Abstract
The accumulation of abnormal protein aggregates represents a universal hallmark of neurodegenerative diseases (NDDs). Post-translational modifications (PTMs) regulate protein structure and function. Dysregulated PTMs may influence the propensity for protein aggregation in NDD-proteinopathies. To investigate this, we systematically reviewed the literature to evaluate effects of PTMs on aggregation propensity for major proteins linked to the pathogenesis and/or progression of NDDs. A search of PubMed, MEDLINE, EMBASE, and Web of Science Core Collection was conducted to retrieve studies that investigated an association between PTMs and protein aggregation in seven NDDs: Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), spinocerebellar ataxias, transmissible spongiform encephalopathy, and multiple sclerosis. Together, 1222 studies were identified, of which 69 met eligibility criteria. We identified that the following PTMs, in isolation or combination, potentially act as modulators of proteinopathy in NDDs: isoaspartate formation in Aβ, phosphorylation of Aβ or tau in AD; acetylation, 4-hydroxy-2-neonal modification, O-GlcNAcylation or phosphorylation of α-synuclein in PD; acetylation or phosphorylation of TAR DNA-binding protein-43 in ALS, and SUMOylation of superoxide dismutase-1 in ALS; and phosphorylation of huntingtin in HD. The potential pharmacological manipulation of these aggregation-modulating PTMs represents an as-yet untapped source of therapy to treat NDDs.
Keywords: neurodegenerative diseases, neurotoxicity, post-translational modifications, protein aggregates
1. Introduction
Neurodegenerative diseases (NDDs) are a major cause of global morbidity and mortality in the elderly, and, with an ever-rising prevalence, represent one of the greatest health challenges of the 21st century. NDDs encompass heterogeneous cerebral proteinopathies, characterised by a progressive loss of vulnerable neurons such that patients present with broad clinical sequelae that includes motor, behavioural, and cognitive deficits [1,2,3]. At autopsy, NDDs can be characterised histopathologically via hallmark intra- or extracellular accumulations of degradation-resistant protein aggregates concentrated to certain brain regions (Table 1). These protein aggregates interfere with neuronal function, and presumably induce toxicity that ultimately drives cell death [2].
Table 1.
Overview of neuropathology, approximate global prevalence rates, and frequency of genetic and sporadic forms of major neurodegenerative diseases [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29].
| NDD | Commonly Mutated Proteins | Primary Region of Damage | Compartment of Aggregate Deposition | Aggregate-Forming Proteins | Global Prevalence | Sporadic Cases | Familial Cases |
|---|---|---|---|---|---|---|---|
| AD | APP, presenilins | Cortex, hippocampus | Extracellular, intracytoplasmic | Aβ (plaques), tau (tangles) | 593:100,000 | >98% | <2% |
| PD | α-synuclein, LRRK2 | Substantia nigra, cortex | Intracytoplasmic | α-synuclein (Lewy bodies) | 1–2:1000 | >90% | <10% |
| HD | Htt | Striatum, basal ganglia | Intranuclear, intracytoplasmic | Htt | 1:10,000 | 3% | 97% |
| ALS | TDP-43, SOD1, c9orf72 | Spinal motor neurons, motor cortex | Intracytoplasmic | SOD1, TDP-43 | 5:100,000 | 90–95% | 5–10% |
| MS | - | Basal ganglia, brainstem | Intracytoplasmic, extracellular | Aβ, tau, APP, bassoon protein | 30.1:100,000 | 80–90% | 10–20% |
| SCAs | ATX1, ATX2, ATX3, CACNA1A, ATX7, TBP, ATN1 | Cerebellum, brainstem | Intranuclear | Atrophin-1, ataxins | 3:100,000 | No data | No data |
| TSEs | PrP | Cortex, brainstem, thalamus, cerebellum | Extracellular | PrP | 1–2:1,000,000 | 85–90% | 10–15% |
Abbreviations: APP, Amyloid precursor protein; LRRK2, Leucine-rich repeat kinase-2; Htt, Huntingtin; TDP-43, TAR DNA-binding protein 43; c9orf72, Chromosome 9 open reading frame 72; SOD1, Superoxide dismutase 1; ATX, ataxins; CACNA1A, Voltage-gated calcium channel subunit α1A; TBP, TATA-binding protein; ATN1, atrophin 1; PrP, Prion protein.
Although the pathogenesis of Huntington’s disease (HD) and spinocerebellar ataxias (SCAs), as well as rare familial forms of other NDDs are influenced by gene mutations that affect protein structure and function, the majority of NDDs arise from a multifactorial idiopathic aetiology.
Numerous drug therapies to treat NDDs have entered clinical trials over the last two decades, but their low success rates underscore substantial gaps in understanding of the molecular mechanisms that drive neurodegeneration in sporadic diseases [1,2,3]. Consequently, there are no specific curative treatments available to reverse or even halt the progression of brain pathology in NDDs, and all therapeutics licensed for treatment merely work at a symptomatic level. Furthermore, the number of patients with these age-related diseases is rising due to increased longevity, and this carries an enormous social and economic global burden. It is therefore crucial to elucidate the molecular mechanisms that trigger protein aggregation and subsequent neurotoxicity in order to identify potential targets for drug development, to resist or possibly reverse disease progression.
1.1. Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common NDD and accounts for 60–80% of all cases of dementia [27,30]. More than 30 million people are currently suffering from AD, and this number is estimated to increase to 115 million by 2050 [31]. Clinically, patients predominantly present with a decline in cognitive function that initially manifests as episodic short-term memory loss, but as the disease progresses, visuospatial, behavioural, and psychiatric disturbances follow and eventually lead to the inability to perform activities of daily living [32]. Diagnosing AD relies heavily on patient history and cognitive function tests such as the Mini Mental State Examination or the Montreal Cognitive Assessment, but screening for plasma and CSF biomarkers and brain imaging have proven helpful ancillary methods [1,33]. Nevertheless, macroscopic confirmation of AD is provided from post-mortem examination, and typified by widespread cortical atrophy primarily within the frontotemporal lobes (including the hippocampus) that leads to an enlargement of the lateral ventricles [1,2,34]. Microscopically, AD pathology is characterised by extracellular plaque deposits of aggregated amyloid-beta peptide (Aβ) as well as intracellular neurofibrillary tangles (NFTs) primarily composed of hyperphosphorylated fibrils of microtubule-associated protein tau [2,34].
Aβ peptide is primarily composed of 42–43 amino acids formed from proteolytic cleavage of the transmembranous amyloid precursor protein (APP) [34,35]. Two proteases that act on APP, a β-secretase and γ-secretase, yield the Aβ40 and Aβ42 isoforms, respectively. Alternatively, APP may be processed by α-secretase via the non-amyloidogenic pathway [35,36,37]. While Aβ is believed to play a number of physiological roles, including regulation of synaptic activity [36,37], in AD, it acquires a toxic gain-of-function, undergoes oligomerisation and aggregation, eventually forming insoluble, fibrillar, senile plaques that are central to neurotoxicity and neurodegeneration [2,38,39].
Human tau protein exists in six isoforms of between 352 and 441 amino acids encoded by the MAPT gene on chromosome 17q21.31. It is localised predominantly to neuronal axons, where its primary function is the stabilisation of microtubules and regulation of neuronal transport [40,41,42]. In AD, abnormally phosphorylated tau adopts an altered conformation that hinders its binding to microtubules and promotes its self-assembly (aggregation) into paired helical filaments (PHFs), the primary component of NFTs [40,41,42]. It is these tau aggregates and Aβ aggregates that have been the subject of targeted therapies [42].
1.2. Parkinson’s Disease
Approximately 1% of individuals over the age of 60 years and close to 5% of the population aged 80 or older suffer from Parkinson’s disease (PD), the second most common NDD and most common movement disorder [43]. PD is characterised by a progressive loss of dopaminergic neurons in the subcortical basal ganglia; specifically, within the substantia nigra pars compacta (SNpc) [1,2,43,44,45]. Since this midbrain region plays a crucial role in fine-tuning motor circuits and facilitating movement, PD manifests clinically with a pathognomonic triad of bradykinesia, rigidity, and resting tremor [1,45]. In addition to nigral degeneration, the primary pathological feature of PD is the presence of intraneuronal proteinaceous inclusions termed Lewy bodies (LBs), primarily composed of α-synuclein [2,46,47].
α-synuclein is a cytosolic 140 amino acid protein encoded by the SNCA gene on chromosome 4q21. Although widely expressed within neurons, it is abundant at presynaptic terminals, indicative of a role in synaptic signalling. A number of rare SNCA point mutations trigger dominant familial (early-onset) forms of PD. Fibrillar forms of α-synuclein have been identified within LBs that accumulate in hereditary and sporadic forms of PD [46,47,48,49]. Oligomeric and/or fibrillar aggregates of α-synuclein may be cytotoxic and trigger neuronal degeneration.
1.3. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal, incurable NDD characterised by the loss of upper and lower motor neurons. ALS typically presents with symptoms of bulbar and spinal dysfunction including muscle weakness, wasting and spasticity, and ultimately, patients may become paralysed and die from respiratory failure [2,50,51]. Pathologically, both sporadic and familial ALS are characterised by marked cytoplasmic protein aggregates in degenerating neurons that harbour ubiquitinated forms of Trans-activation response DNA-binding protein 43 (TDP-43) and the antioxidant enzyme, superoxide dismutase 1 (SOD1) [10,50,51].
TDP-43 is a 414 amino acid protein encoded by the TARDBP gene on chromosome 1 and is normally nuclear resident and involved in the regulation of RNA metabolism. In ALS, cytoplasmic, ubiquitinated, hyperphosphorylated, and truncated forms of TDP-43 accumulate as protein aggregates [52,53,54]. These cytoplasmic aggregates are neurotoxic, produce ALS-like phenotypes, and contribute to a loss of nuclear (functional) TDP-43 [52,53,54,55].
Superoxide dismutase 1 (SOD1) is a 153-amino acid copper- and zinc-dependent metalloenzyme encoded by the SOD1 gene on chromosome 21q22.11. SOD1 functions to scavenge highly reactive superoxide radicals by catalysing their conversion to hydrogen peroxide and molecular oxygen [56]. SOD1 is predominantly cytoplasmic, although nuclear, lysosomal, and mitochondrial residences have also been reported [57]. In SOD1-mediated familial ALS, mutations in the SOD1 gene sequence are thought to affect post-translational processing, rendering the protein prone to misfolding, aggregation, and formation of neuronal inclusion bodies. Misfolded SOD1 is also found in cytoplasmic inclusions in patients with sporadic ALS, as well as other proteins including TDP-43 [58]; suggesting a common pathophysiological mechanism for hereditary and idiopathic ALS.
1.4. Huntington’s Disease
Huntington’s disease (HD) is a rare, autosomal dominant NDD with an average age of onset of 40 years [59]. HD is caused by an expansion of the CAG repeat (≥36) in the Huntingtin (Htt) gene on chromosome 4p16.3, that when translated produces an elongated polyglutamine (polyQ) stretch in the Htt protein [59]. The formation of aggregated Htt within neuronal intranuclear inclusion bodies is a histopathological hallmark of HD [60]. Additionally, the degeneration of GABAergic medium spiny neurons in the striatum that project to other regions of the basal ganglia and thereby modulate central motor circuitries, is central to HD pathology [61]. This is reflected in the clinical picture of HD such that patients present with severe motoric abnormalities including prominent ‘dance-like’ involuntary movements termed chorea [62]. With disease progression, other brain regions undergo degeneration, with an associated array of additional psychiatric, behavioural, and cognitive symptoms.
Htt is a large, 3144-amino acid, monomeric protein required for embryonic neurogenesis that controls a number of nuclear and cytoplasmic homeostatic functions including regulation of synaptic activity [63,64]. In HD, the expanded polyQ stretch of the protein induces production of N-terminal Htt fragments that are prone to misfold and form amyloid-like structures [65,66,67,68]. Furthermore, polyQ tracts have a tendency to form β-pleated sheets and this conformational change directly increases the aggregation propensity of Htt [66,67,68]. These changes result in the assembly of oligomeric structures and eventually the formation of Htt-positive intracellular inclusion bodies that disrupt cellular homeostasis and cause neuronal degeneration [69].
1.5. Spinocerebellar Ataxias
Spinocerebellar ataxias (SCAs) are a heterogeneous group of >27 different autosomal dominant NDDs that share the phenotypical core feature of ataxia and are characterized by degenerative and atrophic changes in the central nervous system, primarily affecting the cerebellum [70]. The most common SCAs belong to the polyQ repeat diseases and are caused by CAG expansion mutations in a number of different genes [71].
1.6. Transmissible Spongiform Encephalopathy
Transmissible spongiform encephalopathies (TSEs), also known as prion diseases, are progressive NDDs that typically present with variable symptomology including dementia and ataxia [72]. They can be classified according to their genetic, acquired, and sporadic aetiology, with the most common TSE being sporadic Creutzfeldt-Jakob disease [23]. TSEs typically present with a pathology that involves neuronal vacuolation (spongiform change), widespread neuronal death, and subsequent gliotic scarring in cerebral grey matter secondary to the accumulation of aberrant, self-propagating prion protein scrapie (PrPsc) [73]. Diagnostic criteria as well as ancillary tests such as electroencephalography or genetic testing facilitate diagnosis, but only at autopsy can a definitive diagnosis be confirmed [74]. Due to the propagating nature of TSEs and lack of disease-modifying or curative treatments, after clinical onset, the disease course is usually short with a median survival of 5 months [75].
The prion gene PRNP on chromosome 20 encodes a human prion glycoprotein, PrPc, of 253 amino acids localised to pre- and postsynaptic terminals in the CNS [76,77,78]. Its precise physiological function has been debated and is considered non-essential [79]: indicative that a toxic gain-of-function rather than loss-of-function of PrPc underlies neurotoxicity in prion diseases [80]. In TSEs, PrPc misfolds and is partially converted from an α-helical to β-sheet structure that is resistant to proteolytic clearance, PrPsc [78,81]. Misfolded PrPsc accumulates in synaptic and axonal processes and forms fibrillar aggregates that are capable of inducing neuronal apoptosis in vitro and in vivo [80,82]. Pathology is thought to be propagated when abnormal PrPsc converts normal PrPc into PrPsc via an autocatalytic mechanism [83].
1.7. Multiple Sclerosis
Multiple Sclerosis (MS) is traditionally classed as a neuroinflammatory disease caused by autoimmune-mediated axonal demyelination within the CNS [84,85]. Clinical presentation is variable since focal inflammatory lesions may affect any CNS structure, and the diagnostic process is therefore based on a combination of clinical history and examination, brain imaging, and blood screening [84]. There are four MS subtypes: relapsing remitting (RRMS), progressive relapsing (PRMS), primary progressive (PPMS) and secondary progressive (SPMS), and each has a unique therapeutic approach and prognosis [84,85].
While most subjects (≈85%) are diagnosed with RRMS at symptomatic onset, the majority of patients will progress to development of highly debilitating SPMS [85]. Post-mortem analyses of MS patients have demonstrated degenerative lesions and significant cerebral atrophy indicative of a NDD. Furthermore, the identification of proteinaceous, oligomeric aggregates, that include Aβ, tau, and APP, as well as deposits in neuronal somata composed of aggregated bassoon (Bsn) protein, may represent a link between neuroinflammation and neurodegeneration in MS [8,12,13].
Bsn is a large, 3926 amino acid, scaffold protein that is part of the presynaptic cytoskeletal matrix, and has multiple roles in mediating synaptic function [12,86]. In MS, Bsn is mis-localised and accumulates in neuronal cells, to induce neurotoxicity and neurodegeneration [12].
1.8. Protein Aggregation in Neurodegeneration
Protein misfolding and accumulation of toxic aggregates has emerged as a central theme of paradigmatic NDDs [87]. Protein turnover is an orchestrated process controlled via a balance of protein synthesis and degradation. Cells are equipped with efficient protein quality control mechanisms such as the ubiquitin-proteasome system and chaperone-mediated autophagy that are able to eliminate aberrant or misfolded proteins [87,88]. However, the capacity or indeed fidelity of these degradative pathways may be compromised with a corresponding accumulation of damaged, misfolded, or aggregated proteins, and associated pathology. Hence the formation of protein aggregates may serve as an initiating step in cellular dysfunction in NDDs, and it is therefore critical to understand the molecular mechanisms that alter the protein aggregation of these NDD-associated proteins.
1.9. Post-Translational Modifications and NDDs
The chemical modifications of proteins during or after their biosynthesis via the covalent attachment of functional groups or proteolytic cleavage at specific amino acids are collectively termed protein post-translational modifications (PTMs). PTMs can be mediated by enzymatic or non-enzymatic means and may be reversible or irreversible. Over 600 different PTMs have been demonstrated experimentally [89]. This plethora of PTMs diversifies the proteome by modulating the structural and functional properties of proteins. Due to their pivotal role in regulating cellular processes and roles in ageing, protein PTMs may need to be strictly controlled, such that dysregulation of PTMs could contribute to disease pathogenesis or progression [90,91].
The major risk factor for developing NDD is age, and this may also correlate with disturbed PTM homeostasis. One could hypothesise that as an individual ages the likelihood of dysregulation of PTMs increases, and if this leads to aberrant modification of susceptible proteins then protein misfolding and aggregation, neurotoxicity, and ultimately neurodegeneration may ensue. Against this backdrop, the identification of the PTMs that drive dysregulated protein function proffer a therapeutic window if the formation of toxic protein aggregates could be obviated. Indeed, recent animal studies have demonstrated that therapies that influence protein PTMs provide a viable means to modulate proteinopathy and associated neurodegeneration [91,92].
The aim of this systematic research was to provide a comprehensive, unbiased analysis of the PTMs that have been reported to affect the aggregation propensity of proteins implicated in the pathology of AD, PD, ALS, HD, SCAs, TSEs, and MS. Mapping of the patterns and number of studies demonstrating aggregation-modulating PTMs will improve our understanding of the extent and site-specificity of aggregation-inducing PTMs, and thereby provide an insight into novel therapeutic targets that may limit pathogenesis and/or disease progression.
2. Methods
A systematic review of the literature was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.1. Search Strategy
From the 7th to 17th September 2019, a systematic electronic database search was conducted on MEDLINE (OvidSP), EMBASE (OvidSP), Web of Science Core Collection, and PubMed to retrieve all experimental studies investigating the effect of PTMs on protein aggregation in the pre-specified NDDs. A combination of controlled vocabulary (MeSH) and free-text keywords were used for each of the four concepts and search terms included: (a) neurodegenerative diseases, nerve degeneration, neurodegenerat *, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease, spinocerebellar ataxia, transmissible spongiform encephalopathy, and multiple sclerosis; (b) protein aggregation, aggreg *; (c) beta-amyloid, tau, alpha-synuclein, TDP-43, superoxide dismutase 1, huntingtin, prions, protein aggregate, inclusion bodies; (d) post-translational modifications, protein processing. The full search strategy for MEDLINE is provided as Supplementary Data S1. Additional studies were identified through bibliography screening of relevant review articles and hand-searching of relevant articles.
2.2. Eligibility Criteria
All search results (n = 1222) were exported into Endnote (Clarivate Analytics) and Excel (Microsoft) for duplicate removal (automatic deduplication and manual checking) and title/abstract screening to identify studies deemed appropriate to the pre-specified inclusion criteria. Included articles were original studies directly investigating the effect of PTMs at residues of human proteins central to pathology in AD, PD, ALS, HD, SCAs, TSEs, or MS for which the PTM had an effect on aggregation behaviour. Studies were excluded if they focused on proteins or NDDs other than those pre-defined, or were published in a language other than English, or performed with non-human proteins or tissue, or were review articles, editorials, or conference abstracts.
2.3. Data Acquisition and Analysis
Eligible publications were read in full and information for the following variables collated as a data extraction spreadsheet: NDD; protein investigated; type of PTM; site of PTM; peptide or protein source; in vitro or in vivo study; assay method for aggregation; overall study findings and conclusion; authors and year of publication.
3. Results
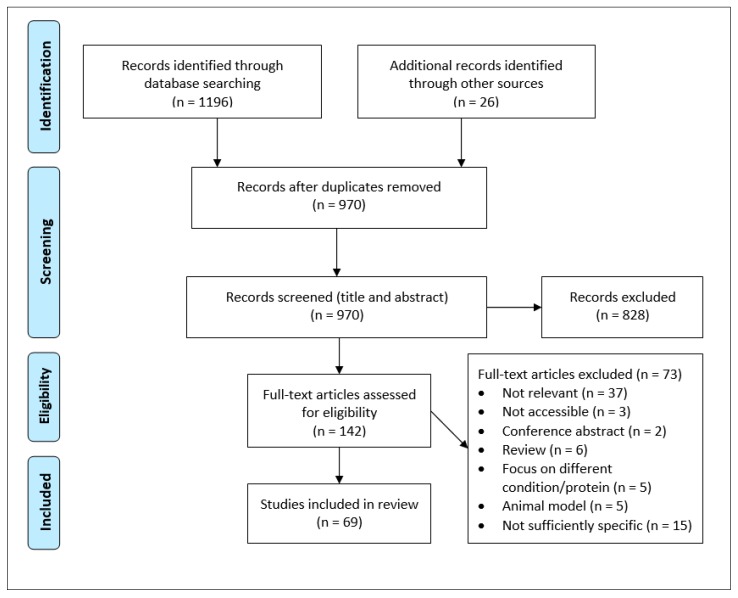
A total of 1196 articles were identified from the database search and an additional 26 potentially relevant papers yielded from hand-searching key papers. Duplicate articles were removed, and then 166 papers excluded based upon title screening, yielding 804 papers for abstract review. Further screening led to the exclusion of 662 articles and left a final 142 papers for full-text assessment. Of these, 73 studies did not meet the pre-defined eligibility criteria and were rejected based on the following grounds: off-topic (n = 37), full-text not accessible (n = 3), conference abstract (n = 2), review article (n = 6), focus on condition/protein not pre-specified in the inclusion criteria (n = 5), animal model (n = 5), lack of specificity (n = 15). The remaining 69 articles fulfilled the inclusion criteria and were included in the final analysis (Figure 1).
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart detailing the stages of study retrieval and selection [94].
Of the 69 included studies, the majority were focused upon PTMs and protein aggregation in AD (n = 28) and then PD (n = 20), 10 studies considered ALS, 7 HD, and 2 studies each examined SCAs and TSEs, respectively. No studies investigating the effect of PTMs on protein aggregation in the context of MS were identified from this search. The most commonly employed method to assess aggregation behaviour was Thioflavin T (ThT) fluorescence for quantitation of in vitro formation of amyloid-like fibrils [93]. Other studies that examined aggregation included the use of Western blotting, immunohistochemistry, sedimentation assays, atomic force microscopy, and transmission electron microscopy. The results for each NDD-related protein are presented and stratified according to effects on: (1) aggregation in general; (2) formation of oligomeric species; (3) formation of fibrillar aggregates; and (4) formation of amorphous aggregates.
3.1. Alzheimer’s Disease
3.1.1. Aβ PTMs and Propensity for Aggregation
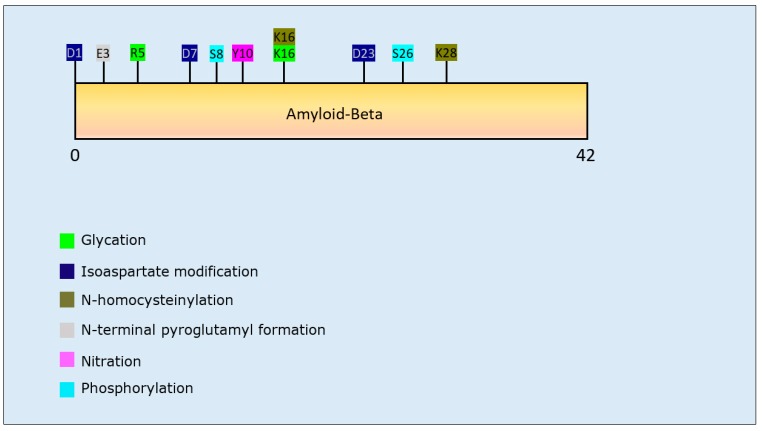
Eleven studies focused upon aggregation behaviour of Aβ. Two studies investigated isoaspartate modification (collectively covering residues D1, D7, and D23) and both reported that it had a pro-aggregation effect, with enhanced formation of Aβ oligomers as well as fibrillar aggregates [95,96]. A similar pro-aggregation effect was observed after N-terminal pyroglutamylation at E3 of Aβ [97]. The effect of phosphorylation on aggregation propensity of Aβ varied for different residues: specifically, S8 phosphorylation increased the formation of oligomeric and high molecular weight species [98,99], while phosphorylation at S26 had an overall inhibitory effect on the formation of large aggregates through stabilising intermediate oligomers [100]. Reduced transition from oligomer to fibril was also observed for N-homocysteinylation at K16 and K28 [101]. The results relating to nitration at Y10 were inconclusive: two studies reported a decrease of Aβ aggregation propensity [102,103], whereas one study reported a pro-aggregation effect [104]. Lastly, glycation at R5 and K16 reduced the formation of fibrillar aggregates [105]. A detailed overview of the effect of each PTM on the aggregation behaviour of Aβ is presented in Table 2.
Table 2.
Summary of β-amyloid PTMs and propensity for protein aggregation.
| Post-Translational Modification | Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| Glycation | R5, K16 | Emendato et al., 2018 [105] | Decrease | - | Decrease | - |
| Isoaspartate formation | D23 | Shimizu et al., 2002 [96] | Increase | - | Increase | - |
| D1, D7, D23 | Fossati et al., 2013 [95] | Increase | Increase | Increase | - | |
| N-Homocysteinylation | K16, K28 | Khodadadi et al., 2012 [101] | Decrease | Increase | Decrease | - |
| N-terminal pyroglutamylation | N-terminal E3 | Schilling et al., 2006 [97] | Increase | Increase | Increase | - |
| Nitration | Y10 | Kummer et al., 2011 [104] | Increase | Increase | Increase | - |
| Y10 | Zhao et al., 2015 [102] | Decrease | Decrease | Decrease | - | |
| Y10 | Guivernau et al., 2016 [103] | Decrease | Increase | Decrease | - | |
| Phosphorylation | S8 | Jamasbi et al., 2017 [98] | Increase | - | Increase | - |
| S8 | Kumar et al., 2011 [99] | Increase | Increase | Increase | - | |
| S26 | Kumar et al., 2016 [100] | Decrease | Increase | Decrease | - |
Interestingly, all PTMs that affected Aβ aggregation were within the first 28 residues of the peptide (Figure 2), leaving the 29–40 C-terminal section less prone to structural alterations.
Figure 2.
Schematic representation of beta-amyloid-42 peptide illustrating PTMs and respective amino acid residues.
3.1.2. Tau PTMs and Propensity for Aggregation
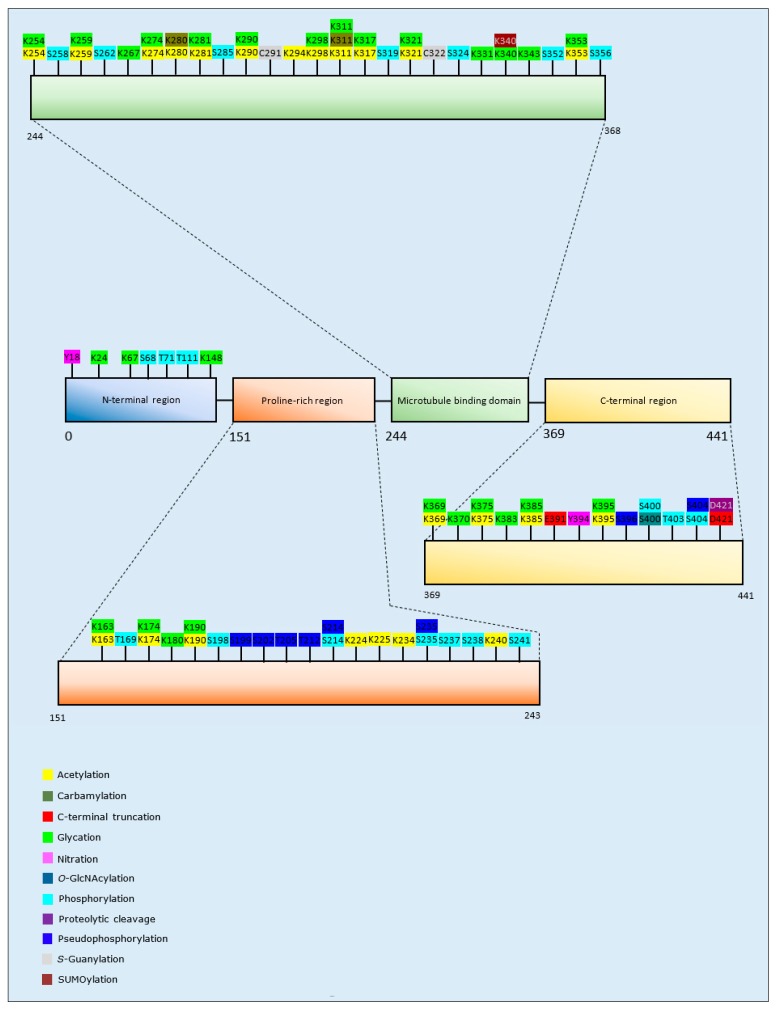
The majority of studies that have investigated PTMs of tau and protein aggregation have focused upon acetylation. Collectively, the results of acetylation analyses have been equivocal; with a demonstration of increased aggregation after acetylation at K280/K281 [106,107,108], but with acetylation at multiple lysine residues, including K280/281, also reported to decrease aggregation and the fibrillation rate of tau [109,110]. Furthermore, K321 acetylation alone or in combination with acetylation at other lysine residues decreased the level of tau aggregates [110,111].
Carbamylation, C-terminal truncation, glycation, proteolytic cleavage, pseudo-phosphorylation (replacement of a phospho-acceptor amino acid with a negatively charged amino acid to mimic phosphorylation), and SUMOylation all primarily increased tau assembly into aggregates [112,113,114,115,116,117,118], with the exception of pseudo-phosphorylation at S235 that significantly reduced tau aggregation [116]. O-GlcNAcylation, S-guanylation, and methylation decreased aggregation [119,120,121]. Likewise, site-specific tau nitration at Y18 and Y394 resulted in the formation of fewer and/or shorter tau filaments compared to the unmodified protein [122]. The effect of phosphorylation of tau and aggregation varied for different isoforms: phosphorylation of 4R2N and 3R1N at specific residues increased tau aggregation, while phosphorylation of 4R0N and 3R2N tau isoforms decreased their aggregation [114]. Additionally, one study identified an ‘acetylation-phosphorylation switch’, whereby acetylation of K321 was able to prevent phosphorylation at S324, a common PTM observed in tau inclusions in patients with AD [111]. A summary of the PTMs of tau that influence aggregation propensity are included as Table 3, and Figure 3.
Table 3.
Summary of Tau PTMs and propensity for protein aggregation.
| Post-Translational Modification | Isoform and Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| Acetylation | 4R2N: K280/K281 | Trzeciakiewicz et al., 2017 [106] | Increase | - | Increase | - |
| 4R2N: K163/K174/K190/K224/K234/K240/K254/K280/K281/K290/K311/K375/K385/K395 | Ferreon et al., 2018 [109] | Decrease | - | Decrease | - | |
| 4R0N: K321, K259/K290/K321/K353, K290/K321, K274 | Carlomagno et al., 2017 [111] | Decrease | - | Decrease | - | |
| 4R2N: K163, K174, K224, K225, K234, K240, K259, K274, K280, K290/K321, K294, K298, K317, K353, K369 | Kamah et al., 2014 [110] | Decrease | - | Decrease | - | |
| 4R2N: K280 | Haj-Yahya and Lashuel, 2018 [107] | Increase | Increase | Decrease | - | |
| 4R, Tau-K18: K163/K280/K281/K369 | Cohen et al., 2011 [108] | Increase | - | Increase | - | |
| Carbamylation | 4R2N: K311, K280, K311/K280 | KrishnaKumar et al., 2018 [112] | Increase | - | Increase | - |
| C-terminal Truncation | 4R2N: D421, E391 | Yin and Kuret, 2006 [113] | Increase | - | Increase | - |
| Glycation | 4R2N: K67, K148, K163, K180, K190, K259, K267, K274, K281, K290, K298, K311, K317, K321, K331, K340, K343, K353, K369, K370, K375, K383, K385, K395 | Liu et al., 2016 [114] | Increase | - | Increase | - |
| 3R2N: K24, K163, K174, K180, K190, K254, K259, K267, K311, K343, K353, K369, K385 | Liu et al., 2016 [114] | Increase | - | Increase | - | |
| Methylation | 4R2N: Multiple residues* | Funk et al., 2014 [121] | Decrease | - | Decrease | - |
| Nitration | 4R2N: Y18, Y394 | Reynolds et al., 2005 [122] | Decrease | - | Decrease | - |
| O-GlcNAcylation | 4R2N: S400 | Yuzwa et al., 2014 [119] | Decrease | - | Decrease | - |
| Phosphorylation | 4R2N: S68, T169, S214, S262, S285, S319, S356, T403 | Liu et al., 2016 [114] | Increase | - | Increase | - |
| 3R1N: T71 | Liu et al., 2016 [114] | Increase | - | Increase | - | |
| 4R0N: T111, S198, S214, S237, S238, S241, S258, S324, S352, S356, S400, S404 | Liu et al., 2016 [114] | Decrease | - | Decrease | - | |
| 3R2N: S235, S237, S324 | Liu et al., 2016 [114] | Decrease | - | Decrease | - | |
| Proteolytic cleavage | 4R2N: D421 | Mead et al., 2016 [115] | Increase | - | - | Increase |
| Pseudo-phosphorylation | 4R2N: S199, S199/S202/T205, T212, S214, T212/S214, S396/S404, | Necula and Kuret, 2004 [116] | Increase | - | Increase | - |
| 4R2N: S235 | Necula and Kuret, 2004 [116] | Decrease | - | Decrease | - | |
| 4R2N: T212 | Chang et al., 2011 [117] | Increase | - | Increase | - | |
| S-Guanylation | 3R2N: C291 | Yoshitake et al., 2016 [120] | Decrease | Decrease | Decrease | - |
| 4R2N: C291, C322 | Yoshitake et al., 2016 [120] | Decrease | Decrease | Decrease | - | |
| SUMOylation | 4R2N: K340 | Luo et al., 2014 [118] | Increase | - | - | - |
* Tau methylation at multiple residues across the protein.
Figure 3.
Schematic representation of tau protein illustrating PTMs and respective amino acid residues.
3.2. Parkinson’s Disease
α-Synuclein PTMs and Propensity for Aggregation
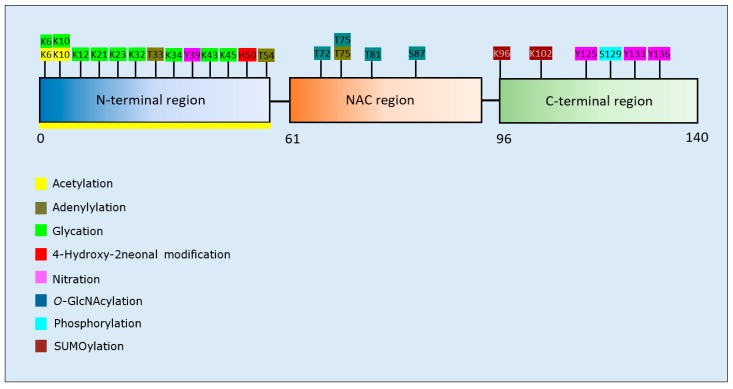
Twenty studies investigated the effects of PTMs on the PD-related protein, α-synuclein. Collectively, acetylation of the N-terminal region of α-synuclein reduced aggregation in four out of five studies [123,124,125,126], and with one study that reported that this PTM increased the propensity of α-synuclein to aggregate [127]. Adenylylation at T33, T54, and T75 reduced α-synuclein aggregation [128]. Glycation at multiple lysine residues increased α-synuclein aggregation and formation of stable oligomers [129]. Increased oligomerization but reduced aggregation was triggered after SUMOylation at K96 and K102 [130]. 4-hydroxy-2-neonal (HNE) modification at H50 resulted in enhanced formation of oligomers, which in two studies reduced the formation of fibrillar aggregates [131,132,133]. O-GlcNAcylation decreased formation of aggregates, and favoured the formation of oligomers after modification at T81 and S87, or when O-GlcNAcylation was performed across the whole protein [134,135]. By contrast, α-synuclein O-GlcNAcylated at T72 formed markedly fewer oligomers compared to the non-modified protein [136]. O-GlcNAcylation at T72 also inhibited S129 phosphorylation [136]; a PTM that increased the formation of α-synuclein aggregates [137,138]. Lastly, nitration of α-synuclein displayed conflicting results regarding effects upon α-synuclein aggregation [131,139,140,141,142]. Although all of these studies demonstrated that nitration increased oligomerisation, this was associated with a reduction or inability to form fibrils [131,139,141]. A summary of the PTMs that influence aggregation propensity of α-synuclein are shown in Table 4.
Table 4.
Summary of α-synuclein PTMs and propensity for protein aggregation.
| Post-Translational Modification | Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| Acetylation | N-terminus | Bartels et al., 2014 [123] | Decrease | - | Decrease | - |
| N-terminus | Kang et al., 2012 [124] | Decrease | - | Decrease | - | |
| N- terminus | Bu et al., 2017 [125] | Decrease | Decrease | Decrease | - | |
| N-terminus | Birol et al., 2019 [127] | Increase | - | Increase | - | |
| N-terminus, K6, K10 | Oliveira et al., 2017 [126] | Decrease | Decrease | Decrease | - | |
| Adenylylation | T33, T54, T75 | Sanyal et al., 2019 [128] | Decrease | - | Decrease | - |
| Glycation | K6, K10, K12, K21, K23, K32, K34, K43, K45 | Vicente et al., 2017 [129] | Increase | Increase | Decrease | Increase |
| 4-Hydroxy-2-neonalModification | H50, and other Lys residues | Qin et al., 2006 [133] | Decrease | Increase | Decrease | - |
| H50, and other Lys residues | Xiang et al., 2013 [131] | Decrease | Increase | Decrease | - | |
| H50 | Xiang et al., 2015 [132] | - | Increase | - | - | |
| Nitration | Y39, Y125, Y133/Y136 | Burai et al., 2015 [139] | Decrease | Increase | Decrease | Increase |
| Y39, Y125, Y133, Y136 | Liu et al., 2011 [140] | - | Increase | - | Increase | |
| Y39, Y125, Y133, Y136 | Hodara et al., 2004 [141] | Increase | - | - | - | |
| Y39, Y125, Y133, Y136 | Souza et al., 2000 [142] | Increase | Increase | - | - | |
| Y39/Y125/Y133/136 | Xiang et al., 2013 [131] | Decrease | Increase | Decrease | - | |
| O-GlcNAcylation | T72 | Levine et al., 2019 [134] | Decrease | - | Decrease | - |
| T75 | Levine et al., 2019 [134] | Decrease | - | Decrease | - | |
| T81 | Levine et al., 2019 [134] | Decrease | Increase | Decrease | - | |
| S87 | Levine et al., 2019 [134] | Decrease | Increase | Decrease | - | |
| T72/T75/T81 | Levine et al., 2019 [134] | Decrease | - | Decrease | - | |
| Muliple sites * | Zhang et al., 2017 [135] | Decrease | Increase | Decrease | - | |
| T72 | Marotta et al., 2015 [136] | Decrease | Decrease | Decrease | - | |
| Phosphorylation | S129 | Fujiwara et al., 2002 [137] | Increase | Increase | Increase | - |
| S129 | Samuel et al., 2016 [138] | Increase | - | Increase | - | |
| SUMOylation | K96, K102 | Krumova et al., 2011 [130] | Decrease | Increase | Decrease | - |
* α-synuclein O-GlcNAcylation at multiple residues across the entire protein was investigated.
Aggregation-related modifications of α-synuclein cluster to discrete regions of the protein, dependent upon the type of PTM (Figure 4). More specifically, acetylation, glycation, and HNE modifications were confined to the N-terminal region, O-GlcNAcylation targeted the NAC region, and SUMOylation, phosphorylation, and nitration were predominantly at the C-terminal region.
Figure 4.
Schematic representation of α-synuclein illustrating PTMs and respective amino acid residues.
3.3. Amyotrophic Lateral Sclerosis
3.3.1. TAR DNA-Binding Protein 43 PTMs and Propensity for Aggregation
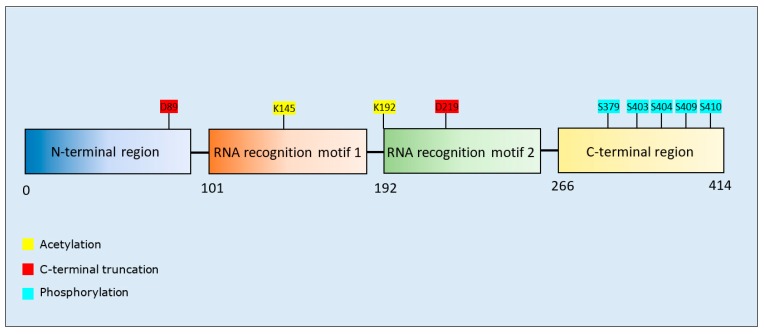
Acetylation of K145 and K192 increased aggregation of TDP-43 [143,144]. C-terminal fragmentation at D89 and D219 produced aggregation-prone TDP-43 fragments (residues 90–414 or residues 220–414, respectively) [145]. Phosphorylation of TDP-43 was detected at multiple sites, including S409/S410, and increased TDP-43 aggregation in two studies [146,147], but decreased the aggregation of C-terminal fragments of TDP-43 [148]. Phosphorylation at multiple serine residues (including S409 and S410) also induced a protective (reduction of aggregation) rather than pathogenic (pro-aggregation) effect [149]. C-terminal fragmentation at D219 produced a protein more prone to phosphorylation at S409/S410 [145], and similarly, acetylation at K145 promoted the accumulation of TDP-43 aggregates hyperphosphorylated at S409/S410 [144] (Table 5). TDP-43 phosphorylation arose primarily at residues clustered within the glycine-rich C-terminus (Figure 5); a protein region susceptible to formation of amyloid-like fibrils [150,151].
Table 5.
Summary of TDP-43 PTMs and propensity for protein aggregation.
| Post-Translational Modification | Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| Acetylation | K145 | Wang et al., 2017 [143] | Increase | - | - | - |
| K145, K192 | Cohen et al., 2015 [144] | Increase | - | - | - | |
| C-terminal fragmentation | D89, D219 | Zhang et al., 2009 [145] | Increase | - | - | - |
| Phosphorylation | S379, S403, S404, S409, S410, S403/S404, S409/S410, S379/S403/S404, S379/S409/S410, S403/S404/S409/S410 | Li et al., 2011 [149] | Decrease | - | - | - |
| S409/S410 | Carlomagno et al., 2014 [146] | Increase | - | Increase | - | |
| S379, S403/404, S409, S410, S409/S410 | Hasegawa et al., 2008 [147] | Increase | Increase | Increase | - | |
| S409/S410 | Brady et al., 2011 [148] | Decrease | - | - | - |
Figure 5.
Schematic representation of TDP-43 illustrating PTMs and respective amino acid residues.
3.3.2. SOD1 PTMs and Propensity for Aggregation
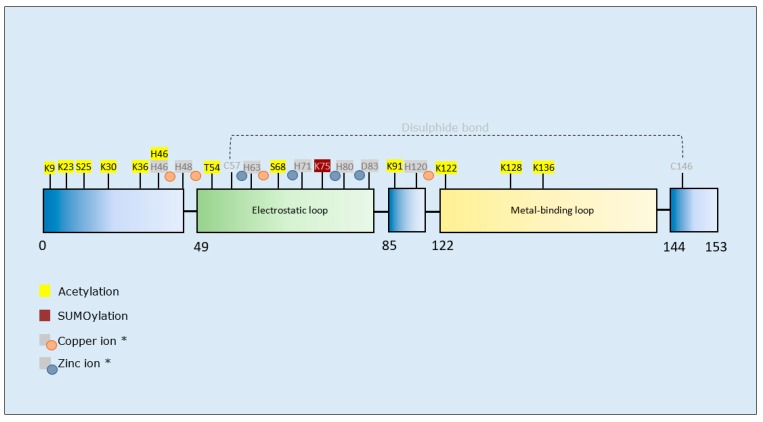
The agent employed for acetylation of SOD1 in vitro as well as the site(s) of acetylation influenced the type of aggregates formed [152]. SUMOylation at K75 increased aggregation of mutant (and wild-type) SOD1 [153,154] (Table 6 and Figure 6).
Table 6.
Summary of SOD1 PTMs and propensity for protein aggregation.
| Post-Translational Modification | Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| Acetylation | K23/K30/K36/H46/K91/K122/K128/K136 (BP *) | Rasouli et al., 2017 [152] | - | - | Decrease | - |
| K36/K128 (BT *) | Rasouli et al., 2017 [152] | - | - | Decrease | Increase | |
| K23/S25/K30/K36/K91/K122/K128/K136 (PM *) | Rasouli et al., 2017 [152] | - | - | Decrease | Increase | |
| K9 (CA*) | Rasouli et al., 2017 [152] | - | - | Increase | - | |
| K9/K23/K30/K36/K91/K122/K136/T54/S68 (GA *) | Rasouli et al., 2017 [152] | - | - | Decrease | Increase | |
| K23/S25/K30/K36/K122/K128/K136 (SA *) | Rasouli et al., 2017 [152] | - | - | Increase | - | |
| SUMOylation | K75 (SUMO3) † | Niikura et al., 2014 [153] | Increase | - | - | - |
| K75 (SUMO1) ‡ | Fei et al., 2006 [154] | Increase | - | - | - |
* Abbreviations: BP, 3,3’,4,4’-biphenyltetracarboxylic dianhydride; BT, benzophenone-3,3’,4,4’-tetracarboxylic dianhydride; PM, pyromellitic dianhydride; CA, citraconic anhydride; GA, glutaric anhydride; SA, succinic anhydride. † Mutant SOD-1 was used in this study. ‡ Wild-type and mutant SOD-1 were used in this study.
Figure 6.
Schematic representation of SOD1 illustrating PTMs and respective amino acid residues. * Due to their importance for SOD1 structural stability, metal-binding sites, and residues involved in disulphide bond formation are also marked.
3.4. Huntington’s Disease
Htt PTMs and Propensity for Aggregation
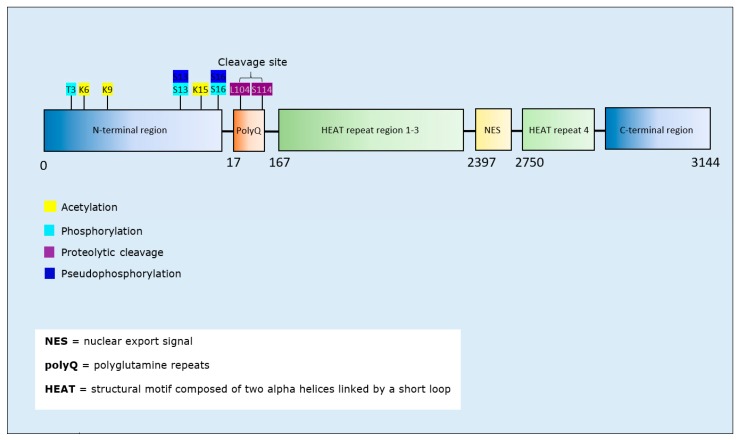
Acetylation of Htt decreased the formation aggregated fibrils [155]. Htt phosphorylation at T3 decreased Htt aggregation [156,157,158], as did phosphorylation at S13 and/or S16 [159,160]. Proteolytic cleavage of mutant Htt generated fragments with an increased tendency to aggregate into nuclear and cytoplasmic inclusion bodies [161] (Table 7). Residues targeted by acetylation, phosphorylation, or proteolytic cleavage were confined to the N-terminal region and polyQ stretch of Htt (Figure 7); regions that play a critical role in Htt aggregation behaviour [161,162].
Table 7.
Summary of huntingtin PTMs and propensity for protein aggregation.
| Post-Translational Modification | Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| Acetylation | K6, K9, K15 ‡ | Chaibva et al., 2016 [155] | Decrease | Decrease | - | |
| Phosphorylation | T3 * | Chiki et al., 2017 [156] | Decrease | - | Decrease | - |
| T3 | Ansaloni et al., 2014 [157] | Decrease | Decrease | Decrease | - | |
| T3 * | Cariulo et al., 2017 [158] | Decrease | - | - | - | |
| S13, S16, S13/S16 † | DeGuire et al., 2018 [159] | Decrease | Increase | Decrease | - | |
| Pseudo-phosphorylation | S13, S16 † | DeGuire et al., 2018 [159] | Decrease | Increase | Decrease | - |
| S13/S16 * | Gu et al., 2009 [160] | Decrease | - | Decrease | - | |
| Proteolytic Cleavage | Cleavage site between residue 104 and 114 * | Lunkes et al., 2002 [161] | Increase | - | - | - |
* Mutant huntingtin protein was used in this study. † Wild-type and mutant huntingtin protein were used in this study. ‡ Wild-type and truncated huntingtin protein were used in this study.
Figure 7.
Schematic representation of huntingtin illustrating PTMs and respective amino acid residues.
3.5. Spinocerebellar Ataxias
Ataxins PTMs and Propensity for Aggregation
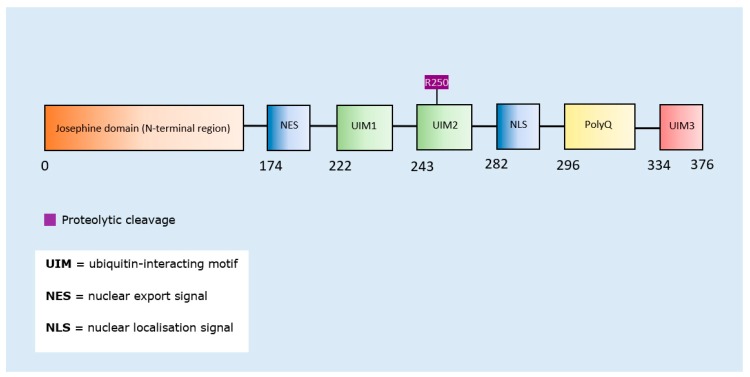
SUMOylation of ataxin-1 (across the whole protein) enhanced aggregation [163], as did proteolytic cleavage of ataxin-3 [164] (Table 8). Since SUMOylation of ataxin-1 was not confined to a specific amino acid residue, a schematic representation was only produced for ataxin-3. The proteolytic cleavage site was mapped to the second ubiquitin-interacting motif domain of the protein (Figure 8).
Table 8.
Summary of ataxins PTMs and propensity for protein aggregation.
| Post-Translational Modification | Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| SUMOylation (ataxin-1) | Multiple residues | Ryu et al., 2010 [163] | Increase | - | - | - |
| Proteolytic Cleavage (ataxin-3) | N-terminal region cleavage near residue 250 | Haacke et al., 2006 [164] | Increase | - | - | - |
Figure 8.
Schematic representation of ataxin-3 illustrating PTMs and respective amino acid residues.
3.6. Transmissible Spongiform Encephalopathies
Prion Protein PTMs and Propensity for Aggregation
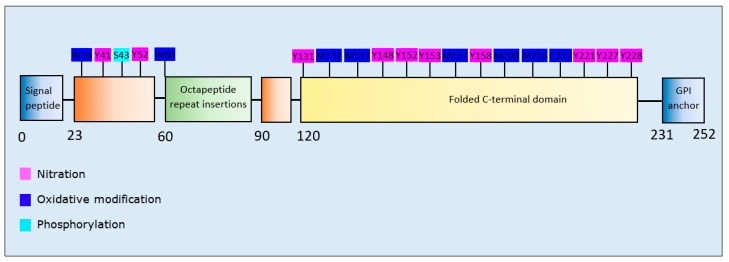
Oxidation and nitration at multiple residues of PrPc increased aggregation propensity with enhanced formation of fibrillar as well as amorphous aggregates [165]. Phosphorylation at S43 of PrPc enhanced its aggregation, with a tendency of the phosphorylated form to produce large fibrillar and fewer amorphous aggregates than unmodified PrPc [166] (Table 9). PrPc residues susceptible to nitrative and oxidative modifications were primarily clustered within the folded C-terminal domain of the protein (Figure 9).
Table 9.
Summary of PrPc PTMs and propensity for protein aggregation.
| Post-Translational Modification | Residues Modified | Author & Year | Aggregation | Formation of Oligomers | Formation of Fibrillar Aggregates | Formation of Amorphous Aggregates |
|---|---|---|---|---|---|---|
| Oxidative modification and Nitration | Oxidation: W34, W60, M157, M209, M216, C217, M132/M137 | Dear et al., 2007 [165] | Increase | - | Increase | Increase |
| Nitration: Y41, Y41/Y52, Y131, Y148, Y152, Y153, Y158, Y221, Y227/Y228 | Increase | - | Increase | Increase | ||
| Phosphorylation | S43 | Giannopoulos et al., 2009 [166] | Increase | - | Increase | Decrease |
Figure 9.
Schematic representation of PrPc illustrating PTMs and respective amino acid residues.
3.7. Multiple Sclerosis
Bsn: Bsn protein was identified only recently as a protein aggregate that may contribute to neurotoxicity in MS [12], and an examination of the influence of PTMs upon Bsn aggregation has yet to be undertaken.
Aβ and tau: The detection of oligomeric aggregates of Aβ and tau, and APP within brain tissue from MS patients [8,13] suggests that for some NDDs, a common subset of potentially toxic protein accumulations exist. The PTMs that influence protein aggregation of Aβ and tau, are detailed in Table 2 and Table 3, respectively, and Figure 2 and Figure 3, respectively. Whether similar PTMs are relevant to the Aβ and tau deposited in brain tissue of MS patients has yet to be determined.
4. Discussion
This review considered PTMs that may influence pathogenic protein aggregation in NDDs. Evidence indicating a role for PTMs in a NDD tended to reflect the prevalence of the disease: with most studies focused upon AD or PD, with relatively sparse literature coverage of PTMs in SCAs or TSEs, and no studies identified that investigated the PTM of Bsn protein in MS. Furthermore, for many of the studies of the PTMs, only a single report of the effect of the PTM has been published. However, for some of the PTMs, at least two independent studies have considered aggregation behaviour in response to a PTM at the same amino acid, and these have been discussed further:
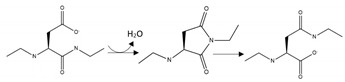
Aβ isoaspartate: Results from Table 2 indicate that isoaspartate formation has a pro-aggregation effect on Aβ. Consistent with a role for this PTM in AD, the levels of isoaspartate in Aβ was significantly higher in brain tissue from patients with AD compared with age-matched controls [167]. Isoaspartate is a non-enzymatic PTM that forms in peptides or proteins from asparagine deamination or aspartic acid isomerisation [168,169]. Isoaspartate formation influences protein structure and function since it results in a kink in the peptide backbone and the addition of a methylene group. Protein levels of isoaspartate are normally restricted in vivo via the action of the enzyme, protein L-isoaspartyl methyltransferase (PIMT) [168,169]. However, for peptides or proteins that become extracellular, including Aβ, inaccessibility to cytosolic or nuclear PIMT activity renders them susceptible to ongoing isoaspartate formation. A therapeutic intervention that prevents the formation of isoaspartate within Aβ and thereby limits its pro-aggregation effect may be of benefit in AD, perhaps through targeted repair of Aβ or increased activity of PIMT.
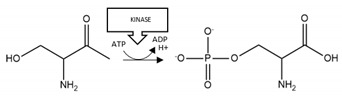
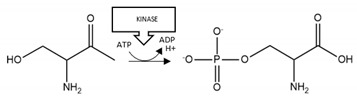
Aβ phosphorylation: The results of Table 2 suggest that there are divergent effects of phosphorylation on Aβ aggregation; such that phosphorylation of S8 stimulated Aβ aggregation, whereas phosphorylation at S26 decreased aggregation. Hence, total phosphorylation of Aβ is unlikely to be an informative marker of aggregation propensity and toxicity. Moreover, site-specific phosphorylation may need to be considered for therapeutic interventions: agents that, for example, enhance phosphatase activity or inhibit kinase activity at S8, or conversely inhibit phosphatase activity and promote kinase activity at S26 may be useful for limiting pathogenic aggregation.
Tau phosphorylation: Although hyperphosphorylation of tau as a trigger for the formation of NFTs and progression of AD has been reported [170], evidence collated in this review suggests that tau phosphorylation and aggregation is only specific to certain tau isoforms (Table 3). Indeed, phosphorylation of the tau isoforms 4R0N and 3R2N resulted in decreased tau aggregation [114]. By contrast, phosphorylation or pseudo-phosphorylation of the longest tau isoform, 4R2N, increased tau aggregation [114,116,117]. Clinical treatments for AD that have utilised kinase inhibitors to prevent tau hyperphosphorylation and aggregation have had limited success [171], and future studies will need to consider those tau isoforms that exhibit increased propensity to aggregate after phosphorylation, as well as the influence of site-specific phosphorylations.
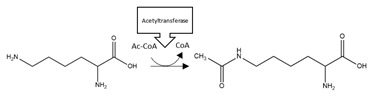
α-synuclein acetylation: Four of five studies reported reduced aggregation of α-synuclein after acetylation within the N-terminal region (Table 4). Acetylation of α-synuclein interferes with internal hydrogen-bonding and induces an α-helical rather than β-pleated sheet structure, and this may be the mechanism for decreased aggregation [124,125]. In contrast, a single study reported increased α-synuclein aggregation after acetylation [127]. Hence, collectively, there may be a beneficial therapeutic anti-aggregation effect upon α-synuclein through targeted N-terminal acetylation.
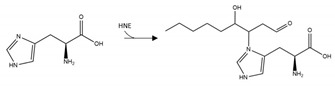
α-synuclein 4-hydroxy-2-neonal (HNE) modification: NDDs are associated with cellular redox stress that can lead to increased lipid peroxidation and formation of the α,β-unsaturated hydroxyalkenal, HNE, capable of covalently adducting α-synuclein [133]. Adduction of α-synuclein by HNE reduced the tendency of α-synuclein to undergo fibrillar aggregation (Table 4) [131,133]. However, whether this could be exploited as a protective modification is equivocal, since HNE modification decreased aggregation by stabilising oligomeric intermediates, and α-synuclein oligomers may also be toxic to neurons [172,173].
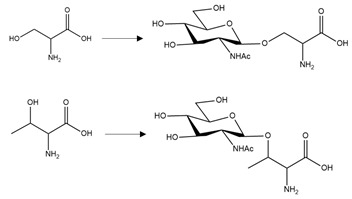
α-synuclein O-GlcNAcylation: Protein glycosylation as O-GlcNAcylation arises from enzymatic O-linked addition of β-N-acetyl-glucosamine at serine or threonine residues and is involved in a number of homeostatic mechanisms. All three studies undertaken to date reported decreased α-synuclein aggregation after O-GlcNAcylation [134,135,136]. Furthermore, interplay between PTMs was reported, with an inhibitory effect of α-synuclein T72 O-GlcNAcylation upon α-synuclein S129 phosphorylation [136]; a PTM that normally enhances α-synuclein aggregation and promotes neuropathology [137,138]. Collectively, these studies suggest a benefit of O-GlcNAcylation that could be manipulated by therapeutic intervention. However, total levels of O-GlcNAcylation were increased in post-mortem tissue from three PD patients compared to control subjects, although specific levels of O-GlcNAcylation of α-synuclein were not determined [174].
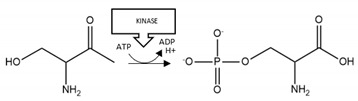
α-synuclein phosphorylation: Phosphorylation at S129 of α-synuclein has a pro-aggregation effect, is detected in LBs, and is associated with proteinopathy in PD [137,138,174]. Hence, there may be therapeutic value in manipulating the levels of this phosphorylation. To that end, a transgenic mouse model of α-synucleinopathy with enhanced dephosphorylation at S129 displayed reduced aggregation and symptomatic improvement [91].
TDP-43 acetylation: Acetylated TDP-43 has been recovered within spinal cord of patients with ALS [144], indicative of its pathological relevance. In support of this supposition, two studies demonstrated enhanced aggregation of TDP-43 following its acetylation (Table 5). Since the acetylated residues (K145 and K192) are within the nucleic acid binding regions (Figure 5), this PTM may reduce RNA-binding of TDP-43 and promote the accumulation of dysfunctional TDP-43 aggregates [144]. Thus, preventing TDP-43 acetylation may provide a new approach for treatment of ALS.
Superoxide Dismutase 1 SUMOylation: SUMOylation of mutant or wild-type SOD1 by SUMO1 and SUMO3 at K75 enhanced its aggregation [153,154]. Thus, preventing SUMOylation of SOD1 may be beneficial to limit progression of fALS. Certainly, inhibition of mutant SOD1 SUMOylation at K75 has proven a successful approach to prevent SOD1 aggregation in neuronal cells [175], and therefore underpins a rationale for translation to animal models.
Huntingtin phosphorylation: Preclinical models of HD and HD patient brain samples display lower phosphorylation levels at T3 of huntingtin compared with controls [158]. Htt phosphorylation at T3 as well as at S13/16 inhibits the formation of aggregates, potentially serving as a protective mechanism against proteinopathic changes (Table 7). Consistent with this proposal, restored N-terminal Htt phosphorylation reversed neurotoxicity in a model of HD [176]. Therefore, modification of the levels of Htt phosphorylation represent a promising target for therapeutic development. Additionally, Htt phosphorylation at T3 prevents phosphorylation at S13/S16 [177]. Nevertheless, since both phosphorylation at T3 and S13/S16 decrease Htt aggregation, it will be of interest to delineate their potential cumulative effects upon Htt aggregation.
A summary of the PTMs whose manipulations may be of therapeutic benefit are included in Table 10.
Table 10.
Summary of PTMs with therapeutic potential and suggested pharmacological interventions.
| Protein | PTM | Residues | Schematic Representation of Modification | Suggested Pharmacological Intervention |
|---|---|---|---|---|
| Aβ | Isoaspartate modification | D1, D7, D23 |
|
Inhibition |
| Aβ | Phosphorylation | S8 |
|
Inhibition |
| Aβ | Phosphorylation | S26 |
|
Enhancement |
| αS | Acetylation | K6, K10, N-terminal region |
|
Enhancement |
| αS | HNE modification | H50 |
|
Inhibition |
| αS | O-GlcNacylation | T72, T75, T81, S87 |
|
Enhancement |
| αS | Phosphorylation | S129 |
|
Inhibition |
| TDP-43 | Acetylation | K145, K192 |
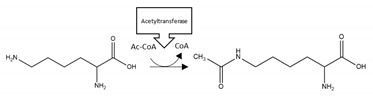
|
Inhibition |
| SOD1 | SUMOylation | K75 |
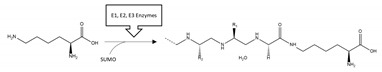
|
Inhibition |
| HTT | Phosphorylation | T3, S13, S16 |
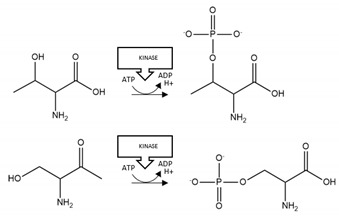
|
Enhancement |
Abbreviations: αS, α-synuclein.
4.1. Study Limitations
The majority of studies investigating the roles of PTMs for proteins that contribute to NDDs have been undertaken in vitro, and it is therefore difficult to make direct inferences to in vivo effects. Furthermore, while we have compiled and summarised the primary literature that has examined the effects of PTMs upon protein aggregation, these studies invariably only consider individual PTMs, and therefore will not reflect the multiple PTMs experienced within a dynamic in vivo system. Hence, analysis of site-specific PTMs by, for example, mass spectrometric means, only provides a snapshot of site occupancy at that juncture. Indeed, PTMs co-exist in vivo and may act in a permissive, reciprocal, antagonising, potentiating, or even synergistic manner to influence overall protein structure and function. Additionally, to consider functional relevance of each PTM to support therapeutic targeting, it is also important to consider the stoichiometry associated with each PTM, and this has not yet been addressed in most of the in vitro or in vivo studies.
Our study has also only focussed upon the influence of the PTM upon protein aggregation, and for which measurements of aggregation were conducted by different means. The majority of studies have utilised ThT fluorescent assays, but other protein separation and imaging systems were also used–refer to Supplementary Data, S2. Furthermore, differences between biological models, protein sources (for example, synthetic or recombinant peptides or proteins), and assay techniques, could account for some of the conflicting studies that describe the effects of PTMs on aggregation behaviour. Furthermore, we have tried to consider different aggregation elements: oligomers, fibrillary, and amorphous aggregates, but some studies have demonstrated reduced aggregate formation via increased stability of oligomeric species. Hence, one must interpret results with caution due to the unresolved debate as to whether oligomers and/or mature aggregates are the primary toxic species [178]. While both oligomers and larger aggregates are known to adopt β-sheet conformations, Aβ and α-synuclein oligomers can be arranged in antiparallel β-pleated sheets, whereas fibrillar aggregates display a parallel β-sheet structure [179,180]. Hence, differences in secondary structures, certainly for Aβ, may ultimately influence neurotoxicity [181]. Lastly, the formation of toxic oligomers or aggregates may not only be a consequence of increased protein aggregation propensity, but also arise from dysfunctional protein degradation pathways.
4.2. Summary and Conclusions
A more comprehensive understanding of the molecular mechanisms that trigger pathogenesis or progression of NDDs is a prerequisite to developing new treatment options. The results of this review highlight that multiple PTMs can alter aggregation potential and thereby contribute to proteinopathies. The future development of therapies to modify PTM profiles for key NDD-related proteins may provide an as yet untapped source of novel drug treatments for NDDs.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/10/4/232/s1.
Author Contributions
Conceptualization, L.-N.S. and W.G.C.; methodology, L.-N.S.; validation, L.-N.S. and W.G.C; formal analysis, L.-N.S. and W.G.C; investigation, L.-N.S. and W.G.C; data curation, L.-N.S. and W.G.C; writing—original draft preparation, L.-N.S. and W.G.C; writing—review and editing, L.-N.S. and W.G.C; supervision, W.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Erkkinen M.G., Kim M.O., Geschwind M.D. Clinical Neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018;10:a033118. doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe M.S., editor. The Molecular and Cellular Basis of Neurodegenerative Diseases: Underlying Mechanisms. Elsevier; London, UK: 2018. Solving the puzzle of neurodegeneration. [Google Scholar]
- 4.Sullivan R., Yau W.Y., O’Connor E., Houlden H. Spinocerebellar ataxia: An update. J. Neurol. 2019;266:533–544. doi: 10.1007/s00415-018-9076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain R., Zubair H., Pursell S., Shahab M. Neurodegenerative diseases: Regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018;8:177. doi: 10.3390/brainsci8090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross C.A., Poirier M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 7.Wan L., Xu K., Chen Z., Tang B., Jiang H. Roles of post-translational modifications in spinocerebellar ataxias. Front. Cell. Neurosci. 2018;12:290. doi: 10.3389/fncel.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David M.A., Tayebi M. Detection of protein aggregates in brain and cerebrospinal fluid derived from multiple sclerosis patients. Front. Neurol. 2014;5:251. doi: 10.3389/fneur.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 10.Blokhuis A.M., Groen E.J.N., Koppers M., van den Berg L.H., Pasterkamp R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ugalde C.L., Finkelstein D.I., Lawson V.A., Hill A.F. Pathogenic mechanisms of prion protein, amyloid-β and α-synuclein misfolding: The prion concept and neurotoxicity of protein oligomers. J. Neurochem. 2016;139:162–180. doi: 10.1111/jnc.13772. [DOI] [PubMed] [Google Scholar]
- 12.Schattling B., Engler J.B., Volkmann C., Rothammer N., Woo M.S., Petersen M., Winkler I., Kaufmann M., Rosenkranz S.C., Fejtova A., et al. Bassoon proteinopathy drives neurodegeneration in multiple sclerosis. Nat. Neurosci. 2019;22:887–896. doi: 10.1038/s41593-019-0385-4. [DOI] [PubMed] [Google Scholar]
- 13.Gehrmann J., Banati R.B., Cuzner M.L., Kreutzberg G.W., Newcombe J. Amyloid precursor protein (APP) expression in multiple sclerosis lesions. Glia. 1995;15:141–151. doi: 10.1002/glia.440150206. [DOI] [PubMed] [Google Scholar]
- 14.Coarelli G., Brice A., Durr A. Recent advances in understanding dominant spinocerebellar ataxias from clinical and genetic points of view. F1000Res. 2018;7:Rev-1781. doi: 10.12688/f1000research.15788.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyasaka T., Watanabe A., Saito Y., Murayama S., Mann D.M.A., Yamazaki M., Ravid R., Morishima-Kawashima M., Nagashima K., Ihara Y. Visualization of newly deposited tau in neurofibrillary tangles and neuropil threads. J. Neuropathol. Exp. Neurol. 2005;64:665–674. doi: 10.1097/01.jnen.0000173890.79058.1d. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa T., Yoshida S., Sugeno N., Kobayashi J., Aoki M. DnaJ/Hsp40 family and parkinson’s disease. Front. Neurosci. 2018;11:743. doi: 10.3389/fnins.2017.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pihlstrøm L., Wiethoff S., Houlden H., Kovacs G.G., Alafuzoff I. Genetics of neurodegenerative diseases: An overview. Handb. Clin. Neurol. 2017;145:309–323. doi: 10.1016/B978-0-12-802395-2.00022-5. [DOI] [PubMed] [Google Scholar]
- 18.Wirths O., Bayer T.A. Neuron Loss in Transgenic Mouse Models of Alzheimer’s Disease. Int. J. Alzheimers Dis. 2010;12:723782. doi: 10.4061/2010/723782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez A., Milà M., Castellví-Bel S., Rosich M., Jiménez D., Badenas C., Estivill X. Maternal transmission in sporadic Huntington’s disease. J. Neurol. Neurosurg. Psychiatry. 1997;62:535–537. doi: 10.1136/jnnp.62.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sim V. BMJ Best Practice. BMJ Publishing Group; London, UK: 2018. [(accessed on 20 September 2019)]. Prion disease. Available online: https://bestpractice.bmj.com/topics/en-gb/484/pdf/484.pdf. [Google Scholar]
- 21.Steenhof M., Stenager E., Nielsen N.M., Kyvik K., Möller S., Hertz J.M. Familial multiple sclerosis patients have a shorter delay in diagnosis than sporadic cases. Mult. Scler. Relat. Discord. 2019;32:97–102. doi: 10.1016/j.msard.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Kraft S., Furtado S., Ranawaya R., Parboosingh J., Bleoo S., McElligott K., Bridge P., Spacey S., Das S., Suchowersky O. Adult onset spinocerebellar ataxia in a Canadian movement disorders clinic. Can. J. Neurol. Sci. 2005;32:450–458. doi: 10.1017/S0317167100004431. [DOI] [PubMed] [Google Scholar]
- 23.Chen C., Dong X.P. Epidemiological characteristics of human prion diseases. Infect. Dis. Poverty. 2016;5:47. doi: 10.1186/s40249-016-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niclis J.C., Pinar A., Haynes J.M., Alsanie W., Jenny R., Dottori M., Cram D.S. Characterization of forebrain neurons derived from late-onset Huntington’s disease human embryonic stem cell lines. Front. Cell. Neurosci. 2013;7:37. doi: 10.3389/fncel.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GBD 2016 Motor Neuron Disease Collaborators Global, regional, and national burden of motor neuron diseases 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:1083–1097. doi: 10.1016/S1474-4422(18)30404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GBD 2016 Multiple Sclerosis Collaborators Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:269–285. doi: 10.1016/S1474-4422(18)30443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GBD 2016 Dementia Collaborators Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tysnes O.B., Storstein A. Epidemiology of Parkinson’s disease. J. Neural. Transm. 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 29.Burré J. The synaptic function of α-Synuclein. J. Park. Dis. 2015;5:699–713. doi: 10.3233/JPD-150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimers Dis. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Wortmann M. Dementia: A global health priority - highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 2012;4:40. doi: 10.1186/alzrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns A., Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 33.Hane F.T., Robinson M., Lee B.Y., Bai O., Leonenko Z., Albert M.S. Recent progress in Alzheimer’s disease research, Part 3: Diagnosis and treatment. J. Alzheimers Dis. 2017;57:645–665. doi: 10.3233/JAD-160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perl D.P. Neuropathology of Alzheimer’s disease. Mt. Sinai. J. Med. 2010;77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouras G.K., Olsson T.T., Hansson O. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics. 2015;12:3–11. doi: 10.1007/s13311-014-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brothers H.M., Gosztyla M.L., Robinson S.R. The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease. Front. Ageing Nurosci. 2018;10:118. doi: 10.3389/fnagi.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson H.A., Peers C. Physiological roles for amyloid beta peptides. J. Physiol. 2006;575:5–10. doi: 10.1113/jphysiol.2006.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lührs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Döbeli H., Schubert D., Riek R. 3D structure of Alzheimer’s amyloid-beta (1–42) fibrils. Proc. Natl. Acad. Sci. USA. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 40.Guo T., Noble W., Hanger D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133:665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina M., Hernández F., Avila J. New features about tau function and dysfunction. Biomolecules. 2016;6:21. doi: 10.3390/biom6020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jouanne M., Rault S., Voisin-Chiret A.S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017;139:153–167. doi: 10.1016/j.ejmech.2017.07.070. [DOI] [PubMed] [Google Scholar]
- 43.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pakkenberg H., Brody H. The number of nerve cells in the substantia nigra in paralysis agitans. Acta Neuropathol. 1965;5:320–324. doi: 10.1007/BF00686528. [DOI] [PubMed] [Google Scholar]
- 45.DeMaagd G., Philip A. Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T. 2015;40:504–532. [PMC free article] [PubMed] [Google Scholar]
- 46.Spillanti M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 47.Venda L.L., Cragg S.J., Buchman V.L., Wade-Martins R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010;33:559–568. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cookson M.R. alpha-Synuclein and neuronal cell death. Mol. Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baba M., Nakajo S., Tu P.H., Tomita T., Nakaya K., Lee V.M., Trojanowski J.Q., Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 50.Oskarsson B., Gendron T.F., Staff N.P. Amyotrophic lateral sclerosis: An update for 2018. Mayo Clin. Proc. 2018;93:1617–1628. doi: 10.1016/j.mayocp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Zarei S., Carr K., Reiley L., Diaz K., Guerra O., Altamirano P.F., Pagani W., Lodin D., Orozco G., Chinea A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015;6:171. doi: 10.4103/2152-7806.169561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hergesheimer R.C., Chami A.A., de Assis D.R., Vourc’h P., Andres C.R., Corcia P., Lanznaster D., Blasco H. The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: A resolution in sight? Brain. 2019;142:1176–1194. doi: 10.1093/brain/awz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prasad A., Bharathi V., Sivalingam V., Girdhar A., Patel B.K. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2019;12:25. doi: 10.3389/fnmol.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D.M.A., Tsuchiya K., Yoshida M., Hashizume Y., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 55.Highley J.R., Kirby J., Jansweijer J.A., Webb P.S., Hewamadduma C.A., Heath P.R., Higginbottom A., Raman R., Ferraiuolo L., Cooper-Knock J., et al. Loss of nuclear TDP-43 in amyotrophic lateral sclerosis (ALS) causes altered expression of splicing machinery and widespread dysregulation of RNA splicing in motor neurones. Neuropathol. Appl. Neurobiol. 2014;40:670–685. doi: 10.1111/nan.12148. [DOI] [PubMed] [Google Scholar]
- 56.Rakhit R., Chakrabartty A. Structure, folding, and misfolding of Cu,Zn superoxide dismutase in amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2006;1762:1025–1037. doi: 10.1016/j.bbadis.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Pansarasa O., Bordoni M., Diamanti L., Sproviero D., Gagliardi S., Cereda C. SOD1 in Amyotrophic Lateral Sclerosis: “Ambivalent” Behavior Connected to the Disease. Int. J. Mol. Sci. 2018;19:1345. doi: 10.3390/ijms19051345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medinas D.B., Rozas P., Martínez Traub F., Woehlbier U., Brown R.H., Bosco D.A., Hetz C. Endoplasmic reticulum stress leads to accumulation of wild-type SOD1 aggregates associated with sporadic amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 2018;115:8209. doi: 10.1073/pnas.1801109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myers R.H. Huntington’s disease genetics. NeuroRx. 2004;1:255–262. doi: 10.1602/neurorx.1.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DiFiglia M., Sapp E., Chase K.O., Davies S.W., Bates G.P., Vonsattel J.P., Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 61.Huang W.J., Chen W.W., Zhang X. Huntington’s disease: Molecular basis of pathology and status of current therapeutic approaches. Exp. Ther. Med. 2016;12:1951–1956. doi: 10.3892/etm.2016.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roos R.A. Huntington’s disease: A clinical review. Orphanet J Rare Dis. 2010;5:40. doi: 10.1186/1750-1172-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nasir J., Floresco S.B., O’Kusky J.R., Diewert V.M., Richman J.M., Zeisler J., Borowski A., Marth J.D., Phillips A.G., Hayden M.R. Targeted disruption of the Huntington’s disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell. 1995;81:811–823. doi: 10.1016/0092-8674(95)90542-1. [DOI] [PubMed] [Google Scholar]
- 64.McKinstry S.U., Karadeniz Y.B., Worthington A.K., Hayrapetyan V.Y., Ozlu M.I., Serafin-Molina K., Risher W.C., Ustunkaya T., Dragatsis I., Zeitlin S., et al. Huntingtin is required for normal excitatory synapse development in cortical and striatal circuits. J. Neurosci. 2014;34:9455–9472. doi: 10.1523/JNEUROSCI.4699-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finkbeiner S. Huntington’s disease. Cold Spring Harb Perspect Biol. 2011;3:a007476. doi: 10.1101/cshperspect.a007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M. Beta conformation of polyglutamine track revealed by a crystal structure of Huntingtin N-terminal region with insertion of three histidine residues. Prion. 2013;7:221–228. doi: 10.4161/pri.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arrasate M., Finkbeiner S. Protein aggregates in Huntington’s disease. Exp. Neurol. 2012;238:1–11. doi: 10.1016/j.expneurol.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sánchez I., Mahlke C., Yuan J. Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature. 2003;421:373–379. doi: 10.1038/nature01301. [DOI] [PubMed] [Google Scholar]
- 69.Davies S.W., Turmaine M., Cozens B.A., DiFiglia M., Sharp A.H., Ross C.A., Scherzinger E., Wanker E.E., Mangiarini L., Bates G.P. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/S0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 70.Ramachandra N.B., Kusuma L. An understanding of spinocerebellar ataxia. Indian J. Med. Res. 2015;141:148–150. doi: 10.4103/0971-5916.155537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paulson H.L. The spinocerebellar ataxias. J. Neuroophthalmol. 2009;29:227–237. doi: 10.1097/WNO0b013e3181b416de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabinovici G.D., Wang P.N., Levin J., Cook L., Pravdin M., Davis J., DeArmond S.J., Barbaro N.M., Martindale J., Miller B.L., et al. First symptom in sporadic Creutzfeldt–Jakob disease. Neurology. 2006;66:286. doi: 10.1212/01.wnl.0000196440.00297.67. [DOI] [PubMed] [Google Scholar]
- 73.Jeffrey M., Scott J., Williams A., Fraser H. Ultrastructural features of spongiform encephalopathy transmitted to mice from three species of bovidae. Acta Neuropathol. 1992;84:559–569. doi: 10.1007/BF00304476. [DOI] [PubMed] [Google Scholar]
- 74.Gupta A., Hirsch N. Prion diseases. Anaesth. Inten. Care Med. 2013;14:407–409. doi: 10.1016/j.mpaic.2013.05.018. [DOI] [Google Scholar]
- 75.Collins S.J., Sanchez-Juan P., Masters C.L., Klug G.M., van Duijn C., Poleggi A., Pocchiari M., Almonti S., Cuadrado-Corrales N., de Pedro-Cuesta J., et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt–Jakob disease. Brain. 2006;129:2278–2287. doi: 10.1093/brain/awl159. [DOI] [PubMed] [Google Scholar]
- 76.Moya K.L., Salès N., Hässig R., Créminon C., Grassi J., Di Giamberardino L. Immunolocalization of the cellular prion protein in normal brain. Microsc. Res. Tech. 2000;50:58–65. doi: 10.1002/1097-0029(20000701)50:1<58::AID-JEMT9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 77.Um J.W., Nygaard H.B., Heiss J.K., Kostylev M.A., Stagi M., Vortmeyer A., Wisniewski T., Gunther E.C., Strittmatter S.M. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 2012;15:1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Acevedo-Morantes C.Y., Wille H. The structure of human prions: From biology to structural models-considerations and pitfalls. Viruses. 2014;6:3875–3892. doi: 10.3390/v6103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bueler H., Fischer M., Lang Y., Bluethmann H., Lipp H.P., DeArmond S.J., Prusiner S.B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 80.Ambadi Thody S., Mathew M.K., Udgaonkar J.B. Mechanism of aggregation and membrane interactions of mammalian prion protein. Biochim. Biophys. Acta Biomembr. 2018;1860:1927–1935. doi: 10.1016/j.bbamem.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 81.Pan K.M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R.J., Cohen F.E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kovacs G.G., Budka H. Molecular pathology of human prion diseases. Int. J. Mol. Sci. 2009;10:976–999. doi: 10.3390/ijms10030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laurent M. Autocatalytic processes in cooperative mechanisms of prion diseases. FEBS Lett. 1997;407:1–6. doi: 10.1016/S0014-5793(97)00310-4. [DOI] [PubMed] [Google Scholar]
- 84.Ghasemi N., Razavi S., Nikzad E. Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017;19:1–10. doi: 10.22074/cellj.2016.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dutta R., Trapp B.D. Relapsing and progressive forms of multiple sclerosis: Insights from pathology. Curr. Opin. Neurol. 2014;27:271–278. doi: 10.1097/WCO.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Altrock W.D., tom Dieck S., Sokolov M., Meyer A.C., Sigler A., Brakebusch C., Fässler R., Richter K., Boeckers T.M., Potschka H. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. doi: 10.1016/S0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 87.Takalo M., Salminen A., Soininen H., Hiltunen M., Haapasalo A. Protein aggregation and degradation mechanisms in neurodegenerative diseases. Am. J. Neurodegener. Dis. 2013;2:1–14. [PMC free article] [PubMed] [Google Scholar]
- 88.Lim J., Yue Z. Neuronal aggregates: Formation, clearance, and spreading. Dev. Cell. 2015;32:491–501. doi: 10.1016/j.devcel.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uniprot Modified Residues in Proteins. [(accessed on 15 March 2020)]; Available online: http://www.uniprot.org/docs/ptmlist.txt.
- 90.Xu H., Wang Y., Lin S., Deng W., Peng D., Cui Q., Xue Y. PTMD: A database of human disease-associated Post-translational Modifications. Genom. Proteom. Bioinform. 2018;16:244–251. doi: 10.1016/j.gpb.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee K.W., Chen W., Junn E., Im J.Y., Grosso H., Sonsalla P.K., Feng X., Ray N., Fernandez J.R., Chao Y., et al. Enhanced phosphatase activity attenuates α-synucleinopathy in a mouse model. J. Neurosci. 2011;31:6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindstedt P.R., Aprile F.A., Matos M.J., Perni M., Bertoldo J.B., Bernardim B., Peter Q., Jiménez-Osés G., Knowles T.P.J., Dobson C.M., et al. Enhancement of the anti-aggregation activity of a molecular chaperone using a rationally designed post-translational modification. ACS Cent. Sci. 2019;5:1417–1424. doi: 10.1021/acscentsci.9b00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xue C., Lin T.Y., Chang D., Guo Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017;4:160696. doi: 10.1098/rsos.160696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fossati S., Todd K., Sotolongo K., Ghiso J., Rostagno A. Differential contribution of isoaspartate post-translational modifications to the fibrillization and toxic properties of amyloid β and the Asn23 Iowa mutation. Biochem. J. 2013;456:347–360. doi: 10.1042/BJ20130652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimizu T., Fukuda H., Murayama S., Izumiyama N., Shirasawa T. Isoaspartate formation at position 23 of amyloid beta peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer’s disease. J. Neurosci. Res. 2002;70:451–461. doi: 10.1002/jnr.10350. [DOI] [PubMed] [Google Scholar]
- 97.Schilling S., Lauber T., Schaupp M., Manhart S., Scheel E., Böhm G., Demuth H.U. On the Seeding and Oligomerization of pGlu-Amyloid Peptides (in vitro) Biochemistry. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- 98.Jamasbi E., Separovic F., Hossain M.A., Ciccotosto G.D. Phosphorylation of a full length amyloid-β peptide modulates its amyloid aggregation, cell binding and neurotoxic properties. Mol. Biosyst. 2017;13:1545–1551. doi: 10.1039/C7MB00249A. [DOI] [PubMed] [Google Scholar]
- 99.Kumar S., Rezaei-Ghaleh N., Terwel D., Thal D.R., Richard M., Hoch M., Mc Donald J.M., Wüllner U., Glebov K., Heneka M.T., et al. Extracellular phosphorylation of the amyloid β-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer’s disease. EMBO J. 2011;30:2255–2265. doi: 10.1038/emboj.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar S., Wirths O., Stüber K., Wunderlich P., Koch P., Theil S., Rezaei-Ghaleh N., Zweckstetter M., Bayer T.A., Brüstle O., et al. Phosphorylation of the amyloid β-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity. Acta Neuropathol. 2016;131:525–537. doi: 10.1007/s00401-016-1546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khodadadi S., Riazi G.H., Ahmadian S., Hoveizi E., Karima O., Aryapour H. Effect of N-homocysteinylation on physicochemical and cytotoxic properties of amyloid β-peptide. FEBS Lett. 2012;586:127–131. doi: 10.1016/j.febslet.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 102.Zhao J., Wang P., Li H., Gao Z. Nitration of Y10 in Aβ1–40: Is it a compensatory reaction against oxidative/nitrative stress and Aβ aggregation? Chem. Res. Toxicol. 2015;28:401–407. doi: 10.1021/tx500353s. [DOI] [PubMed] [Google Scholar]
- 103.Guivernau B., Bonet J., Valls-Comamala V., Bosch-Morató M., Godoy J.A., Inestrosa N.C., Perálvarez-Marín A., Fernández-Busquets X., Andreu D., Oliva B., et al. Amyloid-β peptide nitrotyrosination stabilizes oligomers and enhances NMDAR-Mediated Toxicity. J. Neurosci. 2016;36:11693–11703. doi: 10.1523/JNEUROSCI.1081-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kummer Markus P., Hermes M., Delekarte A., Hammerschmidt T., Kumar S., Terwel D., Walter J., Pape H.C., König S., Roeber S., et al. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron. 2011;71:833–844. doi: 10.1016/j.neuron.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Emendato A., Milordini G., Zacco E., Sicorello A., Dal Piaz F., Guerrini R., Thorogate R., Picone D., Pastore A. Glycation affects fibril formation of Aβ peptides. J. Biol. Chem. 2018;293:13100–13111. doi: 10.1074/jbc.RA118.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trzeciakiewicz H., Tseng J.H., Wander C.M., Madden V., Tripathy A., Yuan C.X., Cohen T.J. A dual pathogenic mechanism links tau acetylation to sporadic tauopathy. Sci. Rep. 2017;7:44102. doi: 10.1038/srep44102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haj-Yahya M., Lashuel H.A. Protein semisynthesis provides access to tau disease-associated post-translational modifications (PTMs) and paves the way to deciphering the tau ptm code in health and diseased states. J. Am. Chem. Soc. 2018;140:6611–6621. doi: 10.1021/jacs.8b02668. [DOI] [PubMed] [Google Scholar]
- 108.Cohen T.J., Guo J.L., Hurtado D.E., Kwong L.K., Mills I.P., Trojanowski J.Q., Lee V.M. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferreon J.C., Jain A., Choi K.J., Tsoi P.S., MacKenzie K.R., Jung S.Y., Ferreon A.C. Acetylation disfavors tau phase separation. Int. J. Mol. Sci. 2018;19:1360. doi: 10.3390/ijms19051360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamah A., Huvent I., Cantrelle F.X., Qi H., Lippens G., Landrieu I., Smet-Nocca C. Nuclear magnetic resonance analysis of the acetylation pattern of the neuronal tau protein. Biochemistry. 2014;53:3020. doi: 10.1021/bi500006v. [DOI] [PubMed] [Google Scholar]
- 111.Carlomagno Y., Chung D.E.C., Yue M., Castanedes-Casey M., Madden B.J., Dunmore J., Tong J., DeTure M., Dickson D.W., Petrucelli L., et al. An acetylation-phosphorylation switch that regulates tau aggregation propensity and function. J. Biol. Chem. 2017;292:15277–15286. doi: 10.1074/jbc.M117.794602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guru KrishnaKumar V., Baweja L., Ralhan K., Gupta S. Carbamylation promotes amyloidogenesis and induces structural changes in Tau-core hexapeptide fibrils. Biochim. Biophys. Acta Gen. Subj. 2018;1862:2590–2604. doi: 10.1016/j.bbagen.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 113.Yin H., Kuret J. C-terminal truncation modulates both nucleation and extension phases of τ fibrillization. FEBS Lett. 2006;580:211–215. doi: 10.1016/j.febslet.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 114.Liu K., Liu Y., Li L., Qin P., Iqbal J., Deng Y., Qing H. Glycation alter the process of Tau phosphorylation to change Tau isoforms aggregation property. Biochim. Biophys. Acta. 2016;1862:192–201. doi: 10.1016/j.bbadis.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Mead E., Kestoras D., Gibson Y., Hamilton L., Goodson R., Jones S., Eversden S., Davies P., O’Neill M., Hutton M., et al. Halting of caspase activity protects tau from MC1-Conformational change and aggregation. J. Alzheimers Dis. 2016;54:1521–1538. doi: 10.3233/JAD-150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Necula M., Kuret J. Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J. Biol. Chem. 2004;279:49694–49703. doi: 10.1074/jbc.M405527200. [DOI] [PubMed] [Google Scholar]
- 117.Chang E., Kim S., Schafer K.N., Kuret J. Pseudophosphorylation of tau protein directly modulates its aggregation kinetics. Biochim. Biophys. Acta. 2011;1814:388–395. doi: 10.1016/j.bbapap.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luo H.B., Xia Y.Y., Shu X.J., Liu Z.C., Feng Y., Liu X.H., Yu G., Yin G., Xiong Y.S., Zeng K., et al. SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Proc. Natl. Acad. Sci. USA. 2014;111:16586–16591. doi: 10.1073/pnas.1417548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yuzwa S.A., Cheung A.H., Okon M., McIntosh L.P., Vocadlo D.J. O-GlcNAc modification of tau directly inhibits its aggregation without perturbing the conformational properties of tau monomers. J. Mol. Biol. 2014;426:1736–1752. doi: 10.1016/j.jmb.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 120.Yoshitake J., Soeda Y., Ida T., Sumioka A., Yoshikawa M., Matsushita K., Akaike T., Takashima A. Modification of Tau by 8-Nitroguanosine 3’,5’-Cyclic Monophosphate (8-Nitro-cGMP): Effects of nitric oxide-linked chemical modification on tau aggregation. J. Biol. Chem. 2016;291:22714–22720. doi: 10.1074/jbc.M116.734350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Funk K.E., Thomas S.N., Schafer K.N., Cooper G.L., Liao Z., Clark D.J., Yang A.J., Kuret J. Lysine methylation is an endogenous post-translational modification of tau protein in human brain and a modulator of aggregation propensity. Biochem. J. 2014;462:77–88. doi: 10.1042/BJ20140372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reynolds M.R., Berry R.W., Binder L.I. Site-Specific nitration differentially influences τ assembly in vitro. Biochemistry. 2005;44:13997–14009. doi: 10.1021/bi051028w. [DOI] [PubMed] [Google Scholar]
- 123.Bartels T., Kim N.C., Luth E.S., Selkoe D.J. N-alpha-acetylation of α-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation. PLoS ONE. 2014;9:e103727. doi: 10.1371/journal.pone.0103727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kang L., Moriarty G.M., Woods L.A., Ashcroft A.E., Radford S.E., Baum J. N-terminal acetylation of α-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci. 2012;21:911–917. doi: 10.1002/pro.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bu B., Tong X., Li D., Hu Y., He W., Zhao C., Hu R., Li X., Shao Y., Liu C., et al. N-Terminal Acetylation Preserves α-Synuclein from Oligomerization by Blocking Intermolecular Hydrogen Bonds. ACS Chem. Neurosci. 2017;8:2145–2151. doi: 10.1021/acschemneuro.7b00250. [DOI] [PubMed] [Google Scholar]
- 126.de Oliveira R.M., Vicente M.H., Francelle L., Pinho R., Szegö É.M., Martinho R., Munari F., Lázaro D.F., Moniot S., Guerreiro P., et al. The mechanism of sirtuin 2-mediated exacerbation of alpha-synuclein toxicity in models of Parkinson disease. PLoS Biol. 2017;15:e2000374. doi: 10.1371/journal.pbio.2000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Birol M., Wojcik S.P., Miranker A.D., Rhoades E. Identification of N-linked glycans as specific mediators of neuronal uptake of acetylated α-Synuclein. PLoS Biol. 2019;17:e3000318. doi: 10.1371/journal.pbio.3000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanyal A., Dutta S., Camara A., Chandran A., Koller A., Watson B.G., Sengupta R., Ysselstein D., Montenegro P., Cannon J., et al. Alpha-Synuclein is a target of fic-mediated adenylylation/ampylation: Possible implications for parkinson’s disease. J. Mol. Biol. 2019;431:2266–2282. doi: 10.1016/j.jmb.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vicente M.H., Szego E.M., Oliveira L.M.A., Breda C., Darendelioglu E., de Oliveira R.M., Ferreira D.G., Gomes M.A., Rott R., Oliveira M., et al. Glycation potentiates alpha-synuclein-associated neurodegeneration in synucleinopathies. Brain. 2017;140:1399–1419. doi: 10.1093/brain/awx056. [DOI] [PubMed] [Google Scholar]
- 130.Krumova P., Meulmeester E., Garrido M., Tirard M., Hsiao H.H., Bossis G., Urlaub H., Zweckstetter M., Kügler S., Melchior F., et al. Sumoylation inhibits alpha-synuclein aggregation and toxicity. J. Cell Biol. 2011;194:49–60. doi: 10.1083/jcb.201010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiang W., Schlachetzki J.C.M., Helling S., Bussmann J.C., Berlinghof M., Schäffer T.E., Marcus K., Winkler J., Klucken J., Becker C.M. Oxidative stress-induced posttranslational modifications of alpha-synuclein: Specific modification of alpha-synuclein by 4-hydroxy-2-nonenal increases dopaminergic toxicity. Mol. Cell. Neurosci. 2013;54:71–83. doi: 10.1016/j.mcn.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Xiang W., Menges S., Schlachetzki J.C., Meixner H., Hoffmann A.C., Schlötzer-Schrehardt U., Becker C.M., Winkler J., Klucken J. Posttranslational modification and mutation of histidine 50 trigger alpha synuclein aggregation and toxicity. Mol. Neurodegener. 2015;10:8. doi: 10.1186/s13024-015-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qin Z., Hu D., Han S., Reaney S., Di Monte D., Fink A. Effect of 4-hydroxy-2-neonal modification on alpha-synuclein aggregation. J. Biol. Chem. 2007;282:5862–5870. doi: 10.1074/jbc.M608126200. [DOI] [PubMed] [Google Scholar]
- 134.Levine P.M., Galesic A., Balana A.T., Mahul-Mellier A.L., Navarro M.X., De Leon C.A., Lashuel H.A., Pratt M.R. α-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson’s disease. PNAS USA. 2019;116:1511–1519. doi: 10.1073/pnas.1808845116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J., Lei H., Chen Y., Ma Y.T., Jiang F., Tan J., Zhang Y., Li J.D. Enzymatic O-GlcNAcylation of alpha-synuclein reduces aggregation and increases SDS-resistant soluble oligomers. Neurosci. Lett. 2017;655:90–94. doi: 10.1016/j.neulet.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 136.Marotta N.P., Lin Y.H., Lewis Y.E., Ambroso M.R., Zaro B.W., Roth M.T., Arnold D.B., Langen R., Pratt M.R. O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson’s disease. Nat. Chem. 2015;7:913–920. doi: 10.1038/nchem.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M.S., Shen J., Takio K., Iwatsubo T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 138.Samuel F., Flavin W.P., Iqbal S., Pacelli C., Sri Renganathan S.D., Trudeau L.E., Campbell E.M., Fraser P.E., Tandon A. Effects of Serine 129 Phosphorylation on α-Synuclein Aggregation, Membrane Association, and Internalization. J. Biol. Chem. 2016;291:4374–4385. doi: 10.1074/jbc.M115.705095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Burai R., Ait-Bouziad N., Chiki A., Lashuel H.A. Elucidating the role of site-specific nitration of α-synuclein in the pathogenesis of parkinson’s disease via protein semisynthesis and mutagenesis. J. Am. Chem. Soc. 2015;137:5041–5052. doi: 10.1021/ja5131726. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y., Qiang M., Wei Y., He R. A novel molecular mechanism for nitrated {alpha}-synuclein-induced cell death. J. Mol. Cell. Biol. 2011;3:239–249. doi: 10.1093/jmcb/mjr011. [DOI] [PubMed] [Google Scholar]
- 141.Hodara R., Norris E.H., Giasson B.I., Mishizen-Eberz A.J., Lynch D.R., Lee V.M., Ischiropoulos H. Functional consequences of alpha-synuclein tyrosine nitration: Diminished binding to lipid vesicles and increased fibril formation. J. Biol. Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 142.Souza J.M., Giasson B.I., Chen Q., Lee V.M., Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 143.Wang P., Wander CM., Yuan C.X., Bereman M.S., Cohen T.J. Acetylation-induced TDP-43 pathology is suppressed by an HSF1-dependent chaperone program. Nat. Commun. 2017;8:82. doi: 10.1038/s41467-017-00088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cohen T.J., Hwang A.W., Restrepo C.R., Yuan C.X., Trojanowski J.Q., Lee V.M.Y. An acetylation switch controls TDP-43 function and aggregation propensity. Nat. Commun. 2015;6:5845. doi: 10.1038/ncomms6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Y.J., Xu Y.F., Cook C., Gendron T.F., Roettges P., Link C.D., Lin W.L., Tong J., Castanedes-Casey M., Ash P., et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc. Natl. Acad. Sci. USA. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carlomagno Y., Zhang Y., Davis M., Lin W.L., Cook C., Dunmore J., Tay W., Menkosky K., Cao X., Petrucelli L., et al. Casein kinase II induced polymerization of soluble TDP-43 into filaments is inhibited by heat shock proteins. PLoS ONE. 2014;9:e90452. doi: 10.1371/journal.pone.0090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hasegawa M., Arai T., Nonaka T., Kametani F., Yoshida M., Hashizume Y., Beach T.G., Buratti E., Baralle F., Morita M., et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann. Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brady O.A., Meng P., Zheng Y., Mao Y., Hu F. Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. J. Neurochem. 2011;116:248–259. doi: 10.1111/j.1471-4159.2010.07098.x. [DOI] [PubMed] [Google Scholar]
- 149.Li H.Y., Yeh P.A., Chiu H.C., Tang C.Y., Tu B.P. Hyperphosphorylation as a defense mechanism to reduce TDP-43 aggregation. PLoS ONE. 2011;6:e23075. doi: 10.1371/journal.pone.0023075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prasad A., Sivalingam V., Bharathi V., Girdhar A., Patel B.K. The amyloidogenicity of a C-terminal region of TDP-43 implicated in Amyotrophic Lateral Sclerosis can be affected by anions, acetylation and homodimerization. Biochimie. 2018;150:76–87. doi: 10.1016/j.biochi.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 151.Yang C., Tan W., Whittle C., Qui L., Cao L., Akbarian S., Xu Z. The C-terminal TDP-43 fragments have a high aggregation propensity and harm neurons by a dominant-negative mechanism. PLoS ONE. 2011;5:e15878. doi: 10.1371/journal.pone.0015878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rasouli S., Abdolvahabi A., Croom C.M., Plewman D.L., Shi Y., Ayers J.I., Shaw B.F. Lysine acylation in superoxide dismutase-1 electrostatically inhibits formation of fibrils with prion-like seeding. J. Biol. Chem. 2017;292:19366–19380. doi: 10.1074/jbc.M117.805283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Niikura T., Kita Y., Abe Y. SUMO3 modification accelerates the aggregation of ALS-linked SOD1 mutants. PLoS ONE. 2014;9:e101080. doi: 10.1371/journal.pone.0101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fei E., Jia N., Yan M., Ying Z., Sun Q., Wang H., Zhang T., Ma X., Ding H., Yao X., et al. SUMO-1 modification increases human SOD1 stability and aggregation. Biochem. Biophys. Res. Commun. 2006;347:406–412. doi: 10.1016/j.bbrc.2006.06.092. [DOI] [PubMed] [Google Scholar]
- 155.Chaibva M., Jawahery S., Pilkington Albert W., Arndt James R., Sarver O., Valentine S., Matysiak S., Legleiter J. Acetylation within the first 17 residues of huntingtin exon 1 alters aggregation and lipid binding. Biophys. J. 2016;111:349–362. doi: 10.1016/j.bpj.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chiki A., DeGuire S.M., Ruggeri F.S., Sanfelice D., Ansaloni A., Wang Z.M., Cendrowska U., Burai R., Vieweg S., Pastore A., et al. Mutant exon1 huntingtin aggregation is regulated by T3 phosphorylation-induced structural changes and crosstalk between T3 phosphorylation and acetylation at K6. Angew. Chem. Int. Ed. Engl. 2017;56:5202–5207. doi: 10.1002/anie.201611750. [DOI] [PubMed] [Google Scholar]
- 157.Ansaloni A., Wang Z.M., Jeong J.S., Ruggeri F.S., Dietler G., Lashuel H.A. One-Pot semisynthesis of Exon 1 of the huntingtin protein: New tools for elucidating the role of posttranslational modifications in the pathogenesis of huntington’s disease. Angew. Chem. Int. Ed. Engl. 2014;53:1928–1933. doi: 10.1002/anie.201307510. [DOI] [PubMed] [Google Scholar]
- 158.Cariulo C., Azzollini L., Verani M., Martufi P., Boggio R., Chiki A., Deguire S.M., Cherubini M., Gines S., Marsh J.L., et al. Phosphorylation of huntingtin at residue T3 is decreased in Huntington’s disease and modulates mutant huntingtin protein conformation. Proc. Natl. Acad. Sci. USA. 2017;114:E10809. doi: 10.1073/pnas.1705372114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.DeGuire S.M., Ruggeri F.S., Fares M.B., Chiki A., Cendrowska U., Dietler G., Lashuel H.A. N-terminal Huntingtin (Htt) phosphorylation is a molecular switch regulating Htt aggregation, helical conformation, internalization, and nuclear targeting. J. Biol. Chem. 2018;293:18540–18558. doi: 10.1074/jbc.RA118.004621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gu X., Greiner E.R., Mishra R., Kodali R., Osmand A., Finkbeiner S., Steffan J.S., Thompson L.M., Wetzel R., Yang X.W. Serines 13 and 16 are critical determinants of full-length human mutant huntingtin induced disease pathogenesis in HD mice. Neuron. 2009;64:828–840. doi: 10.1016/j.neuron.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lunkes A., Lindenberg K.S., Ben-Haïem L., Weber C., Devys D., Landwehrmeyer G.B., Mandel J.L., Trottier Y. Proteases acting on mutant huntingtin generate cleaved products that differentially build up cytoplasmic and nuclear inclusions. Mol. Cell. 2002;10:259–269. doi: 10.1016/S1097-2765(02)00602-0. [DOI] [PubMed] [Google Scholar]
- 162.Raspe M., Gillis J., Krol H., Krom S., Bosch K., van Veen H., Reits E. Mimicking proteasomal release of polyglutamine peptides initiates aggregation and toxicity. J. Cell Sci. 2009;122:3262–3271. doi: 10.1242/jcs.045567. [DOI] [PubMed] [Google Scholar]
- 163.Ryu J., Cho S., Park B.C., Lee D.H. Oxidative stress-enhanced SUMOylation and aggregation of ataxin-1: Implication of JNK pathway. Biochem. Biophys. Res. Commun. 2010;393:280–285. doi: 10.1016/j.bbrc.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 164.Haacke A., Broadley S.A., Boteva R., Tzvetkov N., Hartl F.U., Breuer P. Proteolytic cleavage of polyglutamine-expanded ataxin-3 is critical for aggregation and sequestration of non-expanded ataxin-3. Hum. Mol. Genet. 2006;15:555–568. doi: 10.1093/hmg/ddi472. [DOI] [PubMed] [Google Scholar]
- 165.Dear D.V., Young D.S., Kazlauskaite J., Meersman F., Oxley D., Webster J., Pinheiro T.J., Gill A.C., Bronstein I., Lowe C.R. Effects of post-translational modifications on prion protein aggregation and the propagation of scrapie-like characteristics in vitro. Biochim. Biophys. Acta. 2007;1774:792–802. doi: 10.1016/j.bbapap.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 166.Giannopoulos P.N., Robertson C., Jodoin J., Paudel H., Booth S.A., LeBlanc A.C. Phosphorylation of prion protein at serine 43 induces prion protein conformational change. J. Neurosci. 2009;29:8743–8751. doi: 10.1523/JNEUROSCI.2294-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moro M.L., Phillips A.S., Gaimster K., Paul C., Mudher A., Nicoll J.A.R., Boche D. Pyroglutamate and Isoaspartate modified Amyloid-Beta in ageing and Alzheimer’s disease. Acta Neuropathol. Commun. 2018;6:3. doi: 10.1186/s40478-017-0505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vigneswara V., Cass S., Wayne D., Bolt E.L., Ray D.E., Carter W.G. Molecular ageing of alpha- and Beta-synucleins: Protein damage and repair mechanisms. PLoS ONE. 2013;8:E61442. doi: 10.1371/journal.pone.0061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vigneswara V., Lowenson J.D., Powell C.D., Thakur M., Bailey K., Clarke S., Ray D.E., Carter W.G. Proteomic identification of novel substrates of a protein isoaspartyl methyltransferase repair enzyme. J. Biol. Chem. 2006;281:32619–32629. doi: 10.1074/jbc.M605421200. [DOI] [PubMed] [Google Scholar]
- 170.Neddens J., Temmel M., Flunkert S., Kerschbaumer B., Hoeller C., Loeffler T., Niederkofler V., Daum G., Attems J., Hutter-Paier B. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2018;6:52. doi: 10.1186/s40478-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Congdon E.E., Sigurdsson E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018;14:399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Helwig M., Klinkenberg M., Rusconi R., Musgrove R.E., Majbour N.K., El-Agnaf O.M., Ulusoy A., Di Monte D.A. Brain propagation of transduced alpha-synuclein involves non-fibrillar protein species and is enhanced in alpha-synuclein null mice. Brain. 2016;139:856–870. doi: 10.1093/brain/awv376. [DOI] [PubMed] [Google Scholar]
- 173.Winner B., Jappelli R., Maji S.K., Desplats P.A., Boyer L., Aigner S., Hetzer C., Loher T., Vilar M., Campioni S., et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Saito Y., Kawashima A., Ruberu N.N., Fujiwara H., Koyama S., Sawabe M., Arai T., Nagura H., Yamanouchi H., Hasegawa M., et al. Accumulation of phosphorylated α-synuclein in aging human brain. J. Neuropathol. Exp. Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- 175.Dangoumau A., Marouillat S., Burlaud Gaillard J., Uzbekov R., Veyrat-Durebex C., Blasco H., Arnoult C., Corcia P., Andres C.R., Vourc’h P. Inhibition of Pathogenic mutant SOD1 aggregation in cultured motor neuronal cells by prevention of its sumoylation on lysine 75. Neurodegener. Dis. 2016;16:161–171. doi: 10.1159/000439254. [DOI] [PubMed] [Google Scholar]
- 176.Bowie L.E., Maiuri T., Alpaugh M., Gabriel M., Arbez N., Galleguillos D., Hung C.L.K., Patel S., Xia J., Hertz N.T., et al. N6-Furfuryladenine is protective in Huntington’s disease models by signaling huntingtin phosphorylation. Proc. Natl. Acad. Sci. USA. 2018;115:E7081–E7090. doi: 10.1073/pnas.1801772115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Herrera F., Branco-Santos J., Outeiro T. Threonine 3 regulates Serine 13/16 phosphorylation in the huntingtin exon 1. Matters Sel. 2019 doi: 10.19185/matters.201905000005. [DOI] [Google Scholar]
- 178.Verma M., Vats A., Taneja V. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015;18:138–145. doi: 10.4103/0972-2327.144284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Celej María S., Sarroukh R., Goormaghtigh E., Fidelio Gerardo D., Ruysschaert J.M., Raussens V. Toxic prefibrillar α-synuclein amyloid oligomers adopt a distinctive antiparallel β-sheet structure. Biochem. J. 2012;443:719–726. doi: 10.1042/BJ20111924. [DOI] [PubMed] [Google Scholar]
- 180.Cerf E., Sarroukh R., Tamamizu-Kato S., Breydo L., Derclaye S., Dufrene Y.F., Narayanaswami V., Goormaghtigh E., Ruysschaert J.M., Raussens V. Antiparallel beta-sheet: A signature structure of the oligomeric amyloid beta-peptide. Biochem. J. 2009;421:415–423. doi: 10.1042/BJ20090379. [DOI] [PubMed] [Google Scholar]
- 181.Simmons L.K., May P.C., Tomaselli K.J., Rydel R.E., Fuson K.S., Brigham E.F., Wright S., Lieberburg I., Becker G.W., Brems D.N., et al. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol. Pharmacol. 1994;45:373–379. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.