Abstract
Immune‐related adverse events (irAEs) are often seen during immune‐checkpoint inhibitor (ICI) treatment of various malignancies. Endocrine irAEs including thyroid dysfunctions are the most common irAEs, but their biomarkers remain unclear. In order to identify individuals who are susceptible to thyroid irAE for earlier diagnosis and appropriate follow‐up, the current study is aimed to investigate biomarkers of thyroid irAE. Herein, patients with advanced malignant diseases who received ICIs treatment were prospectively studied. Clinical and laboratory examination, thyroid function, and autoantibodies were evaluated at baseline, and every 4 wk after first treatment with ICIs. Cytokines/chemokines were measured at baseline and at 4 wk. In vivo effects of ICIs on experimental autoimmune thyroiditis were evaluated. Twenty‐six patients with malignant diseases who received ICIs treatment were enrolled in the study. Patients were divided into two groups: those who developed thyroid irAE, and those without irAEs. Comparing the two groups, early increase (≤4 wk) in serum thyroglobulin (Tg) levels and thyroid autoantibodies was seen in thyroid irAE (P < .05). Notably, higher levels of serum IL‐1β, IL‐2, and GM‐CSF at baseline, and early decrease of IL‐8, G‐CSF, and MCP‐1 were significantly associated in the development of thyroid irAE (P < .05). In vivo effects of anti‐PD‐1 antibody on deterioration of mice experimental thyroiditis were seen. In conclusion, early change in Tg, thyroid autoimmunity, and cytokine levels might indicate development of thyroid irAE. Pre‐existing thyroid autoimmunity might be involved with the development of thyroid irAE. Potential application of these factors as surrogate biomarkers for tumor therapy was indicated.
Keywords: cytokine, immune‐related adverse events, immunotherapy, thyroglobulin, thyroid dysfunction
Abbreviations
- CTLA‐4
cytotoxic T‐lymphocyte‐associated antigen 4
- HLA
human leukocyte antigen
- ICI
immune‐checkpoint inhibitor
- irAEs
immune‐related adverse events
- NSCLC
non‐small cell lung cancer
- PD‐1
programmed cell death protein 1
- RCC
renal cell carcinoma
- Tg
thyroglobulin
- TSH
thyroid stimulating hormone
1. INTRODUCTION
Immune checkpoints, which consist of cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4), programmed death protein 1 (PD‐1), and ligand for PD‐1 (PD‐L1) play an indispensable role in anti‐tumor immunity, anti‐infection, and autoimmunity.1, 2, 3 Monoclonal antibodies to immune checkpoints are referred to as immune‐checkpoint inhibitors (ICIs). ICIs are novel agents for the treatment of various malignancies.1 They promote T‐cell‐mediated cytotoxicity directed to cancer cell antigens. Approximately 20%‐30% of patients with malignancies have been found to be responders to ICIs.1, 4 Meanwhile, immune‐related adverse events (irAEs) are often seen during ICIs treatment. The reported incidence is more than 50% for any‐grade irAEs due to ICIs treatment.1 IrAEs include dermatological, gastrointestinal, hepatic, neurological, and endocrine disorders.1, 4, 5, 6, 7, 8, 9 In endocrine organs, irAEs of the pituitary gland, the thyroid gland, the parathyroid glands, the adrenal glands, and the pancreas (type 1 diabetes mellitus) have been reported.1, 4, 5, 6, 7, 8, 9 Thyroid dysfunctions (thyrotoxicosis and hypothyroidism) are the most common irAEs and are reported to occur in 5%‐50% of patients, but their biomarkers remain unclear.1, 4, 6, 9
Positive correlation between thyroid irAE and anti‐tumor effects has been reported.10 Therefore, for continuation and appropriate use of ICIs, identification of individuals who are susceptible to thyroid irAE for earlier diagnosis is warranted.
The current study aims to investigate predictive and sensitive biomarkers in thyroid irAE. Prospective clinical observational research focused on early change of biomarkers and animal research were conducted. Novel biomarkers were identified including thyroid‐related proteins and cytokines.
2. MATERIALS AND METHODS
2.1. Patients
Patients with advanced malignant diseases (malignant melanoma, gastric cancer, renal cell carcinoma, urothelial cancer, and non‐small cell lung cancer) who received ICIs treatment were recruited at Wakayama Medical University between September 2017 and September 2019. Treatment with ICIs included that with anti‐PD‐1 antibody (nivolumab or pembrolizumab), with anti‐CTLA‐4 antibody (ipilimumab), or by combination therapy of ipilimumab and nivolumab. ICI agents were intravenously administrated as follows: pembrolizumab, 2 mg/kg every 3 wk, nivolumab; 3 mg/kg every 2 wk, combination therapy; 1 mg/kg nivolumab in combination with 3 mg/kg ipilimumab, every 3 wk for the first four doses, followed by a second phase in which nivolumab monotherapy was administrated; 3 mg/kg, every 2 wk.
Minimum patient follow‐up period was 12 mo, the longest was 24 mo. Patients who had infectious diseases or who were treated with glucocorticoid before 8 wk, those who transferred to other hospital before 8 wk, and those who had iodide contrast enhancement CT immediately before thyroid tests were excluded from the study. For assessment of immune‐related adverse events (irAEs), the description and grading scales of NCI Common Terminology Criteria for Adverse Events version 3.0 were used. After the 24‐mo follow‐up period, patients were divided into two groups: those with thyroid irAE (IR) and those without any irAEs, including thyroid irAEs (non‐IR) (eg gastrointestinal disorders, hepatobiliary disorders, nervous system disorders, and skin disorders), and analyzed. Written informed consent was obtained from all patients. The study protocol was approved by the Wakayama Medical University Institutional Ethical Review Board, and was conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Data and sample collection
Clinical manifestations were recorded at every hospital visit. Peripheral blood samples were collected from patients at baseline (0 wk) (before treatment [BT]), 4 wk after first ICIs treatment: immediate before third ICIs treatment (after treatment [AT]), and at 4 wk intervals. Cytokines/chemokines were measured BT and AT. Serum samples were centrifuged at 1200 g at 4C for 15 min and stored at −80°C until measurement.
2.3. Thyroid function tests and laboratory examinations
Serum free triiodothyronine (fT3), free thyroxine (fT4), thyrotropin (TSH), and thyroglobulin (Tg) levels were measured by chemiluminescence immunoassay (Roche Diagnostics, Germany). The reference ranges were defined as follows: 2.3‐4.0 pg/mL, 0.9‐1.7 ng/dL, 0.5‐5.0 μIU/mL, and <33.7 ng/mL, respectively. Anti‐thyrotropin autoantibody (TRAb) was determined by enzyme‐linked immunosorbent assay (ELISA) (Cosmic). Thyroglobulin autoantibodies (TgAb) and thyroid peroxidase autoantibodies (TPOAb) were measured with an electrochemiluminescent immunoassay (TOSOH). Normal values were defined as follows: TRAb <1.0 IU/L; TgAb <28 IU/mL; TPOAb <16 IU/mL. The number of eosinophils and the platelet count in blood, and serum levels of sodium were also recorded. These factors BT, AT, and the ratio of AT/BT were evaluated.
2.4. Definition of thyroid irAE
Thyroid irAE were defined as follows: (a) increased serum fT4 and decreased TSH levels: overt thyrotoxicosis [Tox]; (b) normal fT4 and decreased TSH levels: subclinical thyrotoxicosis [S‐Tox]; (c) decreased fT4 and increased TSH levels: overt hypothyroidism [Hypo]; and (d) normal fT4 and increased TSH levels: subclinical hypothyroidism [S‐Hypo].
2.5. Cytokine and chemokine analysis
Functional cytokine and chemokine levels were measured using Bio‐Plex Pro Human 17‐plex panes (interleukin [IL]‐1β, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8, IL‐10, IL‐12, IL‐13, IL‐17, interferon [IFN]‐γ, tumor necrosis factor [TNF]‐α, granulocyte colony‐stimulating factor [G‐CSF], granulocyte–macrophage colony‐stimulating factor [GM‐CSF], monocyte chemoattractant protein [MCP]‐1, and macrophage inflammatory protein [MIP]‐1b) (Bio‐Rad Laboratories) according to the manufacturer's protocols. Bio‐Plex Manager software was used for data analysis. IL‐33 was measured using a Human IL‐33 Cytokine Domain Detection Kit based on the sandwich ELISA system (MBL). Each assay was performed in duplicate. Fluorescence intensity (Fi) values were derived from the discovery assay and are in direct proportion to reflect the amount of proteins in the samples. Fi values were log2 transformed for analysis.11
2.6. Mice
HLA‐DR3 mice have human transgene of HLA‐DR3, and mice MHC class II were knocked out in them.12 Experimental autoimmune thyroiditis (EAT) is induced in the mice immunized to human Tg (hTg), therefore the mice were used in the study.12 The expression of transgenes and knocked out mouse MHC class II genes were tested by flow cytometry using mouse monoclonal antibody for HLA‐DR (L227, ATCC) and by polymerase chain reaction of genomic DNA. Near equal numbers of male and female mice at 6‐14 wk of age were used for study. Mice were maintained in conventional conditions. Animal care and all experimental procedures were performed in accordance with the Guidelines for Animal Experiments of Wakayama Medical University, with the approval of the Institutional Animal Care and Use Committee.
2.7. Induction of mouse EAT and antibody treatment
For induction of EAT, mice were immunized intravenously on d 0 and d 7 with 50 µg of hTg, followed by 10 µg of LPS 3 h later.12, 13
Anti‐murine PD‐1 Ab (clone 4H2) is a chimeric rat Ab with a murine IgG1, which was provided by Ono Pharmaceutical Company. Ab was given as intraperitoneal (ip) injections of 20 mg/kg on d 0, and 10 mg/kg three times at 6 d intervals.14
2.8. Histological examinations
Mice were sacrificed on d 42 after immunization. Thyroids glands were removed and fixed in 10% formalin and embedded in paraffin. Sections from each tissue (5 μm) were stained with hematoxylin and eosin. The extent of thyroiditis was scored on sections cut in each lobe at five levels through the gland. The scoring system used was as follows: 0 = no infiltration, 0.5 = small perivascular foci of infiltration, 1.0 = interstitial accumulation of inflammatory cells distributed between two or more follicles, 2.0 = one to two foci of inflammatory cells, 3.0 = 10%‐40% of the thyroid replaced by inflammatory cells; and 4.0 = more than 40% of the thyroid replaced by inflammatory cells.13 Three to five sections were scored by an observer who was blinded to the treatment. The score for each mouse was the total for all sections divided by the number of sections observed. The average score for thyroiditis for each group is the mean and 2 SD of all mice in the group.
2.9. Immunohistochemical analyses of the mice thyroid glands
Immunohistochemical analyses were performed with Vector statin ABC kits according to the manufacturer's manual. Formalin‐fixed, paraffin‐embedded tissue sections of mice thyroid glands were immunostained with anti‐CD3 (CD3‐12, ab11089, abcam) (1:200 dilution), anti‐CD19 (C1C3, GTX101512, GeneTex, Inc.) (1:500 dilution), or anti‐CD11c (E‐AB‐70017, Elabscience Biotechnology Co., Ltd) (1:1000 dilution) antibodies.
2.10. Measurement of serum mouse Tg
Serum mouse Tg levels at sacrifice were measured using mouse Tg quantitative ELISA kit according to the protocol provided by the company (CUSABIO TECHNOLOGY, LLC).
2.11. Measurement of mice serum TgAb
Titer of the TgAb in mouse sera was tested by ELISA at sacrifice. The wells of 96‐well plates were coated with 50 μL of a 10 μg/mL solution of hTg in 0.1 M carbonate/bicarbonate, pH 9.6, and incubated overnight at 4°C. After washing, the reactive sites were blocked with 8.3% skimmed milk for 2 h at room temperature. 50 μL of serum samples diluted in PBST + 1% BSA (1:1000) were then added, and plates were incubated for 3 h at room temperature. Plates were washed, and secondary antibody HRP‐conjugated goat anti‐mouse IgG was added for 1 h at room temperature. Substrate was added, and the reaction was stopped at 15 min. Optical density was measured at absorbance 450 nm.
2.12. Statistical analysis
Differences between two individual groups were analyzed by Fisher’s exact test using 2 × 2 contingency tables. Mann‐Whitney U‐tests were used to compare two individual groups or paired samples between two groups. Spearman’s rank correlation coefficient (rs) was determined to assess the correlation between two variables. Values among more than two groups were ascertained by Kruskal‐Wallis test and corrected by Bonferroni’s method.
Statistical analyses were performed using JMP software, version 14 (SAS Institute Inc.). A P‐value <.05 was considered to be statistically significant.
3. RESULTS
3.1. Patients
During the follow‐up period, 66 patients were recruited and we enrolled 26 subjects. The procedure for patient enrollment is shown in Supporting Information Figure S1. Based on the inclusion and exclusion procedures, 13 patients with thyroid irAE (IR group), and those without organs with irAE during the follow‐up period (non‐IR group, n = 13) were analyzed. Baseline demographic data are listed in Table 1. Twenty‐four patients received anti‐PD‐1 therapy (nivolumab n = 18, and pembrolizumab n = 6), and two patients took combination therapy of nivolumab and anti‐CTLA‐4 therapy (ipilimumab). The overall incidence of thyroid irAE was 19.6% (13/66), consistent with that in previous reports.1, 4, 6, 9, 10
Table 1.
Patients background in the study
| Group | Number | Age | Gender (M/F) | Treatment of ICI | Malignancy |
|---|---|---|---|---|---|
| Non‐IR | 13 | 67.6 ± 9.9 | 10M/ 3F | Nivo 11, Pem 2 | MM 3, GC 5, RCC 3, URO 2 |
| IR | 13 | 71.5 ± 14.1 | 9M/ 4F | Nivo 7, Pem 4, Ipi/Nivo 2 | MM 7, GC 2, RCC 1, URO 2, NSCLC 1 |
| Total | 26 | 69.5 ± 12.6 | 19M/ 7F | Nivo 18, Pem 6, Ipi/Nivo 2 | MM 10, GC 7, RCC 4, URO 4, NSCLC 1 |
| P‐value | N/A | NS | NS | N/A | N/A |
Abbreviations: F, female; GC, gastric cancer; Ipi/Nivo, combination of ipilimumab and nivolumab; IR, patients with thyroid irAE; M, male; MM, malignant melanoma; N/A, not applicable; Nivo, nivolumab; Non‐IR, patients without thyroid irAE; NS, not significant; NSCLC, non‐small cell lung cancer. Age is represented as mean ± SD; Pem, pembrolizumab; RCC, renal cell carcinoma; URO, urogenital cancer.
Age and gender were not significantly different between the two groups. Treatments of ICI and malignancies are shown in Table 1.
3.2. Clinical characteristics of thyroid irAE
Patients who developed thyroid irAE are clinically described in Table 2 and in Figure S1. All 13 patients developed thyroid irAE within 8 wk. Among them, seven patients developed grade 1 thyrotoxicosis, and four of them spontaneously recovered within 2 mo. Three out of the seven patients had subsequent hypothyroidism. Two patients of grade 1 subclinical hypothyroidism all spontaneously recovered. But the remaining four patients in grade 2 hypothyroidism underwent L‐T4 therapy throughout. Markedly, the four patients who were taking moderate dosage of L‐T4 therapy (≥75 μg) had either high titer of TgAb (>200 IU/mL) and TPOAb (>200 IU/mL) prior to ICIs treatment (patients 3, 5, and 8) or increase of Tg after ICI therapy (patients 3, 5, 8, and 13). All four patients showed early increase of two or three of TgAb, TPOAb, or Tg. Regarding other organ irAEs, intestinal pneumonitis occurred in two patients, and two patients developed encephalitis after 8 wk. TRAb levels were not shown, because all samples were below the normal range (<1.0 IU/L).
Table 2.
Description of the patients who developed thyroid irAE
| Patient | Age | Gender | Thyroid irRAE | Thyroid irAE grade | Time at onset | Other irAEs | Initial treatment | Current treatment | Treatment of ICI | Malignancy | TSH | Tg | TgAb | TPOAb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | AT | AT/BT | BT | AT | AT/BT | BT | AT | AT/BT | BT | AT | AT/BT | |||||||||||
| 1 | 42 | Male | S‐hypo | G1 | 4 wk | None | none | Nivo | GC | 3.72 | 8.10 | 2.18 | 9.1 | 9.8 | 1.08 | <10 | <10 | 1.00 | 9.7 | 14.0 | 1.44 | |
| 2 | 64 | Male | S‐hypo | G1 | 4 wk | None | none | Nivo | GC | 1.91 | 5.50 | 2.88 | 11.5 | 17.0 | 1.48 | <10 | <10 | 1.00 | <9 | <9 | 1.00 | |
| 3 | 90 | Male | Hypo | G2 | 8 wk | T4 25 μg | T4 87.5 μg | Pem | MM | 2.88 | 4.59 | 1.59 | 3.6 | 4.7 | 1.31 | >4000 | >4000 | 1.00 | 233.3 | 256.5 | 1.10 | |
| 4 | 66 | Female | Hypo | G2 | 4 wk | T4 25 μg | T4 25 μg | Nivo | MM | 4.27 | 6.03 | 1.41 | 16.7 | 55.4 | 3.32 | <10 | <10 | 1.00 | <9 | 10.0 | 1.11 | |
| 5 | 90 | Male | Tox | G1 | 4 wk | None | T4 100 μg | Pem | NSCLC | 1.00 | 0.01 | 0.01 | 1.1 | 7.9 | 7.18 | 283.4 | 332.8 | 1.17 | >600 | >600 | 1.00 | |
| 6 | 64 | Male | Tox | G1 | 8 wk | IP, 8w | None | none | Nivo | MM | 1.16 | 1.27 | 1.09 | 15.0 | 16.4 | 1.09 | <10 | <10 | 1.00 | <9 | 15.7 | 1.96 |
| 7 | 90 | Female | Tox | G1 | 4 wk | None | none | Nivo | MM | 3.70 | 0.15 | 0.04 | 3.3 | 23.6 | 7.15 | 53.5 | 356.0 | 6.65 | <9 | <9 | 1.00 | |
| 8 | 73 | Male | Tox | G1 | 4 wk | None | T4 150 μg | Pem | URO | 3.89 | 0.02 | 0.01 | 47.6 | 51.4 | 1.08 | 892.0 | 1146.0 | 1.28 | 403.0 | >600 | 1.49 | |
| 9 | 90 | Female | Hypo | G2 | 4 wk | T4 25 μg | T4 25 μg | Nivo | MM | 4.41 | 16.10 | 3.65 | 57.5 | 83.7 | 1.46 | <10 | <10 | 1.00 | 15.9 | 13.3 | 0.84 | |
| 10 | 61 | Male | Tox | G1 | 4 wk | None | None | Pem | URO | 0.50 | 0.12 | 0.24 | 7.7 | 32.9 | 4.27 | 13.2 | 12.2 | 0.92 | 20.0 | 12.6 | 0.63 | |
| 11 | 63 | Female | Tox | G1 | 8 wk | IP, 8w | None | None | Ipi/Niv | MM | 1.23 | 0.54 | 0.44 | 6.8 | 6.2 | 0.91 | <10 | 35.0 | 3.50 | 9.9 | 15.0 | 1.52 |
| 12 | 68 | Male | Hypo | G2 | 4 wk | Encephalitis, 8w | T4 25 μg | T4 25 μg | Nivo | MM | 1.55 | 6.03 | 3.89 | 5.4 | 5.8 | 1.07 | <10 | <10 | 1.00 | <9 | <9 | 1.00 |
| 13 | 69 | Male | Tox | G1 | 4 wk | Encephalitis, 8w | None | T4 75 μg | Ipi/Niv | RCC | 1.30 | 0.15 | 0.12 | 13.5 | 1385.0 | 102.59 | <10 | 240.8 | 24.08 | <9 | <9 | 1.00 |
Abbreviations: <9, value less than 9; <10, value less than 10; >4000, value more than 4000; >600, value more than 600; GC, gastric cancer; Hypo, hypothyroid; IP, interstitial pneumonitis; Ipi/Nivo, combination of ipilimumab and nivolumab; MM, malignant melanoma; Nivo, nivolumab; NSCLC, non‐small cell lung cancer; Pem, pembrolizumab; RCC, renal cell carcinoma; S‐hypo, subclinical hypothyroid; T4, dosage of L‐T4 supplemental therapy (per d); Tg, thyroglobulin (ng/ml); TgAb, anti‐thyroglobulin autoantibody (IU/ml); Tox, thyrotoxicosis; TPOAb, anti‐TPO autoantibody (IU/ml); URO, urogenital cancer.
3.3. Predictive and diagnostic factors of thyroid irAE
Comparison of non‐IR and IR groups is shown in Figure 1A‐C and Table S1. Early changes: AT/BT in serum Tg levels were strongly associated with the development of thyroid irAE (Figure 1A) (P = .01). The increase of Tg was also shown in the IR group by paired U‐test (P = .007). Moreover, TgAb (Figure 1B) and TPO (Figure 1C) titers AT were had more significantly increase in the IR group than in the non‐IR group (P = .012, and P = .048, respectively).
Figure 1.
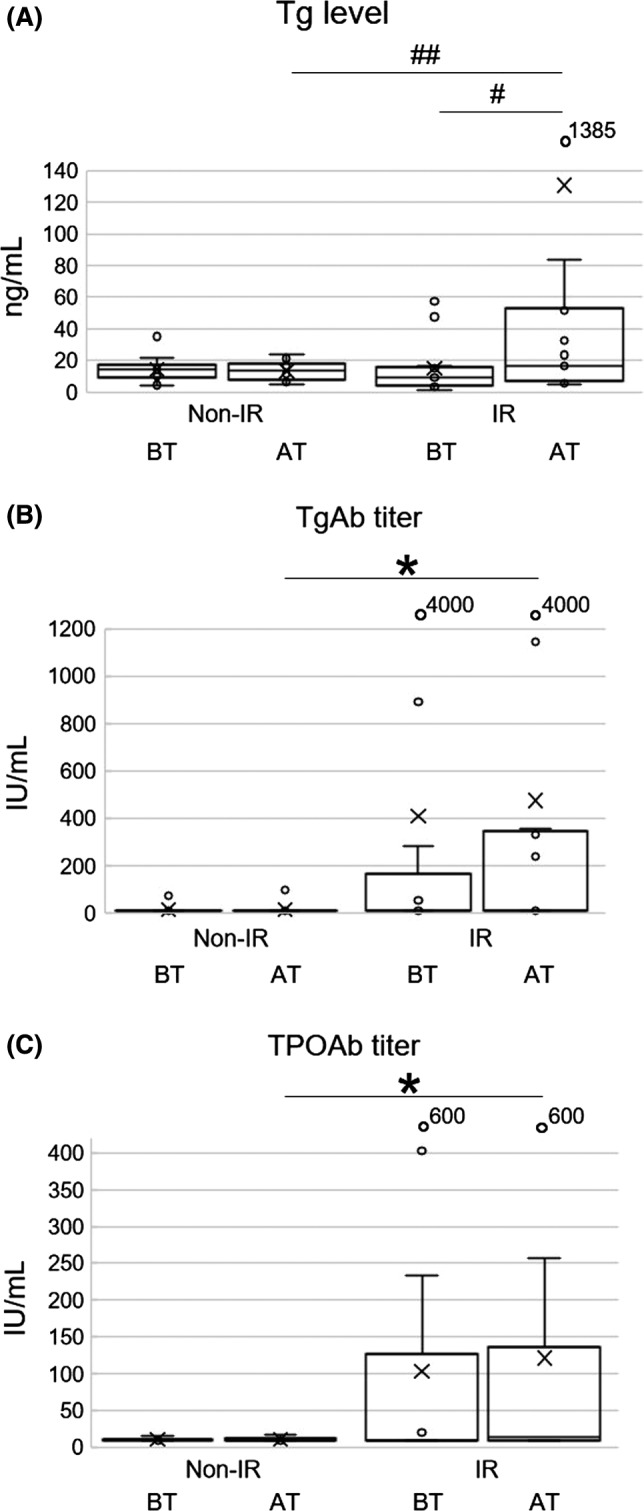
Box‐and‐whisker plots are shown. Minimum, lower quartile, median, upper quartile, and maximum levels are shown as indicated. Average levels are shown as ‘x’. Levels or titers of each factors at baseline (BT) and 4 wk after first ICI treatment (AT) are shown (A‐C). A, Serum Tg levels in the IR group were significantly increased from BT to AT (# P < .05). In addition, the ratio of AT/BT in the IR group was also greater than that of the non‐IR group (## P < .05). B, TgAb titers at AT in the IR group were significantly higher than those in the non‐IR group (*P < .05). C, TPOAb titers at AT in the IR group were significantly higher than those in the non‐IR group (*P < .05)
3.4. Cytokine and chemokine levels
Among the 18 cytokines and chemokines, the levels of IL‐33 (pro‐Th2 cytokine) were below detection levels and therefore eliminated from the analysis. Th1 cytokines (IL‐2, IL‐12 and IFN‐γ), Th2 cytokines and chemokines (IL‐4, IL‐5, IL‐6, IL‐13, and MCP‐1), regulatory T‐cell cytokine; IL‐10, Th17 cytokine; IL‐17, inflammatory cytokines (IL‐1β, TNF‐α); and other cytokines and chemokines (IL‐7, IL‐8, G‐CSF, GM‐CSF, MIP‐1b) were evaluated (Figure 2A‐G, Table S2). The overall levels of AT and BT, and ratio of AT/BT in all cytokines/chemokines between the two groups were compared. The log2 transformed Fi levels of BT in the IR group were significantly greater than those in the non‐IR group (mean ± SD; non‐IR, 7.31 ± 1.52, and IR, 7.60 ± 1.51, respectively, P = .042). In comparison of the paired values from BT to AT in IR group, the log2 transformed Fi levels in AT were significantly lower than those in BT (BT, 7.60 ± 1.51, and AT, 7.37 ± 1.42, respectively, P < .001). The ratio of AT/BT in the IR group was significantly lower than that in the non‐IR group (non‐IR, 1.00 ± 0.04, and IR, 0.97 ± 0.06, respectively, P < .001; Table S2).
Figure 2.
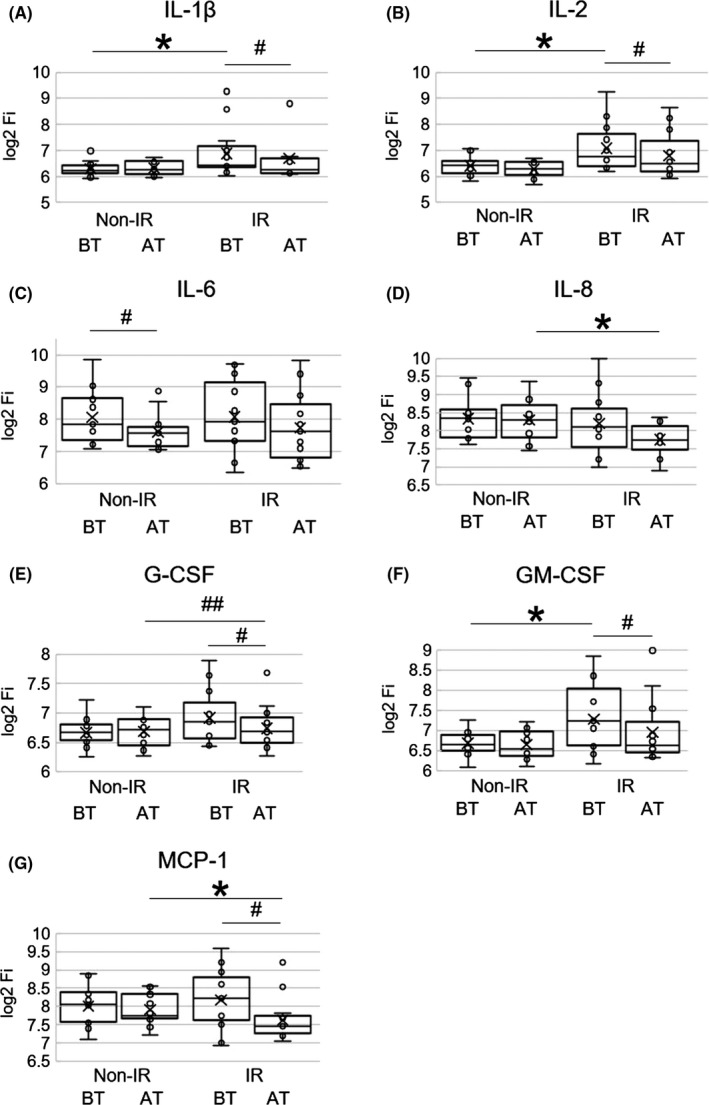
Box‐and‐whisker plots are shown. Fluorescence intensity (Fi) values of each cytokine or chemokine were measured. Log2 transformed Fi values are shown on the y axis.11 Results at baseline (BT) and 4 wk after first ICIs treatment (AT) in each group are represented. Minimum, lower quartile, median, upper quartile, and maximum levels are shown as indicated. Average levels are shown as ‘x’. A, Serum IL‐1β values at BT in the IR group were significantly higher than those in the non‐IR group (*P < .05). Serum IL‐1β values in the IR group were significantly decreased from BT to AT (# P < .05). B, Serum IL‐2 values at BT in the IR group were significantly higher than those in the non‐IR group (*P < .05). Serum IL‐2 values in the IR group were significantly decreased from BT to AT (# P < .05). C, Serum IL‐6 values in the non‐IR group were significantly decreased from BT to AT (# P < .05). D, Serum IL‐8 values at AT in the IR group were significantly lower than those in the non‐IR group (*P < .05). E, Serum G‐CSF values in the IR group were significantly decreased from BT to AT (# P < .05). The ratio of AT/BT was significantly different between the two groups (## P < .05). F, Serum GM‐CSF values at BT in the IR group were significantly higher than those in the non‐IR group (*P < .05). Serum GM‐CSF values in the IR group were significantly decreased from BT to AT (# P < .05). G, Serum MCP‐1 values at AT in the IR group were significantly lower than those in the non‐IR group (*P < .05). Serum MCP‐1 values in the IR group were significantly decreased from BT to AT (# P < .05)
Of those, basal levels at BT of IL‐1β, IL‐2, and GM‐CSF were significantly higher in the IR group than those in the non‐IR group. Conversely, 4 wk after first ICIs treatment (AT) levels of IL‐8 and MCP‐1 were significantly decreased in the IR group compared with those in the non‐IR group. The early (BT to AT) reduction of G‐CSF in the IR group was also significant compared with that of the non‐IR group.
3.5. Correlation among thyroid irAE and cytokines/ chemokines
In order to investigate the potential effects on each factor, correlation analysis among early changes (the ratio of AT/BT) was performed (Table S3A,B). Significant strong positive correlations (rs > 0.7 and P < .0001) were observed in cytokines/chemokines as follows; IL‐4 and IL‐13, IL‐4 and G‐CSF, IL‐5 and G‐CSF, IL‐5 and IFN‐γ, IL‐8 and TNF‐α, IL‐12 and TNF‐α, IL‐13 and G‐CSF, IL‐17, and MIP1‐b. Interestingly, early change of IL‐10 was shown to be significantly and negatively associated with that of TPOAb titer, and positively associated with platelet counts (rs = −0.43, P = .0287 and rs = 0.49, P = .0117, respectively). Increase of TSH also had significant positive correlation with platelet counts (rs = 0.45, P = .0251).
3.6. In vivo effect of anti‐PD‐1 antibody on the development of EAT
Anti‐mouse‐PD‐1 antibody as well as hTg was given to the ‘humanized’ HLA‐DR3 transgenic mice. Although thyroid function was not different among the three groups (Figure S2A), EAT was successfully induced in the mice immunized to hTg, and anti‐PD‐1 antibody significantly strengthened the inflammation in the thyroid gland (Figure S2B).
Serum mouse Tg levels were also elevated in mice immunized to hTg, and anti‐PD‐1 antibody significantly increased the Tg levels (Figure S2C). Moreover, TgAb production which represent B‐cell immunity to hTg was also seen in the mice immunized to hTg, and with anti‐PD‐1 antibody (Figure S2D).
Representative mice thyroid glands in hematoxylin and eosin staining are shown in Figure S3A. A control mouse showed no thyroiditis (left). A Tg‐immunized mouse developed score 1 thyroiditis (middle, arrow), and a Tg‐immunized and anti‐PD‐1 antibody‐treated mouse developed score 2 thyroiditis (right, arrow).
Mice thyroid glands were also examined for expression of CD3, CD19, and CD11c (Figure S3B‐D, respectively). Few thyroid‐infiltrating mononucleocytes were stained with‐CD3 antibody (mostly T‐cells) in mice immunized to Tg or Tg with anti‐PD‐1 antibody (Figure S3B, middle and right, respectively), but no staining was observed in the control mouse (Figure S3B, left). Thyroid‐infiltrating mononucleocytes were mildly stained with anti‐CD19 antibody (mostly B‐cells) in mice immunized to Tg or Tg with anti‐PD‐1 antibody (Figure S3C, middle and right, respectively), but no staining was observed in the control mouse (Figure S3C left). Thyroid‐infiltrating mononucleocytes were mildly stained with anti‐CD11c antibody (mostly dendritic cells) in mice immunized to Tg or Tg with anti‐PD‐1 antibody (Figure S3D, middle and right, respectively), but no staining was observed in the control mice (Figure S3D left).
4. DISCUSSION
Multiple novel biomarkers for thyroid irAE were found in the clinical study, and are summarized in Table 3. Moreover, deterioration of mice thyroiditis by anti‐PD‐1 antibody was seen in the in vivo study.
Table 3.
Summary of the biomarkers of thyroid irAE identified in the study
| Factors significantly increased 4 wk after initial ICI treatment in thyroid irAE |
| Tg, TgAb, TPOAb |
| Factors significantly higher at baseline in thyroid irAE |
| IL‐1β, IL‐2, GM‐CSF |
| Factors significantly decreased 4 wk after initial ICI treatment in thyroid irAE |
| IL‐8, G‐CSF, MCP‐1 |
Abbreviations: G‐CSF, granulocyte colony‐stimulating factor; GM‐CSF, granulocyte–macrophage colony‐stimulating factor; MCP‐1, monocyte chemoattractant protein; Tg, thyroglobulin; TgAb, anti‐thyroglobulin autoantibody; TPOAb, anti‐TPO autoantibody.
Notably, early increase in serum Tg levels could be very useful for prediction of thyroid irAE. Tg is a thyroid‐specific protein produced by thyrocytes.15 Due to inflammation in the thyroid, in cases with thyroiditis including those induced by ICIs, leakage of Tg may occur. Thus, the early increase of serum Tg seemed to be associated with thyroid irAE.15 Additionally, early increase of TgAb and TPOAb also seemed to be associated with thyroid irAE. It was previously reported that either high titer of TgAb or TPOAb prior to ICI therapy appeared to be related to thyroid irAE.4, 6, 9 Similar to those reports, baseline positivity of thyroid autoantibodies was seen in the current three patients with persistent moderate hypothyroidism. The greater overall cytokines/chemokines levels of BT in IR than those in non‐IR group may suggest activation of immunity at basal level in IR group, and thus may be related to the development of thyroid irAE. The reduction of overall ratio of AT/BT in IR group suggested that ICIs bound to the PD‐1 or CTLA‐4 molecules on the surface of T‐cell might subsequently inhibit production of the cytokines/chemokines. Comparison of BT and AT of cytokines/chemokines in the two groups revealed that significantly higher levels of pretreatment IL‐2 were associated with thyroid irAE. IL‐2 stimulates CD8+ T‐cells and thus is used as a cancer immunotherapy,16 and increase of IL‐2 was reported to be an indicator of anti‐tumor activity.11 During the use of IL‐2, thyroid dysfunction occurs (thyrotoxicosis, 0%‐7%; hypothyroidism, 35%‐47%, and positivity of thyroid autoantibody, 10%‐60%) possibly because IL‐2‐activated CD8+ T‐cells attack not only tumor cells, but also thyroid cells.17 IL‐2 stimulates secretions of IL‐1β, TNF‐α, and IFN‐γ, and levels of those cytokines also increased in the current study, suggesting Th1 predominance.17 Since such a Th1‐skewed balance is also observed in cases of Hashimoto’s thyroiditis (HT),15 higher levels of IL‐2 at baseline may be related to the development of thyroid irAE. In addition, early reduction of MCP‐1, a Th2 chemokine in the IR group suggested increased Th1/Th2 balance, which is consistent with the above findings. Increase of IL‐1β has also been reported to increase the risk of HT.18 Interestingly, administration of GM‐CSF was reported to induce autoimmune hypothyroidism; this was observed in higher baseline levels of GM‐CSF in our study.19 Four wk after the first ICI treatment, levels of IL‐8 were significantly decreased in the IR group. It is interesting that previous reports mentioned that early decrease of IL‐8 was related to favorable prognosis of malignant melanoma with anti‐PD‐1 therapy.20 Solid evidence supports a tumor‐promoting role of IL‐8 in several human cancers.11, 21 However, the role of serum levels of IL‐8 in patients with autoimmune thyroid diseases remain unclear.22 Yamazaki, et al reported that higher baseline levels of IL‐6, IL‐10, and IFN‐γ were better prognosis markers of malignant melanoma (MM) with anti‐PD‐1 therapy, and those cytokines were similarly higher in our study.23 Increased levels of G‐CSF, GM‐CSF, IL‐12, IL‐1β, IL‐2, and IL‐13 in the IR group were further consistent with findings in a previous report of patients with irAEs.11 Therefore, thyroid irAE may have a similar mechanism to other organ irAEs.
In the correlation analysis of early change of each factor, a regulatory T‐cell cytokine, IL‐10, was negatively associated with TPOAb. Therefore, the reduction of IL‐10 might be associated with TPOAb increase, suggesting development of autoimmunity in thyroid irAE.
We previously reported that thrombocytopenia might be a possible biomarker for thyrotoxicosis with decreased TSH.8 Assuming that the decrease of IL‐10 and also of TSH may relate to decrease of platelet count, the early decrease in platelet count may predict thyroid irAE.
Considering the strong positive correlation of G‐CSF and IL‐4/IL‐5/IL‐13, the reduction of G‐CSF might be related to the reduction of Th2 cytokine activities, which also suggest increasing Th1/Th2 balance in thyroid irAE.
In addition to the predictive factors mentioned above, human leukocyte antigen (HLA) molecules may play an important role in the development of thyroid irAE. HLA‐DR3 has been reported as a major predisposing allele for autoimmune thyroid diseases.24 Thyroid autoantigen as well as tumor‐associated antigen/neoantigen could be presented with HLA on the surface of antigen‐presenting cells. Pathogenic T‐cells recognize the epitope and immune reactions develop. Vita et al25 reported homology of tumor‐associated antigen NY‐ESO‐1 with thyroid by anti‐PD‐1 antibody, anti‐PD‐1 antibody might strengthen the B‐ and T‐cell immunity in the autoantigens in panels of HLA class I and class II binding motifs. Assuming that the deterioration of mouse EAT occurred thyroid gland. Therefore, mechanisms of thyroid irAE can be partly explained by cross presentation of thyroid autoantigen and tumor‐associated antigen/neoantigen.
Several wk after immunization of hTg, EAT was induced, and anti‐PD‐1 antibody strengthened the thyroiditis. Serum mouse Tg levels and anti‐hTg antibody titers were also elevated in EAT mice, and anti‐PD‐1 antibody significantly strengthened those. Assuming that Tg levels and TgAb titers were increased in patients with thyroid irAE 4 wk after administration of anti‐PD‐1 antibody, anti‐PD‐1 antibody may deteriorate EAT, and also play an important role in the development of human thyroid irAE.
In the current EAT mice immunized to Tg or Tg with anti‐PD1 antibody, thyroid‐infiltrating mononuclear cells appeared to consist of dendritic cells (CD11c+), B‐cells (CD19+). T‐cells (CD3+) were slightly less. We speculated that antigen presentation and antibody production might be important rather than T‐cell‐mediated immunity in the current EAT mice model. Similarly, Zha et al26 reported B‐cells predominance in the mononucleocytes infiltration seen in human chronic thyroiditis, however, further investigations are required to determine histological feature of ICI‐induced thyroiditis in human or mice. Interestingly, it was reported that B‐cells and tertiary lymphoid structures were also seen in the malignant tumors of the responders during ICIs treatment.27
Positive relation of incidence of thyroid irAE and feasible outcome of lung cancer was reported.10 The factors found in the current study (Table 3) might be surrogate biomarkers for tumor therapy. However, since our study consists of small numbers and mixed tumors, further studies are needed to establish positive correlation between the incidence of thyroid irAE and anti‐tumor activities. Moreover, the differences between the patients with spontaneous resolution of thyroid irAE and those in whom it persisted remain unclear.
In conclusion, early change in Tg levels might indicate development of thyroid irAE, and pre‐existing and early change of thyroid autoimmunity were associated with the development of thyroid irAE, and. In addition, higher levels of serum IL‐1β, IL‐2, and GM‐CSF at baseline, and early decrease of IL‐8, G‐CSF, and MCP‐1 were found to be important in thyroid irAE.
CONFLICT OF INTEREST
Hiroaki Akamatsu received lecture fees from AstraZeneca, Eli Lilly, Chugai Pharmaceutical Co, Ltd., and Taiho Pharmaceutical, and received research funds from MSD KK. Isao Hara received scholarship endowments from Ono Pharmaceutical. Takashi Akamizu received scholarship endowments from Ono Pharmaceutical. The remaining authors declare no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
A written informed consent was obtained from all patients. This study was approved by the Wakayama Medical University Hospital Ethics Committee (No. 1987). All the animal procedures were performed according to the guidelines of the Wakayama Medical University Ethics Committee for animal welfare and the Japan Ministry of Health.
Supporting information
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3A
Table S3B
ACKNOWLEDGMENTS
We are indebted to Dr. Professor Nobuyuki Yamamoto (Department of Respiratory Medicine and Clinical Oncology), Dr. Yuko Ishida and Dr. Professor Toshikazu Kondo (Department of Forensic Medicine), and Dr. Yusuke Yamashita and Dr. Shinobu Tamura (Department of Hematology) at Wakayama Medical University for technical assistance. We also thank members of The First Department of Medicine, Department of Dermatology, Department of Urology, Second Department of Surgery, and Department of Respiratory Medicine and Clinical Oncology, Wakayama Medical University for cooperating on the research. We acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center, Wakayama Medical University. This work was partially supported by Takeda Science Foundation, and Grants‐in‐Aid for Scientific Research 26461385.
Kurimoto C, Inaba H, Ariyasu H, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune‐checkpoint inhibitors. Cancer Sci. 2020;111:1468–1477. 10.1111/cas.14363
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blank CU, Enk A. Therapeutic use of anti‐CTLA‐4 antibodies. Int Immunol. 2015;27:3‐10. [DOI] [PubMed] [Google Scholar]
- 3. Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813‐824. [DOI] [PubMed] [Google Scholar]
- 4. Inaba H, Ariyasu H, Okuhira H, et al. Endocrine dysfunctions during treatment of immune‐checkpoint inhibitors. Trends. Immunotherapy. 2018;2:1‐5. 10.24294/ti.v2i2.606 [DOI] [Google Scholar]
- 5. Shiba M, Inaba H, Ariyasu H, et al. Fulminant type 1 diabetes mellitus accompanied by positive conversion of anti‐insulin antibody after the administration of anti‐CTLA‐4 antibody following the discontinuation of anti‐PD‐1 antibody. Intern Med. 2018;57:2029‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inaba H, Ariyasu H, Takeshima K, Iwakura H, Akamizu T. Comprehensive research on thyroid diseases associated with autoimmunity: autoimmune thyroid diseases, thyroid diseases during immune‐checkpoint inhibitors therapy, and immunoglobulin‐G4‐associated thyroid diseases. Endocr J. 2019;66:843‐852. [DOI] [PubMed] [Google Scholar]
- 7. Inaba H, Ariyasu H, Iwakura H, et al. Comparative analysis of HLA between idiopathic and Anti‐PD‐1 antibody induced Isolated ACTH deficiency: a pilot study. Clin Endocrinol (Oxf). 2019;91;786‐792. 10.1111/cen.14082 [DOI] [PubMed] [Google Scholar]
- 8. Ariyasu H, Inaba H, Ota T, et al. Thyrotoxicosis and adrenocortical hormone deficiency during immune‐checkpoint inhibitor treatment for malignant melanoma. Vivo. 2018;32:345‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arima H, Iwama S, Inaba H, et al. Management of immune‐related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J. 2019;66:581‐586. [DOI] [PubMed] [Google Scholar]
- 10. Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune‐related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14:e0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune‐related toxicity in melanoma patients receiving anti‐PD‐1‐based immunotherapy. Clin Cancer Res. 2019;25:1557‐1563. [DOI] [PubMed] [Google Scholar]
- 12. Kong YC, Lomo LC, Motte RW, et al. HLA‐DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA‐DRB1*0301 (DR3) gene. J Exp Med. 1996;184:1167‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guimaraes VC, Quintans J, Fisfalen ME, et al. Suppression of development of experimental autoimmune thyroiditis by oral administration of thyroglobulin. Endocrinology. 1995;136:3353‐3359. [DOI] [PubMed] [Google Scholar]
- 14. Terawaki S, Chikuma S, Shibayama S, et al. IFN‐α directly promotes programmed cell death‐1 transcription and limits the duration of T cell‐mediated immunity. J Immunol. 2011;186:2772‐2779. [DOI] [PubMed] [Google Scholar]
- 15. Akamizu T, Amino N. Hashimoto’s thyroiditis In: Feingold KR, Anawalt B, Boyce A, et al., Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2000‐2017. July 17. PMID: 25905412 [Google Scholar]
- 16. Jiang T, Zhou C, Ren S. Role of IL‐2 in cancer immunotherapy. Oncoimmunology. 2016;25(5):e1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torino F, Barnabei A, Paragliola R, Baldelli R, Appetecchia M, Corsello SM. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid. 2013;23:1345‐1366. [DOI] [PubMed] [Google Scholar]
- 18. Sun L, Zhang X, Dai F, et al. Elevated interleukin‐1β in peripheral blood mononuclear cells contributes to the pathogenesis of autoimmune thyroid diseases, especially of Hashimoto thyroiditis. Endocr Res. 2016;41:185‐192. [DOI] [PubMed] [Google Scholar]
- 19. Hoekman K, Wagstaff J, Pinedo HM, von Blomberg‐van der Flier BM, Drexhage HA. Reversible thyroid dysfunction during treatment with GM‐CSF. Lancet. 1991;338:541‐542. [DOI] [PubMed] [Google Scholar]
- 20. Sanmamed MF, Perez‐Gracia JL, Schalper KA, et al. Changes in serum IL‐8 levels reflect and predict response to anti‐PD‐1 treatment in melanoma and non‐small‐cell lung cancer patients. Ann Oncol. 2017;28:1988‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kantola T, Klintrup K, Väyrynen JP, et al. Stage‐dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rotondi M, Coperchini F, Chiovato L. CXCL8 in thyroid disease: from basic notions to potential applications in clinical practice. Cytokine Growth Factor Rev. 2013;24:539‐546. [DOI] [PubMed] [Google Scholar]
- 23. Yamazaki N, Kiyohara Y, Uhara H, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017;108:1022‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inaba H, De Groot LJ, Akamizu T. Thyrotropin receptor epitope and human leukocyte antigen in Graves' disease. Front Endocrinol (Lausanne). 2016;7;1‐9. 10.3389/fendo.2016.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vita R, Guarneri F, Agah R, Benvenga S. Autoimmune thyroid disease elicited by NY‐ESO‐1 vaccination. Thyroid. 2014;24:390‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zha B, Huang X, Lin J, Liu J, Hou Y, Wu G. Distribution of lymphocyte subpopulations in thyroid glands of human autoimmune thyroid disease. J Clin Lab Anal. 2014;28:249‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3A
Table S3B
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


