Abstract
Recent studies have raised the possibility of a role for lipoproteins, including high-density lipoprotein cholesterol (HDLc), in abdominal aortic aneurysm (AAA). The study was conducted in plasmas from 39 large size AAA patients (aortic diameter > 50 mm), 81 small/medium size AAA patients (aortic diameter between 30 and 50 mm) and 38 control subjects (aortic diameter < 30 mm). We evaluated the potential of HDL-mediated macrophage cholesterol efflux (MCE) to predict AAA growth and/or the need for surgery. MCE was impaired in the large aortic diameter AAA group as compared with that in the small/medium size AAA group and the control group. However, no significant difference in HDL-mediated MCE capacity was observed in 3 different progression subgroups (classified according to growth rate < 1 mm per year, between 1 and 5 mm per year or >5 mm per year) in patients with small/medium size AAA. Moreover, no correlation was found between MCE capacity and the aneurysm growth rate. A multivariate Cox regression analysis revealed a significant association between lower MCE capacity with the need for surgery in all AAA patients. Nevertheless, the significance was lost when only small/medium size AAA patients were included. Our results suggest that MCE, a major HDL functional activity, is not involved in AAA progression.
Keywords: abdominal aortic aneurysm, cardiovascular disease, cholesterol efflux, HDL, apoA-I, aortic diameter, growth rate, need for surgery, reverse cholesterol transport
1. Introduction
An abdominal aortic aneurysm (AAA) is defined as a permanent dilatation of the abdominal aorta above the threshold of a diameter of 30 mm, as determined using imaging techniques [1]. The prevalence of AAA ranges from 4–8% in men and 0–2% in women, based on population screening and large-scale randomized controlled trials [2,3]. The estimated prevalence of AAA in the US is over one million, with approximately half of AAA cases being women, nonsmokers and aged less than 65 years [4]. AAA is generally asymptomatic, and progressive aneurismal dilation is finally associated with the severe consequences of aortic rupture. To prevent AAA rupture, surgical repair is indicated when the aortic diameter exceeds 55 mm. Other than the AAA diameter, factors such as age, sex, body size and image characteristics should be considered in AAA evaluations [5]. For smaller aneurysms (30–50 mm), follow-up to monitor the growth rate is mandatory to estimate the median growth (mm per year) and/or rupture risk [6]. In these patients, there is no definitive pharmacological treatment. Recent studies showed that the use of statins and low-dose aspirin were associated with lower AAA growth rates and decreased progression [7,8]. However, major randomized double-blind trials are scarce, and no official recommendation regarding medical treatment exists in current guidelines [6,9].
The AAA pathophysiology remains unclear, but it is believed to be associated with alterations in the connective tissue in the aortic wall, mainly due to the high proteolytic activity produced by both infiltrating and resident cells, leading to a decrease in the amount of elastin [10]. A large number of exogenous immune cells, including neutrophils, lymphocytes and macrophages, infiltrate into the aortic tissue, eliciting a significant immune inflammatory response in the AAA wall. These inflammatory cells may enhance smooth muscle cell (SMC)-mediated secretion of matrix metalloproteinases, thereby impairing the stability and mechanical properties of the aortic wall, resulting in destruction of the medial extracellular matrix [11]. The AAA wall is also characterized by the presence of cholesterol crystals, which also induce inflammasome activation [12]. In this context, cholesterol accumulation enhances macrophage differentiation toward a pro-inflammatory state [13].
Among well-established risk factors for AAAs, dyslipidemia has grown in importance since a recent genetic meta-analysis and a Mendelian randomization study demonstrated the potential causal association of lipoproteins with this condition [14,15]. In a prospective study cohort of AAA patients, we found significantly decreased apolipoprotein A-I (apoA-I) (the main protein of high-density lipoprotein (HDL) particles) concentration, and plasma HDL cholesterol (HDLc) concentration predicted the aneurysmal growth rate [16]. However, strong evidence indicates that circulating HDLc levels may only represent a surrogate marker of atherogenesis. The ability of HDL to induce macrophage cholesterol efflux (MCE) is considered one of the main atheroprotective functions of HDL [17]. We recently reported that AAA patients showed impaired HDL-mediated MCE, which could be mechanistically linked to AAA. This inverse association was confirmed after adjusting for age, statin use, plasma lipids, apoA-I and HDLc levels [18]. However, this study included only AAA patients with a large aortic diameter (>50 mm). The association between HDL-mediated MCE and AAA progression has never been evaluated. In this study, we aimed to evaluate HDL-mediated MCE in a cohort of AAA patients with small/medium aortic diameters as a tool to test the potential of MCE to predict AAA growth and/or the need for surgery.
2. Materials and Methods
2.1. Study Design and Participants
All samples were obtained from a Danish cohort derived from the Viborg Vascular (VIVA) trial (URL: http://www.clinicaltrials.gov. Unique identifier: NCT00662480). The trial was approved by the regional ethics committee on Health Research Ethics (M20080025) on 28 March 2008. All the subjects gave informed consent. The study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki. One hundred and fifty-eight male patients aged 64–74 years with different AAA sizes were randomly selected and classified into three groups according to their aortic diameter, which was measured by abdominal ultrasound: a large size group (aortic diameter > 50 mm; n = 39, based in the US Aneurysm Detection and Management study), small/medium size group (aortic diameter between 30 and 50 mm; n = 81) and control group (aortic diameter < 30 mm; n = 38). This subset was selected from a large collection of plasmas from the VIVA trial [19], and HDLc/apoA-I levels as well as other clinical parameters were similar to the complete collection.
The large size group was referred for a computed tomography scan and vascular assessment. The small/medium size group underwent medical monitoring for clinical control to check for diameter expansion. Monitoring consisted of ultrasonographic follow-up of the aortic diameter (a minimum of two follow-ups in a 5-year period) to obtain a linear growth rate per year. Based on the rate, the patients were divided into three subgroups: low progression (growth rate of < 1 mm per year; n = 26), medium progression (growth rate between 1 and 5 mm per year; n = 29) and high progression (growth rate of > 5 mm per year; n = 26). The patients were assigned to surgery according to increases in the aortic diameter and evaluation of clinical parameters.
2.2. Lipid, Apolipoprotein and Lipoprotein Analyses
Whole blood samples were collected in Vacutainer® tubes and fractionated by centrifugation at 1300× g for 15 min at room temperature to obtain plasma. Plasma was aliquoted into 1.5 mL tubes and kept frozen at −80 °C until analysis. Plasma total cholesterol and triglyceride (TG) concentrations were determined enzymatically using commercial kits and a COBAS 501c autoanalyzer (Roche Diagnostics, Rotkreuz, Switzerland). ApoA-I levels were determined by an immunoturbidimetric assay (Roche Diagnostics). HDLc levels were measured in plasma obtained after precipitation of apoB-containing lipoprotein particles with phosphotungstic acid and magnesium ions (Roche Diagnostics). Low-density lipoprotein (LDL) cholesterol levels were calculated with the Friedewald equation.
2.3. Macrophage Cholesterol Efflux Assays
The MCE capacity of apoB-depleted plasma samples (equivalent to 5% of plasma containing mature HDL, nascent preβ-HDL particles and HDL regulatory proteins) was determined using J774.A1 [3H]-cholesterol-labeled murine macrophages according to a previously described protocol [18,20]. Briefly, macrophages were seeded and grown for two days in the Roswell Park Memorial Institute (RPMI) growth medium. Macrophages were then incubated for 48 h with a loading medium containing 1 µCi of radiolabeled cholesterol/well. The cells were washed and incubated with a serum-free medium supplemented with fatty acid-free Bovine serum albumin (BSA) for 18 h to allow equilibration of the radiolabeled cholesterol with the intracellular cholesterol pools. After equilibration, the medium was removed, and the cell cultures washed. The macrophages were then incubated for 4 h in the presence of apoB-depleted plasma (equivalent to 5% of plasma), after which cholesterol efflux was determined and expressed as ([3H]-cholesterol medium)/([3H]-cholesterol cells medium) × 100. The samples were assayed in duplicate in five independent batches using six-well plates. To minimize the effects of intraplate variation, both AAA and control samples were included in each experiment.
2.4. Statistical Analysis
Data are presented as mean ± standard deviation (SD) for continuous variables and as frequencies and percentages for categorical variables. A chi-square test was used to compare the categorical data between groups. The normality of the data was analyzed using the Kolmogorov–Smirnov and D’Agostino and Pearson omnibus test. A one-way analysis of variance (ANOVA) test was used to compare the continuous variables, and Tukey’s post-test was used for comparing differences among groups. Correlations between variables were analyzed using Pearson’s correlation analysis. Multivariate lineal regression models were used to explore the association between efflux and the aortic baseline diameter and growth rate, adjusting for potential confounders. A multivariate Cox regression, analyzing tertiles as a categorical variable using the upper tertile as reference, was performed to explore the association between efflux and time to surgery, adjusting for potential confounders. Clinical confounders were chosen based on previous evidence that associated some clinical parameters and statin use with AAA. The statistical software R (http://www.r-project.org) and GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA) were used to perform all statistical analyses. A p-value < 0.05 was considered to represent a significant difference in all the analyses.
3. Results
The clinical and plasma biochemical characteristics of the patients and controls are shown in Table 1. The body mass index (BMI) and diastolic blood pressure (DBP) of the AAA patients were significantly higher than those of the control group, whereas the ankle-brachial index (ABI) was lower than that of the control group. No differences among the groups were observed in terms of smoking habits, diabetes, arterial hypertension, a history of cardiovascular events, use of statins and use of low-dose aspirin. As previously reported [16,18], the apoA-I concentrations in the AAA patients at presentation were significantly lower than those in the control group. Only patients with large size AAAs presented with significantly lower total cholesterol concentrations as compared with those in the control group, and this was concomitant with lower concentrations of LDL cholesterol. Patients with small/medium size AAAs had higher concentrations of very-low density lipoprotein VLDL cholesterol and TG. There was no significant difference in HDLc concentrations when this parameter was compared among the three groups.
Table 1.
Clinical and plasma biochemical parameters of the abdominal aortic aneurysm (AAA) patients and controls.
| Parameters | Large Size (n = 38) |
Small/Medium Size (n = 81) |
Control (n = 39) |
ANOVA or Chi-Square p-Value |
|---|---|---|---|---|
| Age (y) | 69.71 ± 2.88 | 69.65 ± 2.81 | 68.87 ± 2.69 | ns |
| BMI (%) | 28.03 ± 2.42 † | 27.41 ± 3.48 † | 25.80 ± 2.55 | <0.01 |
| Total cholesterol (mmol/L) | 4.52 ± 0.71 † | 4.87 ± 0.88 | 5.24 ± 0.77 | <0.001 |
| TG (mmol/L) | 0.98 ± 0.40 # | 1.44 ± 0.66 † | 1.10 ± 0.36 | <0.001 |
| ApoA-I (g/L) | 1.53 ± 0.28 † | 1.59 ± 0.32 † | 1.80 ± 0.32 | <0.001 |
| HDLc (mmol/L) | 1.09 ± 0.43 | 1.09 ± 0.40 | 1.22 ± 0.42 | ns |
| LDLc (mmol/L) | 2.98 ± 0.81 † | 3.13 ± 0.90 | 3.52 ± 0.89 | <0.05 |
| VLDLc (mmol/L) | 0.45 ± 0.19 # | 0.65 ± 0.26 † | 0.50 ± 0.16 | <0.001 |
| Aortic diameter (mm) | 62.52 ± 15.35 †,# | 36.35 ± 4.54 † | 18.16 ± 2.90 | <0.001 |
| DBP (mm Hg) | 91.00 ± 13.62 † | 88.00 ± 12.30 † | 80.97 ± 11.36 | <0.01 |
| Lowest ABI | 0.99 ± 0.11 † | 0.95 ± 0.19 † | 1.10 ± 0.09 | <0.001 |
| Smoking | 8 (2%) | 32 (41%) | 15 (40%) | ns |
| Diabetes | 3 (8%) | 10 (13%) | 4 (8%) | ns |
| Arterial hypertension | 15 (40%) | 41 (52%) | 23 (61%) | ns |
| Previous CVD | 1 (18%) | 11 (14%) | 7 (3%) | ns |
| Statin use | 16 (42%) | 41 (52%) | 20 (53%) | ns |
| Low-dose aspirin | 8 (50%) | 35 (44%) | 19 (22%) | ns |
BMI = body mass index; TG = triglycerides; ApoA-I = apolipoprotein A–I; HDLc = High-density lipoprotein cholesterol; LDLc = low-density lipoprotein cholesterol; VLDLc = very low-density lipoprotein cholesterol; DBP = diastolic blood pressure; ABI = ankle brachial index; CVD = cardiovascular disease (acute myocardial infarction, angina or stroke); ANOVA = analysis of variance. Results expressed as mean ± standard deviation (SD). † p < 0.05 compared to the control group, # p < 0.05 compared to small/medium size, ns = non-significant.
The clinical and plasma biochemical characteristics of the small/medium size AAA groups, classified as subjects with low, medium and high AAA progression, are shown in Supplementary Materials Table S1. No significant differences were found for lipid, apoA-I and lipoprotein levels and almost all of the studied clinical parameters when they were compared among the three AAA progression groups, thereby indicating that these parameters were not related with AAA progression, at least in our sub-cohort of the VIVA trial. The only exception was the lower use of statins and aspirin in the high progression subgroup.
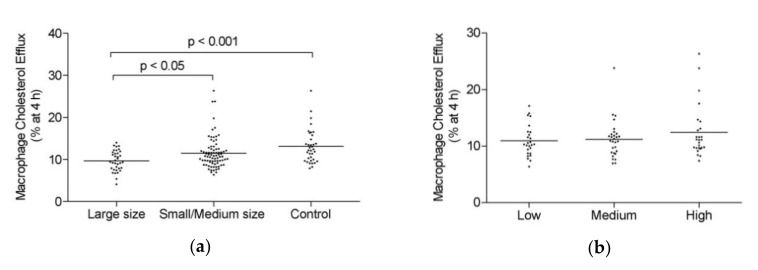
The ability of apoB-depleted plasma to induce MCE was evaluated in all the groups. MCE was impaired in the large size AAA group as compared with that in the small/medium size AAA group and the control group (Figure 1a). However, no significant differences in HDL-mediated MCE capacity were observed when the different AAA progression subgroups were compared (Figure 1b).
Figure 1.
High-density lipoprotein (HDL)-mediated macrophage cholesterol efflux (MCE) capacity. (a) Large size abdominal aortic aneurysm (AAA) (n = 38), small/medium size AAA patients (n = 81) and control group (n = 39). (b) Small/medium size AAA group based on progression: low (n = 26), medium (n = 29) and high (n = 26) progression. Scatter dot blots are shown, and the line represents the mean. Differences were assessed using Tukey’s multiple comparison test.
A significant linear trend was detected among the indicated groups after a one-way ANOVA analysis was performed (R square = 0.1010; p < 0.0001). The association between MCE capacity and AAA remained significant after adjusting for age, BMI and DBP (Supplementary Materials Table S2).
Univariate Pearson correlation tests revealed that the MCE capacity correlated negatively with the aortic baseline diameter and BMI and positively with apoA-I and HDLc in all subjects (Supplementary Materials Table S3). However, after adjusting for potential confounders, such as age, smoking, BMI, statin use and DBP, the MCE capacity did not correlate with the aortic baseline diameter (Table 2). Furthermore, when Pearson’s correlation tests were performed only in the AAA patients, the significant correlation between the MCE capacity and aortic diameter disappeared, whereas BMI and the apoA-I and HDLc concentrations remained significant (Supplementary Materials Table S3).
Table 2.
Multivariate linear regression of the aortic baseline diameter and macrophage cholesterol efflux (MCE) capacity in all subjects, adjusted for age, body mass index (BMI), smoking, statin use and diastolic blood pressure (DBP).
| Coefficients | |||
|---|---|---|---|
| Model | Standardized Coefficients | t | p-Value |
| Beta | |||
| Age | 0.050 | 0.634 | 0.527 |
| BMI | 0.145 | 1.622 | 0.107 |
| Smoke | 0.027 | 0.325 | 0.745 |
| Statins | 0.103 | 1.273 | 0.205 |
| DBP | 0.275 | 3.457 | 0.001 |
| MCE capacity | −0.115 | −1.295 | 0.198 |
| Dependent variable: aortic baseline diameter | |||
Moreover, no significant correlation was found between the MCE capacity and AAA growth rate in the small/medium size group (Table 3), even after adjusting for potential confounders (Supplementary Materials Table S4). Importantly, in the small/medium size AAA group, the positive associations between MCE capacity and apoA-I and HDLc concentrations remained significant, as well as the negative association with BMI.
Table 3.
Univariate correlations between high-density lipoprotein (HDL)-mediated macrophage cholesterol efflux (MCE) and abdominal aortic aneurysm (AAA) growth rate, apolipoprotein A-I (apoA-I), high-density lipoprotein cholesterol (HDLc) and body mass index (BMI) in the small/medium size AAA group.
| Growth Rate | ApoA-I | HDLc | BMI | |
|---|---|---|---|---|
| MCE capacity in small/medium size AAA group | 0.11 (−0.11–0.32) |
0.36 (0.16–0.54) |
0.37 (0.16–0.55) |
−0.36 (−0.54–(−0.15)) |
| p-value | ns | <0.001 | <0.001 | <0.01 |
Results expressed as r Pearson coefficient (95% confidence interval), ns = non-significant.
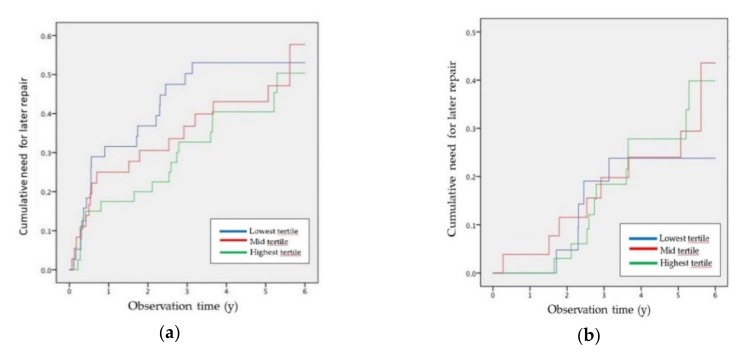
A multivariate Cox regression analysis was also conducted across HDL-mediated MCE tertiles to evaluate the association of the MCE capacity with the need for surgery, adjusted for potential confounders (smoking, a history of cardiovascular disease, use of low-dose aspirin, statins or angiotensin-converting enzyme inhibitors, DBP, BMI, lowest ABI and initial AAA diameter). When all the AAA patients were included (small/medium and large size groups), the need for surgical repair hazard ratio was significant after adjusting for potential confounders (Figure 2a and Supplementary Materials Table S5). However, this analysis did not reveal significant associations between the MCE capacity and the need for surgical repair when only small/medium size AAA patients were considered (Figure 2b and Supplementary Materials Table S6).
Figure 2.
Cox regression of the cumulative need for surgery based on high-density lipoprotein HDL-mediated macrophage cholesterol efflux (MCE) tertiles in all AAA patients (a) and in small/medium size AAA patients (b). The observation time to surgery is represented in years. The cumulative need for later repair represents the need for surgery over the follow-up. The lowest MCE tertile is shown in blue, the mid tertile is shown in green and the highest tertile is shown in red.
4. Discussion
Recent reports support the concept that lipoproteins play a role in the pathogenesis of AAAs [14,15,21]. In agreement with our previous results in AAA subjects with large AAA diameter, LDLc and apoA-I were downregulated in the late stages of the disease [18]. However, cholesterol transported by HDL did not change and it is not considered a good surrogate marker of the lipoprotein antiatherogenicity. We recently demonstrated that that HDL-facilitated MCE, one of the potential main surrogate markers of HDL function, was impaired in large size AAA patients [18]. In this study, in a larger cohort of AAA patients, we confirmed that HDL-facilitated MCE was downregulated in large size AAA patients compared with that of controls and small/medium size AAA patients. In a previous study, we also demonstrated oxidative modifications in some apoA-I residues of HDLs isolated from AAA tissue (>50 mm) obtained after surgery [22]. These apoA-I modifications were closely associated with reduced HDL-mediated MCE capacity in vitro and in vivo [22]. Myeloperoxidase-induced modification of apoA-I is mainly responsible for the loss of apoA-I cholesterol acceptor activity in AAAs by affecting the conformational stability of apoA-I and enhancing apoA-I displacement from HDLs and, therefore, catabolism [23]. Thus, oxidative modifications in apoA-I residues could partially explain the downregulation of HDL-mediated MCE capacity, particularly in late AAA stages.
Since we demonstrated that HDL-mediated MCE capacity was impaired in the late stages of AAA, a clinically relevant question is whether HDL-mediated MCE capacity is associated with the progression of the disease in small/medium size AAA patients. In this study, we evaluated the ability of apoB-depleted plasma, which includes mature HDL, nascent preβ-HDL particles and HDL regulatory proteins, to induce MCE in a cohort of AAA patients at different stages of AAA evolution. To our knowledge, our study is the first prospective study to evaluate the association of HDL-mediated MCE capacity with both the AAA growth rate and/or need for surgical repair in small/medium size AAA patients. When we evaluated the MCE capacity in the small/medium size AAA group, there were no differences in AAA progression, at least in terms of aneurysm growth. As the growth rate can be an incomplete reflection of the real progression of aneurysm [24], we also investigated the association of HDL-mediated MCE capacity with the time to surgery. When all the AAA patients were included (small/medium and large size groups), and after adjusting for potential confounders, the need for surgical repair was associated to lower MCE capacity. However, significance was lost when only small/medium size AAA patients were included. This was interpreted as a biased HR estimation caused by the inclusion of large size AAA patients, due to the impaired MCE capacity of these patients, whose surgical repair was already indicated. New cohort analyses with a larger number of events and more uniformity (for example, including only subjects with small AAAs between 30 and 40 mm) would be needed, however, to further confirm this point.
Overall, our results indicate that determining HDL function using MCE as a surrogate is irrelevant in terms of predicting AAA progression and need for surgical repair, as is also the case of HDLc. Indeed, HDLs display a wide variety of pleiotropic effects including antioxidant, anti-inflammatory, anti-protease, anti-thrombotic, anti-infectious, anti-apoptotic and vasodilatory roles that may be involved in AAA development. It should be noted that the injection of reconstituted discoidal HDLs reduced experimental AAA formation [25]. In line with these findings, we previously demonstrated that the injection of an apoA-I mimetic peptide (D4F) and overexpression of the main anti-inflammatory/antioxidant HDL enzyme paraoxonase (PON) 1 inhibited experimental AAA progression [16,26]. Furthermore, the serum activity of PON1 was reduced in a small cohort of AAA patients [26]. These results suggest that other HDL functions beyond MCE, such as their antioxidant and anti-inflammatory properties, may be significant determinants of AAA development and would warrant further study.
The present study has some limitations. We used a single sample collected to measure MCE. It would be interesting to test the variation of MCE in two sequential samples of the same small/medium AAA patients at two time points of their follow-up (at least on those who progress and would not require surgery) to evaluate whether the changes in MCE are correlated to changes in aortic diameter. In addition, it is important to note that to obtain correlation with AAA growth or time to surgery, we previously tested the whole VIVA cohort [27,28], so in this case, lack of power could also account for the obtained negative results. However, considering alpha = 0.05 and power = 80%, it was estimated that a difference of 2% in MCE could be detected by studying a minimum of 20 subjects in each group.
5. Conclusions
HDL-mediated MCE capacity was impaired in large size AAA patients but a lower MCE was not associated with the AAA growth rate and/or the need for surgical repair in small/medium size AAA patients, suggesting that this major HDL functional activity may not be mechanistically involved in AAA progression.
Acknowledgments
The English grammar and language was corrected by Scribendi (https://www.scribendi.com).
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/4/662/s1, Table S1: Clinical and biochemical parameters of small/medium size AAA patients, Table S2: One-way ANCOVA of MCE in all subjects adjusted for age, BMI and DBP, Table S3: Univariate correlations in all subjects between HDL-mediated macrophage cholesterol efflux (MCE) capacity and aortic diameter, apoA-I, HDLc and BMI, Table S4: Multivariate lineal regression of Growth Rate with MCE capacity in small/medium size AAA group adjusted for age, BMI, smoke, statins and DBP, Table S5: Multivariate Cox regression analysis of 5-year predictors of need to surgery in all AAA patients. MCE tertiles were analyzed as a categorical variable using the upper tertile as reference, Table S6: Multivariate Cox regression analysis of 5-years predictors of need to surgery in small/medium AAA patients. MCE tertiles were analyzed as a categorical variable using the upper tertile as reference.
Author Contributions
Conceptualization, F.B.-V., J.L.M.-V. and J.C.E.-G.; experimental procedures, M.C., I.F.-A. and D.S.; formal statistical analysis, M.C., D.d.G.-C. and J.S.L.; writing—original draft preparation, M.C. and M.T.; writing—review and editing, L.M.B.-C., J.C.E.-G., F.B.-V. and J.L.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly funded by the Instituto de Salud Carlos III and FEDER “Una manera de hacer Europa” grants FIS 16-00139 (to J.C.E-G), FIS 18-00164 (to F.B.-V. and M.T.), Spanish MINECO (SAF2016-80843-R) and La Caixa Foundation (HR17-00247). CIBERDEM and CIBERCV are Instituto de Salud Carlos III projects.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hirsch A.T., Haskal Z.J., Hertzer N.R., Bakal C.W., Creager M.A., Halperin J.L., Hiratzka L.F., Murphy W.R., Olin J.W., Puschett J.B., et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Ashton H.A., Buxton M.J., Day N.E., Kim L.G., Marteau T.M., Scott R.A., Thompson S.G., Walker N.M. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 3.Moll F.L., Powell J.T., Fraedrich G., Verzini F., Haulon S., Waltham M., van Herwaarden J.A., Holt P.J., van Keulen J.W., Rantner B., et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011;41(Suppl. 1):S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Kent K.C., Zwolak R.M., Egorova N.N., Riles T.S., Manganaro A., Moskowitz A.J., Gelijns A.C., Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 5.Wanhainen A. How to define an abdominal aortic aneurysm—Influence on epidemiology and clinical practice. Scand. J. Surg. 2008;97:105–109; discussion 109. doi: 10.1177/145749690809700204. [DOI] [PubMed] [Google Scholar]
- 6.Wanhainen A., Verzini F., Van Herzeele I., Allaire E., Bown M., Cohnert T., Dick F., van Herwaarden J., Karkos C., Koelemay M., et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019;57:8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Periard D., Guessous I., Mazzolai L., Haesler E., Monney P., Hayoz D. Reduction of small infrarenal abdominal aortic aneurysm expansion rate by statins. Vasa. 2012;41:35–42. doi: 10.1024/0301-1526/a000161. [DOI] [PubMed] [Google Scholar]
- 8.Lindholt J.S., Bjorck M., Michel J.B. Anti-platelet treatment of middle-sized abdominal aortic aneurysms. Curr. Vasc. Pharmacol. 2013;11:305–313. doi: 10.2174/1570161111311030005. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y.D., Liu Z.J., Ren J., Xiang M.X. Pharmacological Therapy of Abdominal Aortic Aneurysm: An Update. Curr. Vasc. Pharmacol. 2018;16:114–124. doi: 10.2174/1570161115666170413145705. [DOI] [PubMed] [Google Scholar]
- 10.Sakalihasan N., Limet R., Defawe O.D. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 11.Michel J.B., Martin-Ventura J.L., Egido J., Sakalihasan N., Treska V., Lindholt J., Allaire E., Thorsteinsdottir U., Cockerill G., Swedenborg J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajamaki K., Lappalainen J., Oorni K., Valimaki E., Matikainen S., Kovanen P.T., Eklund K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison S.C., Holmes M.V., Burgess S., Asselbergs F.W., Jones G.T., Baas A.F., van’t Hof F.N., de Bakker P.I.W., Blankensteijn J.D., Powell J.T., et al. Genetic Association of Lipids and Lipid Drug Targets With Abdominal Aortic Aneurysm: A Meta-analysis. JAMA Cardiol. 2018;3:26–33. doi: 10.1001/jamacardio.2017.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng L.C., Roetker N.S., Lutsey P.L., Alonso A., Guan W., Pankow J.S., Folsom A.R., Steffen L.M., Pankratz N., Tang W. Evaluation of the relationship between plasma lipids and abdominal aortic aneurysm: A Mendelian randomization study. PLoS ONE. 2018;13:e0195719. doi: 10.1371/journal.pone.0195719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burillo E., Lindholt J.S., Molina-Sanchez P., Jorge I., Martinez-Pinna R., Blanco-Colio L.M., Tarin C., Torres-Fonseca M.M., Esteban M., Laustsen J., et al. ApoA-I/HDL-C levels are inversely associated with abdominal aortic aneurysm progression. Thromb. Haemost. 2015;113:1335–1346. doi: 10.1160/TH14-10-0874. [DOI] [PubMed] [Google Scholar]
- 17.Lee-Rueckert M., Escola-Gil J.C., Kovanen P.T. HDL functionality in reverse cholesterol transport—Challenges in translating data emerging from mouse models to human disease. Biochim. Biophys. Acta. 2016;1861:566–583. doi: 10.1016/j.bbalip.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Lopez D., Cedo L., Metso J., Burillo E., Garcia-Leon A., Canyelles M., Lindholt J.S., Torres-Fonseca M., Blanco-Colio L.M., Vazquez J., et al. Impaired HDL (High-Density Lipoprotein)-Mediated Macrophage Cholesterol Efflux in Patients With Abdominal Aortic Aneurysm-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2018;38:2750–2754. doi: 10.1161/ATVBAHA.118.311704. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Carrio J., Lindholt J.S., Canyelles M., Martinez-Lopez D., Tondo M., Blanco-Colio L.M., Michel J.B., Escola-Gil J.C., Suarez A., Martin-Ventura J.L. IgG Anti-High Density Lipoprotein Antibodies Are Elevated in Abdominal Aortic Aneurysm and Associated with Lipid Profile and Clinical Features. J. Clin. Med. 2019;9:67. doi: 10.3390/jcm9010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escola-Gil J.C., Lee-Rueckert M., Santos D., Cedo L., Blanco-Vaca F., Julve J. Quantification of In Vitro Macrophage Cholesterol Efflux and In Vivo Macrophage-Specific Reverse Cholesterol Transport. Methods Mol. Biol. 2015;1339:211–233. doi: 10.1007/978-1-4939-2929-0_15. [DOI] [PubMed] [Google Scholar]
- 21.Forsdahl S.H., Singh K., Solberg S., Jacobsen B.K. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromso Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Lopez D., Camafeita E., Cedo L., Roldan-Montero R., Jorge I., Garcia-Marques F., Gomez-Serrano M., Bonzon-Kulichenko E., Blanco-Vaca F., Blanco-Colio L.M., et al. APOA1 oxidation is associated to dysfunctional high-density lipoproteins in human abdominal aortic aneurysm. EBioMedicine. 2019;43:43–53. doi: 10.1016/j.ebiom.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy A.J., Woollard K.J., Hoang A., Mukhamedova N., Stirzaker R.A., McCormick S.P., Remaley A.T., Sviridov D., Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 24.Thompson S.G., Brown L.C., Sweeting M.J., Bown M.J., Kim L.G., Glover M.J., Buxton M.J., Powell J.T. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: Implications for surveillance intervals and their cost-effectiveness. Health Technol. Assess. 2013;17:1–118. doi: 10.3310/hta17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torsney E., Pirianov G., Charolidi N., Shoreim A., Gaze D., Petrova S., Laing K., Meisinger T., Xiong W., Baxter B.T., et al. Elevation of plasma high-density lipoproteins inhibits development of experimental abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2012;32:2678–2686. doi: 10.1161/ATVBAHA.112.00009. [DOI] [PubMed] [Google Scholar]
- 26.Burillo E., Tarin C., Torres-Fonseca M.M., Fernandez-Garcia C.E., Martinez-Pinna R., Martinez-Lopez D., Llamas-Granda P., Camafeita E., Lopez J.A., Vega de Ceniga M., et al. Paraoxonase-1 overexpression prevents experimental abdominal aortic aneurysm progression. Clin. Sci. (Lond.) 2016;130:1027–1038. doi: 10.1042/CS20160185. [DOI] [PubMed] [Google Scholar]
- 27.Lindholt J.S., Kristensen K.L., Burillo E., Martinez-Lopez D., Calvo C., Ros E., Martin-Ventura J.L., Sala-Vila A. Arachidonic Acid, but Not Omega-3 Index, Relates to the Prevalence and Progression of Abdominal Aortic Aneurysm in a Population-Based Study of Danish Men. J. Am. Heart Assoc. 2018;7:e007790. doi: 10.1161/JAHA.117.007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Garcia C.E., Burillo E., Lindholt J.S., Martinez-Lopez D., Pilely K., Mazzeo C., Michel J.B., Egido J., Garred P., Blanco-Colio L.M., et al. Association of ficolin-3 with abdominal aortic aneurysm presence and progression. J. Thromb. Haemost. 2017;15:575–585. doi: 10.1111/jth.13608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.