Abstract
Inactivated hemagglutinating virus of Japan envelope (HVJ‐E) has an antitumor effect and tumor immunity. We undertook an open‐label, phase I, dose‐escalation study in patients with castration‐resistant prostate cancer (CRPC) to determine the safety and efficacy of intratumoral and s.c. injection of HVJ‐E (GEN0101). Patients with CRPC, who were resistant to or unable to receive standard of care, were included. GEN0101 was injected directly into the prostate and s.c. in two 28‐day treatment cycles. The primary end‐points were to evaluate the safety and tolerability of GEN0101 and determine its recommended dose. The secondary end‐points were to analyze the antitumor effect and tumor immunity. Three patients received 30 000 mNAU GEN0101 and 6 received 60 000 mNAU. There was no dose‐limiting toxicity, and the recommended dose of GEN0101 was defined as 60 000 mNAU. Radiographically, 1 patient had stable disease and 2 had progressive disease in the low‐dose group, whereas 5 patients had stable disease and 1 had progressive disease in the high‐dose group. Three patients in the high‐dose group showed reduction in lymph node metastasis. Prostate‐specific antigen increase rates in the high‐dose group were suppressed more than those in the low‐dose group. Natural killer cell activity was enhanced in 2 patients of the low‐dose group and in 5 patients in the high‐dose group. In conclusion, intratumoral and s.c. injections of GEN0101 were well‐tolerated and feasible to use. The study is registered with the UMIN Clinical Trials Registry (no. UMIN000017092).
Keywords: antitumor effect, castration‐resistant prostate cancer, NK cell, phase I clinical study, Sendai virus
Inactivated hemagglutinating virus of Japan envelope (HVJ‐E) has an antitumor effect and tumor immunity. We undertook an open‐label, phase I, dose‐escalation study in patients with castration‐resistant prostate cancer (CRPC). Intratumoral and s.c. injections of GEN0101 were well‐tolerated and feasible to use; antitumor effects were observed in patients with CRPC.
![]()
1. INTRODUCTION
Recently, several treatment options for castration‐resistant prostate cancer have been developed, and new therapy using antiandrogen drugs is under clinical trial.1
The US Preventive Services Task Force recommended against prostate‐specific antigen (PSA) screening in 2012, and the incidence of prostate cancer in the USA decreased thereafter. However, the incidence of metastatic prostate cancer has been increasing rapidly since 2012.2 Therefore, the development of new therapies became an urgent need. Hemagglutinating virus of Japan (HVJ) is a mouse parainfluenza virus, with a single‐stranded RNA, belonging to the family Paramyxoviridae. Hemagglutinating virus of Japan is also known as Sendai virus, and has the ability to fuse with cells. Ultraviolet irradiation of HVJ results in fragmentation of the virus RNA genome while fusion activity of the virus envelope remains intact. Hemagglutinating virus of Japan, inactivated by UV irradiation (HVJ‐envelope HVJ‐E]), loses its replication activity while retaining the fusion ability of its envelope. Additionally, HVJ‐E has the ability of direct tumor killing in specific tumors. It fuses with prostate cancer cell lines through GD1a ganglioside on the cell surface, and the fragmented viral RNA is recognized by cytoplasmic RNA receptor and retinoic acid‐inducible gene‐I (RIG‐I), and induces type‐1 interferon, resulting in apoptosis of prostate cancer cells.3 Hemagglutinating virus of Japan‐envelope also enhances antitumor immunity.4 It induces the activation of natural killer (NK) cells and cytotoxic T cells through dendritic cells, which in turn are activated by HVJ‐E, and suppresses regulatory T cells.5, 6
Previously, we undertook phase I/II clinical studies in patients with castration‐resistant prostate cancer (CRPC) to determine the safety and efficacy of intratumoral and s.c. injection of HVJ‐E (3000 mNAU and 10 000 mNAU).7 The HVJ‐E injections were effective in 1 patient, with complete response in PSA levels, out of 6 patients with CRPC who were resistant to docetaxel. Patients treated with 10 000 mNAU GEN0101 did not show dose‐limiting toxicity, and higher dose of GEN0101 could be more effective against CRPC. Currently, several new drugs against CRPC, such as the new generation of antiandrogens, abiraterone acetate, and cabazitaxel, have been developed. As patients with CRPC might eventually become resistant to these new agents, it is necessary to develop treatments with new mechanisms. In this report, an open‐label, phase I, dose‐escalation study is presented in patients with CRPC, to test the safety and efficacy of higher dose of GEN0101.
2. MATERIALS AND METHODS
2.1. Study protocols
To determine the recommended dose of GEN0101, a modified 3 + 3 dose‐escalation design was used. First, 3 patients were assigned to the low‐dose group of GEN0101 (30 000 mNAU). After the assessment of safety and tolerability of 30 000 mNAU GEN0101 by an independent data monitoring committee, the next 3 patients were assigned to the high‐dose group of 60 000 mNAU GEN0101. Once the safety and tolerability of 60 000 mNAU were confirmed by the independent data monitoring committee, an additional 3 patients were assigned to the high‐dose group.
GEN0101 was injected directly into the prostate on day 1, transrectally guided by ultrasound, after 6‐core prostate needle biopsy was carried out. Subcutaneous injections of GEN 0101 to the inguinal region were carried out on days 5, 8, and 12 in 28‐day treatment cycles. Treatment was repeated over 2 cycles (Figure 1). The primary end‐points were to evaluate the safety and tolerability of GEN0101 and determine the recommended dose. The secondary end‐points were to analyze the antitumor effect and tumor immunity.
Figure 1.
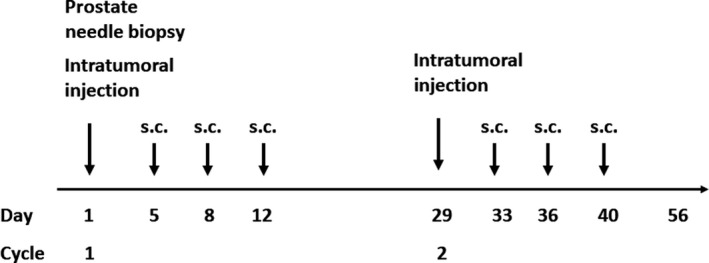
Patients with castration‐resistant prostate cancer underwent transrectal ultrasound‐guided injection of GEN0101 (inactivated hemagglutinating virus of Japan envelope [HVJ‐E]) into the prostate on day 1, followed by s.c. injection of HVJ‐E on days 5, 8, and 12. Patients underwent 2 cycles of GEN0101 treatment
2.2. GEN0101
GEN0101 was manufactured by GenomIdea (Osaka, Japan). Hemagglutinating virus of Japan was irradiated by UV radiation after the treatment of β‐propiolactone, resulting in the fragmentation of virus RNA genome. Inactivated HVJ‐E was purified by 4 repeats of column chromatography, followed by lyophilization. GEN0101 was stored at 4°C and was diluted in distilled water before the injection.
2.3. Patients
Patients with CRPC, who were resistant to or refused standard of care, were enrolled in this study. Castration‐resistant prostate cancer was defined by the elevation of serum PSA levels under androgen deprivation therapy, and serum testosterone levels less than 50 ng/mL. Inclusion criterion was an ECOG performance status of either 0 or 1. Patients undergoing radiotherapy for localized prostate cancer were also included. Exclusion criteria were as follows, and excluded patients who: were allergic to GEN0101, had brain metastasis from prostate cancer, underwent radical prostatectomy, received chemotherapy within 3 weeks, received radiation therapy or immune therapy within 6 weeks, received immunosuppressive agents or had autoimmune disease, or experienced malignant diseases other than prostate cancer within 5 years.
The protocol was approved by the institutional review board of Osaka University Hospital. Written informed consent was obtained from each patient. The study was registered with UMIN Clinical Trials Registry (no. UMIN000017092).
2.4. GEN0101 preparation
2.4.1. Assessment of safety
The NCI’s Common Terminology Criteria for Adverse Events version 4.0 was used for the coding of adverse events. Safety was evaluated in the hospital from day 1 to day 14, and at each visit to the hospital at days 21 and 28 per cycle.
2.4.2. Assessment of antitumor efficacy
Serum PSA levels were measured at pretreatment, day 28, and day 56. Computed tomography and bone scintigraphy were undertaken before the treatments, and after cycles 1 and 2, for radiological assessment. Overall response was assessed according to RECIST version 1.1.
2.4.3. Assessment of tumor immunity
Natural killer cell activity, serum interleukin (IL)‐6, and γ‐interferon (IFN‐γ) levels were measured at pretreatment and on days 14, 28, 42, and 56. Antiparainfluenza virus titer was measured as anti‐HVJ‐E Ab at pretreatment and on days 28 and 58. The NK cell activity was evaluated by 51Cr release assay using radiolabeled K562 cells.
3. RESULTS
3.1. Patients
A total of 10 patients with CRPC provided informed consent before enrolling for this study. One patient showed lower limb paralysis due to the rapid progression of spinal metastasis before GEN0101 treatment, and could not be enrolled in the study. A total of 9 patients with CRPC were enrolled between March 2015 and December 2017. The first 3 patients were assigned to the low‐dose group (30 000 mNAU GEN0101), and the subsequent 6 patients were assigned to the high‐dose group (60 000 mNAU GEN0101). Characteristics of the patients are summarized in Table 1. Prostate needle biopsy was undertaken before the injection of GEN0101 into the prostate at day 1. In 2 patients in the low‐dose group, who had earlier received radiotherapy for prostate cancer, prostate needle biopsy showed no malignancy in the specimens. One patient in the high‐dose group, who had received radiotherapy for prostate cancer, and another patient in the same group, who had not received radiotherapy, had no malignant cells in prostate needle biopsy specimens. One patient in the low‐dose group showed disease progression during cycle 1 and stopped the protocol before cycle 2. In the high‐dose group, all patients completed the 2 cycles of GEN0101 treatment (Figure 2).
Table 1.
Characteristics of patients with castration‐resistant prostate cancer treated 30 000 mNAU (low‐dose) or 60 000 mNAU (high‐dose) GEN0101
| Low‐dose group | High‐dose group | Total | |
|---|---|---|---|
| N | 3 | 6 | 9 |
| Age, median (range) | 73 (64‐78) | 67 (54‐74) | 68 (54‐78) |
| PSA, median (range) | 29.4 (15.7 ‐49.6) | 10.2 (1.1‐67.1) | 15.7 (1.1‐67) |
| Gleason score, n (%) | |||
| 7 | 0 (0) | 1 (17) | 1 (12) |
| 8 | 3 (100) | 1 (17) | 4 (44) |
| 9 | 0 (0) | 4 (66) | 4 (44) |
| TNM classification | |||
| T stage, n (%) | |||
| T2 | 1 (34) | 1 (17) | 2 (22) |
| T3 | 2 (66) | 3 (50) | 5 (56) |
| Unknown | 0 | 2 (33) | 2 (22) |
| N stage, n (%) | |||
| N0 | 2 (66) | 1 (17) | 3 (34) |
| N1 | 1 (34) | 5 (83) | 6 (66) |
| M stage, n (%) | |||
| M0 | 1 (34) | 1 (17) | 2 (22) |
| M1a | 0 (0) | 0 (0) | 0 (0) |
| M1b | 2 (66) | 4 (66) | 6 (66) |
| M1c | 0 (0) | 1 (17) | 1 (12) |
| Local radiation therapy, n (%) | 2 (66) | 2 (33) | 4 (44) |
| Previous therapies, n (%) | |||
| Docetaxel | 2 (66) | 1 (17) | 3 (34) |
| Cabazitaxel | 1 (34) | 1 (17) | 2 (22) |
| Enzalutamide | 2 (66) | 4 (44) | 6 (66) |
| Abiraterone | 3 (100) | 3 (34) | 6 (66) |
| Immune therapy | 1 (34) | 2 (33) | 3 (34) |
| Other chemotherapy | 0 (0) | 1 (17) | 1 (12) |
Abbreviation: PSA, prostate‐specific antigen.
Figure 2.
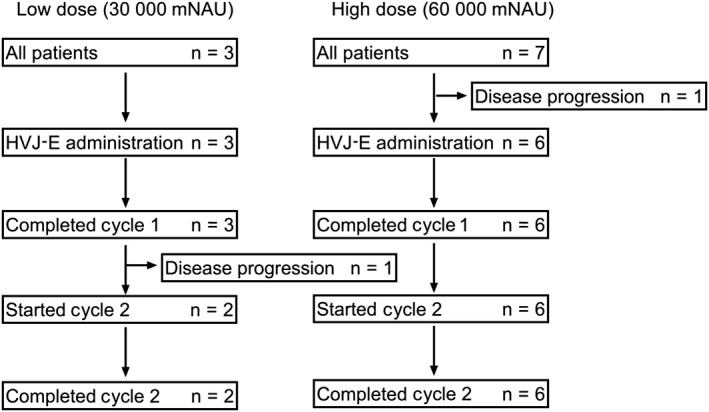
Diagram of patients with castration‐resistant prostate cancer who were enrolled in the low‐dose (30 000 mNAU GEN0101) or high‐dose (60 000 mNAU GEN0101) treatment groups. HVJ‐E, inactivated hemagglutinating virus of Japan envelope
3.2. Safety
None of the 3 patients in the low‐dose group showed dose‐limiting toxicities. Six patients received high‐dose GEN0101 and showed no severe adverse events. The adverse events are summarized in Table 2. One patient in the low‐dose group developed grade 4 thrombocytopenia, which was thought to be caused by the progression of prostate cancer. All 9 patients developed injection site reaction (grade 1). Four patients (3 patients [100%] in the low‐dose group and 1 patient [16.7%] in the high‐dose group) developed grade 1 fever, and 5 patients (83.3%) in the high‐dose group developed grade 2 fever. Grade 3 lymphopenia was reported in 1 patient (33.3%) in the low‐dose group and in 1 patient (16.7%) in the high‐dose group. Four patients in the high‐dose group showed urinary retention. Generally, the reported adverse events were not serious and recovered before the end of the cycles. Treatment with GEN0101 was well tolerated and the recommended dose was determined to be 60 000 mNAU.
Table 2.
Summary of adverse events in patients with castration‐resistant prostate cancer treated 30 000 mNAU (low‐dose) or 60 000 mNAU (high‐dose) GEN0101
| Low dose (n = 3) | High dose (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | |
| Conjunctivitis | 1 | |||||||
| Headache | 1 | |||||||
| Syncope | 1 | |||||||
| Vomiting | 1 | |||||||
| Constipation | 1 | |||||||
| Anal pain | 2 | |||||||
| Erythema multiforme | 1 | |||||||
| Pain in extremity | 1 | |||||||
| Hematuria | 2 | 2 | ||||||
| Incontinence | 1 | |||||||
| Urinary retention | 4 | |||||||
| Injection site reaction | 3 | 6 | ||||||
| Edema, limbs | 1 | |||||||
| Fever | 3 | 1 | 5 | |||||
| Weight loss | 1 | |||||||
| Hypotension | 1 | |||||||
| Anemia | 2 | 1 | ||||||
| Lymphopenia | 1 | 1 | ||||||
| Neutropenia | 1 | |||||||
| Thrombocytopenia | 1 | 1 | ||||||
| Proteinuria | 1 | |||||||
| Alkaline phosphatase increased | 1 | 3 | ||||||
| Aspartate aminotransferase increased | 1 | |||||||
| Hypoalbuminemia | 1 | |||||||
| Fibrinogen decreased | 1 | |||||||
3.3. Antitumor effect
No patient in either of the groups showed a decrease of serum PSA levels for 8 weeks.
The median percentage changes of serum PSA levels from the baseline were 285.3% (range, 93.0‐287.4) in the low‐dose group, and 38.9% (range, 13.9‐155.8) in the high‐dose group (Figure 3). The percentage changes of serum PSA levels were significantly lower in the high‐dose group compared to those in the low‐dose group (P = .047, Mann‐Whitney test). Radiologically, 1 patient (33.3%) in the low‐dose group showed stable disease and 2 patients showed progressive disease. All 6 patients in the high‐dose group showed stable disease, according to the RECIST criteria. Among the 9 patients, 4 in the high‐dose group had lymph node metastasis at baseline. Although the change of lymph node metastasis was less than 30%, 5 lymph node metastases in 3 patients were reduced after 2 cycles of GEN0101 treatment (Figure 4). There was no association between the results of prostate needle biopsy and that of antitumor effect. There was also no association between the results of antitumor effect and previous therapies.
Figure 3.
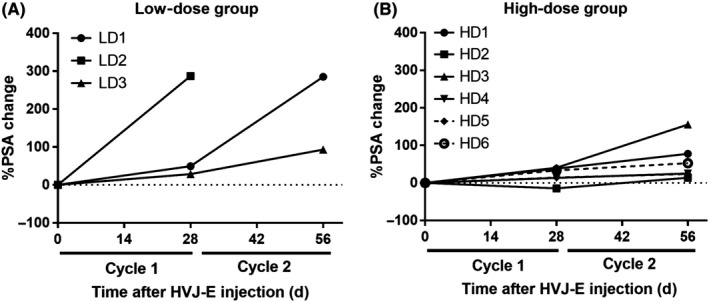
Changes in serum prostate‐specific antigen (PSA) levels from the baseline in patients with castration‐resistant prostate cancer who were enrolled in the low‐dose (LD; 30 000 mNAU GEN0101) or high‐dose (HD; 60 000 mNAU GEN0101) treatment group. A, Low‐dose group (n = 3). B, High‐dose group (n = 6)
Figure 4.
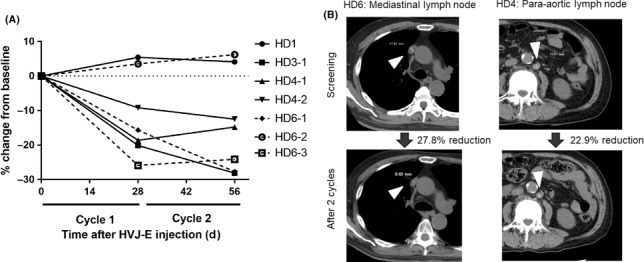
Change in lymph node metastasis during the GEN0101 treatment in patients with castration‐resistant prostate cancer. A, Change in lymph node metastasis in 3 patients in the high‐dose group (HD; 60 000 mNAU GEN0101). B, Computed tomography image of lymph node metastasis (arrowheads). HVJ‐E, inactivated hemagglutinating virus of Japan envelope
3.4. Tumor immunity
Serum anti‐HVJ‐E Ab was elevated by treatment with GEN0101 in all cases (Figure S1). There was no significant change in serum IFN‐γ or IL‐6 levels after treatment with GEN0101 (Figure S1). The NK cell activity was increased by more than 10% in 2 cases in the low‐dose group (66%) and 5 cases in the high‐dose group (83%) (Figure 5).
Figure 5.
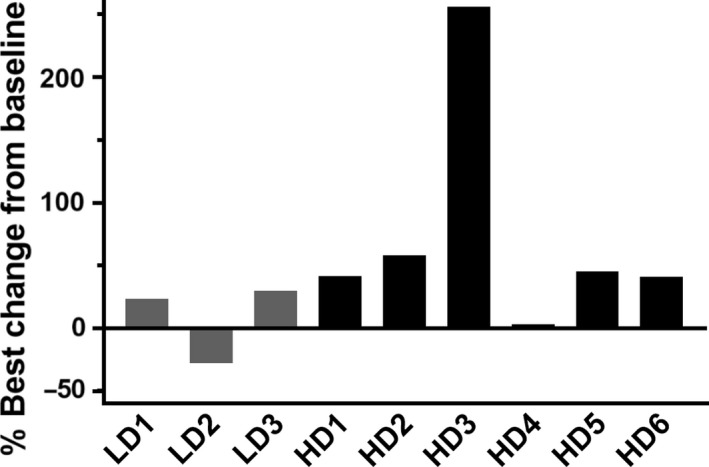
Changes in natural killer cell activity from baseline in patients with castration‐resistant prostate cancer who received low‐dose (LD; 30 000 mNAU) or high‐dose (HD; 60 000 mNAU) GEN0101
4. DISCUSSION
We confirmed the safety and tolerability of GEN0101 at 30 000 mNAU and 60 000 mNAU, without severe adverse events, in patients with CRPC. With the 8‐week administration of GEN0101, patients in the high‐dose group showed reduced rate of PSA rise compared to that in the low‐dose group, although no patients showed a decrease in PSA level. Lymph node metastasis also showed shrinkage, although the assessment was of stable disease with less than 30% shrinkage. Natural killer cell activity was also increased in many cases, especially in the high‐dose group, contrary to results in a previous study. The higher dose of GEN0101 could have induced the activation of NK cells.
The safety profile of 60 000 mNAU GEN0101 showed appreciable tolerance. Therefore, the recommended dose of GEN0101 was determined to be 60 000 mNAU. At this dose, GEN0101 induced grade 2 fever in 5 of 6 patients and grade 3 lymphopenia in 1 of 6 patients. Doses higher than 60 000 mNAU might be tolerated and could induce more tumor immunity and antitumor responses.
A previous phase I/II study had shown the decline of PSA in 1 patient with CRPC, after treatment with 3000 mNAU HVJ‐E.7 However, with higher doses of 30 000 mNAU and 60 000 mNAU GEN0101, no patient showed a decline of serum PSA levels. In contrast, 3 cases with lymph node metastasis showed reduction of metastasis by less than 20%, whereas no case had shown radiological responses in the previous study. In the previous study, patients with CRPC, who were resistant or ineligible for docetaxel, had been enrolled. Currently, enzalutamide, abiraterone acetate, and cabazitaxel are used in patients with CRPC, and those resistant or ineligible for the new agents have been enrolled, with more aggressive disease than in patients involved in the previous study. This phase I clinical study was not planned to follow the patients for more than 2 months, and the GEN0101 treatment was finished at the end of 2 cycles. Continuous treatment might have resulted in PSA decline or partial response of lymph node metastasis. The effect of GEN0101 in overall survival should be evaluated in future.
Direct injection of GEN0101 into the prostate could induce apoptosis of prostate cancer cells. When HVJ‐E was added to the cell culture of DU145, PC3, and LNCaP cell lines, the growth of DU145 and PC3 cells was significantly suppressed, whereas that of LNCaP was not.8 Inactivated hemagglutinating virus of Japan envelope fused with the cell membrane through ganglioside GD1a and sialyl paragloboside (SPG).9 PC3, DU145, and PNT2 had higher amounts of GD1a and SPG. Fusion of PC3 and DU145 with HVJ‐E resulted in apoptosis with the activation of caspases‐8 and ‐9. In a mouse model of murine renal cancer (Renca), local injection of HVJ‐E into tumor tissues induced the upregulation of C‐X‐C motif chemokine ligand 10 (CXCL10) by dendritic cells localized in the tumor; CXCL10 enhanced the infiltration and activation of NK cells. Type I interferon secreted from the HVJ‐E‐stimulated dendritic cells also activated NK cells. In the mouse model, intratumoral injection of HVJ‐E suppressed kidney cancer growth in vivo, which was cancelled by NK cell depletion.6 F‐glycoprotein in HVJ also suppresses regulatory T cells by stimulating IL‐6 production from dendritic cells.5, 10 In this study, serum IL‐6 and IFN‐γ levels were not affected by GEN0101 treatment. It is not concluded whether adaptive immune responses, such as cytotoxic T cells, are activated, because T cell activity was not planned to be measured.
Prostate cancer cells could not be detected by prostate needle biopsy in 4 patients, and the antitumor efficacy of GEN0101 was also not associated with the results of prostate biopsy. Two cases with negative biopsy results had received local radiotherapy previously. Even patients with negative biopsy results showed reduction of lymph node metastasis. As many patients with CRPC have a small prostate, only 6‐core biopsy could be carried out. Although false‐negative biopsy results might have been caused by insufficient sampling, GEN0101 could induce tumor immune response even when it did not exert an antitumor effect directly on prostate cancer cells. GEN0101 induces apoptosis in prostate cancer cells by fusing with prostate cancer cells through GD1a on the cancer cell surface. We had previously confirmed GD1a expression in a few CRPC specimens; however, whether prostate cancer in these patients expressed GD1a remains unknown.
Recently, patients with CRPC who had a high microsatellite instability were shown to have a high response rate to anti‐programmed cell death 1 (PD‐1)/programmed cell death ligand 1 (PD‐L1) therapy, although the frequency of high microsatellite instability in patients with CRPC was low, at approximately only 3%. The combination therapy of GEN0101 with anti‐PD‐1/PD‐L1 could also be an option to explore in the future. Higher dose of GEN0101 or repeated s.c. injections of GEN0101 should be tested in patients with CRPC. Sipuleucel‐T is an FDA‐approved immune therapy for the early stage of prostate cancer. GEN0101 has a mechanism of direct interaction with prostate cancers. GD1a expression was confirmed in hormone‐naïve prostate cancer; GEN0101 might be more effective in localized or metastatic hormone‐naïve prostate cancer with no local therapy administered to the prostate, although several drugs are currently available to prolong their overall survival. Patients with CRPC who are ineligible for taxane chemotherapy might be also candidates for GEN0101 after androgen axis‐targeted therapy. A predictive biomarker, to select the patients who will benefit from GEN0101, should also be explored in future.
In conclusion, GEN0101 has a tolerable safety profile with a limited antitumor effect in patients with CRPC, who were resistant to standard of care.
CONFLICT OF INTEREST
Toshihiro Nakajima is the CEO of GenomIdea. Yasufumi Kaneda is a stock‐holder (0.3%) of GenomIdea. The other authors declare no conflicts of interest.
Supporting information
Fig S1
ACKNOWLEDGMENTS
We thank Yukio Tanaka (Medical Center for Translational Research Osaka University Hospital) for supporting the clinical trial. This study was also supported by the Japan Science and Technology Agency.
Fujita K, Kato T, Hatano K, et al. Intratumoral and s.c. injection of inactivated hemagglutinating virus of Japan envelope (GEN0101) in metastatic castration‐resistant prostate cancer. Cancer Sci. 2020;111:1692–1698. 10.1111/cas.14366
REFERENCES
- 1. Fujita K, Nonomura N. Role of androgen receptor in prostate cancer: a review. World J Mens Health. 2019;37:288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, current, and future incidence rates and burden of metastatic prostate cancer in the United States. Eur Urol Focus. 2018;4:121‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushima‐Miyagi T, Hatano K, Nomura M, et al. TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG‐I/MAVS signaling pathway by nonreplicating Sendai virus particles. Clin Cancer Res. 2012;18:6271‐6283. [DOI] [PubMed] [Google Scholar]
- 4. Hatano K, Miyamoto Y, Nonomura N, Kaneda Y. Expression of gangliosides, GD1a, and sialyl paragloboside is regulated by NF‐B‐dependent transcriptional control of α2,3‐sialyltransferase I, II, and VI in human castration‐resistant prostate cancer cells. Int J Cancer. 2011;129:1838‐1847. [DOI] [PubMed] [Google Scholar]
- 5. Kurooka M, Kaneda Y. Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res. 2007;67:227‐236. [DOI] [PubMed] [Google Scholar]
- 6. Fujihara A, Kurooka M, Miki T, Kaneda Y. Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation. Cancer Immunol Immunother. 2008;57:73‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujita K, Nakai Y, Kawashima A, et al. Phase I/II clinical trial to assess safety and efficacy of intratumoral and subcutaneous injection of HVJ‐E to castration‐resistant prostate cancer patients. Cancer Gene Ther. 2017;24:277‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawaguchi Y, Miyamoto Y, Inoue T, Kaneda Y. Efficient eradication of hormone‐resistant human prostate cancers by inactivated Sendai virus particle. Int J Cancer. 2009;124:2478‐2487. [DOI] [PubMed] [Google Scholar]
- 9. Sadler AJ, Williams BR. Interferon‐inducible antiviral effectors. Nat Rev Immunol. 2008;8:559‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki H, Kurooka M, Hiroaki Y, Fujiyoshi Y, Kaneda Y. Sendai virus F glycoprotein induces IL‐6 production in dendritic cells in a fusion‐independent manner. FEBS Lett. 2008;582:1325‐1329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


