rate of these patients reached 40%. This is probably because patients with medically inoperable stage I‐II disease were included in this cohort, while only patients with stage III disease were included in the cohort of sequential chemoradiotherapy.
Patients with stage IIIA and stage IIIB diseases are potential candidates for chemoradiotherapy. Of 3260 patients with stage IIIA and stage IIIB diseases, 2572 (79%) received thoracic radiotherapy‐based treatment. The remaining 688 patients were probably treated with chemotherapy alone or palliative care alone due to too large tumor volume or complication such as lung fibrosis and pulmonary emphysema. Concurrent chemoradiotherapy was 7.6 times more commonly selected than sequential chemoradiotherapy for these patients. As the concurrent approach was more effective with tolerable toxicity,18 it is preferable to most patients with stage III NSCLC if they have a good general condition.
In conclusion, this study showed the general picture of medically treated lung cancer patients in Japan, including descriptive statistics, treatment choice, and prognosis. About two‐thirds of patients were classified as stage IV disease at the initial diagnosis. Use of EGRT‐tyrosine kinase inhibitors improved the survival of patients with NSCLC significantly. Further analyses of this study are underway to elucidate the characteristics of subgroups and the effect of several clinical and pathological variables on survival.
DISCLOSURE
The authors have no conflicts of interest.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
I would like to thank Editage (http://www.editage.jp) for English language editing. This study was funded by the Japan Lung Cancer Society, Japanese Association for Chest Surgery, Japanese Respiratory Society, Japan Society for Respiratory Endoscopy, and Japanese Association for Thoracic Surgery.
Sekine I, Shintani Y, Shukuya T, et al. A Japanese lung cancer registry study on demographics and treatment modalities in medically treated patients. Cancer Sci. 2020;111:1685–1691. 10.1111/cas.14368
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391:1023‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non‐small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50:227‐234. [DOI] [PubMed] [Google Scholar]
- 5. Sawabata N, Asamura H, Goya T, et al. Japanese Lung Cancer Registry Study: first prospective enrollment of a large number of surgical and nonsurgical cases in 2002. J Thorac Oncol. 2010;5:1369‐1375. [DOI] [PubMed] [Google Scholar]
- 6. Asamura H, Goya T, Koshiishi Y, et al. A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46‐52. [DOI] [PubMed] [Google Scholar]
- 7. Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6:1229‐1235. [DOI] [PubMed] [Google Scholar]
- 8. Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706‐714. [DOI] [PubMed] [Google Scholar]
- 9. Travis WD, Colby TV, Shimosato Y, et al. World Health Organization Histological Typing of Lung and Pleural Tumours, 3rd edn Berlin: Springer; 1999. [Google Scholar]
- 10. Segawa Y, Kiura K, Takigawa N, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non‐small‐cell lung cancer: OLCSG 0007. J Clin Oncol. 2010;28:3299‐3306. [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second‐ and third‐generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non‐small‐cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739‐3745. [DOI] [PubMed] [Google Scholar]
- 12. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018. [DOI] [PubMed] [Google Scholar]
- 13. Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non‐small‐cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290‐3296. [DOI] [PubMed] [Google Scholar]
- 14. Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non‐small‐cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802‐809. [DOI] [PubMed] [Google Scholar]
- 15. Puri V, Crabtree TD, Bell JM, et al. Treatment outcomes in stage I lung cancer: a comparison of surgery and stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1776‐1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I‐II non‐small cell lung cancer. Chest. 2005;128:1461‐1467. [DOI] [PubMed] [Google Scholar]
- 17. Dudani S, Zhu X, Yokom DW, et al. Radical treatment of stage II non‐small‐cell lung cancer with nonsurgical approaches: a multi‐institution report of outcomes. Clin Lung Cancer. 2018;19:e11‐e18. [DOI] [PubMed] [Google Scholar]
- 18. Auperin A, Le Pechoux C, Rolland E, et al. Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28:2181‐2190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


